Chapter 163 Management of Far Lateral Lumbar Disc Herniations
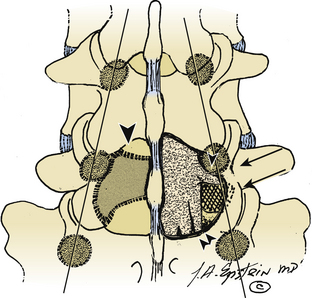
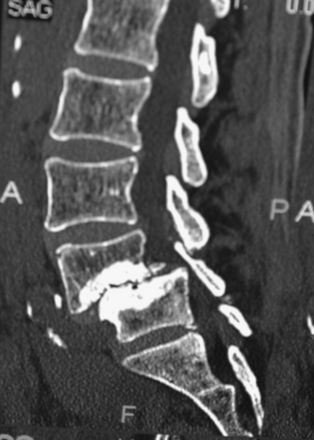
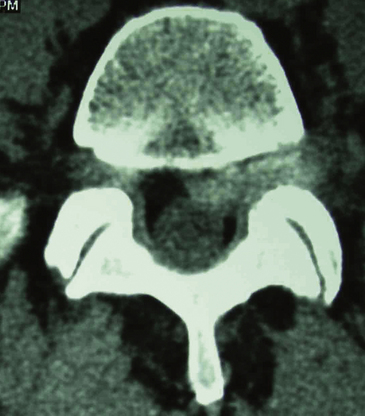
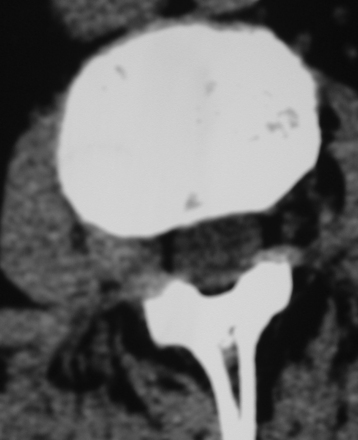
Far lateral disc (FLD) herniations account for between 6.5% and 12% of lumbar disc herniations.1–6 Operative resection techniques include minimally invasive procedures or microendoscopic alternatives, laminotomy, hemilaminectomy, and laminectomy with or without fusion (Figs. 163-1 to 163-9). The latter alternatives require differing degrees of facet resection depending on the location and complexity of the FLD and the accompanying degree and extent of spondylosis and stenosis. Minimally invasive techniques often include significant disruption of the ipsilateral pars and or facet joint, and they are typically performed with posterior lumbar interbody fusion and pedicle–screw instrumentation (open or percutaneous). Laminotomies and laminectomy decompressions rarely include just medial facetectomy or foraminotomy (possible only at the L5–S1 level) or the intertransverse approach, which are used primarily in younger patients who do not have significant attendant stenosis. Where patients have significant stenosis in conjunction with an FLD herniation, a single-level or multilevel laminectomy with focal full facetectomy are required along with non-instrumented and instrumented lumbar fusions. Alternatively, the extreme lateral approach performed either open or endoscopically removes little bone, thereby mitigating the requirement for fusion.
Attendant pathology can include foraminal, far lateral stenosis, degenerative spondylolisthesis, degenerative scoliosis, and/or limbus vertebral fractures. The type and extent of decompression and facet resection must be based on an individual patient’s pathology, because no single technique is universally appropriate.3 Clinical, neurodiagnostic, and surgical alternatives and outcomes for patients undergoing FLD surgery are reviewed.
Materials and Methods
Anatomy
Microdissection of more than 200 cadaver spines revealed that bony structures increasingly overlay the intervertebral foramen, resulting in less available space in the lateral and subarticular recesses as one progresses caudally in the lumber spine (see Figs. 163-1 to 163-9).7 Therefore, progressively more removal of medial bone from the pars interarticularis and facet joint are required at the lower lumbar levels to adequately expose the nerve root and ganglion. Typically, at the L5 and S1 level, where the interpedicular diameter is widest, almost no facet removal is required to expose the superiorly exiting nerve root as it extends into the foramen.
Cadaveric lumbar studies document that the foramen contains four distinct ligaments whose four bands extend radially from the nerve root sleeve.8 The first attaches posteriorly to the overlying facet capsule, two attach respectively to the superior and inferior pedicles, and the fourth is tethered to the disc annulus anteriorly. Additionally, a superolateral arterial arcade, which supplies the exiting nerve root, defines the vasculature of the extraforaminal compartment; to preserve this, dissection should be confined to its inferomedial aspect.9
Incidence and Location of Far Lateral Discs
Far lateral discs account for between 6.5% and 12% of all lumbar herniations and are typically sequestrated fragments found superolateral to the disc space of origin.1,3,10–16 In one series, FLDs were located foraminally (3%) or both intra- and extraforaminally (4%).17 FLD herniations compress the superiorly exiting nerve root and its dorsal root ganglion, producing deficits referable to the cephalad nerve roots. Thus, an FLD herniation at the L3–4 level contributes to L3 root compromise, and far lateral pathology at the L4–5 and L5–S1 levels contributes respectively to L4 and L5 root signs.
The majority of FLD herniations occur at the L3–4 or L4–5 levels, typically followed by L5–S1.2–4,11,14 FLDs rarely occur at the L5–S1 level, where the iliotransverse ligament provides excellent support if the L5 vertebra is located below the intercrestal line. More cephalad involvement at the L1–2 and L2–3 levels are rare, except in one study that cited a 28% incidence of FLDs at these cephalad sites.10 The location of 202 foraminal discs and FLDs in one series included the following levels: L1–2 (1 FLD), L2–3 (9 FLDs), L3–4 (48 FLDs), L4–5 (86 FLDs), and L5–S1 (58 FLDs).1
Clinical and Neurologic Parameters
Patients presenting with FLD herniations are typically in their mid-fifties (range, 19 to 78 years of age), with the ratio of male to female patients varying from 1:1 to 2:1.1–310 Severe radicular pain accompanies dorsal nerve root ganglion compression and inflammation in the far lateral compartment. Although the radicular pain is often excruciating and unremitting, the degree of back pain is significantly less. Patients with FLD compromising the femoral nerve roots (L2–4) complain of pain radiating into the hip, thigh, and medial aspect of the calf. Signs include a positive reverse Lasegue maneuver (femoral stretch test), iliopsoas and/or quadriceps weakness, a diminished or absent patellar response, and appropriate dermatomal sensory loss. As an example, the typical FLD at the L4–5 interspace compressing the L4 nerve root typically results in a positive femoral stretch test, weakness involving the tibial anticus and the extensor hallucis longus, a diminished Achilles response, and decreased pin appreciation in the L4 distribution. The more proximal location of complaints requires differentiation from intrinsic hip or knee pathology. X-ray and MR studies can identify intrinsic hip or knee disease, and arterial Doppler tests can help define whether vascular claudication is present.
Other Pathology Contributing to Far Lateral Root Compromise
Limbus Vertebral Fractures
Fractures of the vertebral limbus with or without lateral, foraminal, or far lateral stenosis or disc herniation can contribute to nerve root compression.18,19 There are four types of limbus vertebral fractures, I to IV. Type I fractures consist of a cortical rim separation of the posterior vertebral end plate, which extends across the full width of the disc space, resulting in central and foraminal stenosis. Type II fractures include both cortical and cancellous elements, with predominant midline intrusion on the thecal sac. Type III fractures are often found foraminally or far laterally, and may be noncalcified cartilaginous (III-A) or ossified (III-B). Type IV fractures involve the full sagittal length of the vertebral body, from one disc space to the next, and are often located centrally, resulting in significant thecal sac and nerve root compromise. Any of these fractures can arise from the cephalad or caudad vertebral end plates at any specified level.
Removal of limbus vertebral fractures located foraminally or far laterally often necessitates a full facetectomy to adequately expose the nerve root over its entire course.18 Resection first warrants removing the annulus from within the interspace. Next, morcellation of the bony limbus fracture is performed using a down-biting curette, tamp, and mallet technique, followed by delivery of these ossified fragments into the disc space cavity. Exposure for this type of decompression and resection may also be accomplished using microscopic endoscopic approaches with simultaneous fusion combining posterior lumbar interbody fusion and percutaneous pedicle screw instrumentation techniques. Nevertheless, manipulation and resection of the limbus fracture may be more readily and in many circumstances more safely accomplished with a larger exposure.
Stenosis and Spondylosis
Lumbar spinal stenosis and FLDs can coexist.8,12,19 In one series, FLDs were accompanied by stenosis, and surgical procedures were altered or extended in 72% of these older patients when magnetic resonance imaging (MRI) or noncontrast computed tomography (CT) studies were supplemented with myelo-CT examinations.2 An and colleagues similarly noted that 50 patients with FLDs and stenosis also required more extended decompressions.10 When Epstein reported on 857 patients undergoing surgery for lumbar stenosis, 40 patients demonstrated FLDs, and 5 exhibited far lateral stenosis.12,13,19
Degenerative Spondylolisthesis
Grade I degenerative spondylolisthesis or olisthy is most often encountered at L4–5, followed in descending order of frequency by the L3–4, L2–3, and the L5–S1 levels.15,16,20 Grade I olisthy is defined by a 25% slip of the vertebral body width and is usually limited by a locking of sagittally oriented, hypertrophied posterior facet joints.20 Disc herniations are encountered between 4.3% and 20% of the time with degenerative spondylolisthesis.
FLD herniations occurring at the level of a slip require a full unilateral facetectomy with instrumented fusion to prevent progressive instability in patients younger than 65 years, but many older than 65 years may be managed successfully with in-situ posterolateral intertransverse fusion.3,15 Good to excellent results are cited in up to 80% of patients.
Spondylolisthesis with Spondylolysis
When spondylolysis accompanies spondylolisthesis, the exiting nerve root becomes maximally compressed beneath the mobile and fractured pars interarticularis. Here, safe removal of an extruded or sequestrated disc fragment requires exposure of the nerve root along its entire intracanalicular, foraminal, and extreme lateral course. Many of these patients require simultaneous instrumented fusions, by either open pedicle screw-and-rod constructs or endoscopic posterior lumbar interbody fusions with percutaneous application of instrumentation. Good to excellent outcomes are typically reported in 80% to 85% of cases.16
Neurodiagnostic Evaluations
Magnetic Resonance Scans
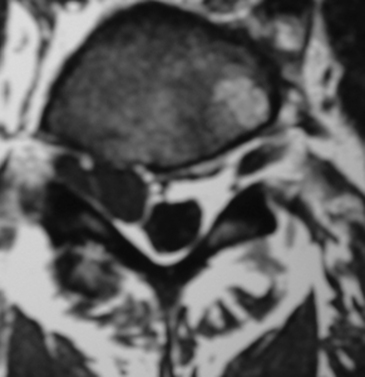
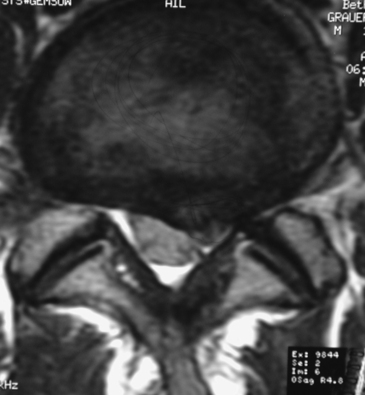
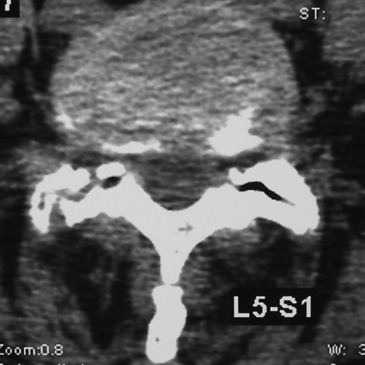
On MRI studies, far lateral soft disc herniations are located lateral to the pedicles and appear isointense or hypointense. Obliteration of the normally hypointense fat pad surrounding the dorsal root ganglion can signal the presence of an FLD herniation. Additionally, the presence of degenerative spondylolisthesis, spondylolisthesis and spondylolysis, or degenerative scoliosis can result in unilateral or bilateral foraminal or far lateral root compromise secondary to soft disc or calcified or ossified spondylosis. MRI studies document the multiple types of foraminal and far lateral nerve root compression (Figs. 163-10 to 163-12). However, CT better documents bony or ossified pathology, providing a direct hyperdense image, rather than the hypointense signal seen on MRI.
Gadolinium DTPA (diethylenetriamine-pentaacetic acid) enhanced MRI helps differentiate FLDs from other enhancing neoplasms.21 Postoperative enhanced MRI scans can distinguish between scar formation (enhancing) and recurrent disc herniations (nonenhancing).22
CT and Myelo-CT Scans
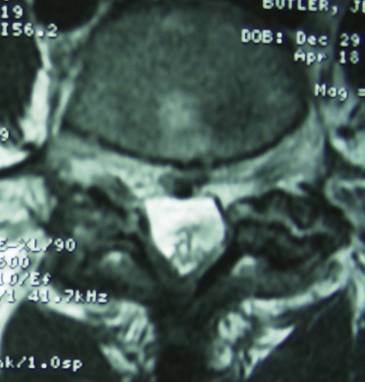
CT studies, with multiplanar reformations (two-dimensional to three-dimensional) or enhanced with iodine-based dyes, demonstrate foraminal and/or FLD herniations combined with spondylotic changes (Figs. 163-13 through 163-16).5,21 Soft FLDs can appear isodense, and accompanying limbus vertebral fractures, facet arthropathy, olisthy, and scoliosis appear hyperdense on CT-based studies. Additional intrathecal contrast used for myelo-CT studies better define central, lateral, and proximal foraminal stenosis. However, beyond the neural foramen, where the root is no longer invested with a subarachnoid sleeve, the addition of contrast does not provide any further imaging advantages. Noncontrast CT–based and myelo-CT studies better define the extent of stenosis when compared with MRI, and these added findings often significantly affect operative procedures performed in the elderly.2 Although CT discography can facilitate the demonstration of a far lateral lesion by revealing extravasation of dye far laterally, it is increasingly rarely used.
Conservative Management
The efficacy of conservative management in patients with FLD herniations, using steroidal and nonsteroidal medications, epidural steroid injections, and physiotherapy remains controversial. In one series of 17 patients, symptoms resolved in 12 (71%) without surgery.23 In another study, transforaminal injections of local anesthesia and depo-steroids provided immediate relief in 27 of 30 of patients and long-term relief in 22 of 28 patients; only 3 subsequently required surgery.24 Nevertheless, other studies show that only 10% of patients with FLD herniations can be successfully managed without surgery.3,4 The more severe the pain syndrome and the neurologic symptoms and signs, the more likely patients will choose surgery.
Critical Adjuncts to Far Lateral Disc Surgery
A randomized, controlled trial documented the efficacy of administering corticosteroids preoperatively and bupivacaine intraoperatively during lumbar disc surgery.25 The anti-inflammatory mechanism of the steroids includes the inhibition of phospholipase A2, which plays a critical role in alleviating pain production. Before the incision was made, the skin was infiltrated with 10 ml of 1% lidocaine with 1:200,000 adrenaline. At the time of wound closure, an additional 20 ml of 0.9% saline was given to the patients in group I (22 patients) and 20 ml of 0.25% bupivicaine in group II (22 patients). Postoperative pain medications were markedly reduced for the first 12 hours for those receiving the bupivicaine, and no significant differences were observed in heart rate or blood pressure.
Different Facet Resection Techniques
Medial Facetectomy
Excision of the medial 25% of the facet joint in conjunction with a laminotomy, hemilaminectomy, or laminectomy can facilitate resection of a proximal foraminal FLD herniation, but typically only at the L5–S1 level (see Fig. 163-3). However, only limited foraminal exposure is provided by this exposure, particularly in the presence of significant degenerative changes. Blind passage of angulated down-biting curettes or Woodson dissectors into the foramen and far lateral compartment without further facet excision can inadvertently damage the cephalad-exiting foraminal nerve root and should therefore be avoided.
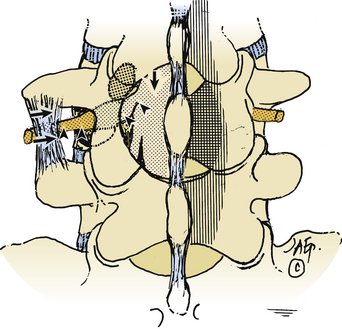
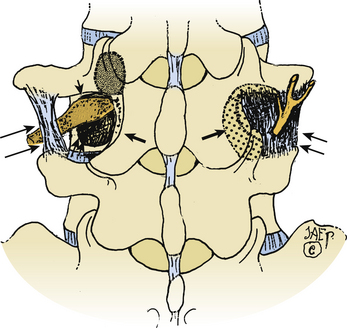
Full Facetectomy
Far lateral stenosis that is attributed to spondylosis, degenerative spondylolisthesis, spondylolisthesis with lysis, scoliosis, limbus fractures, or other degenerative pathology can require a complete unilateral or bilateral facetectomy to achieve sufficient far lateral exposure (see Figs. 163-4 and 163-5). The full facetectomy, familiar to most surgeons, offers the safest exposure and most complete visualization of the cephalad foraminally exiting nerve root along its entire course; this technique has the lowest incidence of inadvertent root injury and of retained disc fragments. Radiographically documented instability with unilateral facetectomy is relatively rare. Only 1 of 41 patients in Garrido and Connaughton’s series undergoing FLD excision with full facetectomy required fusion.5 In Epstein’s initial series, only 1 of 60 patients with FLDs also undergoing full unilateral facet removal later warranted a fusion.2 Of the 4 of 170 subsequent patients undergoing FLD surgery who required fusions, all 4 demonstrated preoperative grade I degenerative spondylolisthesis at the L4–5 level, the level of their FLD.3
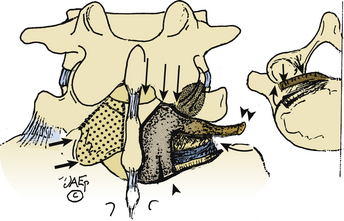
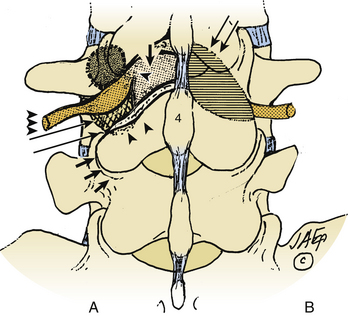
Trans-Pars Technique
The trans-pars approach to FLD herniations is fraught with deterrents (see Fig. 163-8). First, it requires that the far laterally exiting nerve root be isolated using a laminotomy at the superior interspace. Second, it has to be followed laterally, extending below the superior pedicle, thus requiring sacrifice of the pars interarticularis. Third, it is followed foraminally and far laterally by removing the entire inferior articular facet. Major disadvantages of this approach include the lack of access to the disc medially, increasing the chances of recurrent or residual disc, disrupting the pars and facet joint, and increasing instability. Of the 170 patients undergoing FLD surgery in my series, this approach was only employed once.3
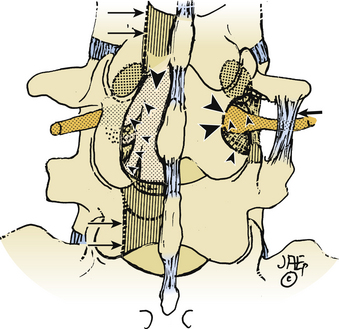
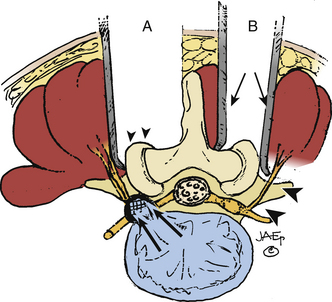
Isolated Extraforaminal (Extreme Lateral) Approach
The extreme lateral or extraforaminal exposure, using midline or paramedian muscle-splitting approaches, directly exposes the far lateral compartment (see Figs. 163-6 and 163-7).5,17,26 Excising the intertransversarii ligament and fascia exposes the cephalad nerve root exiting along a diagonal superomedial to inferolateral course. Using a cadaveric model consisting of 15 human L3 and L4 lumbar vertebrae, it was determined that excision of one fourth of the lateral pars interarticularis minimally increases the stress on the residual parts, thereby markedly reducing the risk of a delayed fracture. Alternatively, if the lateral half of the pars (thickest portion) is removed, the risk of weakening or spontaneous fracture markedly increases.27
The extraforaminal technique is chosen where pathology is confined to the far lateral compartment.17 In one series in which 25 FLDs were excised using the muscle-splitting approach, 2-year postoperative results revealed 48% excellent, 32% good, and 20% fair or poor results.28 Interestingly, postoperative low back pain and persistent dysesthesias constituted the major complaints that could not be radiographically attributed to instability. In another series of 40 patients undergoing extraforaminal resection of far lateral lesions, pain relief was achieved in 85% of patients; their success was attributed to preserved stability associated with limited bony decompression and facet excision.17 In a third series using a microsurgical far lateral approach to foraminal and extraforaminal FLD in 202 cases, outcomes (Macnab classification) were 31% excellent, 42% good, 20% fair, and 7% poor.1 In a fourth series of 25 patients using extreme lateral approaches to FLDs (no medial bone resection), postoperative neuropathic pain typically resolved over a 4- to 6-week period, and Visual Analog Pain Scale sores (7.7 preoperatively, 4.2 postoperatively) and Oswestry Scores (50.7% preoperatively and 34.7% postoperatively) improved.29
For patients who have had previous surgery, the isolated extraforaminal exposure provides access to the FLD and spondylosis while avoiding epidural scarring. However, limitations of this technique include a higher disc-recurrence rate because the disc space is not evacuated medially. In the study by Porchet’s group, 11 of 202 FLDs excised extraforaminally recurred: 4 in the same extreme lateral location, 5 paramedially, and 2 contralaterally.1 An added disadvantage is the lack of access to foraminal and intracanalicular spondyloarthrotic pathology (e.g., recurrent stenosis, ossification of the yellow ligament).
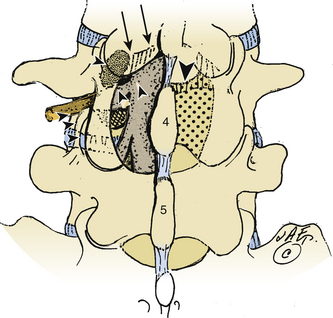
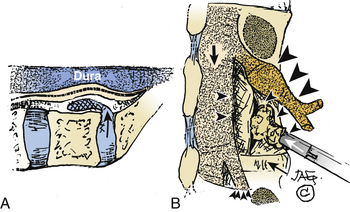
Intertransverse Technique: Medial Facetectomy and Extraforaminal Exposure
The intertransverse exposure combines the medial facetectomy with the extreme lateral approach (see Figs. 163-1 and 163-2).30 Medially, the thecal sac and proximally exiting nerve root are exposed, allowing evacuation of the disc and decompression of lateral recess stenosis. The pars interarticularis and the mid portion of the facet joint are preserved. The extraforaminal exposure includes removal of the lateral-most aspect of the superior articular facet. Advantages of the intertransverse technique include simultaneous medial and far lateral exposure, limiting the risk of disc recurrence or an exacerbation of postoperative symptoms attributable to lateral recess compromise.30,31
The extent of facet resection required to perform the intertransverse technique procedure raises concerns regarding the concurrent need for stabilization. Some studies have demonstrated that the risk of instability was not significantly altered by the varying degrees of facet resection. Postacchini and colleagues found under 50% (group I), 50% to 75% (group II), and 76% to 100% (group III).31
Endoscopic Techniques
Transforaminal arthroscopic and endoscopic techniques can be employed for foraminal and FLD excision. In one series using a transforaminal percutaneous endoscopic discectomy technique for foraminal FLD excision, 47 patients were followed for 18 months, and 85% exhibited good or excellent outcomes, but 11% showed poor results and later required open operations at the same level.32 A second transforaminal endoscopic series involved the removal of 100 FLDs (of 533 total discs), and observed a 91% success rate.33 Also, using a transforaminal percutaneous endoscopic discectomy technique for the resection of foraminal and FLD herniations, excellent to good outcomes were attained in 85% of 47 patients an average of 18 months postoperatively.6 Although 90% returned to work, 11% of patients required second open procedures to address residual same-level disease. In a fourth series consisting of 110 transforaminal endoscopic procedures over a 6-year period (2-year minimum follow-up), MacNab’s criteria showed an excellent to good postoperative outcome in 95% of cases.33 However, in a more recent series of percutaneous endoscopic discectomies performed in 66 patients (foraminal FLDs), mostly at the L3–4 (28%) and L4–5 levels (64%), problems were encountered in 9 patients (13.l6%): In 2 patients, disc material could not be accessed and required open surgery, 3 patients needed reoperation for inadequate disc removal (3 to 6 months), and in 4 patients nerve roots were damaged owing to direct operative trauma (2 patients) or owing to trocar placement (2 patients).34
Anterolateral Retroperitoneal Approach
Anterolateral retroperitoneal approaches to far lateral lumbar disc herniations commence with a left-sided incision above the 12th rib, followed by retroperitoneal dissection, retraction of soft tissues and the psoas muscle, and exposure at the appropriate level. Accompanying anterior lumbar interbody fusion may be completed with titanium cages or allograft bone dowels. If performed laparoscopically, less perineural and peridural scar forms, and the posterior elements remain undisturbed. Disadvantages include the morbidity of the transabdominal, retroperitoneal, or laparoscopic approaches, the risk of an anterior meningocele, poor visualization of the medial portion of the nerve root thereby risking root damage, and/or a retained or recurrent disc fragment. In Weinhert and Wiechert’s study using the lateral retroperitoneal approach to deal with FLD, 171 patients had modified anterior interbody fusions and 26 had total disc replacements; the average intraoperative blood loss was 100 ml, and the pseudarthrosis rate was 5%.35 In a second series employing a retroperitoneal laparoscopic lateral approach to 22 lumbar FLDs, facet joints and posterior elements were undisturbed, and the exposure was optimal for L5–S1 lesions or those near the conus at the L1–2 level.36
Fusion
Fusion Requirements
As the extent of facet resection increases, so does the risk of instability, warranting spinal fusion. Whereas the extraforaminal exposure does not compromise the facet joint, the medial facetectomy excises 25% of the medial facet joint, the intertransverse approach sacrifices 25% of the medial facet and up to 25% or more of the superolateral facet joint (the amount varies according to the level of involvement), and the full facetectomy sacrifices the entire inferior articular facet and the medial portion of the superior articular facet. Despite the varying degrees of facet resection, several studies have shown full facet resection only rarely results in the need for secondary stabilization.2,3,5
Percutaneous Pedicle Screw Fixation Techniques
FLD excision may include percutenaous pedicle screw-and-rod fixation. In the series of Khoo and colleagues, microendoscopic posterior lumbar discectomy, interbody fusion, and percutaneous pedicle screw-and-rod instrumentation (METRx, Tangent, and Sextant [Sofamor Danek, Memphis, TN]) techniques were used to perform routine and FLD excision.37 Foley and Gupta noted that standardized techniques for pedicle screw instrumentation in the lumbar region involved extensive exposures and muscle dissection, and they offered a new device for percutaneous placement of pedicle screw instrumentation.38 They developed an extension sleeve for remote manipulation of a polyaxial screw head and engagement of the screw-locking mechanism. The Sextant device also allowed a precut contoured rod to be placed between two screw heads percutaneously. Of the 12 patients in the original study (6 men and 6 women; age range, 23 to 68 years), 10 had attendant spondylolisthesis and 2 had nonunions associated with prior fusions. Ten single-level fusions (six at L5–S1, three at L4–5, and one at L2–3) and two two-level fusions (L3–5, L4–S1) were performed. When followed from 10 to 19 months (mean, 13.8 months), all successfully fused.
Resorbable Posterior Interbody Fusion Devices
Lowe and Coe performed 60 posterior transforaminal lumbar interbody fusions employing a resorbable polymer cage combined with autogenous bone graft in patients with degenerative lumbar disease.39 Although outcome studies are still pending, this procedure might prove particularly useful for the excision of FLDs where unilateral facetectomy and fusion are required.
Interbody Fusion Cages
Lumbar fusion can also be attained using interbody fusion cages placed either anteriorly (laparoscopically; anterior lumbar interbody fusion) or posteriorly (endoscopically; posterior lumbar interbody fusion). However, close evaluation of the results of these procedures reveals significant attendant morbidities. McAfee and colleagues reviewed 20 patients who required reoperations secondary to the placement of interbody fusion cages (Bagby and Kuslich BAK cage, Sulzer-Spine Technologies, Minneapolis, MN) and Ray threaded fusion cages (Surgical Dynamics, Norwalk, CT).40 Eight of the failed cages were placed anteriorly and 12 were placed posteriorly; the average interval between first and second procedures was 32 weeks (range, 1 to 156 weeks). Failure correlated with inadequate initial annular distraction, some cages that were too small, cerebrospinal fluid fistula, pseudomeningocele, use of local bone graft rather than iliac crest graft, anterior insertion too laterally resulting in symptoms of an FLD, and failure to define the midline during anterior surgery. All 20 patients had reexploration posteriorly at the level of presumed nerve root compression. In four instances, cages had migrated into the spinal canal. Although in five patients sites were considered fused on preoperative studies, pseudarthrosis was confirmed intraoperatively. Nine patients required posterior fusion with pedicle screw instrumentation.
The feasibility of implanting dual cages in the lumbar spines of 150 Asian men using 13 mm, 15 mm, and 17 mm cylindrical interbody fusion cages and based on preoperative MR studies, was evaluated by Wong and colleagues.41 The use of paired cages from the L3–4 through the L5–S1 levels without full facetectomy was explored; they concluded that without sacrificing the facets, no lumbar segment could accommodate dual cages. Furthermore, even with a unilateral facetectomy, only a few select individuals could receive dual cages at any of these levels. Indeed, most patients required full bilateral facetectomy for paired cage placement, significantly “iatrogenically” affecting stability at each of the involved levels.
Different morbidity rates have been reported following the placement of interbody fusion cages. In one series involving 67 posterior lumbar interbody fusions performed using titanium-threaded cages, 15% of patients (10 patients) developed cerebrospinal fluid fistulas, 25% required transfusions (average blood loss, 670 ml), 4.5% experienced minor wound problems, and one expired.42 Three months postoperatively, 42% exhibited significant low back pain that persisted in 15% over the first postoperative year; additional surgery was required in 20% of patients for persistent back pain or instability and consisted of pedicle screw instrumentation. Other morbidities included a permanent motor deficit accompanied by sexual dysfunction (one patient), postoperative pseudarthrosis (ten patients), lucency around the implant (seven patients), and posterior cage migration (two patients).
Resorbable Anterior Interbody Fusion Devices
In-vitro biomechanical studies have been performed to evaluate the efficacy of anterior stabilization on cage-assisted lumbar interbody fusions using a multilevel human cadaveric spine model.43 Three spinal procedures were analyzed employing harvested bone, bilateral multilevel cages, and cages with bioabsorbable anterior plates. Testing included flexion-extension, lateral bending, and axial rotation movements. Anterior resorbable plating decreased local motion and increased stiffness at the level of stabilization, thereby promoting fusion.
Beta Tricalcium Phosphate for Posterolateral Fusions
Posterolateral lumbar in-situ and instrumented fusions are increasingly being performed using an artificial bone graft expander, beta tricalcium phosphate (B-TCP).44–46 When looking at 40 patients undergoing multilevel laminectomies (average 3.7 levels), dynamic x-ray and CT-confirmed fusion occurred in 26 of 27 patients undergoing one-level fusion and in 11 of 13 patients undergoing two-level instrumented fusions; only one patient was sufficiently symptomatic to warrant secondary fusion.47 Evaluation of in-situ posterolateral fusions using B-TCP revealed in 60 and 75 patients respectively 15% and 17.3% frequencies of dynamic x-ray or CT documented fusion.44,45
Preoperative Psychometric Testing Used for Patient Selection
Preoperative patient selection for routine and FLD surgery should include an assessment for depression, because these symptoms and signs can negatively affect surgical outcomes. In one study, the predictive value of psychometric testing was evaluated in 50 patients before routine lumbar disc surgery; the series included 14 female patients and 36 male patients who averaged 40 years of age.44 The Back Depression Inventory, the State-Trait Anxiety Inventory, and the Pain Visual Analogue Scale were all used preoperatively and at 3 and 12 months postoperatively. Two years following surgery, 37 patients were pleased with their outcomes, 10 were not, and 3 could not be located. Those with histories of prior surgery exhibited more depression and were less satisfied with secondary surgical results; they also experienced greater anxiety and more postoperative pain. Using a combination of three questionnaires might successfully predict patients who would be dissatisfied with postoperative results (78%) and those who would be content with postoperative results (76%). The three instruments together could serve as useful screening tools for selecting optimal surgical candidates.
Outcomes of Far Lateral Disc Surgery
Different success rates have been reported following varied procedures used to address FLDs. Darden and colleagues and Siebner and Deckler observed an overall 85% rate of good to excellent results following extraforaminal surgery for FLDs.17,28 Porchet and colleagues observed excellent and good scores in 73% of patients undergoing microsurgical foraminal and FLD resections.1 Using transforaminal percutaneous endoscopic diskectomy techniques, Lew and colleagues noted excellent and good responses in 85%, with Ditsworth reporting a 95% success rate.1,6,33 Using the intertransverse, full facetectomy, and medial facetectomy approaches respectively for FLD excision, Epstein encountered 79%, 70%, and 68% good to excellent outcomes.3,4 Hodges and colleagues found mean preoperative and postoperative Oswestry Scores were 50.7% and 34.5% respectively.29
SF-36 outcomes analysis following 40 instrumented posterolateral lumbar fusions performed with B-TCP revealed moderate or marked improvement at 1 postoperative year on five of eight SF-36 Health Scales: Physical Function, Bodily Pain, Vitality, Social Function, and Role Emotional47 SF-36 outcomes at 1 and 2 years following 75 in-situ posterolateral fusions using B-TCP revealed comparable maximal improvement at both 1 and 2 years postoperatively on six of eight SF-36 Health Scales (exceptions: General Health, Mental Health).45
Far Lateral Disc Procedures
Clinical Data
Between 1984 and 1994, 170 of Epstein’s patients had FLD surgery.3 Patients were followed a mean of 5 years, averaged 55 years of age, and included 112 men and 58 women. The majority had subclinical symptoms for more than 2 years, but almost all experienced a subacute or acute exacerbation within 6 months of neurosurgical evaluation. On preoperative physical examination patients exhibited atrophy (31 patients) and positive mechanical findings: ipsilateral knee contractures (143 patients), positive femoral stretch tests (reverse Lasegue maneuvers) (145 patients), and positive Lasegue maneuvers (159 patients). Motor deficits (126 patients), reflex abnormalities (167 patients), and radicular sensory abnormalities (135 individuals) were commonly observed. However, evidence of massive central canal compromise and cauda equina syndromes were rarely seen (7 patients).
Flexion-Extension X-rays, Definition of Instability, and Secondary Fusions
Baseline and 3-month postoperative dynamic films were used to assess spinal instability. Where greater than 4 mm of active motion was demonstrated or where there was “fish-mouthing” at the involved level, patients were deemed radiographically unstable. In four patients with preoperative grade I olisthy at the L4–5 level who additionally had full facetectomy performed at L4–5 for the excision of lateral discs, fusions were secondarily required as instability was confirmed on both 3- and 6-month postoperative dynamic radiographs. The first patient in 1986 was managed with a Hibbs fusion and the three subsequent patients underwent pedicle screw–rod instrumentation. Currently, patients with olisthy at the level of FLD herniations receive fusion primarily using either open or percutaneous techniques.
Surgical Procedures
Lumbar spondyloarthrosis, stenosis, degenerative spondylolisthesis, and scoliosis accompanied FLD herniations in 36 of 170 patients.3 Laminectomies and more extensive facet resections were required to address the greater pathology; full facetectomy (58%), the intertransverse approach (medial facetectomy combined with extreme lateral approach 25%), and medial facetectomy alone (17%). In 134 patients, less-severe stenosis was managed with more-restricted facet resections: full facetectomy (38%), medial facetectomy (24%), and intertransversectomy (37%).
Interestingly, 25 of 170 patients required second procedures and 6 required third operations following initial FLD surgery. These additional operations included the removal of recurrent lateral discs and FLDs (15 patients), decompression of recurrent far lateral stenosis (15 patients), and in one instance, resection of a previously undiagnosed neurofibroma.3 The average interval between first and second operations was 49 months (range, 40 months to 7.5 years).
Common Errors In Far Lateral Disc Surgery
Frequent mistakes in FLD surgery include operating at the wrong level, making an incorrect diagnosis (e.g., diabetic amyotrophy), operating on the wrong side, improper patient selection (prohibitive comorbidities), and the failure to recognize far lateral stenosis. Far lateral stenosis, with or without FLDs, may be variously attributed to ossification of the yellow ligament, degenerative spondylolithesis or spondylolysis, limbus vertebral fractures, and lastly, but significantly, synovial cysts.48,49 When synovial cysts extrude into the spinal canal, they impinge on the cephalad foraminally or far laterally exiting nerve root, often extending well into the distal foramen, as well as on the inferiorly exiting nerve root in the lateral recess. However, although both the clinical symptoms and signs appear similar, different signal characteristics of the cyst fluid on MRI (hyperintense on T2), and MRI and CT documentation of cyst continuity with the contiguous facet joint help differentiate synovial cysts from foraminal discs or FLD. Nevertheless, in some cases, both entities appear together and must be simultaneously addressed. In Epstein’s study of synovial cysts, 45 occurred in conjunction with stenosis, and 35 occurred with stenosis or spondylolisthesis.48 Patients required average 3.8 and 3.5 level laminectomies, respectively, and demonstrated increases in postoperative slip in 5 of the 45 with stenosis alone and in 11 of the 35 with the original grade I slip (to grade II). In both groups improvement on the SF-36 Physical Function Scale of +44 and +38 points, respectively, were observed. When dealing with synovial cysts alone, with stenosis, with spondylolisthesis, and/or with an FLD, the full facetectomy exposure is often the safest and best approach, because by following the cephalad root all the way from the proximal interspace (lateral recess) through its foraminal or far lateral course, it will not be inadvertently damaged or sacrificed.
Outcome Study of Far Lateral Disc Surgery Using SF-36
Patient-based outcome measures are increasingly employed to assess surgical outcomes and set standards for operative policy.13 The Medical Outcomes Trust Short Form (SF-36) is a well-tested patient-based outcome instrument that has been successfully employed for more than two decades in more than 260 medical and surgical settings. It is easily administered in 15 minutes over the telephone or in the office. The SF-36 measures eight Health Scales reflecting different dimensions of outcome: Physical Function, Role Physical, Bodily Pain, General Health, Vitality, Social Function, Role Emotional, and Mental Health. Thirty-six generic questions are rated on graded scales. From these data, raw scores are calculated for each of the eight Health Scales and are then converted to a transformed scale (0% to 100%) (raw score minus lowest possible raw score for that health scale, divided by the raw score range multiplied by 100). Difference scores also take into account patients’ normative data based on age and sex obtained from 2474 individuals in the general U.S. population.
The SF-36 was completed by 76 (45%) of 170 patients in our FLD series from 1984-1994.3,4,14 One individual performed telephone interviews, and 100% of those contacted successfully completed the questionnaire. The average period between the last visit to the surgeon (the time of the surgeon-based assessment) and the administration of the questionnaire was 2.8 years (standard deviation, 2.3), with a range of 0.6 to 10.2 years. Those completing the outcome questionnaires averaged 60 years of age, and included 43 men and 33 women.
Along with the SF-36 patient-based outcome measure, the surgeon’s assessment of outcome, employing Odom’s criteria, was also obtained. The surgeon, an average of 9.1 months following surgery, last examined 76 patients, and their outcomes were categorized as excellent (32 patients, 42%), good (24 patients, 31.5%), fair (12 patients, 16%), and poor (8 patients, 11%). The median time between the operation and the surgeon’s outcome analysis was 4.5 months (average, 9.1 months): 75% were at less than 9 months and 90% were at less than 22 months, and the longest interval between surgery and final clinical assessment was 75 months. There was a statistically non-significant correlation (Spearman rank-order r, 0.13) between the surgeon’s evaluation and the time since surgery, the trend lending toward better outcome ratings for increased time since surgery.
An H.S., Vaccaro A., Simeone F.A., et al. Herniated lumbar disc in patients over the age of fifty. J Spinal Disord. 1990;3(2):143-146.
Darden B.V., Wade J.F., Alexander R., et al. Far lateral disc herniations treated by microscopic fragment excision. Techniques and results. Spine. 1996;20(13):1500-1505.
Epstein B.S., Epstein J.A., Jones M.D. Degenerative spondylolisthesis with an intact neural arch. Radiol Clin North Am. 1977;15:227-239.
Epstein J.A., Epstein N.E. Lumbar spondylosis and spinal stenosis. In: Wilkins R.H., Rengachary S.S. Neurosurgery. Ed 2. New York: McGraw-Hill; 1996:3831-3840.
Epstein N.E. An analysis of noninstrumented posterolateral lumbar fusions performed in predominantly geriatric patients using lamina autograft and beta tricalcium phosphate. Spine J. 2008;8(6):882-887.
Epstein N.E. Fusion rates and SF-36 outcomes after multilevel laminectomy and noninstrumented lumbar fusions in a predominantly geriatric population. J Spinal Disord Tech. 2008;21(3):159-164.
Epstein N.E. A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19(6):424-429.
Epstein N.E. Lumbar laminectomy for the resection of synovial cysts and coexisting lumbar spinal stenosis or degenerative spondylolisthesis: an outcome study. Spine. 2004;29(9):1049-1055.
Epstein N.E. Lumbar synovial cysts: a review of diagnosis, surgical management and outcome assessment. J Spinal Disord Tech. 2004;17(4):321-325.
Epstein N.E. Decompression in the surgical management of degenerative spondylolisthesis: advantages of a conservative approach in 290 patients. J Spinal Disord. 1998;11(2):116-122.
Epstein N.E. Evaluation of varied surgical approaches used in the management of 170 far-lateral lumbar disc herniations: indications and results. J Neurosurg. 1995;83:648-656.
Epstein N.E. Review article: different surgical approaches to far lateral lumbar disc herniations. J Spinal Disord. 1995;8(5):383-394.
Epstein N.E. Lumbar surgery for 56 limbus fractures, emphasizing non calcified type II lesions. Spine. 1992;17:1489-1496.
Epstein N.E., Epstein J.A. Surgery for spinal stenosis. In: Wiesel S.W., Weinstein J.N., Herkowitz H. The Lumbar Spine. Ed 2. Philadelphia: W.B. Saunders; 1996:737-757.
Epstein N.E., Epstein J.A., Carras R., Hyman R. Far lateral lumbar disc herniations and associated structural abnormalities. An evaluation in 60 patients of the comparative value of CT, MRI, and myelo-CT in diagnosis and management. Spine (US). 1990;15(6):534-539.
Epstein N.E., Epstein J.A. Lumbar decompression for spinal stenosis: surgical indications and techniques with and without fusion. In: Frymoyer J.W., Ducker T.B., Kostuik J.P., Whitecloud T.S. The Adult Spine. Ed 2. Philadelphia: Lippincott-Raven; 1997:2055-2088.
Epstein N.E., Hood D.C. A comparison of surgeon’s assessment to patient’s self analysis (Short Form 36) after far lateral lumbar disc surgery: an outcome study. Spine. 1997;22:2422-2428.
Foley K.T., Gupta S.K. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg. 2002;97(1 Suppl):7-12.
Garrido E., Connaughton P.N. Unilateral facetectomy approach for lateral lumbar disc herniation. J Neurosurg. 1992;76(2):342-343.
Jane J.A., Haworth C.S., Broaddus W.C. A neurosurgical approach to far-lateral disc herniation. Technical note. J Neurosurg. 1990;72(1):143-144.
McAfee P.C., Cunningham B.W., Lee G.A., et al. Revision strategies for salvaging or improving failed cylindrical cages. Spine. 1999;24(20):2147-2153.
Postacchini F., Cinotti G., Guimna S. Microsurgical excision of lateral lumbar disc herniation through an interlaminar approach. J Bone Joint Surg Br. 1998;80(2):201-207.
Siebner H.R., Faulhauer K. Frequency and specific surgical management of far lateral lumbar disc herniations. Acta Neurochir (Wien). 1990;105(3-4):124-131.
Wiltse L.L., Bateman J.G., Hutchinson R.H. The paraspinal sacrospinalis-splitting approach to the lumbar spine. J. Bone Joint Surg Am. 1960;50:919-921.
1. Porchet F., Chollet-Bornand A., de Tribolet N. Long-term follow up of patients surgically treated by the far-lateral approach for foraminal and extraforaminal lumbar disc herniations. J Neurosurg (US). 1999;90(suppl 1):59-66.
2. Epstein N.E., Epstein J.A., Carras R., Hyman R. Far lateral lumbar disc herniations and associated structural abnormalities. An evaluation in 60 patients of the comparative value of CT, MRI, and myelo-CT in diagnosis and management. Spine (US). 1990;15(6):534-539.
3. Epstein N.E. Evaluation of varied surgical approaches used in the management of 170 far-lateral lumbar disc herniations: indications and results. J Neurosurg. 1995;83:648-656.
4. Epstein N.E. Review article: different surgical approaches to far lateral lumbar disc herniations. J Spinal Disord. 1995;8(5):383-394.
5. Garrido E., Connaughton P.N. Unilateral facetectomy approach for lateral lumbar disc herniation. J Neurosurg. 1992;76(2):342-343.
6. Lew S.M., Mehalic T.F., Fagone K.L. Transforaminal percutaneous endoscopic discectomy in the treatment of far-lateral and foraminal lumbar disc herniations. J Neurosurg (US). 2001;94(suppl 2):216-220.
7. Schlesinger S.M., Frankhauser H., de Tribolet N. Microsurgical anatomy and operative technique for extreme lateral lumbar disc herniations. Acta Neurochir (Wien). 1992;118(3-4):117-129.
8. Grimes P.F., Massie J.B., Garfin S.H. Anatomic and biomechanical analysis of the lower lumbar foraminal ligaments. Spine. 2000;25(16):2009-2014.
9. Viswanathan R., Swamy N.K., Tobler W.E., et al. Extraforaminal lumbar disc herniations microsurgical anatomy and surgical approaches. J Neurosurg (US). 2002;96(2):206-211.
10. An H.S., Vaccaro A., Simeone F.A., et al. Herniated lumbar disc in patients over the age of fifty. J Spinal Disord (US). 1990;3(2):143-146.
11. Ebeling U., Reulen H.J. Are there typical localization of lumbar disc herniations? A prospective study. Acta Neurochir (Wien). 1992;117(3-4):143-148.
12. Epstein J.A., Epstein N.E. Lumbar spondylosis and spinal stenosis. In: Wilkins R.H., Rengachary S.S. Neurosurgery. Ed 2. New York: McGraw-Hill; 1996:3831-3840.
13. Epstein N.E., Epstein J.A. Surgery for spinal stenosis. In: Wiesel S.W., Weinstein J.N., Herkowitz H. The Lumbar Spine. Ed 2. Philadelphia: W.B. Saunders; 1996:737-757.
14. Epstein N.E., Hood D.C. A comparison of surgeon’s assessment to patient’s self-analysis (Short Form 36) after far lateral lumbar disc surgery: an outcome study. Spine. 1997;22:2422-2428.
15. Epstein N.E. Decompression in the surgical management of degenerative spondylolisthesis: advantages of a conservative approach in 290 patients. J Spinal Disord. 1998;11(2):116-122.
16. Epstein N.E. Primary fusion for the management of “unstable” degenerative spondylolisthesis. NeuroOrthopaedics. 1998;23:45-52.
17. Siebner H.R., Faulhauer K. Frequency and specific surgical management of far lateral lumbar disc herniations. Acta Neurochir (Wien) Austria. 1990;105(3-4):124-131.
18. Epstein N.E. Lumbar surgery for 56 limbus fractures, emphasizing non calcified type II lesions. Spine. 1992;17:1489-1496.
19. Epstein N.E., Epstein J.A. Lumbar decompression for spinal stenosis: surgical indications and techniques with and without fusion. In: Frymoyer J.W., Ducker T.B., Kostuik J.P., Whitecloud T.S. The Adult Spine. Ed 2. Philadelphia: Lippincott-Raven; 1997:2055-2088.
20. Epstein B.S., Epstein J.A., Jones M.D. Degenerative spondylolisthesis with an intact neural arch. Radiol Clin North Am. 1977;15:227-239.
21. Winter D.D., Munk P.L., Helms C.A. CT and MR of lateral disc herniation: typical appearance and pitfalls of interpretation. Can Assoc Radiol J (Canada). 1989;40(5):256-269.
22. Glickstein M.F., Sussman S.K. Time-dependent scar enhancement in magnetic resonance imaging of the postoperative lumbar spine. Skeletal Radiol. 1991;20(5):333-337.
23. Rust M.S., Olivero W.C. Far lateral disc herniations: the results of conservative management. J Spinal Disord. 1999;12(2):138-140.
24. Weiner B.K., Fraser R.D. Foraminal injection for lateral lumbar disc herniation. J Bone Joint Surg Br. 1997;79(5):804-807.
25. Mirzai H., Tekin I., Alincak H. Perioperative use of corticosteroid and bupivacaine combination in lumbar disc surgery: a randomized controlled trial. Spine. 2002;27(4):343-346.
26. Wiltse L.L., Bateman J.G., Hutchinson R.H. The paraspinal sacrospinalis—splitting approach to the lumbar spine. J. Bone Joint Surg Am. 1960;50:919-921.
27. Ivanov A.A., Faizan A., Ebraheim N.A., et al. The effect of removing the lateral part of the pars interarticularis on stress distribution to the neural arch in lumbar foraminal microdecompression at L3–L4 and L4–L5: anatomic and finite element investigations. Spine. 2007;32(22):2462-2466.
28. Darden B.V., Wade J.F., Alexander R., et al. Far lateral disc herniations treated by microscopic fragment excision. Techniques and results. Spine. 1996;20(13):1500-1505.
29. Hodges S.D., Humphreys S.C., Eck J.C., et al. The surgical treatment of far lateral L3–L4 and L4–L5 disc herniations. A modified technique and outcomes analysis of 25 patients. Spine. 1999;24(12):1243-1246.
30. Jane J.A., Haworth C.S., Broaddus W.C. A neurosurgical approach to far-lateral disc herniation. Technical note. J Neurosurg. 1990;72(1):143-144.
31. Postacchini F., Cinotti G., Guimna S. Microsurgical excision of lateral lumbar disc herniation through an interlaminar approach. J Bone Joint Surg Br. 1998;80(2):201-207.
32. Lew S.M., Mehalic T.F., Fagone K.L. Transforaminal percutaneous endoscopic discectomy in the treatment of far-lateral and foraminal lumbar disc herniations. J Neurosurg. 2001;94(suppl 2):216-220.
33. Ditsworth D.A. Endoscopic transforaminal lumbar discectomy and reconfiguration: a postero-lateral approach into the spinal canal. Surg Neurol. 1998;49(6):588-597.
34. Sasani M., Ozer A.F., Oktenoglu T., et al. Percutaneous endoscopic discectomy for far lateral lumbar disc herniations: Prospective study and outcome in 66 patients. Minim Invasive Neurosurg. 2007;50(2):91-97.
35. Weinhert K., Wiechert K. Microsurgical anterior approaches to the lumbar spine for interbody fusion and total disc replacement. Neurosurgery. 2002;51(suppl 5):159-165.
36. Dezawa A., Yamane T., Mikami H., et al. Retroperitoneal laparoscopic lateral approach to the lumbar spine: a new approach, technique, and clinical trial. J Spinal Disord. 2000;13(2):138-143.
37. Khoo L.T., Palmer S., Laich D.T., et al. Minimally invasive percutaneous lumbar interbody fusion. Neurosurgery. 2002;51(suppl 5):166-171.
38. Foley K.T., Gupta S.K. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg. 2002;97(suppl 1):7-12.
39. Lowe T.G., Coe J.D. Resorbable polymer implants in unilateral transforaminal interbody fusion. J Neurosurg. 2002;97(suppl 4):464-467.
40. McAfee P.C., Cunningham B.W., Lee G.A., et al. Revision strategies for salvaging or improving failed cylindrical cages. Spine. 1999;24(20):2147-2153.
41. Wong H.K., Goh J.C., Goh P.S. Paired cylindrical interbody cage fit and facetectomy in posterior lumbar interbody fusion in an Asian population. Spine. 2001;26(5):572-577.
42. Elias W.J., Simmons N.E., Kaptain G.J., et al. Complications of posterior lumbar interbody fusion when using a titanium threaded cage device. J Neurosurg. 2000;93(suppl 1):42-45.
43. Di Angelo D.J., Scifert J.L., Kitchel S., et al. Bioabsorbable anterior lumbar plate fixation in conjunction with cage-assisted anterior interbody fusion. J Neuorsurg. 2002;97(suppl 4):447-455.
44. Epstein N.E. An analysis of noninstrumented posterolateral lumbar fusions performed in predominantly geriatric patients using lamina autograft and beta tricalcium phosphate. Spine J. 2008;8(6):882-887.
45. Epstein N.E. Fusion rates and SF-36 outcomes after multilevel laminectomy and noninstrumented lumbar fusions in a predominantly geriatric population. J Spinal Disord Tech. 2008;21(3):159-164.
46. Kjellby-Wendt G., Styf J.R., Carlsson S.G. The predictive value of psychometric analysis in patients treated by extirpation of lumbar intervertebral disc herniations. J Spinal Disord. 1999;12(5):375-379.
47. Epstein N.E. A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19(6):424-429.
48. Epstein N.E. Lumbar laminectomy for the resection of synovial cysts and coexisting lumbar spinal stenosis or degenerative spondylolisthesis: an outcome study. Spine. 2004;29(9):1049-1055.
49. Epstein N.E. Lumbar synovial cysts: a review of diagnosis, surgical management, and outcome assessment. J Spinal Disord Tech. 2004;17(4):321-325.