Chapter 201 Management of Entrapment Neuropathies
Stewart1 defines entrapment neuropathies as “compression neuropathies that occur at specific places where nerves are confined to narrow anatomic pathways and therefore are particularly susceptible to constricting pressures.” The cause of precipitation of symptoms is always local and can be due to either internal or external factors. External factors, such as an increase in pressure in an osteomuscular tunnel (e.g., on the ulnar nerve at the elbow), can leave the gliding properties of the perineurium completely altered even when removed. Causes are either internal to the nerve itself due to systemic conditions (hereditary susceptibility to pressure, alcoholism, diabetes mellitus, uremia, carcinoma) or extraneural (presence of a mass in the osteomuscular tunnel, a particular anatomic configuration due to previous fractures or variations like anomalous muscles). The vast majority of cases operated on at our institution (Peripheral Nerve Injury Unit, Royal National Orthopaedic Hospital in London) are complications or failures referred from other centers where operations for “entrapment neuropathies” were previously performed.
Carpal tunnel syndrome provides a very good example, although it is not the topic of our chapter. It is the most common of all entrapment syndromes, and as such it is therefore possible to reveal the underlying cause of such a compression. Despite this, Rosenbaum and Ochoa2 showed that in a combined series of 2705 patients, a quarter of them had a systemic disease, 321 had rheumatoid arthritis, 166 were diabetic, and 94 had hypothyroidism. This is not to say that all these procedures should have been avoided, but that often the most common entrapment neuropathy should be considered a manifestation of systemic disease rather than a focal isolated problem.
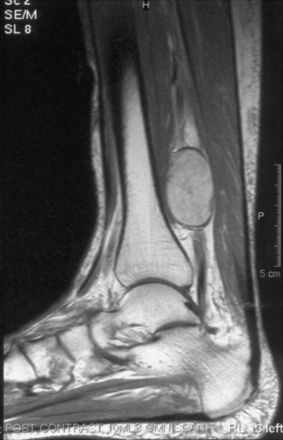
Magnetic resonance imaging (MRI) and ultrasound can easily determine whether there is a mass creating compression, such as a lipoma or intrinsic neural lesions (Fig. 201-1), or whether there is entrapment.
The Physiopathology of Chronic Nerve Compression
The process often begins with the interference of the nerve’s blood supply. Lundborg3 described the fascicular vascular unit, endoneural and perineural vascular systems linked segmentally to the mobile epineural vascular network. The integrity of this connection clearly depends on the ability with which the nerve glides in its surrounding connective tissues and the fascicles glide in the epineurium. Any restriction of excursion in either plane could interfere with blood supply. Rydevik et al.4 observed changes in both intra- and extra-fascicular microcirculation on applying a graded compression on the tibial nerve of rabbits. They found that at 20 to 30 mm Hg, there was already interference with the vascular flow, and at 60 to 80 mm Hg pressure there was no blood flow. Sunderland5 thought that the entire mechanism behind carpal tunnel syndrome was intrafascicular anoxia caused by obstruction of venous return. He stated that this produced edema and further increase in pressure.
There is, however, a peculiar anatomic lesion that characterizes chronic entrapment: the disappearance of myelin within the carpal tunnel. In 1963, Thomas and Fullerton6 analyzed a postmortem specimen of a patient with proven carpal tunnel syndrome and found that the myelin disappeared under the transverse ligament but reappeared distally. It was then clear that the focal slowing of nerve conduction was due to focal demyelination. In 1973, Ochoa and Marotte7 detected myelin changes extending also proximal and distal to the compression site. They described “resembling tadpoles, being bulbous at one end and tapered at the other” with a constant polarization (the bulb pointing away from the wrist proximally) but reversed distally (the bulb pointing away again). They also observed that with time, demyelination and remyelination occurred and eventually axons got interrupted with consequent degeneration of the distal segment. At electron microscopy they showed that “internal myelin lamellae had slipped away at the tapered ends and that these displaced lamellae had buckled at the bulbous ends.” Again Ochoa and Marotte7 stated that this phenomenon was simply a slippage of myelin lamellae followed by disintegration of the contorted myelin at the bulbs. Two years later these findings in the guinea pig were confirmed in human median nerves at the wrist and ulnar nerves at the elbow by Neary et al.8 They also found an enlargement in these nerves from increase in connective tissue. Pham and Gupta9 recently showed that Schwann cell proliferation and apoptosis in early phases of the disorder is the cellular mechanism behind the demyelination. In the area of the injury these proliferating cells downregulate myelin proteins, leading to local demyelination and remyelination. The changes occur before any axonal loss. The axons are in fact the last to be affected and the larger ones are the first to suffer, while unmyelinated fibers are more resistant until late in the course of the entrapment.
Management of Compressed Nerves
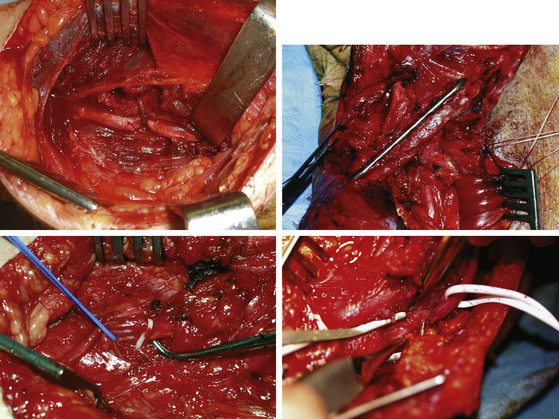
Initial nonoperative management includes simple measures such as avoidance of exacerbating activities, and use of splints and injections of steroids around the inflamed nerve. If these methods are unsuccessful, surgery is indicated. Surgical exposure must be adequate. Normal nerve proximal and distal to the lesion must be visualized. The dissection of the nerve must in fact be carried out from healthy tissue to the pathologic to better define the site and the nature of the lesion and therefore deal with it appropriately. All too often failures are due to insufficient decompression because of poor visualization of the nerve throughout its course. Before separating the nerve from the compressing structure, electrophysiologic evaluation may be useful. After releasing the compression, palpation of the lesion is very useful to an experienced surgeon. A nerve “woody” in texture is of course less likely to recover from simple decompression and neurolysis. Sometimes after opening or partial excision of the thickened epineurium, the texture of the nerve becomes softer (Fig. 201-2). Internal neurolysis should always be very careful and limited to the segment involved. The perineurium should rarely if ever be violated, and never when it is unscarred. Nerves in scarred beds should be placed in better positions without tension.10 Every possible attempt to restore the normal glide of the nerve is to be made at the time. To this end, in cases of previous failed decompression or recurrent symptoms it may even be necessary to consider the use of local soft tissue flaps or free flaps to achieve this. We think that as a general rule surgical decompression should aim to achieve a complete release of all the adhesions around its entire circumference to visualize the nerve throughout its course. External neurolysis should be reserved for cases where the texture of the nerve is hardened and internal neurolysis in the few cases where there is no conduction across the lesion and a “woody” nerve is present.
Postoperative care is centered on avoidance of formation of new scar and adhesions between the nerve and the surrounding tissues. Early mobilization is therefore recommended where possible. If tendons and muscles have been sectioned and repaired during the procedure the mobilization could be started very gently after 1 week.10
Ulnar Nerve at the Elbow
The possibility of many anatomic variations must be taken into account when considering ulnar nerve lesions. The Martin-Gruber anastomosis, in which motor fibers from the median reach the ulnar nerve and within it the small muscles in the hand, is reported between 10% and 44%.11 There are also possible variations in which the small muscles of the hand are completely innervated by the median nerve rather than by the ulnar nerve. For the first dorsal interosseous muscle, this possibility is reported at about 10%.11
Compression, either acute or subacute, occurs with direct trauma or with long continued pressure. This is what happens in the unconscious patient, in those confined to bed or partly immobile, and in those who have lost a large amount of weight over a very short period of time. The local swelling increases the compression between the arcuate ligament and the medial aspect of the elbow joint, producing the localized mononeuropathy. The same thing can happen without external compression or trauma. Osborne12 showed how with flexion of the elbow, the “ligament’s grip” on the ulnar nerve is tightened. Marginal osteophytosis or chronic effusion in the joint with bulging of the capsule or valgus deformity may increase the liability for compression at this particular level. Campbell et al.13 showed by intraoperative electroneurography that the usual site was the retrocondylar groove followed by the cubital tunnel itself. Laxity of the arcuate ligament may contribute to recurrent subluxation of the nerve over the medial epicondyle with flexion of the elbow. There are also rare causes of compression like “snapping” of the medial head of triceps14 or the presence of an anomalous anconeus epitrochlearis.15
Surgical Techniques
During simple decompression,16 which is adequate in most cases, the exposure of the nerve and its decompression by release of the arcade of the flexor carpi ulnaris are performed. The ulnar nerve is traced out through either an anterior semicircular incision centered on the medial epicondyle (author’s preference) or a rather linear incision basically on the course of the nerve. The nerve is identified proximal to the elbow behind the intermuscular septum and then followed distally. It is checked for any form of compression or tether along its course until the entrance in the flexor carpi ulnaris tunnel. Great attention is required to avoid damage to the branch to flexor carpi ulnaris at this point. In cases of severe deformity, the presence of significant osteophystosis, or recurrent subluxation or persistent symptoms following simple decompression exists, and then transposition is required. This will require a longer incision because of the necessity of releasing the intermuscular septum proximally, and the arcuate ligament distally, thus preventing a tether to the nerve once transposed. An incomplete release of these two structures is in fact one of the most frequent causes of failure and the need for reintervention following transposition. We recommend subcutaneous transposition, putting two or three sutures between the subcutaneous fat and the underlying aponeurosis of the flexor origin in order to retain the nerve in its new course. Immediate mobilization of the arm is recommended to avoid formation of scarring and tethering once transposed.
The submuscular transposition requires division of the superficial flexor muscles about 2 to 3 cm distal to the medial epicondyle, and any tendinous connections are divided to provide a straight bed for the nerve. The aponeurosis over the flexor origin is repaired with interrupted sutures, and immobilization is guaranteed with a right angle plaster for 2 weeks. Although previously advised by Seddon17 and more recently Janjua et al.,18 we do not advise submuscular transposition, having seen constriction, adhesion, and distortion of the nerve in the submuscular and intramuscular bed.
Ulnar Nerve at the Wrist
In some cases the compression can be at the level of the hook of the hamate, and sensation is normal throughout the fingers. Intrinsic motor paralysis is usually complete but hypothenar muscles can be spared. In pure motor involvement, pain is not a consistent feature. When the whole nerve is compressed, pain is localized at the wrist and radiates down to the ulna via two fingers. These lesions are more frequently the results of ganglia19 or of occupational compression. Other causes can be previous trauma with or without alteration of the anatomy at the level of the Guyon’s canal or even external compressions by crutches.
Median Nerve in the Axilla
This can occur because of pressure secondary to improper use of crutches in the axilla or as a consequence of trauma. Spinner and Spinner10 also described infraclavicular median nerve entrapment caused by anomalous vascular perforators or vascular arches in six patients treated surgically. Subclavian dynamic arteriography was helpful in confirming the median nerve penetration. With the arm at the side, the arterial system was visualized, whereas in abduction was not.
Other cases of compression of the median nerve at the axilla have been attributed to the presence of Langer’s muscle,20 which arises from the latissimus dorsi’s tendon insertion and terminates on the tendon of pectoralis major. We personally have found the presence of this muscle compressing the median nerve 6 months after shoulder dislocation, and following decompression a full resolution of the complete lesion was achieved after only 3 months.
Surgical Techniques
The anterior divisions of the upper and middle trunks join together to form the anterolateral cord. From this two branches arise: the lateral pectoral nerve and nerve to coracobrachialis. The musculocutaneous nerve and the lateral component of the median nerve then continue as its two terminal divisions. The anterior division of the lower trunk runs separately and gives origin to the medial pectoral nerve and medial cutaneous nerve of arm and forearm as branches, and to ulnar nerve and medial component of median nerve as terminal divisions. All three posterior divisions join together to form the posterior cord from which the thoracodorsal and subscapular nerve branches arise and the radial nerve and axillary nerve as terminal divisions.
Median Nerve in the Arm
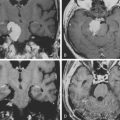
Lesions at the level of the arm are very rare and normally due to trauma (Fig. 201-3) or tourniquets used for achieving a blood-free field during surgery.
In 1% of the population there is a supracondylar process about 5 cm proximal to the medial epicondyle. Normally detected at routine x-ray and asymptomatic, it can sometimes be connected to the medial epicondyle by the Struther’s ligament. Behind the ligament, the artery and the median nerve can be kinked.21–25 Sensory and motor disturbances are present in different degrees. The pain is the main feature with involvement of the elbow, volar aspect of forearm, and sensory distribution of the median nerve in the hand.
Median Nerve at Pronator Arcade
This condition is very rarely due to anatomic variations but rather to repetitive physical activity with the forearm pronated. Initially pain occurs on the volar aspect of the forearm and characteristically increases during pronation against resistance, radiating also proximally. Pain and sensory disturbances are present along the territory of the nerve but motor deficits are exceptionally rare, although patients often complain about writer’s cramp. The median nerve is compressed between pronator teres and the fibrous arch of the flexor digitorum superficialis.26–28
Electrophysiology is not very helpful because of its relative normality, although cases of slowing of velocity across the lesion have been described.29 Operative findings include hypertrophy of the pronator teres muscle, passage of the median nerve under both heads of pronator teres, anomalous origin of the pronator teres, a thick fibrous band running through the muscle, or fibrous thickening of the edge of the flexor digitorum superficialis.30
Anterior Interosseous Syndrome
The anterior interosseous nerve arises from the median nerve beneath the flexor digitorum superficialis and runs in an oblique and radial direction. It supplies the flexor digitorum profundus for the index and middle and the flexor pollicis longus. It was first described by Kiloh and Nevin31 in 1952 and is characterized by pain in the proximal forearm and weakness of flexion of the distal joint of the thumb and of the index so that the patient is unable to effectively pinch the thumb and the index together (pinch or circle sign). Sensory deficits are absent. Electrophysiology may contribute to the diagnosis by comparing the motor latencies with the normal side, and in advanced cases by showing denervation signs in the affected muscles. Paralysis of the median nerve is incomplete, and it may be misleading in localizing the site of the lesion. The most difficult differential diagnosis is brachial plexus neuropathy and, rarely, anterior interosseous neuropathy. Other than finding more diffuse neurologic involvement when the brachial plexus is involved, the key is in the history where excruciating pain in the shoulder and arm lasts for a couple of weeks always precedes the deficit if a viral infection is the cause.
Surgical Techniques
The curvilinear incision across the elbow starts in the distal arm, and then runs transversely at the elbow crease and finishes in the proximal forearm between pronator teres and brachioradialis. The nerve is beneath the radial edge of pronator teres. To increase ability to move the median nerve during the dissection, the many motor branches present at this level must be mobilized, and the pronator teres can be mobilized or even resected. The flexor digitorum superficialis can then be opened longitudinally to follow the nerve distally and remove any sort of compression.
Radial Nerve at the Arm
Spontaneous radial nerve compression at this level may occur at the level of the lateral head of triceps.32,33 A common characteristic in the history is strenuous muscular exercise. Other causes, such as previous trauma, mass lesions, and external compression (Saturday night palsy), can be responsible. Spinner and Spinner10 described 12 cases of compression of the radial nerve in the axilla due to vascular penetration. Deficits are wrist drop with lack of extension of all fingers and sensory deficit in the dorsal ulna aspect of the thumb and first web space. If the lesion is at the level of the humeral groove, there will also be a lack of sensation in the dorsal aspect of the forearm due to the involvement of the posterior cutaneous nerve of the forearm. If the triceps is involved as well, then the lesion will be even higher.
Posterior Interosseous Nerve
The posterior interosseous nerve has been described as compressed by a fibrous band at the radiocapitellar joint34 by a portion of Henry’s vascular leash or by the proximal edge of extensor carpi radialis brevis. However, the most frequent site for compression is Frohse’s arcade, that is, the proximal edge of supinator.35–37 Entrapment may occur spontaneously, but the cause is often to be found in masses compressing the nerve or related to trauma.38
Surgical Techniques
Henry39 described the anterior approach to the posterior interosseous nerve, and Thompson40 the posterior approach. At times, particularly in trauma, they can be combined.
Spinner and Spinner10 have suggested the anterior approach if there is involvement of the supinator on the electromyography, and the posterior if the supinator is spared. If the proximal edge of the supinator is the potential site of entrapment, it would not be identifiable through a posterior approach.
Superficial Radial Nerve
The superficial radial nerve can be compressed at two levels. The first site is at the fascial edge of brachioradialis.41 The second is about 8 cm proximal to the radial styloid42 or at the styloid itself where it is superficial and very vulnerable to any sort of external compression (tight surgical gloves, watches, any constrictive band).
Lateral Cutaneous Nerve of Thigh
The symptoms are paresthesia in the anterolateral aspect of the thigh but very often pain of severe intensity and burning characteristics in the same area with associated sensory deficit. If they do not disappear spontaneously in a few weeks or months, then surgical release is indicated.43
Surgical Techniques
The incision is curvilinear and centered over the anterior iliac spine. The nerve is exposed medial to the tendon of the sartorious muscle and inferior to the inguinal ligament. The many anatomic variations should be always considered. Aszmann et al. described five different kinds44: type A (4%) posterior to the anterior superior spine, across the iliac crest; type B (27%) anterior to the anterior superior iliac spine and superficial to the origin of the sartorius muscle but within the substance of the inguinal ligament; type C (23%) medial to the anterior superior iliac spine, ensheathed in the tendinous origin of the sartorius muscle; type D (26%) medial to the origin of the sartorius muscle located in an interval between the tendon of the sartorius muscle and thick fascia of the iliopsoas muscle deep to the inguinal ligament; and type E (20%) most medial and embedded in loose connective tissue, deep to the inguinal ligament, overlying the thin fascia of the iliopsoas muscle, and contributing the femoral branch of the genitofemoral nerve. The nerve should be decompressed by neurolysis with incision of the iliacus fascia and the inguinal ligament and by dealing with the compression if related to the anatomic variation. We do not advise the resection of the nerve as previously suggested,45,46 because formation of a new painful neuroma may lead to recurrence of symptoms.
Common Peroneal Nerve
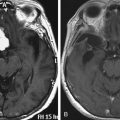
The nerve may be affected by external compression as in cases of prolonged bedrest, anesthesia, coma, tight plaster casts, or generally any sort of compression applied at this level. The deficit also may be caused by internal compression, such as entrapment after fractures of the fibular neck or ganglion of the tibiofibular joint (Fig. 201-4), which finds its way into the nerve through the articular branch of nerve.47,48 Other less common causes are post-traumatic false and true aneurysms or sometimes also Baker’s cysts.49,50 Foot drop is the key feature in the symptoms with associated sensory deficit of various degrees in the territory of the nerve.
Surgical Techniques
The patient is positioned either in a lateral or prone position. A curvilinear incision starting in the popliteal fossa, behind the tendon of the biceps muscle, and extending distally to follow the nerve as far as its divisions. The popliteal fascia is then opened longitudinally behind the tendon of biceps and the nerve identified. The nerve is followed distally as far as the compression site, which is most frequently as it passes around the fibular neck. The success of treatment is dependent on duration of the symptoms and deficit, and in cases of compression due to masses, the ability to remove the lesion creating the compressive force47 (Fig. 201-4).
Tibial Nerve at the Ankle
Compression of the nerve in its course before the tarsal tunnel is mainly due to the presence of masses that may be intra- or extra-neural (Fig. 201-1). This is a very challenging diagnostic problem.51 In its course within the tarsal tunnel, the nerve may be compressed due to post-traumatic fibrosis or a cyst arising from the adjacent tendons. The condition may also be spontaneous and slowly progressive. In these cases, it is most often associated with obesity, decreased elasticity of collagen, or a progressive acquired flat foot deformity in adults.52
Symptoms may include pain in the sole of the foot, positivity at percussion test behind the medial malleolus, and sensory deficit in the sole of the foot. Only rarely can weakness in the intrinsic muscles of the foot be detected.53
Surgical Techniques
The incision is longitudinal along the course of the tibial nerve behind the medial malleolus about 10 cm long. The nerve is identified proximal to the posterior tibial artery and vein, underneath the flexor retinaculum and posterior to the tendon of tibialis posterior muscle. The tarsal tunnel should be divided into a proximal zone, which extends from the retinaculum to the origin of the abductor muscle, and a distal zone, which begins at this level and continues through the muscle.54 Decompression of the nerve must therefore be carried out along the entire length of the tunnel, but as Mullick and Dellon suggested, it must include decompression of the calcaneal branch and medial and lateral plantar nerves in their respective tunnels.55 Early mobilization is essential to avoid formation of new scarring.
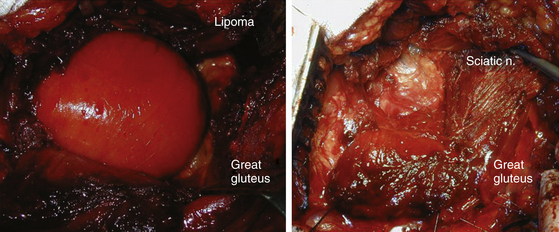
Sciatic Nerve
The sciatic nerve leaves the pelvis through the sciatic notch behind the piriformis muscle. The nerve is vulnerable to fractures of the ilium and sacrum as soon as it exits the sciatic notch. Posterior dislocation of the femur or fracture dislocation of the hip may contuse or compress it, masses displace it (Fig. 201-5), and endometrial implant56 can cyclically irritate it. Potential spontaneous entrapment at the piriformis level is still debated. Solheim et al.57 credited Yeoman58 with the first suggestion that the piriformis muscle had a role in the production of sciatica. Robinson59 described two cases of sciatica apparently caused by hypertrophy of the piriformis and relieved by the surgical release of the muscle. Stewart60 clarified the diagnosis criteria. A clinical picture of focal lesion of the sciatic nerve with electrophysiologic confirmation of a focal lesion, radiologic exclusion of other compression along the course of the nerve, appropriate findings at operation and resolution of the symptoms after the surgery were needed for the diagnosis. Compression of the sciatic nerve in the buttock by varicose gluteal veins have been identified by Bendszuz et al.61 Two of three patients found relief with surgery, and the third with carbamazepine. We concur with Tiel, who asked for renaming of the condition to “nonlocalizing sciatica” and restriction of surgical resection of the piriformis to cases where all other treatments have failed.62
Pain may be relieved after injections of local anesthetics and corticosteroids or botulinum as described by Childers et al.63 and Fishman et al.,64 and lately as introduced by Huerto and colleagues,65 using ultrasonography and electric stimulation.
Surgical Techniques
The exposure of the sciatic nerve at this level can be obtained either by splitting the fibers of the gluteus maximus muscle or by the more classic detachment of the tendon of the same laterally at its insertion on the femur. In the first case the incision is oblique, following the direction of the muscle fibers, about 10 cm in length, and centered on the neck of the femur, in the second case, a “question mark”–shaped incision is used to follow the glutei insertion on the femur and the sciatic nerve in the buttock.66 In this last approach, it is essential to identify the posterior cutaneous nerve of the thigh on opening the fascia to avoid injury to this structure.
“Iatropathic Entrapments”
Any nerve can become “entrapped” following trauma. Therefore, in any case where previous surgery has been performed, the possibility of iatropathic entrapment should be considered (Fig. 201-6). The symptoms may vary from paresthesia without motor involvement to excruciating pain with no motor function present. In any of these situations, and particularly when pain is present, surgical decompression gives the only possibility of abolishing pain and restoring function.
Al-Qattan M.M., Husband J.B. Median nerve compression by the supracondylar process: a case report. J Hand Surg. 1991;16b:101-103.
Cravens G., Kline D.G. Posterior interosseous nerve palsies. Neurosurgery. 1990;27:397-402.
Dellon A.L., Mackinnon S.E. Radial-sensory nerve entrapment in the forearm. J Hand Surg. 1986;11:199-205.
Goldner J.L., Hall R.L.. Nerve entrapment syndromes of the low back and lower extremities, Omer G.E.Jr., Spinner M., Van Beek A.L. Management of Peripheral Nerve Problems, 2nd ed, Philadelphia: WB Saunders, 1998.
Janjua R.M., Fernandez J., Tender G., Kline D.G. Submuscular transposition of the ulnar nerve for the treatment of cubital tunnel syndrome. Neurosurgery. 2008;63:321-324. discussion 324-325
Kiloh L.G., Nevin S. Isolated neuritis of the anterior interosseous nerve. BMJ. 1952;19:850-851.
Mannan K., Altaf F., Maniar S., et al. Cyclical sciatica: endometriosis of the sciatic nerve. J Bone Joint Surg Br. 2008;90:98-101.
Mullick T., Dellon A.L. Results of decompression of four medial ankle tunnels in the treatment of tarsal tunnels syndrome. J Reconstr Microsurg. 2008;24:119-126.
Narakas A.D. Compression and traction neuropathies about the shoulder and arm. In: Gelberman R.H., editor. Operative Nerve Repair and Reconstruction. Philadelphia: JB Lippincott; 1991:1149-1175.
Nawabi D.H., Sinisi M. Schwannoma of the posterior tibial nerve: the problem of delay in diagnosis. J Bone Joint Surg Br. 2007;89:814-816.
Neary D., Ochoa J., Gilliatt R.W. Sub-clinical entrapment neuropathy in man. J Neurol Sci. 1975;24:283-298.
Ochoa J., Marotte L. The nature of the nerve lesion caused by chronic entrapment in the guinea-pig. J Neurol Sci. 1973;19:491-495.
Osborne G.V. The surgical treatment of tardy ulnar neuritis. J Bone Joint Surg. 1957;39:782.
Pham K., Gupta R. Understanding the mechanisms of entrapment neuropathies. Review article. Neurosurg Focus. 2009;26:E7.
Rydevik B., Lundborg G., Ragge U. Effects of graded compression on intraneural blood flow. J Hand Surg. 1981;6:3-12.
Seddon H.J. Surgical Disorders of the Peripheral Nerves, 2nd ed. Edinburgh: Churchill Livingstone; 1975.
Spinner M., Spinner R.J.. Management of nerve compression lesions of the upper extremity, Omer G.E.Jr., Spinner M., Van Beek A.L. Management of Peripheral Nerve Problems, 2nd ed, Philadelphia: WB Saunders, 1998.
Spinner M. The arcade of Frohse and its relationship to the posterior interosseous nerve paralysis. J Bone Joint Surg. 1968;50:809-812.
Spinner R.J., Atkinson J.L., Scheithauer B.W., et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99:319-329.
Sunderland S. The nerve lesion in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1976;39:615-626.
Thomas P.K., Fullerton P.M. Nerve fibre size in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1963;26:520-527.
Tiel R.L. Other median nerve compression syndromes. In: Kaye A.H., Black P.McL. Operative Neurosurgery. London: Churchill Livingstone, 2000.
Tiel R.L. Piriformis and related entrapment syndromes: myth & fallacy. Neurosurg Clin North Am. 2008;19:623-627.
1. Stewart J.D. Proximal median neuropathies: electromyographic and clinical correlation. Muscle Nerve. 1993;16:321-322.
2. Rosenbaum R.B., Ochoa J.L. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. Oxford: Butterworth Heinemann; 1993.
3. Lundborg G. Vascular systems. In: Nerve Injury and Repair. Edinburgh: Churchill Livingstone; 1988.
4. Rydevik B., Lundborg G., Ragge U. Effects of graded compression on intraneural blood flow. J Hand Surg. 1981;6:3-12.
5. Sunderland S. The nerve lesion in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1976;39:615-626.
6. Thomas P.K., Fullerton P.M. Nerve fibre size in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1963;26:520-527.
7. Ochoa J., Marotte L. The nature of the nerve lesion caused by chronic entrapment in the guinea-pig. J Neurol Sci. 1973;19:491-495.
8. Neary D., Ochoa J., Gilliatt R.W. Sub-clinical entrapment neuropathy in man. J Neurol Sci. 1975;24:283-298.
9. Pham K., Gupta R. Understanding the mechanisms of entrapment neuropathies. Review article. Neurosurg Focus. 2009;26:E7.
10. Spinner M., Spinner R.J.. Management of nerve compression lesions of the upper extremity, Omer G.E.Jr., Spinner M., Van Beek A.L. Management of Peripheral Nerve Problems, 2nd ed, Philadelphia: WB Saunders, 1998.
11. Stohr M., Dawson D.M., Swash M.. Compression neuropathies of peripheral nerves and compartment syndromes, Brandt T., Caplan L.R., Dichgans J., Diener H.C., Kennard C. Neurological Disorders: Course and Treatment, 2nd ed, San Diego: Elsevier, 2003.
12. Osborne G.V. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2:10-13.
13. Campbell J.N., Raja S.N., Meyer R.A., Mackinnon S.E. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89-94.
14. Rolfsen L. Snapping triceps tendon with ulnar neuritis. Report on a case. Acta Orthop Scand. 1970;41:74-76.
15. Dahners L.E., Wood F.M., Hill C. Anconeus epitrochlearis, a rare cause of cubital tunnel syndrome: a case report. J Hand Surg. 1984;9:579-580.
16. Osborne G.V. The surgical treatment of tardy ulnar neuritis. J Bone Joint Surg. 1957;39:782.
17. Seddon H.J. Surgical Disorders of the Peripheral Nerves, 2nd ed. Edinburgh: Churchill Livingstone; 1975.
18. Janjua R.M., Fernandez J., Tender G., Kline D.G. Submuscular transposition of the ulnar nerve for the treatment of cubital tunnel syndrome. Neurosurgery. 2008;63:321-324. discussion 324-325
19. Shea J.D., McClain E.J. Ulnar nerve compression syndromes at and below the wrist. J Bone Joint Surg. 1969;51:1095.
20. Narakas A.D. Compression and traction neuropathies about the shoulder and arm. In: Gelberman R.H., editor. Operative Nerve Repair and Reconstruction. Philadelphia: JB Lippincott; 1991:1149-1175.
21. Struther J. On the processus supra-condyloideus humeri of man. Trans Int Med Congr London. 1881;1:148.
22. Al-Qattan M.M., Husband J.B. Median nerve compression by the supracondylar process: a case report. J Hand Surg. 1991;16b:101-103.
23. Crotti F.M., Mangiagalli E.P., Rampini P. Supracondyloid process and anomalous insertion of pronator teres as sources of median nerve neuralgia. J Neurosurg Sci. 1981;25:41-44.
24. Laha R.K., Dujovny M., De Castro C. Entrapment of median nerve by supracondylar process of the humerus. A case report. J Neurosurg. 1977;46:252-255.
25. Smith R.V., Fisher R.G. Struthers ligament, a source of median nerve compression above the elbow. J Neurosurg. 1973;38:778-779.
26. Seyfarth H. Primary myoses in the muscle pronator teres as a cause of lesion of the nerve medianus (the pronator syndrome). Acta Pshychiatry Neurolog. 1951;74:251-254.
27. Spinner M. Injuries to Major Branches of the Forearm, 2nd ed. Philadelphia: WB Saunders; 1978.
28. Eversmann W.W. Proximal median nerve compression. Hand Clin. 1992;8:307-315.
29. Johnson R.K., Spinner M., Shrewsbury M.M. Median nerve entrapment syndrome in the proximal forearm. J Hand Surg Am. 1979;4:48-51.
30. Tiel R.L. Other median nerve compression syndromes. In: Kaye A.H., Black P.McL. Operative Neurosurgery. London: Churchill Livingstone, 2000.
31. Kiloh L.G., Nevin S. Isolated neuritis of the anterior interosseous nerve. BMJ. 1952;19:850-851.
32. Lotem N., Fried A., Levy M., et al. Radial palsy following muscular effort. A new compression syndrome possibly related to a fibrous arch of the lateral head of the triceps. J Bone Joint Surg. 1971;53:500-506.
33. Lubahn J.D., Lister G.D. Familial radial nerve entrapment syndrome: a case report and literature review. J Hand Surg. 1983;8:297-299.
34. Roles N.C., Maudsley R.H. Radial tunnel syndrome. Resistant tennis elbow as a nerve entrapment. J Bone Joint Surg. 1972;54:499-508.
35. Spinner M. The arcade of Frohse and its relationship to the posterior interosseous nerve paralysis. J Bone Joint Surg. 1968;50:809-812.
36. Spinner M. Injuries to the Major Branches of Peripheral Nerves of the Forearm, 2nd ed. Philadelphia: WB Saunders; 1978.
37. Papadopoulus N., Paraschos A., Pelekis P. Anatomical observations on the arcade of Frohse and other structures related to the deep radial nerve. Anatomical interpretation of deep radial nerve entrapment neuropathy. Folia Morphol. 1989;37:319-327.
38. Cravens G., Kline D.G. Posterior interosseous nerve palsies. Neurosurgery. 1990;27:397-402.
39. Henry A.K. Extensile Exposure, 2nd ed. Baltimore: Williams & Wilkins; 1970.
40. Thompson J.E. Anatomical methods of approach in operations on the long bones of the extremities. Ann Surg. 1918;68:309-329.
41. Wartenberg R. Cheiralgia paresthetica. Z Neurol Psychiatry. 1932;141:145.
42. Dellon A.L., Mackinnon S.E. Radial-sensory nerve entrapment in the forearm. J Hand Surg. 1986;11:199-205.
43. Williams P.H., Trzil K.P. Management of meralgia paresthetica. J Neurosurg. 1991;74:76-80.
44. Aszmann O.C., Dellon E.S., Dellon A.L. Anatomical course of the lateral femoral cutaneous nerve and its susceptibility to compression and injury. Plast Reconstr Surg. 1997;100:600-604.
45. Beresford H.R. Meralgia paresthetica after seatbelt trauma. J Trauma. 1971;11:629-636.
46. Edelson J.G., Nathan H. Meralgia paresthetica. Clin Orthop. 1977;122:255-262.
47. Spinner R.J., Atkinson J.L., Scheithauer B.W., et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99:319-329.
48. Spinner R.J., Scheithauer B.W., Amrami K.K. The unifying articular (synovial) origin of intraneural ganglia: evolution-revelation-revolution. Neurosurgery. 2009;65:115-124.
49. Cobb C.A., Maiel R.H. Ganglion of the peroneal nerve. J Neurosurg. 1974;41:255-259.
50. Garland H., Moorhouse D. Compressive lesions of the external popliteal (common peroneal) nerve. BMJ. 1952;2:1373-1378.
51. Nawabi D.H., Sinisi M. Schwannoma of the posterior tibial nerve: the problem of delay in diagnosis. J Bone Joint Surg Br. 2007;89:814-816.
52. Edwards W.G., Lincoln C.R., Bassett F.H.III, Goldner J.L. The tarsal tunnel syndrome. JAMA. 1969;207:716.
53. Dawson D.M., Hallet M., Wilbourn A.J. Entrapment Neuropathies, 3rd ed. Philadelphia: Lippincott-Raven; 1999.
54. Goldner J.L., Hall R.L.. Nerve entrapment syndromes of the low back and lower extremities, Omer G.E.Jr., Spinner M., Van Beek A.L. Management of Peripheral Nerve Problems, 2nd ed, Philadelphia: WB Saunders, 1998.
55. Mullick T., Dellon A.L. Results of decompression of four medial ankle tunnels in the treatment of tarsal tunnels syndrome. J Reconstr Microsurg. 2008;24:119-126.
56. Mannan K., Altaf F., Maniar S., et al. Cyclical sciatica: endometriosis of the sciatic nerve. J Bone Joint Surg Br. 2008;90:98-101.
57. Solheim L.F., Siewers P., Paus B. The piriformis muscle syndrome. Sciatic nerve entrapment treated with section of the piriformis muscle. Acta Orthop Scand. 1981;52:73-75.
58. Yeoman W. The relation of arthritis of the sacroiliac joint to sciatica. Lancet. 2, 1928. 1119–1112
59. Robinson D.R. Pyriformis syndrome in relation to sciatic pain. Am J Surg. 1947;73:355-358.
60. Stewart J.D. Focal Peripheral Neuropathies, 3rd ed. Philadeplhia: Lippincott Williams and Wilkins; 2000.
61. Bendszuz M., Rieckmann P., Perez J., et al. Painful vascular compression syndrome of the sciatic nerve caused by gluteal varicosities. Neurology. 2003;61:985-987.
62. Tiel R.L. Piriformis and related entrapment syndromes: myth & fallacy. Neurosurg Clin North Am. 2008;19:623-627.
63. Childers M.K., Wilson D.J., Gnatz S.M., et al. Botulinum toxin type A use in piriformis muscle syndrome: a pilot study. Am J Phys Med Rehabil. 2002;81:751-759.
64. Fishman L.M., Anderson C., Rosner B. BOTOX and physical therapy in the treatment of piriformis syndrome. Am J Phys Med Rehabil. 2002;81:936-942.
65. Huerto A.P., Yeo S.N., Ho K.Y. Piriformis muscle injection using ultrasonography and motor stimulation—report of a technique. Pain Physician. 2007;10:687-690.
66. Henry A.K. Extensile Exposure, 2nd ed. Edinburgh: E & S Livingstone; 1957.