Chapter 183 Management of Occult Spinal Dysraphism in Adults
Embryologic Considerations
Differentiating the extremes of normal from subtle pathologic states is one of the most difficult aspects of managing patients who are at risk for TCS. When TCS was first described, clinically devastated patients with extreme caudal displacement of the conus medullaris into the sacrum from a thickened and unyielding filum terminale were easily differentiated from normal.1,2 However, the widespread use of magnetic resonance imaging (MRI) has allowed physicians to attempt to define a “normal” level of the conus medullaris in an adult. For some clinicians, below the L1–2 disc space is abnormally low, whereas for others, below the inferior border of L2 is abnormally displaced. This definition is confounded by the fact that in some cases, the conus medullaris, which normally has an anteroposterior diameter ranging from 5.0 to 8.0 mm, gently tapers into a very thick filum rather than demonstrating an obvious caudal tip.
The filum terminale and the distal conus medullaris are thought to arise through the process of secondary neurulation. Distally, the intradural filum terminale travels to fuse with the dorsal dura mater, usually in the midline, and then continues within a dural sheath as the coccygeal ligament to the dorsal coccyx. Historically, in the term infant, the inferior tip of the conus medullaris was described as being located at the L2–3 interspace in 98% of cases and at the L3 level in 1.2% of cases. Presumably, by 3 months of age the tip of the conus achieved its adult position by “ascending” to the L1–2 interspace. However, Wilson and Prince3 concluded that a conus positioned at L2–3 should be considered normal at any age. Reimann and Anson4 compiled both their data and those from three other large series (a total of 801 adult spinal cords) to find that the conus medullaris is found above the L2–3 disc level in approximately 94% of cases, whereas the mean conus lies at the lower third of the L1 vertebra. Saifuddin and colleagues5 have also found that the mean termination of the conus is at the lower third of the L1 vertebra. It would be logical then to assume that tight fixation of the cord by the filum during in utero development would result in the most caudal position of the conus but that lesser degrees of tension would allow some cephalad migration. It might even be possible that mild tension on a relatively elastic cord could result in a relatively normal position of the conus. In such a case, symptoms, possibly secondary to persistent microtrauma, might not occur until adult life.
Presentation and Physical Examination
Although they harbor similar, or identical, pathology, adults with OSD tend to present with a different array of signs and symptoms than children. In children, cutaneous stigmata such as focal hypertrichosis, pigmented nevi, subcutaneous lumbosacral masses, and skin dimples are often observed. However, in the adult OSD population, these signs are present in only 36% of cases.6 Similarly, foot deformities are found in only 37% of adult patients.6
In the adult population, there is a slight female preponderance of congenital TCS, with ratios of 1:1.3 to 1:2.6, and the mean age at presentation is approximately 37 years.7–10 Although there is no universally accepted classification system that stratifies adults with TCS, van Leeuwen and colleagues10 proposed that the origin of tethering might reasonably correlate with surgical outcomes and should be taken into account during surgical planning. Lee and colleagues11 found satisfactory concordance between their experience and this classification scheme. Van Leeuwen’s scheme included four groups of TCS: post-repair myelomeningocele, tight filum terminale or filum terminal lipoma, Conus lipoma/lipomyelomeningocele, and split cord malformation (SCM).
The most common presenting complaint in adults with OSD is pain, which is encountered in as many as 80% of patients.6 The most common type of pain is insidious nonradicular back pain.6,9,12,13 A thorough history might reveal that this pain has been present since childhood. Pang and Wilberger9 proposed three pathophysiologic mechanisms that led to the acute presentation of symptoms in 61% of their adult population with TCS. These mechanisms were transient stretching of the conus medullaris, narrowing of the spinal canal due to degenerative disease, and direct trauma. The added ischemia or mechanical distortion associated with these events is postulated to reveal symptoms in an already injured or stressed caudal spinal cord.
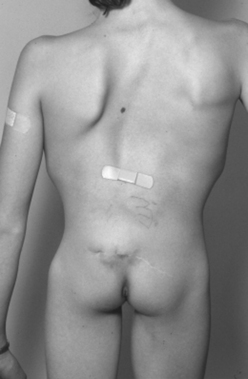
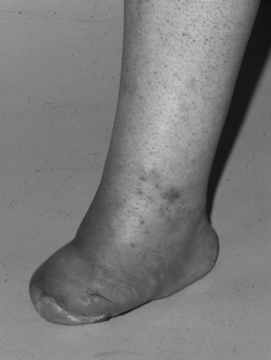
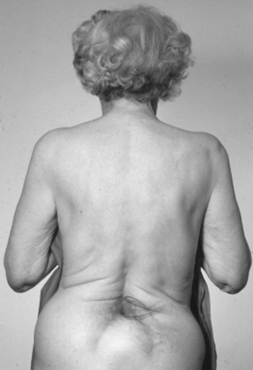
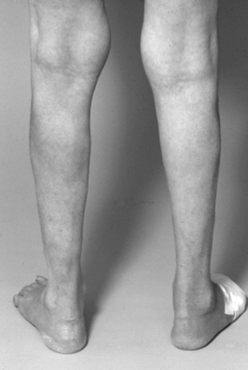
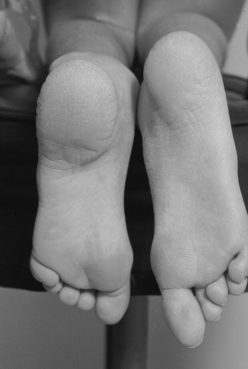
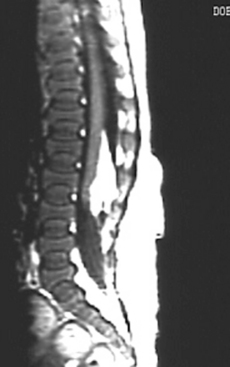
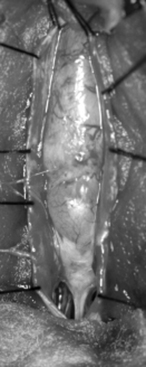
Adults also present with lower extremity symptoms, most commonly pain or weakness, or both. The weakness that results from distal spinal cord fixation is classically associated with both upper and lower motor neuron signs. Less common presenting signs and symptoms include changes in bowel and bladder function (approximately 69%6), scoliosis (8% to 31%,6,11 Fig. 183-1), and loss of lower extremity sensation resulting in foot ulceration (Fig. 183-2).
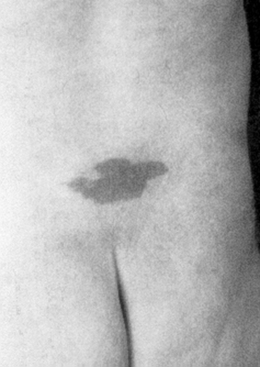
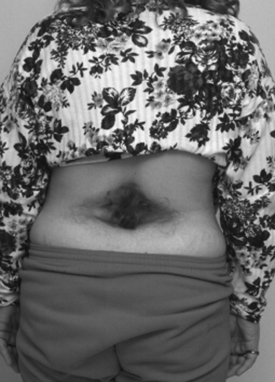
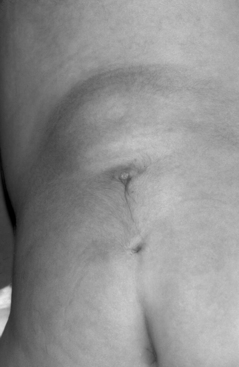
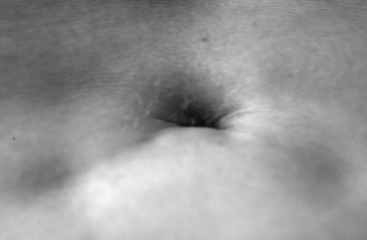
The physical examination findings that can be expected in a patient with TCS can be divided into cutaneous lesions, lower extremity findings, and changes in rectal tone. Although they may be encountered in the adult, the most telling findings (e.g., cutaneous stigmata and lower extremity asymmetry) are more common in the pediatric population. The cutaneous signatures of OSD include midline capillary hemangioma, subcutaneous lipoma, dermal sinus tract, caudal appendage, focal hirsutism, and atretic meningocele (Figs. 183-3 through 183-8). Cutaneous hemangiomas that may be common in other parts of the body are unusual in the lumbosacral region (see Fig. 183-3). In this area, especially in the midline, they have been associated with OSD and are often found in conjunction with other skin findings, such as focal hirsutism and subcutaneous lipomas. However, capillary hemangiomas are thought to be the least sensitive indicator of intradural pathology, with an approximately 10% incidence of associated intradural anomalies when seen in the lumbar region.14 Focal hirsuitism (see Fig. 183-4) should alert the clinician to the possibility of an underlying SCM or atretic meningocele (see Fig. 183-5).

FIGURE 183-5 A female patient with two cutaneous signatures. Note the area of hypertrichosis and a centered atretic meningocele.
Typical lower extremity findings result from a combination of upper and lower motor neuron weakness in the legs without upper extremity symptoms. Such findings can manifest as decreased muscle bulk (Fig. 183-9) with increased or pathologic reflexes, and should alert the examiner to the possibility of TCS. Other findings include progressive arching of the feet and lower extremity size discrepancies (Fig. 183-10). The clinician should evaluate for hypalgesia of the lower extremities in patients with potential TCS. This sensory disturbance is often found in a patchy distribution.
Radiographic Assessment
Computed Tomography
CT offers greater detail regarding any osseous abnormalities and is generally indicated before surgical intervention, especially in the adult population where the diagnosis might not be straightforward. In certain cases, CT myelography may be indicated, because it can provide greater anatomic detail in cases of SCM.
Pathologic Subtypes of Occult Spinal Dysraphism
Tight and Fatty Filum Terminale
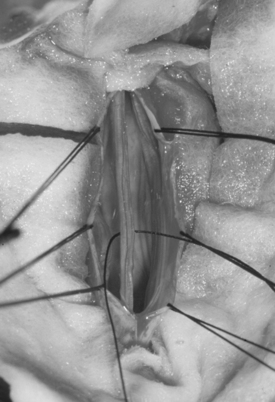
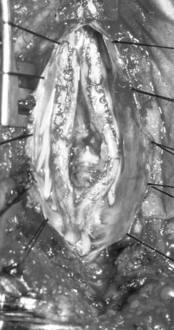
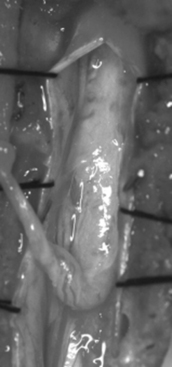
The filum terminale is a structure usually less than 2 mm in diameter. If it is insufficiently elastic (e.g., abnormally thick or fat laden), this can limit the ability of the spinal cord to move cephalad with growth or movement, placing stress on the distal aspect of the conus medullaris (Figs. 183-11 to 183-13). Terminal syrinx, neurenteric cyst, dermal sinus tract and intramedullary dermoid, and SCM can all have an associated tight filum terminale, which must be sought at the time of surgical exploration.1,2,15,16 Lipomatous infiltration ranges from small accumulations of fat in an otherwise normal filum to extensive involvement of the distal spinal cord. Incidental fat within the filum is seen in approximately 3.7% to 17% of the normal adult population.17 In one series, fat was found in 91% of patients with a tethered spinal cord.18 The lack of denticulate ligaments inferior to the origin of the T12 nerve allows relative mobility when caudally directed force is present in this region. Moreover, we have found that the denticulate ligaments, even cephalically, do little to abort either cranial or caudal traction on the spinal cord.19 By definition, the tight or fatty filum terminale originates below the level of the lowest nerve root origins. Additionally, this entity is not associated with a dorsal dural defect. These characteristics decrease the risk of neurologic injury or CSF leak when surgical detethering is performed.
Split Cord Malformation
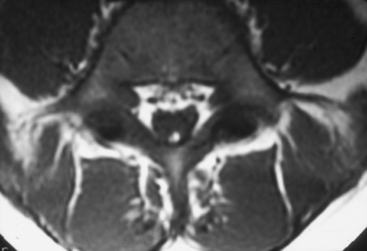
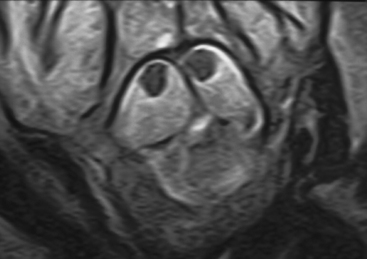
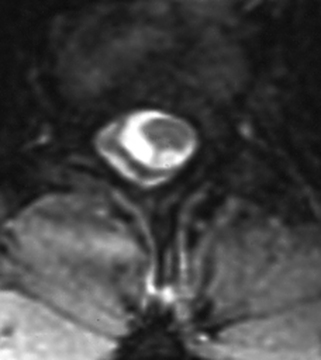
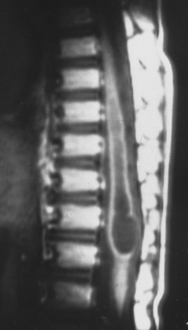
Current nomenclature divides SCMs into types I and II.20,21 The correlation between hypertrichosis and SCM is probably higher than any other single or combined cutaneous abnormality and underlying spinal cord lesion. The two dominant radiographic characteristics of a type I lesion are double dural tubes and an osseous or osseocartilaginous midline septum (Figs. 183-14 and 183-15). The type I septum almost always extends from the posterior surface of the vertebral body to the corresponding neural arch. Rostral to the septum, the hemicords may be separate for as many as seven vertebral levels. In this region, the two hemicords reside within a single thecal sac. The type II SCM is characterized by a single thecal sac.21 The cord may be tethered by nonfunctional nerve roots exiting from the medial aspect of either hemicord or by nonosseous median septum.
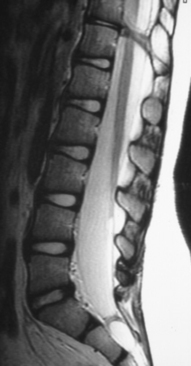
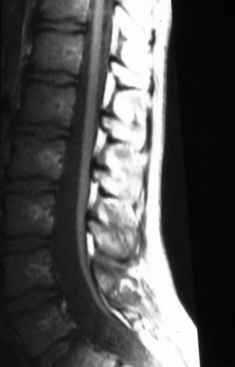
FIGURE 183-15 T2-weighted sagittal magnetic resonance imaging of the lumbosacral spine of the patient described in Figure 183-14. Note the bony septation dividing the spinal canal into two compartments.
Between type I and II SCMs, there is a certain degree of crossover regarding these two features. Thus the SCM cannot always be classified radiographically before surgery. When discernible, the type II septum is usually at the caudal extreme of the split cord, where the hemicords appear to be closely opposed with the septum and with each other before rejoining into a single structure. In general, the hemicords in a type II lesion are much closer together and the split segment is much shorter than in a type I lesion.
At least one unrelated tethering lesion (i.e., fatty filum) is found in many lumbosacral and lower thoracic SCMs and in a much smaller number of cervical SCMs. The entire neural axis should be studied radiographically and if a second tethering lesion is found, it should be considered for treatment.7 The surgical outcome is generally excellent for SCM. The overall surgical morbidity is low but slightly higher with type I lesions (Fig. 183-16). The radiographic data on the split lengths when correlated with age, lesion type, and level of the septum are compatible with the hypothesis of an endomesenchymal tract transfixing the neural plate during early development of the neural tube.21
Lipomyelomeningocele
The name lipomyelomeningocele emphasizes the two key characteristics of this lesion, which are adipose tissue within and/or adjacent to the spinal cord and an adjacent dural defect. Because of pathoanatomic variability, the literature classifying spinal lipomas includes multiple methods.22–24 Chapman initially divided spinal lipomas into three categories and Pang further described them as dorsal, transitional, and caudal (terminal).24,25 Dorsal lipomas are unique in that the insertion of the fibroadipose stalk into the spinal cord occurs in an isolated segmental region, with normal cord and dura both above and below the lesion.25 The rostral portion of the fibroadipose stalk of transitional lipomas inserts onto and blends with the dorsal surface of the conus medullaris (Fig. 183-17). Unlike the dorsal lipoma, however, the transitional lipoma remains intercalated with the nervous tissue all the way to the terminal aspect of the conus, where it joins with the filum, if present. Thus there is no normal spinal cord distal to the lipoma. The conus is commonly twisted within the caudal dural sac, which is often underdeveloped, infiltrated by fat, or frankly absent, complicating surgical closure. These lesions, therefore, present a unique surgical challenge and demand familiarity with the relevant anatomy. Terminal lipomas differ from dorsal and transitional lipomas by inserting into the caudal extremity of the conus or by replacing the filum without any direct infiltration of the spinal cord. There is no blending with the dorsal surface of the spinal cord. All sacral nerve roots leave the conus rostral to the lipoma. Almost invariably, the dura and overlying myofascial layers are intact.
With the exception of terminal lipomas, patients with lipomyelomeningocele almost always have a cutaneous abnormality present from birth and therefore tend to come to clinical attention before adulthood (see Fig. 183-7). There is a female-to-male predominance of approximately 1.5:1.
When patients do not come to medical attention before adulthood, they most commonly present with insidious but progressive signs or symptoms. A small group, however, presents with acute symptoms, usually weakness or urinary incontinence. These are often not reversed with surgical intervention. Symptoms resulting from neurogenic bladder often take the form of incontinence or repeated bouts of urinary tract infection. Rectal incontinence does not occur without accompanying urinary incontinence. Other previously described signs and symptoms also manifest as a result of lipomyelomeningocele.
Neurenteric Cyst
Spinal neurenteric cysts are infrequently reported congenital abnormalities believed to be derived from an abnormal connection between the primitive endoderm and ectoderm. Neurenteric cysts are not confined to the spinal column (Figs. 183-18 through 183-20) but may be found within the brain, mediastinum, abdomen, or pelvis or even in a subcutaneous location. Intraspinal neurenteric cysts represent 0.3% to 0.5% of all spinal “tumors.”26 They are, in fact, not tumors, which differentiates them from teratomas. Instead, these lesions are more similar to hamartomas, that is, displaced nests of endodermally derived tissue. The terminology for these lesions is problematic, because they have been reported as neurenteric cysts, enterogenous cysts, enteric cysts, gastrocytomas, dorsal enteric fistulas, split notochord syndrome, and teratoid cysts. Part of the challenge in naming these cysts is that they are not uniform, but instead likely represent a spectrum of lesions. A consistent association between neurenteric cysts and other forms of OSD has not been reported. A neurenteric cyst is classically reported as a solitary intradural, extramedullary lesion in the cervical region, located anterior or anterolateral to the spinal cord. The second most common location is thoracic, followed by lumbosacral. Osseous abnormalities, if present, are likely to involve the anterior spinal column. There is a male predominance of approximately 2:1. The most challenging technical problem for the surgeon results from the obligation to remove the entire wall of the cyst to prevent its reaccumulation.26
Dermal Sinus Tract/Dermoid
Dermal sinus tracts and associated dermoid tumors represent a common source of TCS in the pediatric population but are exceedingly rare in adults. In some cases, however, a patient has an incompletely treated DST that becomes symptomatic during adulthood. Therefore, symptoms consistent with TCS with a history of a “minor” surgical procedure during childhood should alert the clinician to the possibility of an incompletely treated DST or dermoid. Dermal sinus tracts can be encountered from the tip of the nose to the coccyx. In children, these entities are seen most commonly in the lumbosacral region (see Fig. 183-8), although many are appreciated in the occipital area. Cranial dermal sinus tracts normally travel inferiorly toward the deeper neural structures, whereas lumbosacral tracts ascend from their superficial origin to their deeper destination. Coccygeal tracts or “pits” end before the thecal sac and are regarded as innocent. An approximate incidence of coccygeal pits is 4% of the neonatal population27 and that of DST has been estimated at 1/2500 births.28 Although seemingly benign structures, these small communications between the superficial and deep derivatives of the ectoderm can result in tragic insult to the central nervous system if not adequately addressed. Perhaps reflecting a common ontogenic disorder, dermal sinus tracts are seen in 15% to 40% of split cord malformations. Approximately 60% of these tracts violate the spinal subarachnoid space and, of these, approximately one half attach to the conus medullaris, cauda equina, or filum terminale (Fig. 183-21). The literature supports a small male predominance. Connections between the skin and underlying structures usually occur in the midline but rarely are found in a paravertebral location. Common cutaneous findings seen in conjunction with dermal sinus tracts include flat capillary hemangiomas, focal hirsutism, and subcutaneous lipomas. In children, signs of local infection and/or drainage of debris or fluid from the surface of a tract should be considered suspicious for a DST.7,14
Meningocele Manqué
Tethering bands from the spinal cord to the surrounding tissue, known as meningocele manqué, are a rare anomaly. Meningocele manqué is found in association with multiple other elements of spinal dysraphism, most classically with SCM and myelomeningocele. In both of these cases, the bands must be divided in order to complete detethering. The bands themselves are generally composed of nonfunctional neural tissue with dorsal root ganglion cells. They often penetrate the dura of an associated bony median septum or simply exit dorsally or, rarely, ventrally. On occasion, they pierce the dura and terminate on the undersurface of the lamina. The surgeon should be aware of this condition when removing lamina during an operation on a patient with OSD so as to not inadvertently put tension on the underlying cord or cauda equina. Meningocele manqué may be identified with MRI (Fig. 183-22) (linear and isointense with the spinal cord on T1-weighted images) but is often not appreciated owing to the small size of the bands. The bands are, however, easily demonstrable at operation.
Terminal Syrinx
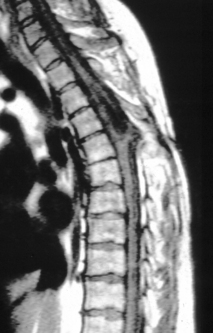
The term terminal syrinx denotes a cavity found within the distal third of the spinal cord (Fig. 183-23). A terminal syrinx is seen in 10% to 30% of patients with TCS and is usually a secondary result of other pathologic processes causing TCS. Symptoms resulting from the syrinx, however, can bring the patient to medical attention before those resulting directly from the primary lesion. Although most classically seen with a syrinx, any intramedullary spinal cord lesion can interrupt or stretch crossing spinothalamic fibers in the anterior commisure of the spinal cord. This results in a dissociated and suspended sensory loss, particularly with diminished pain and temperature appreciation and preservation of position sense and light touch, because the latter fibers do not cross in the spinal cord.
Conus in “Normal” Position with Symptoms of a Tethered Cord
Under most circumstances, the term tethered spinal cord implies an abnormally positioned conus medullaris. As discussed earlier, the inferior limit considered normal is not universally agreed upon. In the adult population, there is not a significant literature discussing patients who present with symptoms and signs consistent with TCS but imaging demonstrating the conus medullaris to be at a position accepted as “normal.” Several authors have established the presence of this entity in the pediatric population. 29–32 Multiple series have shown that incontinence in children who have essentially normal MRI scans of the lumbosacral region and usually have cystometrographic findings indicating a neurogenic bladder (hyperreflexic detrusor muscle) can be successfully treated by sectioning of the filum terminale.29–31,33–35 We strongly recommend that clinicians consider not only imaging findings but also clinical history, physical examination, and physiologic tests (e.g., urodynamics) when treating adult and pediatric patients who might have TCS.
Surgical Decision Making In Adult Tethered Cord Syndrome
In the pediatric population, surgical intervention for the treatment of OSD is widely accepted. There is no established consensus regarding the role of prophylactic surgery for adults with OSD. However, as demonstrated by Rajpal and colleagues,13 adults typically present with progressive symptoms that cannot be expected to abate in the absence of surgical intervention. Although progressive pain or neurologic deficits are indications for surgical intervention in the context of a suspected tethered spinal cord, some adult patients choose not to pursue surgical treatment.12 The natural history of this subpopulation is not well defined.
The use of intraoperative physiologic monitoring in the adult TCS population has been promoted in multiple reports.36–38 The combination of somatosensory evoked potentials with continuous and evoked electromyography can provide a high degree of sensitivity and specificity for identifying intraoperative neurologic injury during complex tethered cord release in adults37 and should be considered. The use of a synthetic or autologous dural graft is left to the discretion of the surgeon. We favor a split lumbodorsal fascia graft, which is readily available in almost all patients.
Surgical Outcome
The likelihood of significant reversal of lost neurologic function in adults with TCS is not entirely predictable, but in general, longer duration and greater severity of a deficit are associated with a lower likelihood of postoperative resolution. Overall, rates of surgical success are comparable to those reported in pediatric series.8 Recent literature reviews by Aufschnaiter and colleagues6 and Lapsiwala and Iskandar8 demonstrated that pain improved in 81% to 89% of patients and stabilized in an additional 15% to 17%. Sensorimotor symptoms improved in approximately 50% of patients and stabilized in 25% to 50%. With regard to back pain specifically, reported rates of postoperative improvement range from 50% to 83%.9,12,13 Although improvement in bowel and bladder function is unpredictable,6,39 reported rates are as high as 38.5%.9 Worse outcomes have been associated with SCM and lipomyelomeningocele.6,10 Consistent with previous findings, patients with a longer duration of symptoms, previous surgery, or rapidly progressive motor weakness also had worse outcomes. There is a nontrivial rate of symptom recurrence following an initial response to surgery, with rates ranging from 5% to 17%, depending upon the specific symptom.13
The most common surgical complication is postoperative CSF leak, with reported rates ranging from 7.5% to 12%6,10,39 Rates of postoperative neurologic deterioration range from 2.2% to 4.5%6,37 and overall complication rates are approximately 11.5%.6
Aufschnaiter K., Fellner F., Wurm G. Surgery in adult onset tethered cord syndrome (ATCS): review of literature on occasion of an exceptional case. Neurosurg Rev. 2008;31:371-383. discussion 384
Chapman P.H. Congenital intraspinal lipomas: anatomic considerations and surgical treatment. Childs Brain. 1982;9:37-47.
Iskandar B.J., Fulmer B.B., Hadley M.N., Oakes W.J. Congenital tethered spinal cord syndrome in adults. Neurosurg Focus. 2001;10:e7.
Lee G.Y., Paradiso G., Tator C.H., et al. Surgical management of tethered cord syndrome in adults: indications, techniques, and long-term outcomes in 60 patients. J Neurosurg Spine. 2006;4:123-131.
Pang D. Spinal cord lipomas. In: Pang D., editor. Disorders of the Pediatric Spine. New York: Raven Press; 1995:175-201.
Pang D. Split cord malformation: Part II: clinical syndrome. Neurosurgery. 1992;31:481-500.
Pang D., Dias M.S., Ahab-Barmada M. Split cord malformation: Part I: a unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 1992;31:451-480.
Pang D., Wilberger J.E.Jr. Tethered cord syndrome in adults. J Neurosurg. 1982;57:32-47.
Rajpal S., Tubbs R.S., George T., et al. Tethered cord due to spina bifida occulta presenting in adulthood: a tricenter review of 61 patients. J Neurosurg Spine. 2007;6:210-215.
1. Garceau G.J. The filum terminale syndrome (the cord-traction syndrome). J Bone Joint Surg Am. 1953;35:711-716.
2. Jones P.H., Love J.G. Tight filum terminale. AMA Arch Surg. 1956;73:556-566.
3. Wilson D.A., Prince J.R. John Caffey award. MR imaging determination of the location of the normal conus medullaris throughout childhood. AJR Am J Roentgenol. 1989;152:1029-1032.
4. Reimann A., Anson B.J. Vertebral level of termination of the spinal cord with report of a case of sacral cord. Anat Rec. 1944;88:127-138.
5. Saifuddin A., Burnett S.J., White J. The variation of position of the conus medullaris in an adult population. A magnetic resonance imaging study. Spine (Phila Pa 1976). 1998;23:1452-1456.
6. Aufschnaiter K., Fellner F., Wurm G. Surgery in adult onset tethered cord syndrome (ATCS): review of literature on occasion of an exceptional case. Neurosurg Rev. 2008;31:371-383. discussion 384
7. Iskandar B.J., Fulmer B.B., Hadley M.N., Oakes W.J. Congenital tethered spinal cord syndrome in adults. Neurosurg Focus. 2001;10:e7.
8. Lapsiwala S.B., Iskandar B.J. The tethered cord syndrome in adults with spina bifida occulta. Neurol Res. 2004;26:735-740.
9. Pang D., Wilberger J.E.Jr. Tethered cord syndrome in adults. J Neurosurg. 1982;57:32-47.
10. van Leeuwen R., Notermans N.C., Vandertop W.P. Surgery in adults with tethered cord syndrome: outcome study with independent clinical review. J Neurosurg. 2001;94:205-209.
11. Lee G.Y., Paradiso G., Tator C.H., et al. Surgical management of tethered cord syndrome in adults: indications, techniques, and long-term outcomes in 60 patients. J Neurosurg Spine. 2006;4:123-131.
12. Duz B., Gocmen S., Secer H.I., et al. Tethered cord syndrome in adulthood. J Spinal Cord Med. 2008;31:272-278.
13. Rajpal S., Tubbs R.S., George T., et al. Tethered cord due to spina bifida occulta presenting in adulthood: a tricenter review of 61 patients. J Neurosurg Spine. 2007;6:210-215.
14. Chapman P.. Surgical management of occult spinal dysraphism, Schmidek H., editor. Schmidek & Sweet Operative Neurosurgical Techniques, 4th ed., Vol 2. Philadelphia: WB Saunders, 2000;1897-1907.
15. Love J.G., Daly D.D., Harris L.E. Tight filum terminale. Report of condition in three siblings. JAMA. 1961;176:31-33.
16. McLone D. Occult dysraphism and the tethered spinal cord. In: Choux M., Concezio D.R., Hockley A., Walker M. Pediatric Neurosurgery. Philadelphia: Churchill-Livingstone; 1999:61-78.
17. Warder D.E. Tethered cord syndrome and occult spinal dysraphism. Neurosurg Focus. 2001;10:e1.
18. McLendon R.E., Oakes W.J., Heinz E.R., et al. Adipose tissue in the filum terminale: a computed tomographic finding that may indicate tethering of the spinal cord. Neurosurgery. 1988;22:873-876.
19. Tubbs R.S., Salter G., Grabb P.A., Oakes W.J. The denticulate ligament: anatomy and functional significance. J Neurosurg. 2001;94:271-275.
20. Pang D. Split cord malformation: Part II: clinical syndrome. Neurosurgery. 1992;31:481-500.
21. Pang D., Dias M.S., Ahab-Barmada M. Split cord malformation: part I: a unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 1992;31:451-480.
22. Arai H., Sato K., Okuda O., et al. Surgical experience of 120 patients with lumbosacral lipomas. Acta Neurochir (Wien). 2001;143:857-864.
23. Bulsara K.R., Zomorodi A.R., Villavicencio A.T., et al. Clinical outcome differences for lipomyelomeningoceles, intraspinal lipomas, and lipomas of the filum terminale. Neurosurg Rev. 2001;24:192-194.
24. Chapman P.H. Congenital intraspinal lipomas: anatomic considerations and surgical treatment. Childs Brain. 1982;9:37-47.
25. Pang D. Spinal cord lipomas. In: Pang D., editor. Disorders of the Pediatric Spine. New York: Raven Press; 1995:175-201.
26. Rauzzino M.J., Tubbs R.S., Alexander E.3rd, et al. Spinal neurenteric cysts and their relation to more common aspects of occult spinal dysraphism. Neurosurg Focus. 2001;10:e2.
27. Elton S., Oakes W.J. Dermal sinus tracts of the spine. Neurosurg Focus. 2001;10:e4.
28. Radmanesh F., Nejat F., El Khashab M. Dermal sinus tract of the spine. Childs Nerv Syst. 2010;26(3):349-357.
29. Kondo A., Gotoh M., Kato K., et al. Treatment of persistent enuresis. Results of severing a tight filum terminale. Br J Urol. 1988;62:42-45.
30. Selcuki M., Coskun K. Management of tight filum terminale syndrome with special emphasis on normal level conus medullaris (NLCM). Surg Neurol. 1998;50:318-322. discussion 322
31. Selcuki M., Unlu A., Ugur H.C., et al. Patients with urinary incontinence often benefit from surgical detethering of tight filum terminale. Childs Nerv Syst. 2000;16:150-154. discussion 155
32. Warder D.E., Oakes W.J. Tethered cord syndrome and the conus in a normal position. Neurosurgery. 1993;33:374-378.
33. Khoury A., Balcom A., McLorie G.A., Churchill B.M. Clinical experience in urological involvement with tethered cord syndrome. In: Yamada S., editor. American Association of Neurological Surgeons. Park Ridge, IL: Tethered Cord Syndrome; 1996:89-98.
34. Khoury A.E., Hendrick E.B., McLorie G.A., et al. Occult spinal dysraphism: clinical and urodynamic outcome after division of the filum terminale. J Urol. 1990;144:426-428. discussion 428-429, 443-424
35. Kondo A., Kato K., Kanai S., Sakakibara T. Bladder dysfunction secondary to tethered cord syndrome in adults: is it curable? J Urol. 1986;135:313-316.
36. Paradiso G., Lee G.Y., Sarjeant R., Fehlings M.G. Multi-modality neurophysiological monitoring during surgery for adult tethered cord syndrome. J Clin Neurosci. 2005;12:934-936.
37. Paradiso G., Lee G.Y., Sarjeant R., et al. Multimodality intraoperative neurophysiologic monitoring findings during surgery for adult tethered cord syndrome: analysis of a series of 44 patients with long-term follow-up. Spine. 2006;31:2095-2102.
38. Quinones-Hinojosa A., Gadkary C.A., Gulati M., et al. Neurophysiological monitoring for safe surgical tethered cord syndrome release in adults. Surg Neurol. 2004;62:127-133. discussion 133-135
39. Klekamp J., Raimondi A.J., Samii M. Occult dysraphism in adulthood: clinical course and management. Childs Nerv Syst. 1994;10:312-320.