Chapter 144 Management of Infections After Craniotomy
Incidence
Infection rates after craniotomy as reported during the 20th century are shown in Table 144–1.1–24 Such percentages, however, must be viewed with skepticism, given the great variation in obtaining and conducting data. The most reliable data are obtained in connection with research projects, in which an investigator, using strictly specified criteria, scrutinizes all patient records, complete with questionnaires after discharge. Inclusion criteria, detection methods, and cohort base may vary (e.g., number of operations, number of patients operated on, number of patients discharged, or number of patients admitted) from study to study. A continuous infection registry run by a hygienist, although not as reliable, is more useful for practical purposes because it allows detection of changes from year to year and even from month to month; for comparison between hospitals, it is of less value. The registry is important in keeping track of bacterial strains and resistance patterns, with special reference to highly resistant strains, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus.
| Author | Year | Percentage |
|---|---|---|
| von Eiselsberg and Ranzi1 | 1913 | 12 |
| Tooth2 | 1913 | 12 |
| Olivecrona3 | 1934 | 15 |
| Cushing and Eisenhardt4 | 1938 | 2 |
| Cairns5 | 1939 | 2 |
| Woodhall et al6 | 1949 | 1 |
| Wright7 | 1966 | 6 |
| Skultety and Nishioka8 | 1966 | 2 |
| Balch9 | 1967 | 5 |
| Green et al10 | 1974 | 3 |
| Chou and Erickson11 | 1976 | 3 |
| Cruse12 | 1977 | 2 |
| Quadery et al13 | 1977 | 6 |
| Chan and Thompson14 | 1984 | 7 |
| Jomin et al15 | 1984 | 3 |
| Vlahov et al16 | 1984 | 7 |
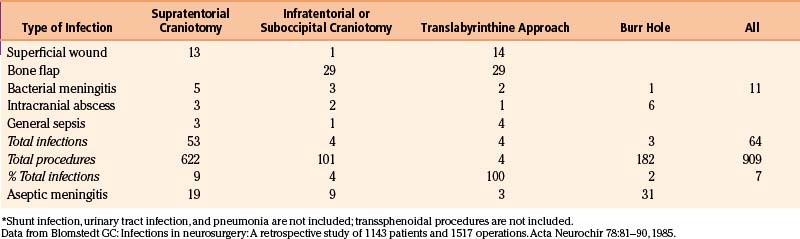
| Blomstedt17 | 1985 | 8 |
| Tenney et al18 | 1985 | 4 |
| van Ek et al19 | 1986 | 8 |
| Rasmussen et al20 | 1990 | 6 |
| Gaillard and Gilsbach21 | 1991 | 9 |
| Mindermann et al22 | 1993 | 9 |
| NNIS 23 | 1996 | 1 |
| Korinek AM 24 | 1997 | 4 |
∗ When the article deals with prophylactic antibiotics, the infection rate for the group without antibiotics is given in this table. All figures are rounded to the nearest full number.
Factors Affecting Infection Rate
Infection rates fell sharply after the introduction of antisepsis and asepsis, but complete eradication of infections seems impossible to achieve. Human defense mechanisms usually have the edge over bacteria always present in the environment, and infection occurs as a result of a faltering balance among defenses, bacterial quantity, and bacterial virulence. Defenses are weakened with age, poor physical or mental condition, devitalized tissue, and foreign bodies. The quantity of bacterial contamination increases with large surgical fields, with reoperation, with long operating times, with inadequate aseptic technique, and with poor ventilation of the operating room.25 Efforts to pinpoint single factors predisposing to infection are rarely convincing: Isolation of one particular factor may be possible in laboratory conditions but is nearly always unreliable in a clinical situation; the impact of most factors is so small that extremely large groups are required to show statistical significance. Many recommendations are difficult to prove, such as that a shunt operation should be done as the first procedure in the morning, that only a minimum of traffic should be allowed in the operating room, and that infected patients should be operated on as the last procedure of the day. In fact most measures usually turn out to be nonsignificant when singled out. External CSF drainage is, however, a factor shown to predispose to infection.26–27 Most hospitals have their own routines, and although different, one may be just as good as the other. Nonetheless, it is probably advantageous to have a fairly rigorous scheme to be followed by all and taught to newcomers, the central issue being to minimize contamination.
Postoperative Cerebrospinal Fluid Leak
Cerebrospinal fluid (CSF) leak after surgery and operation in a patient with a concurrent non-CNS infection are, according to a retrospective study by Mollman and Haines25 on 9202 neurosurgical patients, factors increasing the risk of infection. In a retrospective study by Blomstedt17 of 1039 intracranial procedures (shunts excluded), postoperative CSF leak was also found to be the only highly significant risk factor: Of 15 patients with a CSF leak, 6 developed infection versus 54 patients among 1024 patients without a CSF leak (p = .0002, Fischer’s exact test). Every effort should be made to avoid CSF leak and to seal the leak if it occurs. If the dura cannot be completely closed, dural repair can usually be done with tissue obtained within the operating field, such as periosteum or fascia or, when big patches are required, fascia lata. Fat is quite efficient, especially for drilled-out cavities in the pneumatized bone. Fibrin glue resorbs too quickly to allow natural membranes to form, but it is helpful for fixation of the patch.28 The risk of accidental introduction of prions or other infectants through dura substitutes prepared from animals or cadavers has been reduced considerably through preparations of completely acellular material. Because dural substitutes are avascular, contamination during surgery can result in an infection that is difficult to eradicate. This haven for bacteria is, however, time limited when biodegradable material is used. 29,30,31,32
If CSF leak occurs after surgery, lumbar taps or draining may be helpful. Lumbar drainage causes reduction of CSF pressure, reducing the flow of CSF and allowing the fistula to scar. CSF drainage can, however, cause retrograde flow through the fistula, leading to pneumocephalus and an increased risk of infection. In such cases, early surgical repair is advisable. Persistent CSF leak can also result from low levels of factor XIII.33 In difficult cases, especially after repeated operations and radiotherapy, a microvascular tissue transplant may be necessary to seal the leak. If possible, a locally harvested piece of muscle, with the vasculature intact, is convenient, but a piece of anterior serratus muscle and its vessels can often be anastomosed to the superficial temporal artery and vein.34
Perioperative corticosteroids are widely used in connection with craniotomy to reduce postoperative brain swelling. These agents can have a negative effect on the healing of dura mater and arachnoid.35
The value of prophylactic antimicrobials with a CSF leak is debated.36 If one decides to use an antimicrobial in these circumstances, an agent is selected that is effective against the most likely organisms in the affected area (e.g., if leaking through the nose, the flora of the upper respiratory tract, such as Diplococcus pneumoniae and Haemophilus influenzae; if leaking through the skin, Staphylococcus epidermidis, S. aureus, and Propionibacterium acnes).17 Because CSF fistula increases the risk of intracranial infection substantially, antimicrobial prophylaxis seems justified, despite the lack of evidence of its effectiveness. In cases of CSF rhinorrhea, we use an oral penicillin, which covers most bacteria in the nose but causes few resistance problems. In CSF leak through the skin, we use a first-generation cephalosporin. The use of prophylactic antibiotics in this concept is controversial because the flora can change. When deciding to use prophylaxis, the regimen should be brief and the spectrum should be narrow.
Prophylactic Antimicrobials in Craniotomy
The value of antimicrobial prophylaxis in clean craniotomy is well documented in several controlled studies,21,22,37–44 although other studies do not support the prophylactic use of antimicrobials in this circumstance.24–45 A 2006 study indicates that prophylaxis indeed protects against wound infection but has no effect on postoperative meningitis.46 Negative effects of antimicrobial prophylaxis are possible induction of resistant organisms, inadvertent slackening of aseptic discipline, idiosyncratic reactions to the drug, and expense of the drug. Because the recommended regimen involves a single perioperative dose, only the issue of slackened aseptic discipline and the rare idiosyncratic reaction are relevant. Most investigators use a second-generation or third-generation cephalosporin, vancomycin, or fusidic acid. Because the prophylaxis is targeted at the most likely organisms, an antimicrobial with a fairly narrow spectrum can be selected. In our study,40 we chose vancomycin for three reasons: (1) At the time, bone flap infection was the commonest infection in the department (Table 144–2), and vancomycin covered well the likely agents staphylococci and P. acnes; (2) vancomycin was used sparingly; and (3) resistance against vancomycin was extremely rare, although resistance was reported before our study was finished.47 This single-dose vancomycin regimen brought about a substantial reduction of bone flap infections.40 Circumstances have changed since the mid 1980s in that the use of vancomycin has increased and resistance is reported more often. However, in our institution the prophylaxis in craniotomies is unchanged because no increase in the rate of bone flap infections have occurred. There is no antimicrobial agent that can be recommended universally. Each neurosurgical department needs to establish its types of infections, infection rate (prophylactic antimicrobial treatment is recommended if it is greater than 3%), and the likely bacteria, and the routines must be changed from time to time, if need be.
Postcraniotomy Infections
Central Nervous System Defenses Against Infections
Bacteria do not readily cross the blood-brain barrier and blood-CSF barrier, but when these defenses are breached, the CSF is ill equipped to mount an efficient defense against bacteria. Such a defense requires high-affinity or type-specific antibodies. The complement system reacts with the antigen-bacteria complex to induce lysis, and opsonins combine with bacteria and bacterial fragments and render them susceptible for phagocytosis by polymorphonuclear cells. In normal CSF, the levels of specific antibodies, complement, and opsonic proteins are low or absent, and polymorphonuclear cells are slow to reach the site of infection. If bacteria multiply rapidly, the opsonin supply can be quickly exhausted. Antimicrobial therapy, especially with a bacteriostatic antimicrobial, falls short in the absence of an efficient host response. The opsonic capacity seems to correlate with the leukocyte count of the infected CSF. A vigorous inflammatory response aids in the fight against infection by increasing penetration of opsonic protein and polymorphonuclear cells, but inflammation also causes cortical damage.
Most bacterial species (e.g., Pneumococcus, Haemophilus, Meningococcus, Klebsiella) that commonly cause meningitis possess a capsule that hampers opsonization, which in this situation is important for bacterial virulence. In contrast, the unencapsulated S. aureus rarely causes meningitis but commonly causes bacteremia, which the encapsulated bacteria rarely do.48–53
Antimicrobial Penetration into Cerebrospinal Fluid
Several factors limit antimicrobial penetration into the CSF. The capillary bed of the choroid plexus and the meninges are nonfenestrated, and all organic compounds must pass through a lipid membrane. The lipid solubility of the antimicrobial agent is a penetration factor. Ionization of the antimicrobial is another factor: The more ionized, the less lipid soluble. The pKa of penicillin G is 2.6, and at physiologic pH, the degree of ionization is high. The pH of plasma is normally 7.4 and of CSF is 7.3, and the pH gradient between plasma and CSF favors outflux of lipid-soluble antimicrobials from the CSF. In purulent meningitis, acid metabolites lower the pH of CSF, which increases this gradient and reduces antimicrobial penetration. Other mechanisms associated with meningeal inflammation increase penetration into the CSF several fold. A high degree of antimicrobial protein binding reduces CSF penetration, as do high molecular weight and complex molecular structure. Antibiotic transportation occurs passively, but there are also active bidirectional transport mechanisms.54–6061
Infections
Bacterial Meningitis
Postoperative bacterial meningitis is not common, but it can cause permanent brain damage or death. External CSF drainage devices are commonly used in the intensive care unit and are risk factors for meningitis (odds ratio [OR], 21.8; p = .001), as are extended antimicrobial treatment and infections elsewhere in the body.62,63,64 Staphylococci do not cause spontaneous meningitis but do produce postoperative meningitis, as do gram-negative bacilli.17,65–67,26,46 A rise in body temperature is common in the postoperative period. If the temperature is very high, persistently high, rising, or fluctuating, particularly with a rising C-reactive protein, stiff neck, deteriorating consciousness, new neurologic symptoms, or a CSF fistula, meningitis should be suspected. If the level of consciousness is altered, a computed tomography (CT) scan is done before lumbar puncture. This is to rule out an abscess, as well as other abnormal findings such as hydrocephalus or bleeding. The interpretation of CSF leukocyte criteria for postoperative meningitis is somewhat arbitrary, especially because CSF at this stage usually contains a lot of red blood cells. In our institution we have used the limit 100 × 106/L with a minimum of 50% polymorphonuclear cells or 400 × 106/L regardless of the polymorphonuclear percentage.68 Because the CSF cultures require 2 to 3 days, an elevated CSF cell count is used to decide the initial line of treatment. Gram stain can aid in the selection of antibiotics, as does the likely route of infection: Airway bacteria (e.g., Diplococcus pneumoniae, H. influenzae, and streptococci) can be expected with a CSF leak through the ear or the nose, whereas staphylococci and gram-negative bacilli are more likely if the leak comes through the skin.17,69,70
When one cannot identify a specific bacterial agent, a third-generation cephalosporin combined with vancomycin is a good first-time regimen.71,72,73 Cefotaxine and ceftriaxone are usually effective against methicillin-sensitive staphylococci, which ceftazidime is not. Ceftazidime is, however, effective against Pseudomonas species. New antimicrobials have in most cases made intraventricular administration unnecessary, but the latter is still relevant if good antibiotic penetration into the CSF is not otherwise achieved. Ceftazidime has been a cornerstone in the treatment of meningitis caused by gram-negative bacteria.
With increasing resistance, carbapenems such as meropenem have provided alternative treatment, as has ampicillin in combination with sulbactam, a β-lactamase inhibitor.71 Unfortunately, gram-negative bacterial strains show increasing resistance against carbapenems, the genetic coding of carbapenemases being often plasmid transferred.74 Aztreonam, a monocyclic β-lactam antimicrobial, penetrates well into the CSF and can be combined with antimicrobials against staphylococci and anaerobic bacteria.75 Against very resistant Acinetobacter and Pseudomonas species, colistin, a polymyxin, may be effective.76
The fluoroquinolones penetrate well into the CSF and are so well absorbed from the intestines that intravenous administration does not offer any advantage over oral administration.77–78 Among fluoroquinolones ofloxacin or levofloxacin and moxifloxacin have a good effect against staphylococci, whereas ciprofloxacin is more potent against Pseudomonas79
Vancomycin has been a reliable antimicrobial against gram-positive cocci but it does not penetrate well into CSF and resistance occurs especially among enterococci. Linezolid, an oxazolidinone, is effective against resistant gram-positive cocci and it penetrates well into the CSF.80 Teicoplanin is a glycopeptide antimicrobial agent that resembles vancomycin in its antibacterial spectrum and penetration into the CNS.
Postoperative Aseptic Meningitis
The terms aseptic meningitis and chemical meningitis refer to nonbacterial postoperative meningitis, not to be confused with viral meningitis, which is sometimes also called aseptic meningitis. Bacterial culture distinguishes postoperative bacterial meningitis from aseptic meningitis; other tests are less reliable,68–81 although very high CSF leukocyte counts and low CSF glucose are said to indicate bacterial meningitis.82 When the bacterial culture is negative, the likely diagnosis is aseptic meningitis, yet there is reluctance to trust a negative culture because a poorly treated bacterial meningitis may be detrimental.83 However, one must not underrate the value of clinical signs pointing toward a bacterial etiology: signs of wound infection, reduced level of consciousness, new neurologic dysfunction, onset of new epileptic seizures, a temperature higher than 39.4 °C, or CSF rhinorrhea or otorrhea.82
In one study on postoperative meningitis, all patients with a CSF leukocyte count indicating meningitis were started on antimicrobial treatment, and on the second or third day, when the cultures were available, those with negative cultures were assigned to a group on continued treatment or to a group in which antibiotic treatment was discontinued. No difference was found between the groups.68 The last two decades, our routine for patients with meningitis has been to stop antimicrobial treatment if the cultures are negative, except when clinical signs, such as those mentioned earlier, warn against it. No randomized study is available to establish the value of corticosteroids in the treatment of postoperative aseptic meningitis, although Carmel and associates84 suggest that corticosteroids may have a beneficial effect.
Postoperative Subdural and Brain Abscess
Postoperative brain abscess is uncommon, and when brain abscess developing after craniotomy is compared with brain abscess of other origin, patients who had undergone craniotomy were notable for older age, less fever, evidence of wound infection, frequent false-negative CT scan, and a high percentage of gram-negative aerobic organisms or skin flora as pathogens.24–85 A postoperative abscess can manifest as persistent fever, as persistent headache, as new neurologic dysfunction, and as progressively disturbed consciousness.86
In a study of 2941 patients who had undergone clean craniotomy, 39 patients developed intracranial abscess or empyema within 3 months of surgery. Of those, 14 had been operated on for a malignant glioma.87 Subdural empyemas are sometimes connected with bone flap infection and sometimes with wound infection after burr hole evacuation of chronic subdural hematomas. In a retrospective study of postoperative infections, there were six abscesses after 1039 intracranial procedures: five subdural and one intracerebral (see Table 144–2).17 The elapsed time from operation to the diagnosis of the abscess had a mean of 31 days. One patient had concomitant meningitis, one a bone flap infection, and one a superficial wound infection. Three of these patients died, one possibly resulting from this complication. One abscess was found at autopsy. The bacteria found in the study were S. epidermidis, S. aureus, and P. acnes—that is, bacteria typical for wound and bone flap infection.
The diagnosis may be difficult because postoperative CT changes or tumor recurrence can mimic infection, but repeated CT scans and the clinical picture usually show progression. Diffusion-weighted magnetic resonance imaging may be beneficial for differentiating between the two.88 Laboratory parameters, such as C-reactive protein or CSF leukocyte count, are not necessarily altered.87–89 The treatment follows the same principles as with spontaneous abscesses90: Antimicrobial treatment might suffice if the abscess is small, but surgical evacuation is usually necessary. Extirpation of the intracerebral abscess together with devitalized tissue is preferred, but the borders may be difficult to assess at an early postoperative stage. The antimicrobial treatment should be adjusted when the culture is available, but because a brain abscess does not usually cause a meningeal reaction, drug penetration may be much less than in meningitis. A third-generation cephalosporin with vancomycin is a good first-line drug.
Superficial Postoperative Cranial Wound Infection
A superficial wound infection in the head has the potential to spread to the bone flap and to the meninges. To avoid wound infection, one must be punctilious in closing the wound: No devitalized tissue should be allowed, wound apposition should be meticulous, and the sutures should be slack enough to allow normal wound swelling. Superficial infections do not readily spread to the meninges if the dura is intact, but the dura is often not watertight, and a CSF collection is often seen underneath the skin. In noninfected cases, spinal drainage can keep the persistently refilling subcutaneous space collapsed to allow the skin to adhere to the skull. With a skin infection, this drainage can promote spread of infection to the intradural space.
If there is frank CSF leak through the skin, a couple of stitches usually seals off the fistula, but suturing an infected skin site or sealing the fistula can aggravate the infection and cause the fistula to grow. Instead, it may be necessary to reopen the wound, repair the dura, and, if possible, débride the wound. Difficult cases can require a vascularized flap to withstand the infection and adhere to the surrounding tissue. Lavish use of antimicrobials should generally be condemned, but the dangers of the spread of infection from superficial to deeper layers after craniotomy is serious enough to warrant their use on clinical suspicion alone until bacterial cultures are available. Patients in relatively poor condition, previously beyond neurosurgical treatment, are now treated, and prolonged stay in the intensive care unit is therefore common. A combination of this and abundant use of antibiotics cause selection of the bacterial flora. Bacteria such as Acinetobacter spp can become endemic in such an environment. Finding the reservoir requires diligence and, to identify specific species, special methods such as amplified ribosomal DNA restriction analysis.91
Postoperative Bone Flap Infection
In craniotomy, the bone is disconnected from the skull, devascularized, and devitalized. This process reduces the bone’s natural resistance to infection and hampers antimicrobials from reaching the infectious focus. A bone flap infection can cause high fever, local suppuration, or a stubborn fistula. The standard treatment is removal of the infected bone flap, followed by cranioplasty using artificial bone substitutes, or sometimes a split bone flap from the contralateral side, months later.92–93 Repeated local débridement with long antimicrobial treatment can make bone removal unnecessary.94 Chou and Erickson11 used closed-suction irrigation with topical antibiotics and managed to save 50% of infected bone flaps. Some surgeons advocate leaving the bone flap attached to a muscle pedicle to preserve blood flow and resistance to infection; however, in a study comparing free with pedicled bone flaps, there was no difference in the rate of infection, but fewer pedicled bone flaps ultimately had to be removed.20
Bergman T. Kinetics of tissue penetration. Scand J Infect Dis. 1978;14(Suppl):36-46.
Blomstedt G.C. Infections in neurosurgery: a retrospective study of 1143 patients and 1517 operations. Acta Neurochir. 1985;78:81-90.
Blomstedt G.C., Kytta J. Results of a randomized trial of vancomycin prophylaxis in craniotomy. J Neurosurg. 1988;69:216-220.
Bruce J.N., Bruce S.S. Preservation of bone flaps in patients with postcraniotomy infections. J Neurosurg. 2003;98:1203-1207.
De Bels D., Korinek A.-M., Bismuth R., et al. Empirical treatment of adult postsurgical nosocomial meningitis. Acta Neurochir. 2002;144:989-995.
Falagas M.E., Kasiakou S.K. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333-1341.
Federico G., Tumbarello M., Spanu T., et al. Risk factors and prognostic indicators of bacterial meningitis in a cohort of 3580 postneurosurgical patients. Scand J Infect Dis. 2001;33:533-537.
Gaillard T., Gilsbach J.M. Intra-operative antibiotic prophylaxis in neurosurgery: a prospective, randomized, controlled study on cefotiam. Acta Neurochir. 1991;113:103-109.
Guzman R., Barth A., Lovblad K.-O., et al. Use of diffusion-weighted magnetic resonance imaging in differentiating purulent brain processes from cystic brain tumors. J Neurosurg. 2002;97:1101-1107.
Jallo G.I., Koslow M., Hanna B.A., et al. Propionibacterium as a cause of postneurosurgical infection in patients with dural allografts: report of three cases. Neurosurgery. 1999;44:1138-1141.
Korinek A.M. Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. Neurosurgery. 1997;41:1073-1081.
Korinek A.M., Baugnon T., Golmard J.L., et al. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2006;59:126-133.
Lietard C., Thébaud V., Besson G., et al. Risk factors for neurosurgical site infections: an 18-month prospective survey. J Neurosurg. 2008;109:729-734.
Maher C.O., Anderson R.E., McClelland R.L., et al. Evaluation of a novel propylene oxide-treated collagen material as a dural substitute. J Neurosurg. 2003;99:1070-1087.
Mindermann T., Zimmerli W., Gratzl O. Randomized placebo-controlled trial of single-dose antibiotic prophylaxis with fusidic acid in neurosurgery. Acta Neurochir. 1993;121:9-11.
Ntziora F., Falagas M.E. Linezolid for the treatment of patients with central nervous system infection. Ann Pharmacother. 2007;41:296-308.
Norrby R. A review of the penetration of antibiotics into CSF and its clinical significance. Scand J Infect Dis. 1978;14(Suppl):296-309.
Parodi S., Lechner A., Osih R., et al. Nosocomial Enterobacter meningitis: risk factors, management, and treatment outcomes. Clin Infect Dis. 2003;37:159-166.
Reddy M., Schoggl A., Reddy B., et al. A clinical study of a fibrinogen-based collagen fleece for dural repair in neurosurgery. Acta Neurochirurgica. 2002;144:265-269.
Sinner S.W., Tunkel A.R. Antimicrobial agents in the treatment of bacterial meningitis. Infect Dis Clin North Am. 2004;18:581-602.
Stendel R., Krischek B., Pietila T.A. Biodegradable implants in neurosurgery. Acta Neurochirurgica. 2001;143(3):237-243.
Tunkel A.R., Hartman B.J., Kaplan S.L., et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
van Ek B., Dijkmans B.A., van Dulken H., et al. Clinical, bacteriological and cost-saving effects of antibiotic prophylaxis in craniotomy. Ned Tijdschr Geneeskd. 1992;136:16-20.
Yang K.-Y., Chang W.-N., Ho J.-T., et al. Postneurosurgical nosocomial bacterial brain abscess in adults. Infection. 2006;34:247-251.
1. von Eiselsberg A.F., Ranzi E. Über die chirurgische Behandlung der Hirn und Rückenmarkstumoren. Arch Klin Chir. 1913;102:309-478.
2. Tooth H.H. The treatment of tumours of the brain, and the indications for operations. Trans First Cong Med London. 1913;8:203-299.
3. Olivecrona H. Die Parasagittalen Meningeome. Leipzig: Georg Thieme; 1934. 141–143
4. Cushing H., Eisenhardt L. Meningiomas, Their Classification, Regional Behaviour, Life History, and Surgical End Results. Springfield, IL: Charles C Thomas; 1938. 733–734
5. Cairns H. Bacterial infections during intracranial operations. Lancet. 1939;1:1193-1198.
6. Woodhall B., Neill R.G., Dratz H.M. Ultraviolet radiation as an adjunct in the control of post-operative neurosurgical infection. Ann Surg. 1949;129:820-825.
7. Wright R.L. Postoperative Craniotomy Infections. Springfield, IL: Charles C Thomas; 1966.
8. Skultety F.M., Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage: section VIII, part 2. The results of intracranial surgery in the treatment of aneurysms. J Neurosurg. 25, 1966. 783–704
9. Balch R.E. Wound infection complicating neurosurgical procedures. J Neurosurg. 1967;26:41-44.
10. Green J.R., Kanshepolsky J., Turkian B. Incidence and significance of central nervous system infection in neurosurgical patients. Adv Neurol. 1974;6:223-228.
11. Chou S.N., Erickson D.L. Craniotomy infections. Clin Neurosurg. 1987;23:357-362.
12. Cruse P.J.E. Infection surveillance. South Med J. 1977;70:48.
13. Quadery L.A., Medlery A.V., Miles J. Factors affecting the incidence of wound infection in neurosurgery. Acta Neurochir. 1977;39:133-141.
14. Chan R.C., Thompson G.B. Morbidity, mortality, and quality of life following surgery for intracranial meningioma. J Neurosurg. 1984;60:52-60.
15. Jomin M., Lesoin F., Lozes G. 500 ruptured and operated intracranial arterial aneurysm. Surg Neurol. 1984;21:13-18.
16. Vlahov D., Montgomery E., Tenney J.H., et al. Neurosurgical wound infections: methodological and clinical factors affecting calculations of infection rates. J Neurosurg Nurs. 1984;16:128-133.
17. Blomstedt G.C. Infections in neurosurgery: a retrospective study of 1143 patients and 1517 operations. Acta Neurochir. 1985;78:81-90.
18. Tenney J.H., Vlahov D., Salcman M., et al. Wide variation in risk of wound infection following clean neurosurgery: implications for perioperative antibiotic prophylaxis. J Neurosurg. 1985;62:243-247.
19. van Ek B., Bakker F.P., van Dulken H., Dijkmans B.A. Infections after craniotomy: a retrospective study. J Infect. 1986;12:105-109.
20. Rasmussen S., Ohrstrom J.K., Westergaard L., Kosteljanetz M. Post-operative infections of osteoplastic compared with free bone flaps. Br J Neurosurg. 1990;4:493-495.
21. Gaillard T., Gilsbach J.M. Intra-operative antibiotic prophylaxis in neurosurgery: a prospective, randomized, controlled study on cefotiam. Acta Neurochir. 1991;113:103-109.
22. Mindermann T., Zimmerli W., Gratzl O. Randomized placebo-controlled trial of single-dose antibiotic prophylaxis with fusidic acid in neurosurgery. Acta Neurochir. 1993;121:9-11.
23. National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996. A report from the National Nosocomial Infections Surveillance (NNIS) System. Am J Infect Control. 1996;24:380-388.
24. Korinek A.M. Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. Neurosurgery. 1997;41:1073-1081.
25. Mollman H.D., Haines S.J. Risk factors for postoperative neurosurgical wound infection: a case control study. J Neurosurg. 1986;64:902-906.
26. Federico G., Tumbarello M., Spanu T., et al. Risk factors and prognostic indicators of bacterial meningitis in a cohort of 3580 postneurosurgical patients. Scand J Infect Dis. 2001;33:533-537.
27. Lietard C., Thébaud V., Besson G., et al. Risk factors for neurosurgical site infections: an 18-month prospective survey. J Neurosurg. 2008;109:729-734.
28. Kassam A., Horowitz M., Carrau R., et al. Use of tisseel fibrin sealant in neurosurgical procedures: incidence of cerebrospinal fluid leaks and cost-benefit analysis in a retrospective study. Neurosurgery. 2003;52:1102-1105.
29. Maher C.O., Anderson R.E., McClelland R.L., et al. Evaluation of a novel propylene oxide–treated collagen material as a dural substitute. J Neurosurg. 2003;99:1070-1087.
30. Jallo G.I., Koslow M., Hanna B.A., et al. Propionibacterium as a cause of postneurosurgical infection in patients with dural allografts: report of three cases. Neurosurgery. 1999;44:1138-1141.
31. Reddy M., Schoggl A., Reddy B., et al. A clinical study of a fibrinogen-based collagen fleece for dural repair in neurosurgery. Acta Neurochirurgica. 2002;144:265-269.
32. von Wild K.R. Examination of the safety and efficacy of an absorbable dura mater substitute (Dura Patch) in normal applications in neurosurgery. Surgical Neurology. 1999;52:418-424.
33. Kawamura A., Tamaki N., Yonezawa K., et al. Effect of factor XIII on intractable CSF leakage after a transpetrosal-approach operation: a case report. No Shinkei Geka. 1997;25:53-56.
34. Saint-Cyr M., Nikolis A., Moumdjian R., et al. Paraspinous muscle flaps for the treatment and prevention of cerebrospinal fluid fistulas in neurosurgery. Spine. 2003;28:86-92.
35. Marion D.W., Janetta P.J. Use of perioperative steroids with microvascular decompression operations. Neurosurgery. 1988;22:353-357.
36. Clemenza J.W., Kaltman S.I., Diamond D.L. Craniofacial trauma and cerebrospinal fluid leakage: a retrospective clinical study. J Oral Maxillofac Surg. 1995;53:1004-1007.
37. Geraghty J., Feely M. Antibiotic prophylaxis in neurosurgery: a randomized controlled trial. J Neurosurg. 1984;60:724-726.
38. Shapiro M., Wald U., Simchen E., et al. Randomized clinical trial of intraoperative antimicrobial prophylaxis of infection after neurosurgical procedures. J Hosp Infect. 1986;8:283-295.
39. Young R.F., Lawner P.M. Perioperative antibiotic prophylaxis for the prevention of postoperative neurosurgical infections: a randomized clinical trial. J Neurosurg. 1987;66:701-706.
40. Blomstedt G.C., Kytta J. Results of a randomized trial of vancomycin prophylaxis in craniotomy. J Neurosurg. 1988;69:216-220.
41. van Ek B., Dijkmans B.A., van Dulken H., van Furth R. Antibiotic prophylaxis in craniotomy: a prospective double-blind placebo-controlled study. Scand J Infect Dis. 1988;20:633-639.
42. Winkler D., Rehn H., Freckmann N., et al. Clinical efficacy of perioperative antimicrobial prophylaxis in neurosurgery: a prospective randomized study involving 159 patients. Chemotherapy. 1989;35:304-312.
43. van Ek B., Dijkmans B.A., van Dulken H., et al. Effect of cloxacillin prophylaxis on the bacterial flora of craniotomy wounds. Scand J Infect Dis. 1990;22:345-352.
44. van Ek B., Dijkmans B.A., van Dulken H., et al. Clinical, bacteriological and cost-saving effects of antibiotic prophylaxis in craniotomy. Ned Tijdschr Geneeskd. 1992;136:16-20.
45. Rocca B., Mallet M.N., Scemama F., et al. Perioperative remote infections in neurosurgery: role of antibiotic prophylaxis. Presse Med. 1992;21:2037-2040.
46. Korinek A.M., Baugnon T., Golmard J.L., et al. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2006;59:126-133.
47. Schwalbe R.S., Stapleton J.T., Gilligan P.H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987;316:927-931.
48. Simberkoff M.S., Moldover N.H., Rahal J.J.Jr. Absence of detectable bactericidal and opsonic activities in normal and infected human cerebrospinal fluids: a regional host defense deficiency. J Lab Clin Med. 1980;95:362-372.
49. Waldvogel F.A. Pathophysiological mechanisms in pyogenic infection: two examples—pleural empyema and acute bacterial meningitis. In: Majno G., Cotran R.S., Kaufman N. Current Topics in Inflammation and Infection. Baltimore: Williams & Wilkins; 1982:115-122.
50. Zwahlen A., Nydegger U.E., Vaudaux P., et al. Complement-mediated opsonic activity in normal and infected human cerebrospinal fluid: early response during bacterial meningitis. J Infect Dis. 1982;145:635-646.
51. Bernhardt L.L., Simberkoff M.S., Rahal J.J.Jr. Deficient cerebrospinal fluid opsonization in experimental Escherichia coli meningitis. Infect Immun. 1981;32:411-413.
52. Giampaolo C., Scheld M., Boyd J., et al. Leukocyte and bacterial interrelationship in experimental meningitis. Ann Neurol. 1981;9:328-333.
53. Tofte R.W., Peterson P.K., Youngki K., et al. Opsonic activity of normal human cerebrospinal fluid for selected bacterial species. Infect Immunol. 1979;26:1093-1098.
54. Norrby R. A review of the penetration of antibiotics into CSF and its clinical significance. Scand J Infect Dis. 1978;14(Suppl):296-309.
55. Sande M.A., Sherertz R.J., Zak O., et al. Factors influencing the penetration of antimicrobial agents into the cerebrospinal fluid of experimental animals. Scand J Infect Dis. 1978;14(Suppl):160-163.
56. Bergman T. Kinetics of tissue penetration. Scand J Infect Dis. 1978;14(Suppl):36-46.
57. Craig W.A., Welling P.G. Protein binding of antimicrobials: clinical pharmacokinetics and therapeutic implications. Clin Pharmacokinet. 1977;2:252-278.
58. Fishman R.A. Blood-brain and CSF barriers to penicillin and related organic acids. Arch Neurol. 1966;15:113-124.
59. Schanker L.S. Passage of drugs into and out of the central nervous system. Antimicrob Agents Chemother. 1965;5:1044-1050.
60. Spector R., Levy P. The transport of gentamicin in the choroid plexus and cerebrospinal fluid. J Pharmacol Exp Ther. 1975;194:82-88.
61. Lutsar I., McCracken H., Friedland I.R. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin Infect Dis. 1998;27:1117-1129.
62. Parodi S., Lechner A., Osih R., et al. Nosocomial Enterobacter meningitis: risk factors, management, and treatment outcomes. Clin Infect Dis. 2003;37:159-166.
63. Huang C.R., Lu C.H., Chang W.N. Adult Enterobacter meningitis: a high incidence of coinfection with other pathogens and frequent association with neurosurgical procedures. Infection. 2001;29:75-79.
64. Lozier A.P., Sciacca R.R., Romagnoli M.F., Connolly E.S.Jr. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51:170-182.
65. Dureux J., Voiriot P., Auque J., et al. Bases of antibiotherapy in neuromeningeal infections. Neurochirurgie. 1988;34:72-82.
66. Fong I.W., Ranalli P. Staphylococcus aureus meningitis. QJM. 1984;53:289-299.
67. Mancebo J., Domingo P., Blanch L., et al. Postneurosurgical and spontaneous gram-negative bacillary meningitis in adults. Scand J Infect Dis. 1986;18:533-538.
68. Blomstedt G.C. Postoperative aseptic meningitis. Acta Neurochir. 1987;89:112-116.
69. Bryce G.E., Nedzelski J.M., Rowed D.W., et al. Cerebrospinal fluid leaks and meningitis in acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1991;104:817.
70. Moore G.F., Nissen A.J., Yonkers A.J. Potential complications of unrecognized cerebrospinal fluid leaks secondary to mastoid surgery. Am J Otol. 1984;5:317-323.
71. Sinner S.W., Tunkel A.R. Antimicrobial agents in the treatment of bacterial meningitis. Infect Dis Clin North Am. 2004;18:581-602.
72. Tunkel A.R., Hartman B.J., Kaplan S.L., et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267-1284.
73. De Bels D., Korinek A.-M., Bismuth R., et al. Empirical treatment of adult postsurgical nosocomial meningitis. Acta Neurochir. 2002;144:989-995.
74. Nordmann P., Cuzon G., Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228-236.
75. Sykes R.B., Bonner D.P. Aztreonam: the first monobactam. Am J Med. 1985;78(Suppl 2A):2-10.
76. Falagas M.E., Kasiakou S.K. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333-1341.
77. Wise R., Lister T., McNulty C.A.M., et al. The comparative pharmacokinetics of five quinolones. J Antimicrob Chemother. 1986;18(Suppl D):71-81.
78. Wolff M., Reginer P., Daldoss C., et al. Penetration of pefloxacin into cerebrospinal fluid of patients with meningitis. Antimicrob Agents Chemother. 1984;26:289-291.
79. Wolfson J.S., Hooper D.C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agent Chemother. 1985;28:581-586.
80. Ntziora F., Falagas M.E. Linezolid for the treatment of patients with central nervous system infection. Ann Pharmacother. 2007;41:296-308.
81. Brown E.M. Infections in neurosurgery: using laboratory data to plan optimal treatment strategies. Drugs. 2002;62:909-913.
82. Forgacs P., Geyer C.A., Freidberg S.R. Characterization of chemical meningitis after neurological surgery. Clin Infect Dis. 2001;32:179-185.
83. Ross D., Rosegay H., Pons V. Differentiation of aseptic and bacterial meningitis in postoperative neurosurgical patients. J Neurosurg. 1988;69:669-674.
84. Carmel P.W., Fraser R.A.R., Stein B.M. Aseptic meningitis following posterior fossa surgery in children. J Neurosurg. 1974;41:44-48.
85. Hlavin M.L., Kaminski H.J., Fenstermaker R.A., White R.J. Intracranial suppuration: a modern decade of postoperative subdural empyema and epidural abscess. Neurosurgery. 1994;34:974-980.
86. Yang K.-Y., Chang W.-N., Ho J.-T., et al. Postneurosurgical nosocomial bacterial brain abscess in adults. Infection. 2006;34:247-251.
87. Vogelsang J.P., Wehe A., Markakis E. Postoperative intracranial abscess: clinical aspects in the differential diagnosis to early recurrence of malignant glioma. Clin Neurol Neurosurg. 1998;100:11-14.
88. Guzman R., Barth A., Lovblad K.-O., et al. Use of diffusion-weighted magnetic resonance imaging in differentiating purulent brain processes from cystic brain tumors. J Neurosurg. 2002;97:1101-1107.
89. Rousseaux M., Lesoin F., Clarisse J., et al. Postoperative abscesses and empyemas: a propos of 13 cases. Neurochirurgie. 1986;32:304-310.
90. Bidzinski J., Koszewski W. The value of different methods of treatment of brain abscess in the CT era. Acta Neurochir. 1990;105:117-120.
91. Chandra R., Kapil A., Sharma P., et al. Identification of Acinetobacter species isolated from clinical specimens by amplified ribosomal DNA restriction analysis. Indian J Med Res. 2002;116:1-4.
92. Bullit E., Lehman R.A.W. Osteomyelitis of the skull. Surg Neurol. 1979;11:163-166.
93. Stendel R., Krischek B., Pietila T.A. Biodegradable implants in neurosurgery. Acta Neurochirurgica. 2001;143(3):237-243.
94. Bruce J.N., Bruce S.S. Preservation of bone flaps in patients with postcraniotomy infections. J Neurosurg. 2003;98:1203-1207.