Management of end-stage heart failure: Heart transplantation and ventricular assist devices
End-stage heart failure
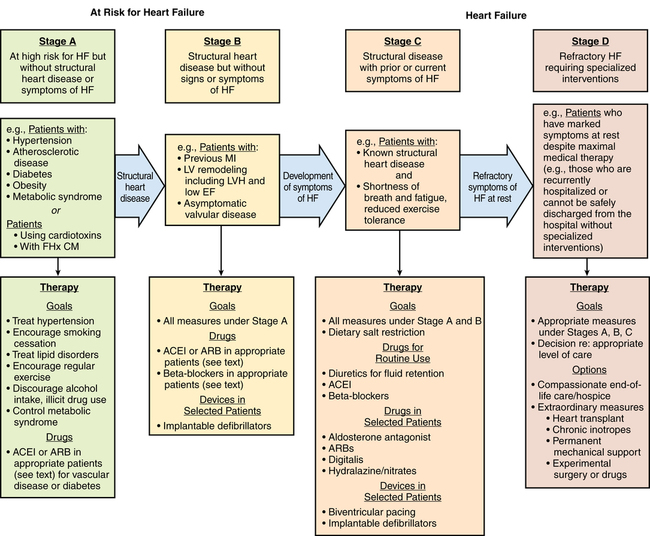
Echocardiography is used to assess ventricular function, to identify structural and functional cardiac abnormalities, and to guide therapy. The American Heart Association classification defines four stages of HF: A through D. Stage D is end-stage HF, (Figure 143-1). Coronary artery disease is the most common cause of both systolic and diastolic failure. Other causes include dilated nonischemic, restrictive, hypertrophic, and stress-induced cardiomyopathy. The most common cause of death is ventricular arrhythmia.
Ventricular assist devices
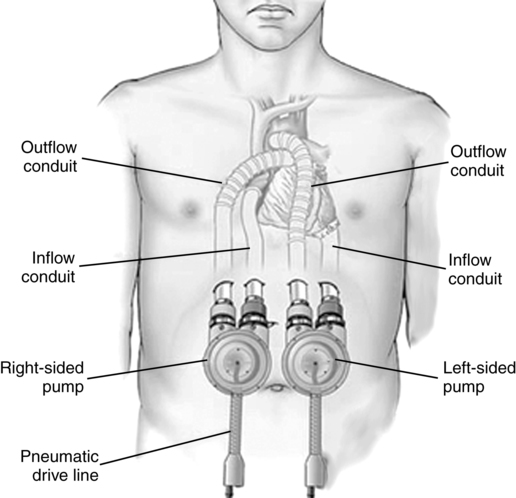
VADs to support the left, right, or both ventricles are either pulsatile or nonpulsatile pumps, located paracorporeally or intracorporeally, and are used as a bridge to recovery (short-term), a-bridge to transplantation, or destination therapy. The first-generation VADs use pulsatile pumps with valves that displace a given volume of blood with every beat. One pulsatile pump is still marketed in the United States, a paracorporeal VAD (pVAD; Thoratec, Pleasanton, CA) for short-term to intermediate-term use in patients who require bridge to transplantation or bridge to recovery (Figure 143-2). Approximately 10% of patients recover sufficient function to be weaned completely from mechanical support. Compared with previous devices, the pVAD allows for greater patient mobility (a portable device is available for patients who leave the hospital) and longer-term use (weeks to months and, in a few cases, years); its use is associated with lower rates of morbidity. Short-term anticoagulation is provided with heparin, whereas long-term anticoagulation requires warfarin and sometimes aspirin.
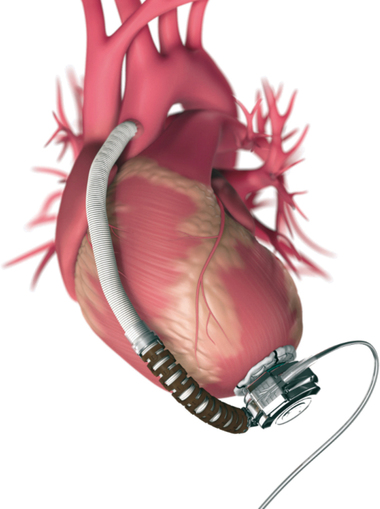
Second-generation VADs are smaller, intracorporeal, nonpulsatile, axial-flow pumps without valves. Third-generation VADs are bearingless and use a combination of magnetically and hydrodynamically suspended impellers. Currently available in the United States are the Heartmate II (Thoratec), a second-generation device approved by the U.S. Food and Drug Administration (FDA) for bridge to transplantation in 2008 and for destination therapy for patients who are not candidates for heart transplantation in 2010 (Figure 143-3), and the HeartWare ventricular assist system (Framingham, MA), a third-generation device approved in 2012 by the FDA as a bridge to transplantation (Figure 143-4).
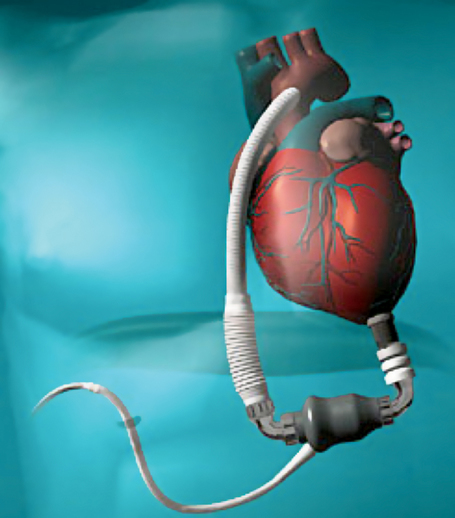
Total artificial heart
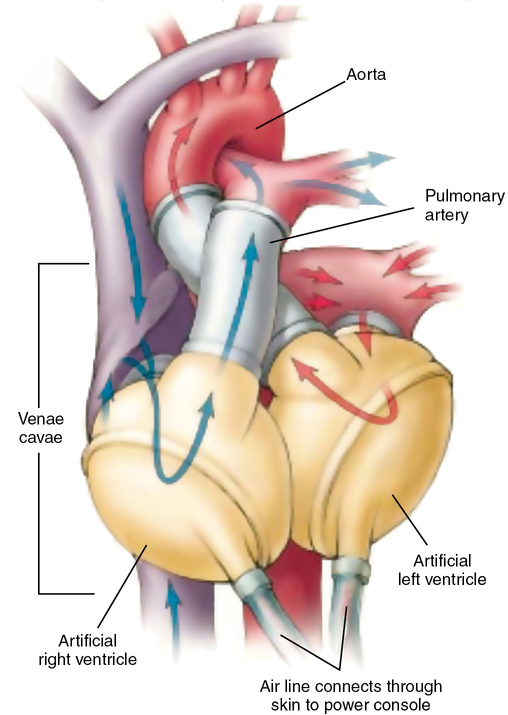
SynCardia (Tucson, AZ) produces two TAHs, each consisting of two independent pulsatile devices (ventricles) that, once the native ventricles are excised, are anastomosed to the native atria with the outflow cannulas inserted into the ascending aorta and pulmonary outflow tract, respectively. The FDA approved the 75-mL TAH (Figure 143-5) as a bridge to transplant in 2004 and designated two humanitarian use device labels for the 50-mL TAH in 2013—destination therapy and pediatric bridge to transplant.