Chapter 199 Management of Median Nerve Compression
Introduction to Median Nerve Compression
Due to their superficial locations, close relationship to neighboring muscles and tendons, and often long and meandering courses, peripheral nerves are frequently subject to physical forces that may result, if applied chronically, in functional impairment or neuropathy. When a nerve is exposed to repetitive stretch, compression, or friction, progressive internal injury may occur and sometimes debilitating symptoms of weakness, paresthesias, or pain will be manifest in the distribution of the affected nerve. The most common occurrence of peripheral nerve compression is that of the median nerve at the wrist, also known as carpal tunnel syndrome (CTS).1 In this chapter, we will characterize the phenomenon of median nerve entrapment not only at the wrist, but also in the arm, at the elbow, and in the proximal forearm. We will describe the clinical features, anatomic findings, and surgical management relevant to compression at each site, including current evidence-based recommendations and technological innovations, such as endoscopy. We hope to provide the reader with both a general understanding of peripheral nerve entrapment and an up-to-date approach to the diagnosis, evaluation, and treatment of median nerve compression syndromes.
Etiology of Nerve Entrapment
The mechanisms underlying peripheral nerve injury due to entrapment are multifactorial and variable in nature, as an individual nerve may be more or less likely to suffer the effects of stretch, pressure, or friction based on its proximity to the skin surface, its axonal density, and the composition of the tissue in which it travels. A relatively superficial nerve that angles sharply around bony prominences, squeezes through packed fibrous tunnels, or is exposed to repetitive intervals of lengthening and shortening, as with flexion and extension across appendicular joints, is at greater risk for developing a compressive neuropathy. Forces may be applied to the nerve in large amounts for short periods of time (acute mechanical compression) or in small amounts on a more chronic basis. Several animal and human studies have demonstrated that early disruption of myelinated fibers, development of extra-axonal edema, and subsequent changes in axoplasmic flow may ultimately lead to a reduction in membrane excitability, axonolysis, and Wallerian degeneration.2–8 Systemic diseases such as diabetes mellitus, advancing patient age, and local vascular insufficiency or venous stasis may also contribute to neuronal ischemia and reduced regenerative capacity that, in combination, can exacerbate neural fibrosis and cause progressive neuropathy.9,10
Clinical Presentation, Diagnosis, and Treatment of Nerve Entrapment
Typically, a patient presenting with symptomatic peripheral nerve compression will relay a history or chief complaint of reduced sensation, paresthesias, pain, and/or weakness in the distribution of the affected nerve that may be worsening over time. Physical examination directed at elucidating alterations in the function of the nerve believed to be involved may reveal a reduction in two-point discrimination and perception of changes in pressure, vibration, or temperature, or decreased power and atrophy of target muscle groups. Specific tests include maneuvers that exacerbate the extent of entrapment, such as manual compression or tapping of an irritable nerve near the skin to cause radiating dysesthesias in the relevant dermatome (Tinel’s sign). In addition to a detailed motor, sensory, and reflex assessment, electromyography (EMG) and nerve conduction velocity (NCV) studies are useful, minimally invasive tools that will potentially confirm or refute suspected deficits discovered on the exam. Findings in entrapment can include prolonged motor or sensory distal latency and delayed or even absent motor or sensory conduction velocity, and these electrical tests will help rule out other diagnoses like motor neuron disease or cervical radiculopathy. Radiographic examinations such as x-ray, computed tomography (CT), and ultrasound (US) may be employed to identify bony abnormalities, soft tissue lesions, or tumors.11 Magnetic resonance imaging (MRI) provides greater detail for peripheral nerve masses such as schwannomas or neurofibromas, while magnetic resonance neurography (MRN) is a newer and more controversial technology that aims to image nerves directly; MRN has shown promise in the evaluation of proximal peripheral nerve compressive lesions (piriformis and thoracic outlet syndromes [TOS]) that may otherwise be difficult to diagnose, but the technique is not yet universally accepted or available.12
Once a diagnosis of peripheral nerve entrapment has been made, treatment is directed at conservative measures that will attempt to reduce the amplitude or frequency of compressive forces on the affected nerve. These steps may include modification of occupational or recreational activities that elicit symptoms, use of splints or immobilizing braces to minimize movements that exacerbate the problem, and prescription of anti-inflammatory medications or corticosteroid injections to reduce nerve edema and irritation. Many patients will experience significant improvement or even resolution of their symptoms with these simple interventions. The peripheral nerve surgeon will be asked to intervene when a patient has failed conservative management but ideally has not yet suffered irreversible loss of neural function. The specific operative approach will be determined by the site of entrapment and requires a familiarity with regional anatomy. Most cases can be completed without risk of major morbidity or further nerve injury, but collaboration with additional specialists, such as vascular or orthopedic surgeons, may be indicated for more complex operations. The basic objective centers on freeing or releasing the compressed nerve from its point of entrapment without traumatizing that nerve or exposing it to prolonged ischemia. Usually, patients recover readily and the desired outcome is achieved. We will discuss multiple aspects of these surgical techniques in greater detail in the paragraphs that follow.
Entrapment of the Median Nerve at the Wrist: Carpal Tunnel Syndrome
Carpal tunnel syndrome (CTS), which results from entrapment of the median nerve under the transverse carpal ligament in the wrist, is the most frequently encountered compressive neuropathy. Accounting for more than 200,000 operative procedures annually in the United States alone, this disorder is estimated to occur in 125 of 100,000 people.1,13–15 Affecting women more commonly than men (ratio of more than 2 to 1), CTS often arises in middle age (40–60 years) and among workers in occupations that involve repetitive use of the hands or wrists.16–18 Although it may result from a congenital narrowing of the carpal tunnel compounded by activities that further reduce the size of the carpal tunnel or exacerbate compression of the median nerve, CTS is associated with a myriad of systemic and local causes. Metabolic etiologies include diabetes mellitus, hyper- or hypo-thyroidism, acromegaly, and pregnancy, with CTS impacting upward of 60% of pregnant women; fortunately, appropriate treatment of the underlying disorders and delivery of the baby will usually achieve resolution of the CTS symptoms.19–21 Traumatic injuries like a Colles fracture that heals improperly, mass lesions such as a ganglion cyst, local infections, edema, and anatomic variants may all increase the volume of the contents of the carpal tunnel and entrap the median nerve.
Surgical Anatomy
The carpal tunnel is a canal with its lateral walls and floor created by the carpal bones and their ligamentous attachments, while its roof is formed by the flexor retinaculum or transverse carpal ligament (TCL). The contents of the carpal tunnel include the nine long digital flexor tendons, synovium, a vascular bundle, and the distal median nerve. The TCL itself is a wide fibrous band that begins 1 cm proximal to the distal wrist crease and ends 3 cm distal to the crease, attaching radially to the trapezium and scaphoid tuberosity and ulnarly to the pisiform and hook of the hamate. The median nerve gives off a palmar cutaneous branch as the main trunk emerges from underneath the FDS tendon approximately 3 to 4 cm before the distal wrist crease. This sensory branch usually exits from the radial or anterolateral surface of the median nerve and then travels lateral to the palmaris longus tendon and superficial to the TCL to innervate the proximal thenar eminence.22,23 The median nerve most commonly passes through the tunnel without branching, and then gives off the recurrent motor or thenar branch as it exits the tunnel. Usually arising from the radial side of the median nerve just distal to the TCL, the recurrent motor branch will then curve back to innervate the abductor pollicis brevis (APB) and opponens pollicis muscles within the thenar mass. The peripheral nerve surgeon must recognize, however, the multiple anatomic variations that exist in the branching pattern of the recurrent motor nerve in order to protect it during exposure and division of the TCL. In 30% of cases, the recurrent branch arises from the ulnar side of the nerve and crosses over the median nerve toward its target; in 20% of cases, it pierces the flexor retinaculum and may be subject to entrapment itself; it may also rarely be duplicated.24,25 The most distal branches of the median nerve proceed to the first two lumbricals and subserve sensation for the palmar surfaces of the thumb and lateral two fingers as well as the radial portion of the ring finger.
Clinical Features
The most common presentation of CTS includes complaints of painful paresthesias or burning, numbness, and tingling in the lateral half of the hand and the radial three fingers. Patients may complain of a deep ache or “pins and needles,” and they importantly note an absence of symptoms in the little finger. Occasionally, pain will radiate proximally into the forearm or even into the arm above the elbow, and patients frequently note an exacerbation with activities involving the hand or wrist. Usually the dysesthesias are worst in the thumb and index finger and classically increase at night after extensive hand use during the day; a patient may often be awakened from sleep with a numb hand that he or she must shake or run under water to obtain relief. Sunderland hypothesized that this phenomenon arises when nocturnal venous stasis activates the symptoms and resolves when shaking improves venous return.26 In more advanced neuropathies, the patient may observe decreased strength in the thenar muscles with concordant wasting.
On physical examination, it is common to appreciate a decreased ability to discriminate light touch or painful stimuli in the radial half of the hand and the first, second, third, and radial half of the fourth digits. Gentle percussion over the median nerve just below the distal wrist crease may elicit tingling dysesthesias in the median nerve distribution (Tinel’s sign). Although supposedly “pathognomonic” for CTS, this test has a sensitivity of only 20% to 70% and specificity of 70% to 83% in several studies.27–29 Forced flexion of the wrist for approximately 60 seconds (Phalen’s sign) may also reproduce the patient’s symptoms in CTS; a positive Phalen wrist flexion test appears to be more specific for the diagnosis than does a positive Tinel’s sign.30,31 We find that simple manual compression over the TCL for 30 to 60 seconds is perhaps the most useful provocative maneuver in CTS. The physician may note a variable amount of thenar atrophy involving the opponens pollicis, APB, or flexor pollicis brevis (FPB) muscles, which will be most obvious when comparing the affected hand to the normal contralateral hand. These muscles can be evaluated by asking the patient to bring the thumb medially to touch the little finger (opponens pollicis) and to abduct the thumb from the plane of the palm against resistance (abductor pollicis).
Electrophysiologic studies are very helpful objective measures in confirming the diagnosis of CTS; research has shown abnormal electrodiagnostic studies in over 90% of patients with clinical CTS.32 The palmar sensory-evoked response test provides the earliest and most sensitive indicator of CTS, that of a prolonged distal sensory latency with decreased amplitude or absence of the evoked response. This study is conducted by stimulating sensory fibers in the palm or fingers and recording over the wrist; as with any test, careful performance and interpretation by an expert in electrophysiology is an absolute requirement, as the results may be affected by numerous patient characteristics such as age, weight, and skin temperature. In more advanced cases, distal motor latency may become prolonged (latency greater than 4 milliseconds across the carpal tunnel is diagnostic) and EMG can show the loss of motor units and the presence of denervation potentials with fibrillations and positive sharp waves in the thenar muscles. EMG will also help differentiate CTS from more proximal median neuropathies, C6–C7 radiculopathies or brachial plexus lesions. The aforementioned radiographic examinations may be necessary if there is any suggestion of a neoplastic or non-neoplastic mass lesion or if the electrodiagnostic studies do not corroborate the diagnosis.
Management
Once the diagnosis of CTS has been confirmed, treatment typically involves conservative measures such as behavioral modification, splinting and anti-inflammatory medications. This is particularly true when the etiology is temporary (pregnancy) or part of a systemic disorder (acromegaly, hypothyroidism, etc.) for which appropriate therapies may be implemented. Occupational or recreational activities that exacerbate the symptoms should be avoided or reduced, a wrist splint that maintains the wrist in slight extension or neutral position may be worn, and corticosteroids can be injected into the carpal tunnel, all of which may be effective strategies to decrease or eliminate symptoms. Some experts caution against steroid injections, however, given their temporary benefit and moderate risk of intraneural injection with consequent injury.33–38
Although some patients with clinical CTS will achieve adequate resolution of symptoms with conservative therapy alone, many will not. Indications for surgical decompression include continued or disabling symptoms in the setting of confirmatory electrodiagnostic studies, evidence of muscle weakness or atrophy, and increased two-point discrimination sensory thresholds. A 2008 Cochrane review compared the efficacy of surgical treatment of CTS with non-surgical treatment and found four randomized controlled trials involving 317 patients with CTS; the conclusion of this meta-analysis was that surgical treatment of CTS relieves symptoms significantly better than does splinting, although it was unclear whether this applies to patients with only mild symptoms.39 A recent randomized, multicenter study compared surgical intervention with conservative management in patients with CTS but no denervation, and surgical treatment produced better outcomes at 12 months follow-up.40 In general, most peripheral nerve surgeons believe operative release of the TCL is a safe and reliable treatment for CTS that is most effective when offered early in the disease course prior to extensive axonal damage and loss. Surgical timing is controversial, but a recent retrospective study of 425 patients with CTS demonstrated that the patients who underwent surgery less than 3 years from the time of initial diagnosis were more than twice as likely to have symptom resolution than patients who underwent surgery more than 3 years after diagnosis.32
Conventional (Open) Carpal Tunnel Release
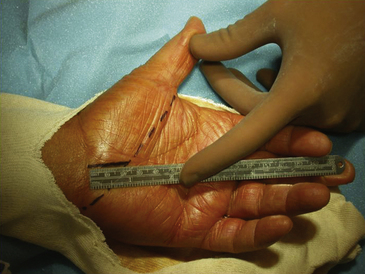
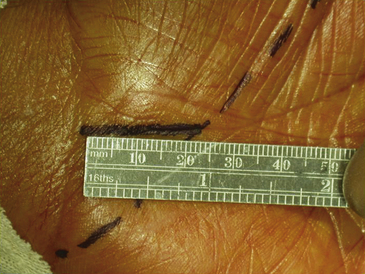
Surgical decompression of the carpal tunnel via transection of the flexor retinaculum was first described by Sir James Learmonth in 1933, and multiple series since have reported patient satisfaction rates of 70% to 96% with the procedure.41 The goal of surgery is to successfully divide the TCL and decompress the median nerve while preserving its recurrent motor and palmar cutaneous branches. The traditional open carpal tunnel release (CTR) is performed using loupe magnification with the patient under locally injected anesthesia (1% lidocaine without epinephrine) with or without mild sedation in an outpatient operating room setting. With the patient positioned supine, the affected arm is abducted and the forearm is supinated on a hand table or an arm board without the use of a tourniquet (to avoid unnecessary ischemia and allow for complete hemostatic control at the conclusion of the case). Following a sterile prep and drape, the incision begins at the distal wrist crease and extends 3 to 4 cm distally in a curvilinear fashion parallel with and approximately 2 mm ulnar to the midpalmar crease (or long axis of the ring finger). The incision ends at or near the level of the distal border of the thumb and may be extended proximally, if necessary, for about 1 cm to create an S-shaped incision. Care must be taken to avoid sectioning branches of the palmar cutaneous nerve during the proximal segment of the skin incision. With experience, the entire incision may be minimized to anywhere from 1.5 to 3 cm in length and still allow for complete division of the TCL (Figs. 199-1 and 199-2).
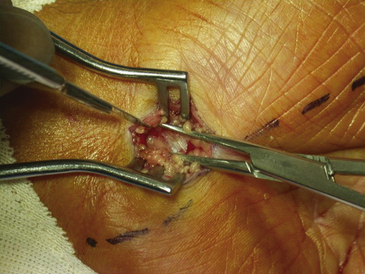
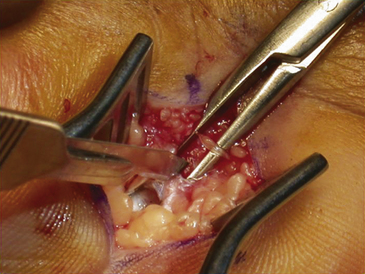
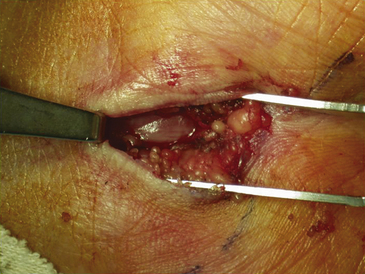
The dissection is then carried down through the subcutaneous fat and the palmar aponeurosis, exposing the TCL, which is aided by the use of small, self-retaining Weitlaner retractors (Fig. 199-3). A no. 15 scalpel is commonly used to start the opening of the firm ligament at its midpoint, revealing, after passing through multiple layers, the underlying median nerve (Fig. 199-4). Once the nerve is exposed, it may be gently dissected away with a No. 4 Penfield blunt dissector or mosquito forceps, and the remainder of the flexor retinaculum may be cut with fine, sharp scissors (iris or tenotomy) or the no. 15 blade; use of the scissors or any other instruments that must be passed beneath the ligament prior to decompression must be done with caution to avoid further compromise of the canal. Additionally, attention must be paid to the fact that the ulnar nerve lies superficial to the TCL and that forceful retraction with the Weitlaner can inadvertently cause compression of the ulnar nerve in Guyon’s canal and result in distal ulnar nerve palsy; intermittently asking the awake patient about numbness or tingling in the little finger will help avoid this complication. Entry of the dissecting instrument into the palmar fat space ensures sufficient distal decompression. A Senn retractor can be employed to provide upward traction on the proximal portion of the incision, and further division of the TCL 1-2 cm proximal to the wrist crease into the deep fascia of the forearm ensures complete proximal decompression (Fig. 199-5). The most common cause of surgical failure is incomplete division of the proximal aspect of the ligament.42 Adequate release of the canal may be confirmed by freely passing the no. 4 Penfield dissector under the wrist and gently palpating both proximally and distally for any residual retinaculum (Fig. 199-6).
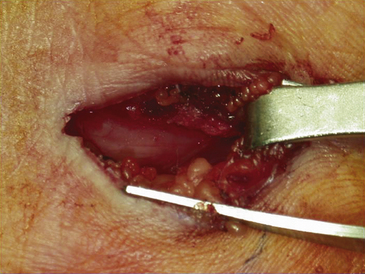
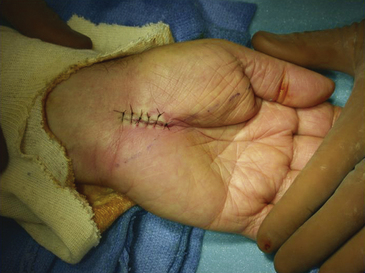
FIGURE 199-6 The median nerve has been freely released distally as well by this mini–carpal tunnel release procedure.
Inspection of the median nerve, which may appear hyperemic, and exploration of the carpal tunnel for evidence of mass lesions or anatomic variants is indicated. There is no proven benefit to separately decompressing the motor branch of the median nerve and numerous studies have shown no improved outcome with routine performance of internal neurolysis of the median nerve during a virgin procedure; in the setting of recurrent disease and repeat surgery in which fibrosis and scarring is prevalent, there may be a role for neurolysis.43 Once the wound has been thoroughly irrigated with sterile saline and meticulous hemostasis is obtained with bipolar coagulation, the fascia and subcutaneous tissues may then be reapproximated with several 3-0 interrupted, inverted Vicryl sutures (Ethicon, Inc., Somerville, NJ). The skin is closed with interrupted or simple running 4-0 nylon sutures; alternatively, the wound may be closed in a single layer of interrupted vertical mattress sutures with a nonabsorbable monofilament suture material (4-0 nylon) (Fig. 199-7). A bulky soft hand dressing is applied, the wrist is not immobilized with a splint, and the entire procedure should be completed within 15 to 30 minutes.
The standard CTR is associated with good to excellent relief of symptoms in 80% of patients, partial relief in 10%, no change in 9%, and worsening of symptoms in 1%.16,18,44 A reduction in pain and numbness of the affected hand is usually the first improvement noted by the patient, often immediately following the release. In a recent study involving 188 CTR procedures, objective measurements of sensory discrimination and motor function demonstrated normal levels of function in 60% and 92% of patients, respectively; a review by Gellman and others revealed improvements in grip and pinch strengths by 12 weeks after surgery.45–48 Several prospective studies have also shown similar improvements in conduction velocities and distal latencies, some as soon as 2 weeks postoperatively, as measured by electrodiagnostic testing.49–51 Complications of the procedure include painful neuroma of the palmar cutaneous nerve, section of the motor branch with subsequent weakness, postoperative fibrosis and hypertrophic or tender scarring, poorly understood pillar pain, wound infections and bow-stringing of the flexor tendons. Patient-related factors that portend the likelihood of unsatisfactory outcomes include systemic metabolic disease (diabetes mellitus, obesity, etc.), severe muscle weakness at the time of surgery, and a lack of response to corticosteroid injections, if attempted, before the operation.51,52
Modifications of the traditional open CTR include the technique described by Paine and Polyzoidis, in which a 1.5-cm transverse incision is made in the wrist crease prior to using the Paine retinaculatome.53 The retinaculatome consists of a small knife that extends perpendicular to a thin footplate, which is passed under the ligament in proximal and distal directions to blindly transect the flexor retinaculum.
Endoscopic (Closed) Carpal Tunnel Release
Endoscopic surgical release of the carpal tunnel has gained popularity in the last decade and both single-portal and biportal techniques have been described. Since its introduction in 1989, six types of endoscopic procedures for the treatment of CTS have been developed, three using a single incision (uniportal) and three using two small incisions (biportal). Chow, Brown, and Okutsu have each reported a large series with excellent results.54–57 The surgery may be done under local anesthesia but many surgeons prefer intravenous sedation and at least a laryngeal mask airway. In a biportal method known as the Brown technique, a tourniquet is applied and a 1-cm incision is made 1-2 cm proximal to the distal wrist crease ulnar to the palmaris longus tendon. The underlying antebrachial fascia is bluntly divided and a synovial elevator is placed distally beneath this fascia and into the carpal tunnel. The synovium is removed from the undersurface of the TCL and an obturator with a slotted cannula is inserted through the carpal tunnel and out approximately 4 cm distal to the distal wrist crease along the third web space. Following removal of the obturator, a 30-degree rigid endoscope is inserted distally and the undersurface of the TCL may be visualized through the slotted end of the cannula. A hook blade is inserted proximally and advanced to the distal end of the flexor retinaculum. The ligament is then divided completely from distal to proximal on the ulnar side of the ligament under direct visualization; this may require more than one pass. It is not recommended that the surgeon attempt to inspect or explore the median nerve. The tourniquet is then deflated, hemostasis is achieved, and the incisions are closed with three simple nylon sutures. A volar splint is applied for the duration of 5 to 7 days and patients typically return to work within 2 to 4 weeks following the procedure.
Reports have demonstrated no difference in long-term outcomes between the open and closed approaches.47,58 A randomized trial performed by Agee et al. showed equal outcomes but faster initial recovery in patients undergoing endoscopic release compared with the traditional open approach.59 Relative contraindications to the endoscopic surgery include rheumatoid arthritis, significant tenosynovitis, recurrent CTS, concurrent ulnar tunnel syndrome or a space-occupying lesion. Many peripheral nerve surgeons who have become very comfortable with the traditional open approach are reluctant to incur the greater expense, steep learning curve, and higher number of serious complications associated with endoscopic carpal tunnel release.
Endoscopic Carpal Tunnel Release Using the Retractor Integrated Endoscope
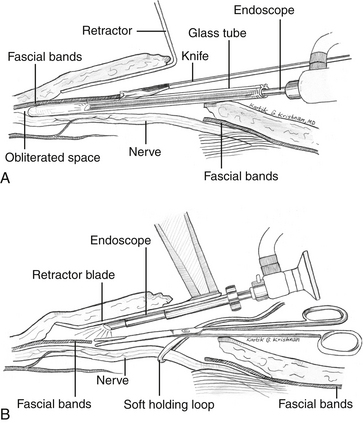
Several years ago we (author KGK) designed an instrument that is nothing other than an endoscope welded to the lower side of the blade of an ergonomically convenient retractor (Fig. 199-8).60,61 This instrument has been used successfully in a variety of surgical operations performed on various extremities, including decompression with or without transposition of peripheral nerves, and harvesting of nerves, tendons, and vessels for grafting purposes.60,61 Specifically, the endoscopic technique of anterior transposition of the ulnar nerve, release of carpal and tarsal tunnels, as well as decompression of Guyon’s canal and the thoracic outlet have been described using this technology. The aforementioned dilator-endoscopic CTR technique (uni- or bi-portal) involves the use of a dilator to blindly expand an already crammed canal, which is then de-roofed under endoscopic scrutiny.62–67 Although these methods have been shown to produce excellent results in experienced hands, as mentioned, the potential exists for erroneous dilation of unintended spaces and subsequent nerve injury, especially owing to initial blind manipulations, the incidence of which is under-reported.62,64,68–72 The retractor-integrated endoscopic technique, on the contrary, does not violate the canal prior to decompression. Instead, the endoscope, which is introduced in a plane above the neural canal, enables a bird’s-eye perspective in addition to high resolution and magnification of the structures to be manipulated (Fig. 199-9).60,61 Thus it is safer than endoscopic techniques requiring the use of a dilator.
Surgical Technique
Aligning the Endoscope
There are two varieties of retractors: one with a narrow blade and another with a broader blade. The narrow retractor is best suited for use in the hand (carpal tunnel and Guyon’s canal) and in the foot (anterior and posterior tarsal tunnel syndromes) (Fig. 199-8). Two different optics are provided in the set: a 0-degree optic that enables straight viewing, and a 30-degree optic that provides an aerial view. The 0-degree optic is preferred for the carpal tunnel release procedure. The 0-degree clear vision endoscopy shaft is fit into the provision made in the retractor and the tip of the optic shaft is approximately one inch behind the tip of the retractor blade. Then the 0-degree optic is aligned into the clear vision shaft snugly and locked with the special mechanism provided. The high-definition camera is next fit to the rear end of the optic, and the cold-light source attached to the provision on the side of the optic. The white balance feature is activated while holding white gauze close to the optic and the endoscope is then ready for use.
Preparation before Bringing in the Endoscope
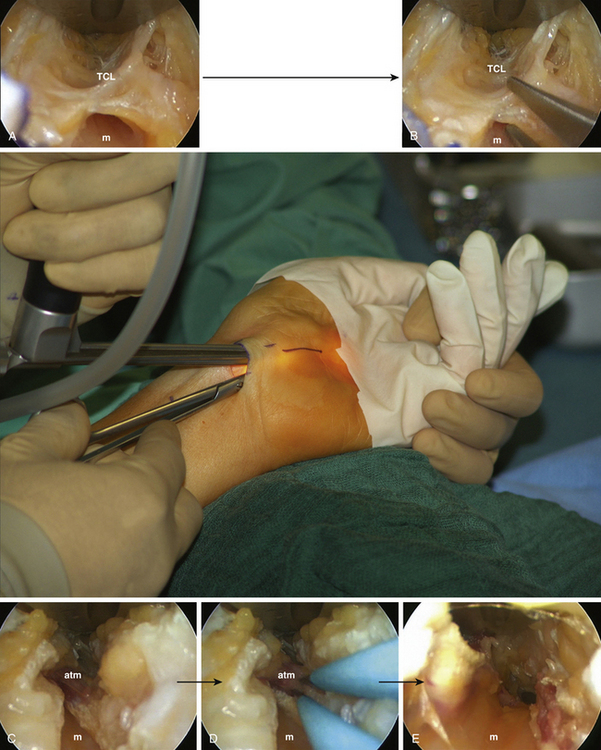
The incision for the endoscopic technique is made in a transverse fashion along the Langer skin tension lines at the wrist crease approximately 10 to 15 mm in length. I (author KGK) use a 2.5× magnifying binocular loupes for the nonendoscopic part of the surgery. Soon after the skin incision is made, one or two superficial veins may be encountered in the subcutaneous plane. These vessels may ideally be bluntly mobilized and moved laterally, but if a rich venous plexus is found (rarely), this may be coagulated with a bipolar cautery and divided. Blunt dissection is carried out with a dry peanut-gauze in the deeper plane until the shiny TCL is visualized. The transverse fibers are split along their natural direction in order to enter the carpal tunnel and visualize the median nerve (Fig. 199-10). Next a 0.5-inch subcutaneous pocket is created below the plane of the superficial fascia both distally and proximally. Importantly, one must be aware that the subcutaneous veins or the venous plexus will be located in a plane above this superficial fascia. Then, the endoscope may be brought into the operative field.
The Endoscopic Technique
The retractor-integrated endoscope is first introduced into the subcutaneous pocket in a distal orientation. The surgeon holds the handle of the retractor-endoscope with his nondominant hand and performs the operative manipulations with his dominant hand (Fig. 199-10). First, the divided TCL is visualized with the endoscope and cut perpendicular to the fiber direction along its ulnar side (Fig. 199-10B). As the surgeon proceeds, more and more of the median nerve is revealed for endoscopic scrutiny (Fig. 199-10A–E). The subcutaneous pocket, into which the retractor-endoscope is introduced, is extended as needed along the course of the nerve under endoscopic guidance for deeper insertion. It is unusual to encounter a venous branch connecting the deep and superficial systems in this area; however, if this occurs, such a vein may be carefully cauterized and divided under endoscopic visualization. In rare instances, an accessory thenar muscle can be seen overlying the TCL (Fig. 199-10B and C) or a median artery overlying the decompressed median nerve. As the surgeon works distally and further transects the TCL along the ulnar border of the median nerve, keeping both the TCL and the median nerve within the field of vision, the previously compressed canal will feel loose (Fig. 199-10E). This denotes complete division of the TCL and further distal release is unnecessary. Typically, this occurs at a point approximately 5 cm from the skin incision (the point of intersection of a medial extension line of the abducted thumb and a straight line extended proximally from the third interdigital space). Next, a blunt Tönnis dissector is introduced into the open canal under endoscopic vision and palpated for completeness of the distal decompression. It should be possible to probe the course of the nerve under endoscopic guidance without any resistance. It is critical to note that careful inspection should be made for anatomic variants of the thenar branch, such as an origin along the ulnar aspect of the median nerve or a course that includes piercing the TCL; each of these variants can be visualized with the retractor endoscopic system.
The skin around the incision is infiltrated with 1% lidocaine and subsequently closed with two or three simple nylon sutures (no subcutaneous stitches are placed). An elastic bandage is applied with mild pressure from the fingers to the distal third of the forearm. Finally, the tourniquet is released and no splints are placed.
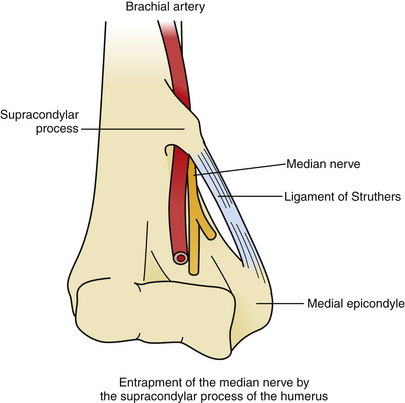
Entrapment of the Median Nerve by the Supracondylar Process of the Humerus
Surgical Anatomy
During the median nerve’s course in the arm, it usually travels below the insertion of the coracobrachialis and above the origin of the pronator teres on the humerus (Fig. 199-11). In 0.7% to 2.7% of the population, however, a supracondylar process is present 5 to 7 cm above the medial epicondyle; the structure protrudes inferiorly, anteriorly, and medially from the humeral surface and connects with the medial epicondyle via a fibrous ligament (Struthers’ ligament). The eponym originates from the discovery by Struthers in 1848 that 3% of his dissected cadavers possessed a supracondylar “spur.”73 The median nerve, brachial artery, and brachial vein must traverse the resulting foramen, and may be subject to compression, particularly when the coracobrachialis inserts on Struthers’ ligament or the pronator teres originates there. Because the median nerve has not yet branched at the level of the supracondylar process, entrapment there affects all median innervated muscles, including flexors and pronators in the forearm, the thenar group in the hand, and the first two lumbricals. Sensory impairment may be seen at the palmar surfaces of the thumb and radial two and a half fingers.
Clinical Features
The most common presenting complaint in a patient with median nerve compression by the supracondylar process of the humerus is weakness in handgrip and pronation of the forearm. He or she may also note some discomfort at the elbow and paresthesias in the aforementioned dermatome, especially in the first three fingers. On examination, the surgeon will usually find objective loss of strength, primarily in the pronator teres, and decreased two-point discrimination. The symptoms may be exacerbated by flexion at the elbow and pronation, while evidence of brachial ischemia may be seen due to compression of the brachial artery.74,75 In some instances, the examiner may be able to palpate the bony process itself, and there may be a Tinel’s sign at this site.
Confirmation of the suspected diagnosis may be made with EMG if it demonstrates denervation potentials in median innervated muscles and if median nerve conduction velocities are reduced across the arm. A critical point is to identify involvement of the pronator teres on electrodiagnostic studies as this will differentiate the diagnosis from common causes of median nerve entrapment that occur more distally in the forearm and wrist. Plain x-rays of the arm are also useful for showing the bony anatomic variant. Chronic pain generated by the proximal median nerve in the arm is usually due to a traumatic injury, predominantly caused by a penetrating or crushing injury (supracondylar humeral fracture), which will be obvious from a careful history.76 Non-traumatic compression may also be caused by slowly expanding masses, many of which are vascular in origin, and that diagnosis will be made with physical exam and radiographic imaging.77,78 It is important to clarify the differential prior to undertaking operative decompression.
Management
Treatment for median nerve entrapment by a supracondylar process of the humerus is straightforward. Once the diagnosis is made, the peripheral nerve surgeon can release the area of compression by removing the supracondylar process and excising Struthers’ ligament. The typical outcome is rapid and long-term relief of symptoms with minimal risk of morbidity.75
Median Nerve Entrapment at the Elbow and in the Forearm
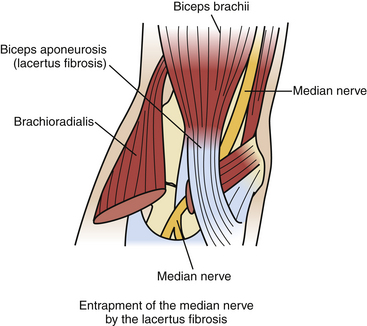
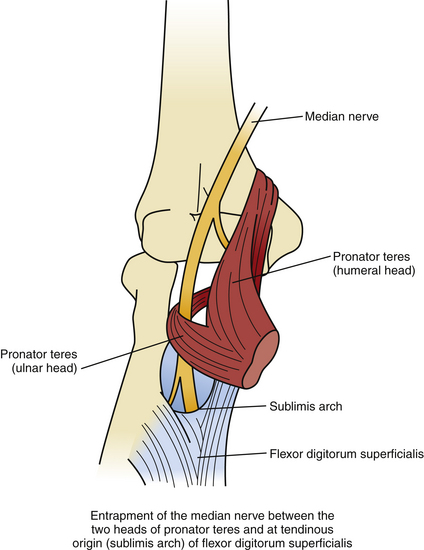
As the median nerve travels from the arm to the forearm, it lies medial to the biceps tendon and brachial artery, anterior to the brachialis muscle and deep to the lacertus fibrosus (biceps aponeurosis, see below) (Fig. 199-12). The median nerve then passes between the superficial or humeral head and deep or ulnar head of the pronator teres muscle, crossing to the lateral side of the ulnar artery. The median nerve then traverses the arch formed by the two heads of the flexor digitorum superficialis muscle and continues distally on the posterior surface of the FDS through the mid-portion of the forearm (Fig. 199-13). At the distal third of the forearm, the median nerve exits from the radial side of the FDS, under the tendon of the palmaris longus and into the carpal tunnel, as described earlier.
Pronator Syndrome
The pronator syndrome is a disorder described by Seyffarth in 1951 that referred to a neuropathy caused by median nerve entrapment as the nerve traversed the pronator teres muscle or FDS arch observed in 17 of his patients.79 Pronator syndrome is a constellation of symptoms with multiple etiologies that occurs most clearly when the median nerve is compressed prior to branching; compression of the median nerve after the branching of the AIN (at the level of pronator teres), creates variable deficits based on the relative degree of injury to the median nerve and its branches.
Surgical Anatomy
There are three potential points of compression:
1. Beneath the lacertus fibrosus. When the lacertus fibrosus is particularly tight or thickened, or if associated with brachialis hypertrophy, entrapment of the median nerve may occur.80–82
2. Between the two heads of the pronator teres muscle or within the muscle itself. A fibrous band within or connecting the two heads of the pronator teres may cause compression of the median nerve, especially following activities or maneuvers that involve repeated pronation and supination.
3. Beneath the tendinous origin of the flexor digitorum superficialis muscle. At the proximal edge of the FDS, a tight fibrous arch (also known as the “sublimis arch”) bridging the heads of the FDS may impinge on the median nerve and result in pronator syndrome. This site (followed by entrapment of the nerve as it passes through pronator teres) is the most common area of median nerve compression above the wrist.83–85
Clinical Features
Although much less common than CTS, the pronator syndrome also presents typically in the fifth decade and is four times more common in women than in men.86 Symptoms often develop over a period of months to years around the onset of a new activity that may incorporate strenuous use of the forearm. Patients complain of an aching pain in the proximal volar forearm and the distal arm, sometimes associated with proximal radiation; the pain is frequently exacerbated by extensive use of the arm, particularly in the setting of forceful pronation or repeated pronation and supination maneuvers.79,87–89 Additionally, patients may note paresthesias and variable insensitivity in the median innervated radial three and a half digits, but especially in the thumb and index finger.80 There should not be a significant component of nocturnal pain or awakening, which is nearly universal in CTS.
Evaluation of the patient with suspected pronator syndrome will reveal tenderness to palpation and sometimes even firmness over the pronator teres muscle.79,85 It is rare to find atrophy of the forearm musculature; a Tinel’s sign over the median nerve at the level of the pronator teres may be positive, but often only after months of compression.82,90 Objective testing for sensory loss and motor weakness have shown inconsistent results and these are not reliable markers for making the diagnosis; if weakness is present, the FPL and APB are involved most prominently. Several provocative tests of the median nerve to assess for proximal entrapment have been shown to be useful, however, and the presence of pain or paresthesias with each maneuver suggests dynamic compression by the associated structure. For example, if the physician provides resistance against neutral forearm pronation as the patient’s elbow is extended, the reproduction of symptoms suggests that the median nerve is compressed by the pronator teres. Alternatively, if the physician resists contraction of the FDS to the long finger and symptoms are evoked, compression of the median nerve by the fibrous arch of the FDS is plausible. Finally, if symptoms occur when the patient’s arm is maximally supinated and flexion of the forearm is resisted with the elbow in a position of more than 120 degrees of flexion, then compression of the median nerve beneath the lacertus fibrosus is the most likely cause.91,92
Unlike in CTS, electrodiagnostic studies are frequently not helpful for confirming the diagnosis of pronator syndrome. Several investigations have shown that slowing of motor and sensory conduction between the wrist and elbow is neither specific nor diagnostic for proximal median nerve entrapment; it has been shown in multiple series that patients with CTS can in fact have slowing of median nerve conduction for different distances proximal to the wrist.93,94 EMG can be used to reveal denervation potentials in median (or branch) innervated muscles and potentially help localize the site of compression.
Before undertaking treatment for possible pronator syndrome, the peripheral nerve surgeon must carefully consider other diagnostic possibilities. Although pronator syndrome and CTS share features including pain in the wrist and forearm, thenar muscle weakness, and paresthesias of the radial three and a half digits, only CTS should have nocturnal pain, positive Tinel’s sign at the wrist, delayed conduction velocity at the wrist, and an absence of dysesthesia in the distribution of the palmar cutaneous nerve (which is likely to be present in pronator syndrome).89 Other confounding disorders include TOS, proximal brachial plexopathies, cervical radiculopathies, and polyneuropathy. A careful history, directed physical examination, and electrodiagnostic studies are critical in ruling out these diagnoses.
Management
Because symptoms of pronator syndrome due to compression of the median nerve between the elbow and wrist are usually caused or exacerbated by specific patient activities, conservative measures are often helpful. Limiting maneuvers that elicit or worsen the symptoms is important. Injection of corticosteroids into the pronator teres muscle has been employed with some success and nonsteroidal anti-inflammatories may provide adequate relief.90
If the symptoms persist, progress, or recur after undergoing reasonable conservative therapy, operative exploration and decompression are appropriate. The preferred approach is an incision that exposes the median nerve at all three possible points of entrapment, essentially incorporating the entire proximal forearm region. Although individual surgeons may vary with regard to the exact incision, most favor a “lazy-S” type of opening that begins just distal to the elbow and extends 6 to 10 cm distally.91 If a Struthers’ ligament is suspected, the incision will have to be extended proximally into the distal arm. Following division of the deep fascia, the median nerve and brachial artery are exposed at the medial edge of the biceps tendon. The lacertus fibrosus is then completely divided in its entirety to follow the median nerve into the forearm. The median nerve is then tracked into the pronator teres, taking care to avoid injuring the proximal motor branches arising from the ulnar side of the nerve. The superficial head of the pronator teres is incised medially, releasing any fibrous bands, in order to visualize the median nerve. The interval between the pronator teres and FCR is then opened to permit more distal dissection, which necessitates division of the FDS fibrous arch, if seen. By the end of this exposure, the median and anterior interosseous nerves will be readily visualized and free from any sites of potential entrapment in the proximal forearm.
Overall, most series have demonstrated 85% to 90% good to excellent outcomes for operative management of pronator syndrome, although there are no universal criteria for objective assessment and evaluation of results.80,85,95 As expected, the authors have concluded that the greatest patient satisfaction is seen in those cases in which a definite anatomic entrapment was identified and decompressed. As in CTS, a longer duration of symptoms and significant motor weakness at the time of surgery portend a worse prognosis.
Anterior Interosseous Syndrome (Kiloh-Nevin Syndrome)
Although quite rare, entrapment of the anterior interosseous nerve is an important diagnosis to consider in the patient presenting with a painful hand of unknown etiology. First described in two patients with isolated neuritis of the AIN by Kiloh and Nevin in 1952 and later investigated by Spinner and others, the AIN syndrome is now a well-recognized entity known to account for approximately 1% of all upper extremity neuropathies.96–98
Clinical Features
The AIN syndrome is a pure motor palsy that may be seen in the setting of forearm fractures or supracondylar fractures of the humerus.99,100 In the former, the mechanism is presumed to be direct trauma to the nerve, whereas in the latter the cause is thought to be a traction injury to the AIN. Additionally, the nerve may be exposed to spontaneous or idiopathic entrapment by fibrous bands or anatomic variants within the forearm.101,102 In these cases, symptoms may arise secondary to chronic compression or following an acute strain on the arm and may include a vague ache in the elbow and forearm as well as a decrease in hand dexterity. Idiopathic AIN palsy may be seen in isolation or as a component of Parsonage-Turner syndrome (neuralgic amyotrophy or brachial neuritis) following a viral illness, inoculation, general anesthesia, or any surgical procedure, and undoubtedly has been confused with idiopathic entrapment.103 Severe spontaneous, self-limited pain in the shoulder girdle or limb is the key element of the history, followed in days to weeks by profound weakness and atrophy with minimal sensory findings. The natural history of this poorly understood syndrome is typically delayed spontaneous improvement, although complete recovery is not universally seen. Surgical decompression of the nerve may be credited for the patient’s recovery. Physical examination will demonstrate variable weakness in the FPL and FDP of the index and middle fingers, which results in the classic “pinch” finding when asking the patient to make an “okay” sign with the thumb and index finger. Because the patient is unable to perform the flexion of the distal interphalangeal joint of the index finger and of the interphalangeal joint of the thumb required by the “okay” maneuver, he instead forms a teardrop outline with his thumb and finger because of extension of both of these joints. The examiner may reveal weakness of the PQ muscle by testing resistance to the forced supination of the forearm with the elbow flexed, though this may be difficult to demonstrate convincingly. EMG of the PQ, FPL, and FDP may demonstrate denervation potentials but the results are inconsistent.104
Management
On the whole, the AIN syndrome typically responds well to conservative therapy. Nearly half of all patients will experience improvement in symptoms after 6 to 12 weeks of simply resting the affected arm and treating with anti-inflammatory medications.10,97 As before, however, if there is no resolution or if symptoms worsen during this time period, surgical exploration and lysis of constricting bands either in the pronator teres near the origin of the AIN or at the tendinous origin of the FDS muscles should be performed.105
Agee J.M., McCarroll H.R., Tortosa R.D., et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17A:987-995.
Bilge T., Yalaman O., Bilge S., et al. Entrapment neuropathy of the median nerve at the level of the ligament of Struthers. Neurosurgery. 1990;27:787-789.
Brown M.G., Keyser B., Rothenberg E.S. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17A:1009-1011.
Dahlin L.B. Aspects on pathophysiology of nerve entrapments and nerve compression injuries. Neurosurg Clin North Am. 1991;2:21-29.
Dawson D.M., Hallet M., Wilbourn A.J. Entrapment Neuropathies, 3rd ed. Philadelphia: Lippincott-Raven; 1999.
Filler A.G., Howe F.A., Hayes C.E., et al. Magnetic resonance neurography. Lancet. 1993;341(8846):659-661.
Hartz C.R., Linscheid R.L., Gramse R.R., et al. The pronator teres syndrome: compressive neuropathy of the median nerve. J Bone Joint Surg Am. 1981;63A:885-890.
Huang J.H., Zager E.L. Mini-open carpal tunnel decompression. Neurosurgery. 2004;54:397-400.
Hudson A.R., Wissinger J.P., Salazar J.L., et al. Carpal tunnel syndrome. Surg Neurol. 1997;47:105-114.
Jarvik J.G., Comstock B.A., Kliot M., et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parellel-group trial. Lancet. 2009;374(9695):1074-1081.
Katz J.N., Larson M.G., Sabra A., et al. The carpal tunnel syndrome: diagnostic utility of the history and physical examination findings. Ann Intern Med. 1990;112:321-327.
Krishnan K.G., Pinzer T., Reber F., et al. Endoscopic exploration of the brachial plexus: technique and topographic anatomy—a study in fresh human cadavers. Neurosurgery. 2004;54(2):401-408. discussion 408-409
Kulick M.I., Gordillo G., Javidi T., et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11:59-66.
Louis D.S., Greene T.L., Noellert R.C. Complications of carpal tunnel surgery. J Neurosurg. 1985;62:352-356.
MacKinnon S.E., Dellon A.L. Surgery of the Peripheral Nerve. New York: Thieme Medical Publishers; 1988.
Ochoa J., Marotte L. The nature of the nerve lesion caused by chronic entrapment in the guinea-pig. J Neurol Sci. 1973;19:491-495.
Omer G.E.Jr., Spinner M. Management of Peripheral Nerve Problems. Philadelphia: WB Saunders; 1980.
Phalen G.S. Reflections on 21 years’ experience with the carpal-tunnel syndrome. JAMA. 1970;212:1365-1367.
Singh S.K., Midha R. Carpal tunnel syndrome. In: Midha R., Zager E.L. Surgery of Peripheral Nerves: A Case-Based Approach. New York: Thieme Medical Publishers, 2008.
Spinner M. The anterior interosseous nerve syndrome: with special attention to its variations. J Bone Joint Surg Am. 1970;52A:84-94.
Stevens J.C., Sun S., Beard C.M., et al. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38:134-138.
Struthers J. On a peculiarity of the humerus and humeral artery. Monthly J Med Sci. 1848;28:264-267.
Sunderland S. Nerves and Nerve Injuries, 2nd ed. New York: Churchill Livingstone; 1978.
Swiggett R., Ruby L.K. Median nerve compression neuropathy by the lacertus fibrosus: report of three cases. J Hand Surg Am. 1986;11A:700-703.
Verdugo R.J., Salinas R.A., Castillo J.L., et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;4:CD001552.
1. Stevens J.C., Sun S., Beard C.M., et al. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38:134-138.
2. Anderson M.H., Fullerton P.M., Gilliatt R.W., et al. Changes in the forearm associated with median nerve compression at the wrist in the guinea-pig. J Neurol Neurosurg Psychiatry. 1970;33:70-79.
3. Fullerton P.M., Gilliatt R.W. Median and ulnar neuropathy in the guinea-pig. J Neurol Neurosurg Psychiatry. 1967;30:393-402.
4. Marotte L.R. An electron microscope study of chronic median nerve compression in the guinea pig. Acta Neuropathol (Berl). 1974;27:69-82.
5. Ochoa J., Marotte L. The nature of the nerve lesion caused by chronic entrapment in the guinea-pig. J Neurol Sci. 1973;19:491-495.
6. Jefferson D., Eames R.A. Subclinical entrapment of the lateral femoral cutaneous nerve: an autopsy study. Muscle Nerve. 1979;2:145-154.
7. Neary D., Ochoa J., Gilliatt R.W. Sub-clinical entrapment neuropathy in man. J Neurol Sci. 1975;24:283-298.
8. Neary C., Eames R. The pathology of ulnar nerve compression in man. Neuropathol Appl Neurobiol. 1975;1:69-88.
9. Dahlin L.B. Aspects on pathophysiology of nerve entrapments and nerve compression injuries. Neurosurg Clin North Am. 1991;2:21-29.
10. Sunderland S. Nerves and Nerve Injuries, 2nd ed. New York: Churchill Livingstone; 1978.
11. Fahr L.M., Sauser D.D. Imaging of peripheral nerve lesions. Orthop Clin North Am. 1988;19:27-41.
12. Filler A.G., Howe F.A., Hayes C.E., et al. Magnetic resonance neurography. Lancet. 1993;341(8846):659-661.
13. Chung K.C., Walters M.R., Greenfield M.L., et al. Endoscopic versus open carpal tunnel release: a cost-effectiveness analysis. Plast Reconstr Surg. 1998;102:1089-1099.
14. Hudson A.R., Wissinger J.P., Salazar J.L., et al. Carpal tunnel syndrome. Surg Neurol. 1997;47:105-114.
15. Levine D.W., Simmons B.P., Kooris M.J., et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75A:1585-1592.
16. Gainer J.V.Jr, Nugent G.R. Carpal tunnel syndrome: report of 430 operations. South Med J. 1977;70:325-328.
17. Phalen G.S. Reflections on 21 years’ experience with the carpal-tunnel syndrome. JAMA. 1970;212:1365-1367.
18. Phalen G.S. The carpal-tunnel syndrome: clinical evaluation of 598 hands. Clin Orthop. 1972;83:29-40.
19. Gould J.S., Wissinger H.A. Carpal tunnel syndrome in pregnancy. South Med J. 1978;71:144-145. 154
20. Massey E.W. Carpal tunnel syndrome in pregnancy. Obstet Gynecol Surv. 1978;33:145-148.
21. Weimer L.H., Yin J., Lovelace R.E., et al. Serial studies of carpal tunnel syndrome during and after pregnancy. Muscle Nerve. 2002;25:914-917.
22. Abdullah A.F., Wolber P.H., Ditto E.W.III. Sequelae of carpal tunnel surgery: rationale for the design of a surgical approach. Neurosurgery. 1995;37:931-936.
23. Rotman M.B., Donovan J.P. Practical anatomy of the carpal tunnel. Hand Clin. 2002;18:219-230.
24. Lanz U. Anatomical variations of the median nerve in the carpal tunnel. J Hand Surg Am. 1977;2:44-53.
25. Bennett J.B., Crouch C.C. Compression syndrome of the recurrent motor branch of the median nerve. J Hand Surg Am. 1982;7:407-409.
26. Sunderland S. The nerve lesion in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1976;39:615-626.
27. Gellman H., Gelberman R.H., Tan A.M., et al. Carpal tunnel syndrome: an evaluation of the provocative diagnostic tests. J Bone Joint Surg Am. 1986;68:735-737.
28. Jarvik J.G., Yuen E., Haynor D.R., et al. MR nerve imaging in a prospective cohort of patients with suspected carpal tunnel syndrome. Neurology. 2002;58:1597-1602.
29. Katz J.N., Larson M.G., Sabra A., et al. The carpal tunnel syndrome: diagnostic utility of the history and physical examination findings. Ann Intern Med. 1990;112:321-327.
30. Seror P. Tinel’s sign in the diagnosis of carpal tunnel syndrome. J Hand Surg. 1987;12B:364-365.
31. Seror P. Phalen’s test in the diagnosis of carpal tunnel syndrome. J Hand Surg (Br). 1989;13B:383-385.
32. Singh S.K., Midha R. Carpal tunnel syndrome. In: Midha R., Zager E.L. Surgery of Peripheral Nerves: a Case-Based Approach. New York: Thieme Medical Publishers, 2008., pp 94-99
33. Kirschberg G.J., Fillingim R., Davis V.P., et al. Carpal tunnel syndrome: classic clinical symptoms and electrodiagnostic studies in poultry workers with hand, wrist, and forearm pain. South Med J. 1994;87:328-331.
34. Koskimies K., Farkkila M., Pyykko I., et al. Carpal tunnel syndrome in vibration disease. Br J Ind Med. 1990;47:411-416.
35. Miller R.F., Lohman W.H., Maldonado G., et al. An epidemiologic study of carpal tunnel syndrome and hand-arm vibration syndrome in relation to vibration exposure. J Hand Surg Am. 1994;19:99-105.
36. Gelberman R.H., Aronson D., Weisman M.H. Carpal-tunnel syndrome: results of a prospective trial of steroid injection and splinting. J Bone Joint Surg Am. 1980;62:1181-1184.
37. Green D.P. Diagnostic and therapeutic value of carpal tunnel injection. J Hand Surg Am. 1984;9:850-854.
38. McConnell J.R., Bush D.C. Intraneural steroid injection as a complication in the management of carpal tunnel syndrome: a report of three cases. Clin Orthop. 1990;250:181-184.
39. Verdugo R.J., Salinas R.A., Castillo J.L., et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev. 2008;4:CD001552.
40. Jarvik J.G., Comstock B.A., Kliot M., et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: a randomised parallel-group trial. Lancet. 2009;374(9695):1074-1081.
41. Louis D.S., Greene T.L., Noellert R.C. Complications of carpal tunnel surgery. J Neurosurg. 1985;62:352-356.
42. Huang J.H., Zager E.L. Mini-open carpal tunnel decompression. Neurosurgery. 2004;54:397-400.
43. Lowry W.E., Follender A.B. Interfascicular neurolysis in the severe carpal tunnel syndrome: a prospective, randomized, double-blind, controlled study. Clin Orthop. 1988;227:251-254.
44. Cseuz K.A., Thomas J.E., Lambert E.H., et al. Long-term results of operation for carpal tunnel syndrome. Mayo Clin Proc. 1966;41:232-241.
45. Osterman A. The double-crush syndrome: cervical radiculopathy and carpal tunnel syndrome. Orthop Clin North am. 1988;19:147-155.
46. Thurston A., Lam N. Results of open carpal tunnel release: a comprehensive, retrospective study of 188 hands. Aust N Z J Surg. 1997;67:283-288.
47. Katz J.N., Keller R.B., Simmons B.P., et al. Maine carpal tunnel study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg Am. 1998;23:697-710.
48. Gellman H., Kan D., Gee V., et al. Analysis of pinch and grip strength after carpal tunnel release. J Hand Surg Am. 14, 1989. 864
49. Shurr D.G., Blair W.F., Bassett G. Electromyographic changes after carpal tunnel release. J Hand Surg Am. 1986;11:876-880.
50. Seror P. Nerve conduction studies after treatment for carpal tunnel syndrome. J Hand Surg (Br). 1992;17:641-645.
51. Aulisa L., Tamburrelli F., Padua R., et al. Carpal tunnel syndrome: indication for surgical treatment based on electrophysiologic study. J Hand Surg Am. 1998;23:687-691.
52. Kulick M.I., Gordillo G., Javidi T., et al. Long-term analysis of patients having surgical treatment for carpal tunnel syndrome. J Hand Surg Am. 1986;11:59-66.
53. Paine K.W.E., Polyzoidis K. Carpal tunnel syndrome: decompression using the Paine retinaculotome. J Neurosurg. 1983;59:1031-1036.
54. Chow J.C.Y. Endoscopic release of the carpal ligament: a new technique for carpal tunnel syndrome. Arthroscopy. 1989;5:19-24.
55. Chow J.C.Y. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990;6:288-296.
56. Brown M.G., Keyser B., Rothenberg E.S. Endoscopic carpal tunnel release. J Hand Surg Am. 1992;17A:1009-1011.
57. Okutsu I., Ninomiya S., Takatori Y., et al. Endoscopic management of carpal tunnel syndrome. Arthroscopy. 1989;5:11-18.
58. Brown R.A., Gelberman R.H., Seiler J.G.III, et al. Carpal tunnel release: a prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75:1265-1275.
59. Agee J.M., McCarroll H.R., Tortosa R.D., et al. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992;17A:987-995.
60. Krishnan K.G., Pinzer T., Reber F., et al. Endoscopic exploration of the brachial plexus: technique and topographic anatomy—a study in fresh human cadavers. Neurosurgery. 2004;54(2):401-408. discussion 408-409
61. Krishnan K.G., Pinzer T., Schackert G. A novel endoscopic technique in treating single nerve entrapment syndromes with special attention to ulnar nerve transposition and tarsal tunnel release: clinical application. Neurosurgery. 2006;59(Suppl 1):ONS89-ONS100. discussion ONS189-ONS100
62. Aroori S., Spence R.A. Carpal tunnel syndrome. Ulster Med J. 2008;77(1):6-17.
63. Groner R., Brunner C.A. Endoscopic methods in peripheral nerve surgery. Krankenpfl J. 1999;37(12):486-490.
64. Kiymaz N., Cirak B., Tuncay I., et al. Comparing open surgery with endoscopic releasing in the treatment of carpal tunnel syndrome. Minim Invasive Neurosurg. 2002;45(4):228-230.
65. Pritsch T., Rosenblatt Y., Carmel A. Carpal tunnel syndrome. Harefuah. 2004;143(10):743-748. 764, 765
66. Serra L., Panagiotopoulos K., Bucciero A., et al. Endoscopic release in carpal tunnel syndrome: analysis of clinical results in 200 cases. Minim Invasive Neurosurg. 2003;46(1):11-15.
67. Szyluk K., Widuchowski J., Jasinski A., et al. Early results of surgical treatment for carpal tunnel syndrome using a single-portal endoscopic method. Ortop Traumatol Rehabil. 2006;8(2):172-181.
68. Assmus H., Dombert T., Staub F. Reoperations for CTS because of recurrence or for correction. Handchir Mikrochir Plast Chir. 2006;38(5):306-311.
69. Barrett S.L. Endoscopic nerve decompression. Clin Podiatr Med Surg. 2006;3:579-595.
70. Kretschmer T., Antoniadis G., Borm W., et al. Iatrogenic nerve injuries. Part 1: frequency distribution, new aspects, and timing of microsurgical treatment. Chirurg. 2004;75(11):1104-1112.
71. Piza-Katzer H., Laszloffy P., Herczeg E., et al. Complications of endoscopic carpal tunnel operations. Handchir Mikrochir Plast Chir. 1996;28(3):156-159.
72. Tennent T.D., Goddard N.J. Carpal tunnel decompression: open vs endoscopic. Br J Hosp Med. 1997;58(11):551-554.
73. Struthers J. On a peculiarity of the humerus and humeral artery. Monthly J Med Sci. 1848;28:264-267.
74. Bilge T., Yalaman O., Bilge S., et al. Entrapment neuropathy of the median nerve at the level of the ligament of Struthers. Neurosurgery. 1990;27:787-789.
75. Laha R.K., Dujovny M., DeCastro S.C. Entrapment of median nerve by supracondylar process of the humerus: case report. J Neurosurg. 1977;46:252-255.
76. MacKinnon S.E., Dellon A.L. Surgery of the Peripheral Nerve. New York: Thieme Medical Publishers; 1988.
77. Ergungor M.F., Kars H.Z., Yalin R. Median neuralgia caused by brachial pseudoaneurysm. Neurosurgery. 1989;24:924-925.
78. Reinstein L., Reid W.P., Sadler J.H. Peripheral nerve compression by brachial artery-basilic vein vascular access in long-term hemodialysis. Arch Phys Med Rehab. 1984;65:142-144.
79. Seyffarth H. Primary myoses in the M. Pronator teres as a cause of lesions of the N. medianus (the pronator syndrome). Acta Psychiatrica Neurologica. 1951;74(suppl):251-254.
80. Hartz C.R., Linscheid R.L., Gramse R.R., et al. The pronator teres syndrome: compressive neuropathy of the median nerve. J Bone Joint Surg Am. 1981;63A:885-890.
81. Martinelli P., Gabollini A.S., Poppi N., et al. Pronator syndrome due to thickened bicipital aponeurosis. J Neurol Neurosurg Psychiatry. 1982;45:181-182.
82. Spinner M., Spencer P.S. Nerve compression lesions of the upper extremity: a clinical and experimental review. Clin Orthop. 1974;104:46-65.
83. Olehnik W.K., Manske P.R., Szerzinski J. Median nerve compression in the proximal forearm. J Hand Surg Am. 1994;19A:121-126.
84. Goldner J.L. Median nerve compression lesions and clinical analysis. Bull J Dis. 1984;44:199-223.
85. Johnson R.K., Spinner M., Shrewsbury M.M. Median nerve entrapment syndrome in the proximal forearm. J Hand Surg. 1979;4:48-51.
86. Johnson R.K., Spinner M. Median nerve compression in the forearm: the pronator tunnel syndrome. In: Szabo R.M., editor. Nerve Compression Syndromes: diagnosis and Treatment. Thorofare, NJ: Slack, 1989., pp 137-151
87. England J.D., Sumner A.J. Neuralgic amyotrophy: an increasingly diverse entity. Muscle Nerve. 1987;10:60.
88. Kopell H.P., Thompson W.A.L. Pronator syndrome: a confirmed case and its diagnosis. N Engl J Med. 1958;259:713-715.
89. Lister G. The Hand: diagnosis and Indications, 3rd ed. New York: Churchill Livingstone; 1993.
90. Morris H.H., Peters B.H. Pronator syndrome: clinical and electrophysiological features in seven cases. J Neurol Neurosurg Psychiatry. 1976;39:461-464.
91. Omer G.E.Jr, Spinner M. Management of Peripheral Nerve Problems. Philadelphia: WB Saunders; 1980.
92. Swiggett R., Ruby L.K. Median nerve compression neuropathy by the lacertus fibrosus: report of three cases. J hand Surg Am. 1986;11A:700-703.
93. Buchthal F., Rosenflack A., Trojaborg W. Electrophysiological findings in entrapment of the median nerve at wrist and elbow. J Neurol Neurosurg Psychiatry. 1974;37:340-360.
94. Cho D.S., MacLean I.C. Pronator syndrome: establishment of electrophysiological parameters. Arch Phys Med Rehabil. 1981;62:531.
95. Werner C.O., Rosen, Thomgren K.G. Clinical and neurophysiologic characteristics of the pronator syndrome. Clin Orthop. 1985;197:231-236.
96. Kiloh L.G., Nevin S. Isolated neuritis of the anterior interosseous nerve. BMJ. 1952;1:250-252.
97. Spinner M. The anterior interosseous nerve syndrome: with special attention to its variations. J Bone Joint Surg Am. 1970;52A:84-94.
98. Stem P.J., Fassler P.R. Anterior interosseous nerve compression syndrome. In: Gelberman R.H., editor. Operative Nerve Repair and Reconstruction. Philadelphia: JB Lippincott, 1991., pp 983-994
99. Spinner M., Schreiber S.N. Anterior interosseous-nerve paralysis as a complication of supracondylar fractures of the humerus in children. J Bone Joint Surg Am. 1969;51:1584-1590.
100. Warren J.D. Anterior interosseous nerve palsy as a complication of forearm fractures. J Bone Joint Surg Br. 1963;45:511-512.
101. Fearn C.B., Goodfellow J.W. Anterior interosseous nerve palsy. J Bone Joint Surg Br. 1965;47:91-93.
102. Rask M.R. Anterior interosseous nerve entrapment (Kiloh-Nevin syndrome): report of seven cases. Clin Orthop. 1979;142:176-181.
103. Dawson D.M., Hallet M., Wilbourn A.J. Entrapment Neuropathies, 3rd ed. Philadelphia: Lippincott-Raven; 1999.
104. Rengachary S.S. Entrapment neuropathies. In: Wilkins R.H., Rengachary S.S. Neurosurgery. New York: McGraw-Hill, 1985., pp1771-1795
105. Lake P.A. Anterior interosseous nerve syndrome. J Neurosurg. 1974;41:306-309.