Chapter 182 Management of Chiari Malformations and Syringomyelia
Definition and History
In 1891, Hans Chiari described three types of cerebellar malformations that were associated with hydrocephalus.1,2 He added a fourth type 5 years later.1,2 The type I Chiari malformation was described as herniation restricted to the tonsils and adjacent cerebellum, which was associated with elongation of these structures into a conical shape. The cerebellar tonsils were atrophic and attached to the dorsal medulla by fibrous adhesions.3 Chiari malformation type II included protrusion of the medulla, fourth ventricle, cerebellar vermis, and cerebellar tonsils into the spinal canal. The insertion of the tentorium was low, the tentorial hiatus widened, and posterior fossa small in these patients.4 This malformation was present at birth and arose in association with myelomeningocele and hydrocephalus. Chiari III malformation consisted of a rare deformity in which the cerebellum herniated through a defect in the suboccipital bone or upper cervical lamina and created an occipitocervical encephalocele. Beaking of the tectum, elongation and kinking of the brain stem, hydrocephalus, and lumbar spina bifida were also present.3 This condition resulted in severe neurologic deficits and early mortality. Chiari type IV malformation was extremely rare and was characterized by severe cerebellar hypoplasia, but with the cerebellum and brain stem remaining within the posterior fossa.5 Mortality in infancy is the rule with this malformation.
In recent years other authors have expanded the Chiari classification system to include other types of lesions involving the hindbrain and cerebrospinal fluid (CSF) pathways at the foramen magnum. The “Chiari 0 malformation” is a condition in which the obex, but not the cerebellar tonsils, are inferiorly located and a syrinx is found within the cervical segments of the spinal cord in an identical anatomic location as syringomyelia associated with Chiari I malformation.6 In this condition a membrane covers the foramen of Magendie or bridges the subarachnoid space, and the volume of the posterior fossa volume is smaller than normal.7,8 The “Chiari 1.5 malformation” is a condition in which cerebellar ectopia is restricted to the cerebellar tonsils, as in Chiari I malformation, but contrary to Chiari’s original description of the Chiari I malformation, the brain stem is also caudally displaced.9
In syringomyelia a cyst forms within the spinal cord and produces myelopathy. The word syrinx is derived from the Greek word for “reed or pipe,” which in classical mythology is the form that the nymph Syrinx assumed to escape pursuit from the Greek god Pan.10 Surgical treatment for syringomyelia was initiated by Abbe and Coley, who performed a syringostomy in 1892.11 In 1938 the Chiari I malformation was first described in adults12 and in a patient without hydrocephalus13 based on intraoperative and postmortem observations.14
In 1950 James Gardner and associates at the Cleveland Clinic recognized the association of the Chiari I malformation with syringomyelia.15 They postulated that the outlets of the fourth ventricle were occluded by the Chiari I malformation and that a water-hammer pulsation was directed from the fourth ventricle, through the obex, and into the central canal of the spinal cord, leading to pulsatile expansion of the central canal to form a syrinx. To reverse this process, Gardner performed a surgical procedure that removed the bone from the posterior aspect of the foramen magnum, opened the fourth ventricle to the subarachnoid space, and plugged the obex.15
In the 1970s Logue introduced a less-invasive alternative to Gardner’s procedure. His procedure consisted of simple bony decompression and expansion of the dura with a tissue graft, and avoidance of opening of the arachnoid membrane and entrance into the subarachnoid space or fourth ventricle. His group performed a clinical study comparing Gardner’s procedure with their procedure of simple decompression and duraplasty and demonstrated that there was no difference in syrinx resolution between the procedures, although Gardner’s operation resulted in a higher complication rate.16,17 Since then, some investigators have advocated a decompressive procedure that opens the arachnoid membrane, removes or shrinks the inferior portion of the cerebellar tonsils, and attempts to enlarge the CSF pathways beyond what is achievable with bony decompression and duraplasty alone.18 Syringomyelia resolves following this latter procedure in about 80% of cases, which is similar to the results reported for simple decompression and duraplasty with preservation of the arachnoid membrane and tonsils (Table 182-1).
Development
In patients with the Chiari I malformation, the bones of the skull base often are underdeveloped, which results in reduced volume of the posterior fossa.19–23 The Chiari I malformation therefore appears to result not from a primary cerebellar anomaly but rather from a smaller-than-normal posterior fossa, the volume of which is inadequate to contain the entire cerebellum; as a result, the cerebellar tonsils are displaced into the cervical spinal canal. Genetic disorders and diseases that affect skull development and reduce intracranial volume can result in the development of secondary Chiari I malformation.24–28 However, most patients with Chiari I malformation do not have an associated disease to explain the development of this condition. In them, environmental and genetic factors presumably contribute to underdevelopment of the posterior fossa and development of the Chiari I malformation.
Chiari type II malformation is associated with myelomeningocele and primary and secondary brain anomalies. Cerebellar gliosis and atrophy occur, along with distortion and hypoplasia of cranial nerve, olivary, and pontine nuclei. Cerebral findings include focal cortical dysplasia, gray heterotopias in the hemispheric white matter and subependymal zone of the lateral ventricles, and thickening of the massa intermedia.3 The posterior fossa in Chiari II malformation is even smaller than in Chiari I malformation. The CSF cisterns are poorly developed in these patients, theoretically because the CSF cisterns did not expand during development because the outlets of the fourth ventricle are obstructed29 and the CSF circulation is down the central canal and into the fistula in the myelomeningocele, rather than into the basilar cisterns.30 Drainage of CSF through the myelomeningocele in utero encourages herniation of the posterior fossa structures through the foramen magnum. Following repair of the myelomeningocele, obstruction of the CSF pathways in the basilar cisterns, and often at the cerebral aqueduct, prevent normal CSF flow, which results in hydrocephalus.30 Ventricular shunting effectively treats hydrocephalus in these patients. Symptomatic syringomyelia can subsequently arise years later in the context of shunt malfunction, spinal cord tethering, or compression of CSF pathways by the malformation at the foramen magnum. Treatment for syringomyelia is directed toward its etiology and includes restoring CSF drainage by shunt revision or craniocervical decompression, relieving tension on the spinal cord by spinal cord untethering, or draining the syrinx directly using a syringopleural or syringoperitoneal shunt. Medullary compression can develop and requires prompt craniocervical decompression.
Syringomyelia occurs as a consequence of another abnormal process. There is general agreement that in syringomyelia the CSF pathways are encroached upon by an underlying condition. Because syrinx fluid is identical in chemical composition to CSF, syrinx formation likely results from a process that increases the movement of CSF into the spinal cord.31 Autopsy and radiographic studies rarely demonstrate a patent central canal in adult patients with syringomyelia,32 so it appears that development of syringomyelia does not occur by expansion of the central canal of the spinal cord by CSF that is transmitted from the fourth ventricle (Gardner’s water hammer theory).
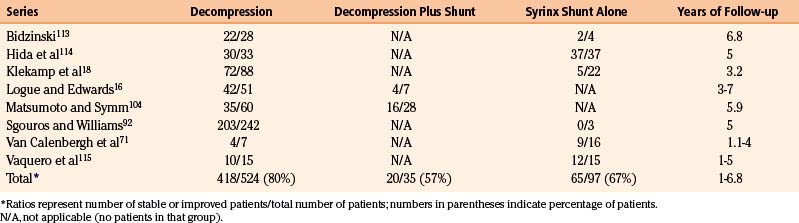
Another mechanism of syrinx formation and progression has been proposed that does not require a patent central canal.33 The mechanism is initiated by the Chiari malformation partially obstructing CSF pathways at the foramen magnum, which prevents the normally rapid efflux and influx of CSF between the head and the spine that compensates for brain expansion and contraction during the cardiac cycle. In lieu of CSF, the cerebellar tonsils are displaced during the cardiac cycle, creating a piston effect on the partially enclosed spinal subarachnoid space that produces enlarged cervical subarachnoid pressure waves, which compress the spinal cord from without, direct CSF into the spinal cord, and cause pulsatile syrinx flow, which leads to syrinx progression (Figs. 182-1 and 182-2).33 A prospective study of patients with Chiari I malformation and syringomyelia that were evaluated before, during, and after surgery provided radiographic and physiologic findings that were consistent with this mechanism.20
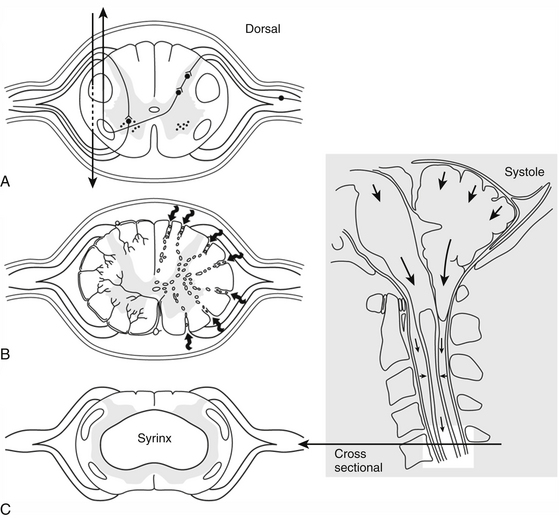
(Reproduced from Heiss JD, Oldfield EH. (February 2010) Syringomyelia and Related Diseases. In: Encyclopedia of Life Sciences: John Wiley & Sons, Ltd: Chichester http://www.els.net/WileyCDA/ElsArticle/refId-a0002208.html, with permission.)
Improved understanding of the pathophysiology of syringomyelia encourages the design and implementation of procedures directed toward eliminating the obstruction of CSF pathways, that is, strategies designed to reverse the pathophysiologic process underlying syringomyelia. Procedures that effectively open the CSF pathways at the foramen magnum, in the case of Chiari I malformation and basilar invagination, or in the spinal canal, in the setting of post-traumatic and postinflammatory syringomyelia, provide effective and lasting treatment of syringomyelia with low morbidity (Figs. 182-3 and 182-4). Since the 1990s the use of syrinx shunts for the treatment of syringomyelia has declined to the extent that shunts are now used only as a last resort.
Clinical Characteristics
In many patients, Chiari I malformation is diagnosed on the basis of non-neurologic symptoms such as suboccipital headache and cough headache; headache and neck pain are typical symptoms in young children.34 Neurologic signs and symptoms arising from the cerebellum, medulla, or central spinal cord are often found in patients with Chiari I malformation, either from direct compression of the cerebellum or medulla at the foramen magnum or from syringomyelia or syringobulbia. Lateral or downbeat nystagmus, ataxia, and reduced gag reflex are neurologic findings consistent with Chiari I malformation. In cases in which neither neurologic findings nor typical symptoms occur, the clinical significance of a mild degree of tonsillar ectopia cannot be determined. Generalized headache, chronic fatigue, and similar symptoms usually have etiologies other than Chiari I malformation. Thus, it is important not to overinterpret the magnetic resonance imaging (MRI) finding of a mild degree of tonsillar ectopia in the absence of syringomyelia.
Prevalence of syringomyelia is not clearly established, but one estimate is that symptomatic syringomyelia occurs in about 8.4 cases per 100,000 population.35 The Chiari I malformation is present in 70% of patients with syringomyelia.18 Basilar invagination causes 10% of cases of syringomyelia.36 Syringomyelia associated with conditions of the spine below the craniocervical junction is known as primary spinal syringomyelia and accounts for about 16% of all cases of syringomyelia. This type of syringomyelia may be posttraumatic, postinflammatory, or compressive.36,37 Patients with posttraumatic syringomyelia present several months or years after the initial trauma.36,38–45 The incidence of syringomyelia following trauma that produces paraplegia was estimated to be 1% to 4% in the pre-MRI era, when it was difficult to detect and could not be detected with noninvasive diagnostic techniques, although with the availability of MRI the incidence is much higher.46
Postinflammatory syringomyelia results from a delayed reaction to chronic meningitis, either infectious or chemical.47–51 Syringomyelia also occurs in association with compression of the CSF pathways by extramedullary tumors or cysts, osteophytes, or herniated intervertebral disks.52–62 Intramedullary spinal cord tumors cause about 4% of the cases of syringomyelia and can do so without narrowing the CSF pathways.36 Syrinx fluid in primary spinal syringomyelia is identical to CSF, whereas syrinx fluid in syringomyelia associated with an intramedullary tumor is highly proteinaceous.31,63,64
Patients with syringomyelia present with symptoms of paralysis, sensory loss, and chronic pain, which most commonly develop during the second through the fifth decades of life. The natural history of syringomyelia is typically one of gradual, stepwise neurologic deterioration over many years.65–67 Although syringomyelia is uncommon, typical signs and symptoms often suggest its diagnosis. The cervical segments of the spinal cord are affected first in syringomyelia associated with the Chiari I malformation, resulting in upper extremity symptoms of pain, weakness, atrophy, and loss of pain and temperature sensation. Early in its course, the signs and symptoms of syringomyelia may be mild and confined to a restricted area of the body. As the syrinx displaces and destroys the central gray matter of the spinal cord, the upper extremities become weak, atrophic, hyporeflexic, and devoid of normal pain and temperature sensation (see Fig. 182-2). Sensory loss is considered to be disassociated, because sensation of pain and temperature is lost, while light touch is preserved, and suspended, because the sensory loss hangs between regions of normal sensation.46 Both sides of the body are usually affected, but asymmetric extension of the syrinx to one side of the spinal cord results in more-severe involvement of the upper extremity on that side.
If left untreated, the upper extremity dysfunction progresses over months to years and eventually is accompanied by spasticity in the lower extremities as the syrinx expands outside the gray matter and into the corticospinal tracts.68 In less-advanced cases, the diagnosis of Chiari I malformation and syringomyelia is often made by MRI while evaluating a patient in whom another, more common condition, such as a herniated cervical disc, cervical spondylosis, or scoliosis, is suspected.34
In untreated patients with Chiari I malformation and syringomyelia, the pace of neurologic deterioration is more rapid initially and slows after the signs of syringomyelia are well established.66 This time course is consistent with an incidence of neurologic deterioration of 10% to 24% per year in studies in the MRI era, in which deterioration is measured early in the disease,69–71 compared with 2% to 3% per year in the pre-MRI era,65,66 when deterioration was measured later in the disease and after definite central myelopathy had developed.
Primary spinal syringomyelia is associated with lesions within the spinal subarachnoid space or with deformity of the spinal canal and is often recognized months or years after spinal trauma or meningitis. It manifests as ascending loss of motor and sensory function, which usually signals involvement of the spinal cord above the original level of injury.32
Diagnostic Evaluation
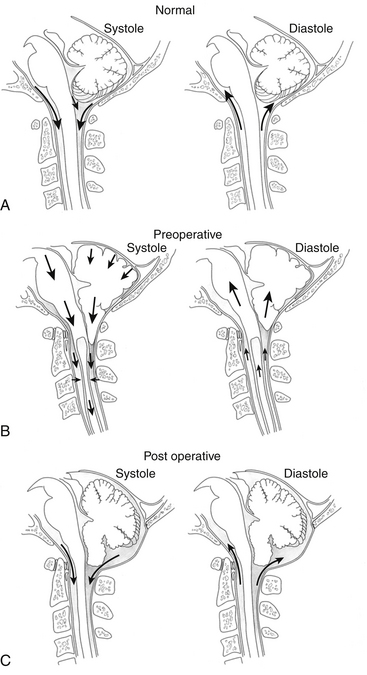
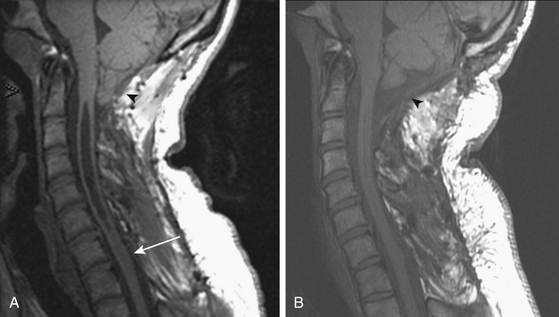
Radiographic evaluation of syringomyelia should include T1- and T2-weighted MRI studies of the brain and cervical and thoracic spine, performed with and without contrast (see Figs. 182-3 and 182-4). Brain MRI detects hydrocephalus or a posterior fossa mass. Lumbar spine MRI may be included if there is consideration of tethering by the filum terminale, although the conus medullaris is usually in its normal position in patients with Chiari I malformation.72,73 MRI clearly defines the presence of syringomyelia and detects associated conditions such as the Chiari I malformation, basilar invagination, an intracranial mass that is causing cerebellar herniation, or a spinal tumor. In general, tonsillar ectopia of 5 mm or less is considered normal,74 although up to 6 mm is normal in those younger than 10 years of age.75 Symptomatic Chiari I malformation patients have a mean tonsillar ectopia of 13 mm, although symptoms have been reported with as little as 3 mm of ectopia.74
Many persons have MRI findings of “incidental Chiari I malformation” and are asymptomatic but meet the radiographic criteria for diagnosis of the condition. One study found that 0.9% of normal adults undergoing MRI studies of the brain have tonsillar herniation extending more than 5 mm below the foramen magnum.76 In a retrospective review of more than 22,000 hospitalized patients, 14% of patients with radiographic findings of Chiari I malformation were clinically asymptomatic.77 In another retrospective series of 68 patients with MRI findings of Chiari I malformation, 30% of patients were asymptomatic. In this study, ectopia over 12 mm was always associated with symptoms.78 Radiographic diagnosis should also take into account Chiari’s original description that the tonsils should be conical rather than round1,2 and the extent of narrowing of the CSF pathways by tonsillar impaction into the foramen magnum. Although cine MRI has helped clarify the pathophysiology of syringomyelia, it is unclear whether phase-contrast cine-MRI (CSF flow study) adds diagnostic information beyond that provided by structural MRI studies.
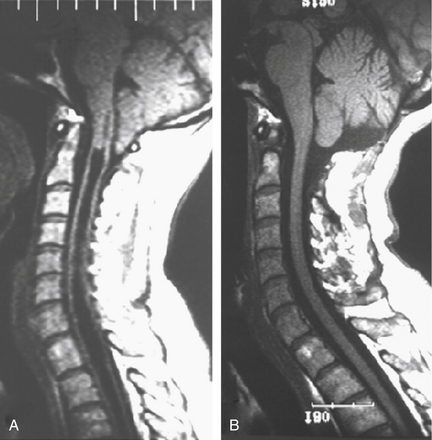
On T1- and T2-weighted MRI, syringomyelia appears as a well-circumscribed intramedullary fluid-filled mass (see Figs. 182-3 and 182-4). Enhancement of the spinal cord indicates the presence of an associated intramedullary tumor. In patients with primary spinal syringomyelia of unknown etiology, axial imaging of the spine using T2-weighted and fast imaging employing steady-state acquisition (FIESTA) MRI sequences might reveal previously undetected arachnoid cysts and arachnoid adhesions (Fig. 182-5, and see Fig. 182-4). Water-soluble CT-myelography has little clinical use in patients with syringomyelia associated with Chiari I malformation, but it is often useful in primary spinal syringomyelia because of its ability to localize and outline the extent of abnormalities within the spinal subarachnoid space. Certain findings on the spinal MRI predict more-rapid neurologic progression, particularly distention of the spinal cord by a syrinx of large diameter (more than 5 mm) and the presence of associated spinal cord edema.79 On the other hand, a narrow syrinx that does not distend the spinal cord might no longer be associated with an active pathophysiologic process and is less likely to cause neurologic progression. Some patients with long-standing symptoms of syringomyelia have a small-diameter syrinx and an atrophic spinal cord. In such cases, a previously distended syrinx has probably collapsed after the Chiari I malformation has been spontaneously disimpacted at the foramen magnum or after the syrinx has created a fissure to the spinal subarachnoid space.80,81 Prominence of the central canal of the spinal cord that does not distend the spinal cord is a normal anatomic variant and does not produce neurologic deficit or require treatment.82
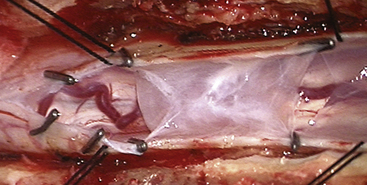
FIGURE 182-5 Intraoperative photograph of the arachnoid cyst that was diagnosed on magnetic resonance images.
To evaluate radiographic outcome of a previous surgical procedure for syringomyelia, MRI scanning can be repeated at 3 to 12 months after the surgical procedure. After successful craniocervical decompression surgery for Chiari I and syringomyelia, the cerebellar tonsils lose their conical shape, the CSF pathways expand at the foramen magnum, and the syrinx decreases to less than 50% of its presurgical diameter.83 After successful laminectomy and duraplasty for primary spinal syringomyelia, the subarachnoid space expands dorsal to the spinal cord, and the syrinx diameter decreases dramatically.
Surgical Management
Operative Treatment of Chiari I Malformation with and without Syringomyelia
Early diagnosis and surgical treatment of syringomyelia is essential to arrest progressive myelopathy and prevent further loss of neurologic function. Modern treatment has low mortality and morbidity. Although surgery is the only treatment for syringomyelia, the symptoms are often present for at least 1 year before surgery is performed.84
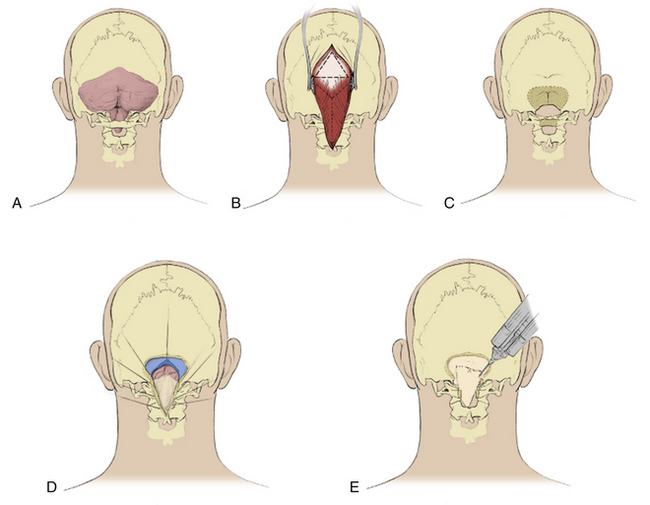
The reported results of various decompressive procedures for treating syringomyelia are identical, and because simple decompression eliminates the pathophysiologic mechanism of syringomyelia, we advocate choosing between the two least-invasive procedures, which are simple craniocervical decompression and duraplasty without opening the arachnoid membrane (Fig. 182-6; see Table 182-1) or decompression of the bone of the posterior lip of the foramen magnum and laminectomy of C1, and occasionally part of C2, with a partial-thickness dural incision.
The patient is positioned prone on silicone gel pads that support the chest and pelvis and that allow the abdomen to hang freely. The neck is flexed slightly. The head is held with the 3-pin Mayfield head holder (OMI Inc., Cincinnati, OH). A midline skin incision is made extending from above the inion to the level of the C2 spinous process. A triangular pericranial graft is obtained from the occipital area measuring 4 to 5 cm in length and width. We free the skin margins from the deep fascia and open the fascia in a Y-shaped incision.85 The Y-shaped incision is preferred to a linear fascial incision because it suspends the suboccipital muscles and prevents them from recessing against the dural graft.
Enough bone should be removed at the foramen magnum to completely decompress the entire posterior surface of the cerebellar tonsils. Cervical laminectomy extends inferiorly enough to expose arachnoid inferior to the tips of the cerebellar tonsils; laminectomy of C1, and often at least the superior part of the lamina of C2, is required. After suboccipital craniectomy and laminectomy, but before opening the dura, ultrasound imaging is performed to assess if bony decompression is adequate to relieve impaction of the cerebellar tonsils and to search for bands and membranes within the cisterna magna and upper cervical subarachnoid space. The dura is opened in the midline at the C1 level with care to avoid injury to the underlying arachnoid. The incision is carried superiorly and split just below the foramen magnum to create a Y-shaped dural opening.85 The cisterna magna expands immediately in almost all cases. Leakage of CSF through a small hole in the cisterna magna is not uncommon and has not resulted in inferior outcomes. The dura is retracted with 4-0 multifilament nylon sutures. The pericranial autograft is sutured to the durotomy margins with a running 4-0 multifilament nylon suture. The graft expands the volume of the posterior fossa and provides a biological membrane that contains the CSF and prevents adhesions to the cerebellum by the suboccipital musculature. Autologous pericranium forms a better seal with the surrounding dura than other graft materials and is not immunogenic, which makes it more suitable as a graft material than fascia lata autograft, pericranial xenograft, or collagen sponge.
Almost all patients respond to simple decompressive surgery.83 In rare patients, opening the dura does not result in expansion of the cisterna magna with CSF, signaling that bands or membranes are binding the arachnoid to the cerebellum and spinal cord. This contingency requires opening of the arachnoid of the cisterna magna, cutting the bands or membranes, and tacking the arachnoid to the durotomy margin. Rarely the syrinx fails to resolve after decompressive surgery, because the pathophysiologic mechanism has not been eliminated; in these cases a second operation usually resolves syringomyelia by correcting conditions that prevented the subarachnoid space posterior to the cerebellar tonsils from expanding, such as inadequate bone removal or an extradural pseudocyst (Fig. 182-7).86–89
In recent years it has become apparent that simply performing the bone decompression and superficially opening the dura (partial thickness) or removing the outer layer of dura results in resolution of the syrinx in most patients, with 5% to 15% failing to respond. The argument of using this approach as the initial procedure is that it reduces the risk of a pseudomeningocele associated with opening the dura and arachnoid, it is usually successful, and these features outweigh the disadvantages of knowing that further surgery to open the dura will be required in 5% to 15% of patients treated in this fashion.90
Syrinx shunting is reserved for rare cases in which arachnoiditis prevents effective opening of the CSF pathways.86,91 Surgical drainage of the syrinx by myelotomy and placement of a shunt within the syrinx was formerly advocated by some as the preferred method for decompressing the syrinx and preventing progression of myelopathy.71 This treatment is based on the premise that the pathophysiology of syringomyelia lies within the spinal cord and that the principal neurologic problem is myelopathy, which results from the syrinx and not the Chiari I malformation. The outcome of this treatment is variable. Shunting procedures have a complication rate of about 16% and a clinical stabilization rate of only 54% at 10 years in experienced hands.92 Placement of a tube into the syrinx requires a myelotomy, and the tube can create traction on the spinal cord (tethering) that leads to delayed spinal cord injury. Furthermore, drainage tubes are prone to obstruction, with about half failing within 4 years, leading to recurrence of the syrinx and the need for additional surgery.92 Correction of the underlying cause of syringomyelia is preferred to syrinx shunting, which is now known to not address the underlying cause of syringomyelia and provides only short-term treatment.
Operative Treatment of Syringomyelia Associated with Spinal Conditions
Standard care of primary spinal syringomyelia consists of surgical approaches that either open obstructed CSF pathways, shunt CSF or syrinx fluid, or remove spinal tumors. The objective of surgical treatment of primary spinal syringomyelia is to eliminate the syrinx and prevent progression of myelopathy.
In most cases a posterior surgical approach to the spine is used. Less frequently, an anterior approach is required to correct anterior deformity or compression of the subarachnoid space. The operation is performed under general anesthesia. If radiographic evaluation shows that the obstruction of the CSF passages around the spinal cord is short (less than 3 spinal segments) and located in one region of the spine, surgery is performed to remove the obstruction, which, when successful, usually results in resolution of syringomyelia.38,45,93
Fluoroscopic guidance is used to center the skin incision on the spinal lesion that is associated with syringomyelia. The muscles over the spine are separated and a laminectomy is performed. Intraoperative ultrasonography to define the subdural anatomy is performed after exposure of the dura. This dura is opened in the midline to extend superior and inferior to the intradural lesion, preserving the underlying arachnoid membrane. Dissection is carried lateral in the subdural plane in places where ultrasonography demonstrated adhesions between the spinal cord and arachnoid membrane. The arachnoid membrane is then opened in the midline superior and inferior to the lesion (arachnoid cyst, arachnoiditis) and tacked with metal clips to the dura (see Fig. 182-5). The dorsal surface of the spinal cord is identified. The area of arachnoiditis is approached in the subarachnoid space from superiorly and inferiorly, with arachnoid bands being cut to free the overlying arachnoid from the spinal cord surface. This frees the remaining dorsal arachnoid membrane from the spinal cord and allows it to be opened and tacked to the dura. Discrete lesions obstructing the subarachnoid space, including tumors and arachnoid cysts, are removed (see Fig. 182-5). A patch is sewn into the dura, which, in addition to the other maneuvers, creates more space for the passage of CSF. Bovine pericardium is used, because harvesting a piece of autologous pericranium requires a separate incision. The wound is closed in layers. Laminectomy, intradural exploration, and expansile duraplasty can effectively relieve pathologic processes such as local arachnoiditis or arachnoid cysts, which can locally obstruct the CSF pathways and produce syringomyelia.
Spinal deformity can also result in syringomyelia and usually requires an anterior surgical approach to remove a posttraumatic kyphosis. Relief of the focal obstructions of the CSF pathways restores CSF flow and resolves syringomyelia in these cases.94
In some cases, the findings of the myelogram and CT scan, or the findings at surgery, indicate that scarring is too widespread for direct surgery to open the CSF pathways. In such cases, a small myelotomy is made and a short piece of shunt tubing is placed within the syrinx. Placing the distal end of the catheter into the adjacent subarachnoid space is not advisable if the catheter would remain inferior to an obstruction in the subarachnoid space. The distal end of the shunt should be placed proximal to the subarachnoid block or in the peritoneal or pleural cavity to bypass the blockage to normal CSF flow and absorption.95 The placement of this tube and the long-term effects of the shunt can damage the spinal cord. A shunt tube tends to plug with time. In addition, the tube does not deal directly with the underlying problem that caused the syringomyelia in the first place.
Some investigators advocate shunting the CSF rather than the syrinx fluid. Their rationale is that in patients with obstruction of the CSF pathways, syrinx fluid arises from the CSF. CSF is shunted from the cerebral ventricles or spinal subarachnoid space to the pleural or peritoneal cavity, where it is absorbed. Ventricular shunting has resulted in improvement in syringomyelia in patients with and without hydrocephalus.96,97 In primary spinal syringomyelia, shunting of CSF from the spinal subarachnoid space has been performed with catheters placed superior to the block of the spinal subarachnoid space.98,99 Reports of shunting of CSF from the subarachnoid space inferior to an obstruction (lumboperitoneal shunting) have been limited to syringomyelia associated with the Chiari I malformation, in which arachnoiditis is less common and the spinal subarachnoid space is less obstructed than in primary spinal arachnoiditis.100–102 Unfortunately, lumboperitoneal shunts can cause herniation of the cerebellar tonsils—acquired Chiari I malformation—which may be associated with syringomyelia.103
Avoiding Complications
Craniocervical decompression has been reported to fail in 10% to 40% of patients.71,104 The most common cause of surgical failure is inadequate removal of bone at the foramen magnum, which results in persistent compression of the cerebellar tonsils and CSF pathways (see Fig. 182-7).
Another common reason for failure is the development of a persistent leak of CSF through the dural graft that results in the formation of a pseudomeningocele dorsal to the dural graft; the pseudomeningocele presses the dura graft anteriorly and creates adhesions between the graft and the underlying cerebellum that obliterates the dorsal CSF pathway.87 Maintaining the arachnoid membrane during craniocervical decompression provides an additional biological barrier to scar formation between the graft or duraplasty suture line and the underlying neural elements. Cerebrospinal leakage through the skin should not occur if the dural closure and the fascial closure are secure, except for cases in which hydrocephalus is present. (Hydrocephalus should be assessed for before surgery because syringomyelia can improve after ventricular shunting in patients with associated hydrocephalus.)96,97 Leakage of CSF through the skin incision is treated initially with skin sutures. If CSF leakage persists, a CT scan should be obtained to evaluate for hydrocephalus and extradural hematoma. External drainage of CSF and/or reoperation to replace or repair the dural graft may be required. Excessive drainage of lumbar CSF can increase tonsillar ectopia.105 Treatment should be prompt because development of meningitis or graft infection can lead to surgical failure from secondary arachnoiditis at the surgical site.
Placing a shunt tube within the syrinx has a reported risk of creating a new neurologic deficit of 20%.106 In addition, shunts can tether the spinal cord, leading to delayed paralysis, can become infected, and have a high rate of obstruction.92,107–109 Because of this, syrinx shunts should only be used in cases of syringomyelia that cannot be treated using an approach that opens CSF pathways at the foramen magnum or within the spinal canal.
Postoperative Care
The results of surgical therapy of syringomyelia can be evaluated noninvasively using MRI. Clinical stabilization or improvement after treatment follows reduction in syrinx diameter.110 MRI scans of the cervical spine and posterior fossa should be performed at 3 to 12 months after surgery to evaluate if CSF pathways have been restored at the foramen magnum and if the syrinx is becoming progressively smaller. Disease stabilization correlates with collapse of a syrinx and reduction in the spinal cord edema as seen on MRI scanning.83,84,111,112 Successful surgical treatment results in disease stabilization and, in some patients, neurologic improvement.
Abbe R., Coley W.B. Syringomyelia, operation, exploration of cord, withdrawal of fluid, exhibition of patient. J Nerv Ment Dis. 1892;17:512-520.
Anderson N.E., Willoughby E.W., Wrightson P. The natural history of syringomyelia. Clin Exp Neurol. 1986;22:71-80.
Boman K., Iivanainen M. Prognosis of syringomyelia. Acta Neurol Scand. 1967;43:61-68.
Brewis M., Poskanzer D.C., Rolland C., Miller H. Neurological disease in an English city. Acta Neurol Scand. 1966;S24:1-89.
Fischbein N.J., Dillon W.P., Cobbs C., Weinstein P.R. The “presyrinx” state: a reversible myelopathic condition that may precede syringomyelia. AJNR Am J Neuroradiol. 1999;20:7-20.
Gardner W.J., Goodall R.J. The surgical treatment of Arnold-Chiari malformation in adults; an explanation of its mechanism and importance of encephalography in diagnosis. J Neurosurg. 1950;7:199-206.
Harding B.N., Copp A.J. Malformations. In: Graham D.I., Lantos P.L. Greenfield’s Neuropathology. London: Arnold; 2002:357-483.
Heiss J.D., Patronas N., DeVroom H.L., et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999;91:553-562.
Holly L.T., Batzdorf U. Slitlike syrinx cavities: a persistent central canal. J Neurosurg. 2002;97:161-165.
Iskandar B.J., Hedlund G.L., Grabb P.A., Oakes W.J. The resolution of syringohydromyelia without hindbrain herniation after posterior fossa decompression. J Neurosurg. 1998;89:212-216.
Klekamp J., Batzdorf U., Samii M., Bothe H.W. The surgical treatment of Chiari I malformation. Acta Neurochir (Wien). 1996;138:788-801.
Klekamp J., Batzdorf U., Samii M., Bothe H.W. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86:233-240.
Levy E.I., Heiss J.D., Kent M.S., et al. Spinal cord swelling preceding syrinx development. Case report. J Neurosurg. 2000;92:93-97.
Logue V., Edwards M.R. Syringomyelia and its surgical treatment—an analysis of 75 patients. J Neurol Neurosurg Psychiatry. 1981;44:273-284.
Milhorat T.H., Bolognese P.A., Nishikawa M., et al. Association of Chiari malformation type I and tethered cord syndrome: preliminary results of sectioning filum terminale. Surg Neurol. 2009;72:20-35.
Milhorat T.H., Chou M.W., Trinidad E.M., et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005-1017.
Munshi I., Frim D., Stine-Reyes R., et al. Effects of posterior fossa decompression with and without duraplasty on Chiari malformation–associated hydromyelia. Neurosurgery. 2000;46:1384-1389. discussion 1389-1390
Oldfield E.H., Muraszko K., Shawker T.H., Patronas N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3-15.
Sacco D., Scott R.M. Reoperation for Chiari malformations. Pediatr Neurosurg. 2003;39:171-178.
Santoro A., Delfini R., Innocenzi G., et al. Spontaneous drainage of syringomyelia. Report of two cases. J Neurosurg. 1993;79:132-134.
Sgouros S., Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1-10.
Sgouros S., Williams B. Management and outcome of posttraumatic syringomyelia. J Neurosurg. 1996;85:197-205.
Vernooij M.W., Ikram M.A., Tanghe H.L., et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821-1828.
Vinters H.V. Neuropathology of syringomyelia. In: Batzdorf U., editor. Syringomyelia: Current Concepts in Diagnosis and Treatment. Baltimore: Williams & Wilkins; 1991:35-58.
Wetjen N.M., Heiss J.D., Oldfield E.H. Time course of syringomyelia resolution following decompression of Chiari malformation type I. J Neurosurg Pediatr. 2008;1:118-123.
1. Chiari H. Über Veränderungen des Kleinhirns infolge von congenitaler Hydrocephalie des Grosshirns (Concerning changes in the cerebellum due to hydrocephalus of the cerebrum). Deutsche Medizinische Wochenschrift. 1891;17:1172-1175.
2. Chiari H. Über Veränderungen des Kleinhirns, des Pons und der Medulla oblongata in Folge von congenitaler Hydrocephalie des Grosshirns (Concerning changes in the cerebellum, pons, and medulla oblongata due to hydrocephalus of the cerebrum). Denkschriften der Akademie der Wissenschaften in Wien. Mathematisch-Naturwissenschaftliche Klasse. 1896;63:71-116.
3. Harding BN, Copp AJ: Malformations. In: Graham DI, Lantos PL (eds). Greenfield’s Neuropathology. Arnold, London, pp 357-483
4. Marin-Padilla M., Marin-Padilla T.M. Morphogenesis of experimentally induced Arnold–Chiari malformation. J Neurol Sci. 1981;50:29-55.
5. Caviness V.S. The Chiari malformations of the posterior fossa and their relation to hydrocephalus. Dev Med Child Neurol. 1976;18:103-116.
6. Iskandar B.J., Hedlund G.L., Grabb P.A., Oakes W.J. The resolution of syringohydromyelia without hindbrain herniation after posterior fossa decompression. J Neurosurg. 1998;89:212-216.
7. Tubbs R.S., Elton S., Grabb P., et al. Analysis of the posterior fossa in children with the Chiari 0 malformation. Neurosurgery. 2001;48:1050-1054. discussion 1054-1055
8. Bogdanov E.I., Heiss J.D., Mendelevich E.G., et al. Clinical and neuroimaging features of “idiopathic” syringomyelia. Neurology. 2004;62:791-794.
9. Tubbs R.S., Iskandar B.J., Bartolucci A.A., Oakes W.J. A critical analysis of the Chiari 1.5 malformation. J Neurosurg. 2004;101:179-183.
10. Gutmann L. Personal history: the pipes of Pan. Neurology. 2005;64:1483-1484.
11. Abbe R., Coley W.B. Syringomyelia, operation, exploration of cord, withdrawal of fluid, exhibition of patient. J Nerv Ment Dis. 1892;17:512-520.
12. McConnell A.A., Parker H.L. A deformity of the hind-brain associated with internal hydrocephalus. Its relation to the Arnold-Chiari Malformation. Brain. 1938;61:415-429.
13. Aring C.D. Cerebellar syndrome in an adult with malformation of the cerellum and brain stem (Arnold-Chiari deformity), with a note on the occurrence of “torpedoes” in the cerebellum. Brain. 1938;1:100-109.
14. Bejjani G.K. Definition of the adult Chiari malformation: a brief historical overview. Neurosurg Focus. 2001;11:E1.
15. Gardner W.J., Goodall R.J. The surgical treatment of Arnold-Chiari malformation in adults; an explanation of its mechanism and importance of encephalography in diagnosis. J Neurosurg. 1950;7:199-206.
16. Logue V., Edwards M.R. Syringomyelia and its surgical treatment—an analysis of 75 patients. J Neurol Neurosurg Psychiatry. 1981;44:273-284.
17. Gardner W.J. Hydrodynamic mechanism of syringomyelia: its relationship to myelocele. J Neurol Neurosurg Psychiatry. 1965;28:247-259.
18. Klekamp J., Batzdorf U., Samii M., Bothe H.W. The surgical treatment of Chiari I malformation. Acta Neurochir (Wien). 1996;138:788-801.
19. Badie B., Mendoza D., Batzdorf U. Posterior fossa volume and response to suboccipital decompression in patients with Chiari I malformation. Neurosurgery. 1995;37:214-218.
20. Heiss J.D., Patronas N., DeVroom H.L., et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999;91:553-562.
21. Milhorat T.H., Chou M.W., Trinidad E.M., et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005-1017.
22. Nishikawa M., Sakamoto H., Hakuba A., et al. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. J Neurosurg. 1997;86:40-47.
23. Nyland H., Krogness K.G. Size of posterior fossa in Chiari type 1 malformation in adults. Acta Neurochir (Wien). 1978;40:233-242.
24. Cinalli G., Spennato P., Sainte-Rose C., et al. Chiari malformation in craniosynostosis. Childs Nerv Syst. 2005;21:889-901.
25. Fujisawa H., Hasegawa M., Kida S., Yamashita J. A novel fibroblast growth factor receptor 2 mutation in Crouzon syndrome associated with Chiari type I malformation and syringomyelia. J Neurosurg. 2002;97:396-400.
26. Lemar H.J.Jr., Perloff J.J., Merenich J.A. Symptomatic Chiari-I malformation in a patient with acromegaly. South Med J. 1994;87:284-285.
27. Ammerman J.M., Goel R., Polin R.S. Resolution of Chiari malformation after treatment of acromegaly. Case illustration. J Neurosurg. 2006;104:980.
28. Caldarelli M., Novegno F., Vassimi L., et al. The role of limited posterior fossa craniectomy in the surgical treatment of Chiari malformation type I: experience with a pediatric series. J Neurosurg. 2007;106:187-195.
29. Gardner W.J. Rupture of the neural tube. Arch Neurol. 1961;4:1-7.
30. Cameron A.H. The Arnold-Chiari and other neuro-anatomic malformations associated with spina bifida. J Path Bact. 1957;73:195-211.
31. Hankinson J. Syringomyelia and the surgeon. Mod Trends Neurol. 1970;5:127-148.
32. Foster J.B., Hudgson P. The pathology of communicating syringomyelia. In: Barnett H.J.M., Foster J.B., Hudgson P. Syringomyelia. Philadelphia: W.B. Saunders; 1973:79-103.
33. Oldfield E.H., Muraszko K., Shawker T.H., Patronas N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3-15.
34. Dauser R.C., DiPietro M.A., Venes J.L. Symptomatic Chiari I malformation in childhood: a report of 7 cases. Pediatr Neurosci. 1988;14:184-190.
35. Brewis M., Poskanzer D.C., Rolland C., Miller H. Neurological disease in an English city. Acta Neurol Scand. 1966;S24:1-89.
36. Williams B. Pathogenesis of syringomyelia. In: Batzdorf U., editor. Syringomyelia: Current Concepts in Diagnosis and Treatment. Baltimore: Williams & Wilkins; 1991:59-90.
37. Batzdorf U. Primary spinal syringomyelia: a personal perspective. Neurosurg Focus. 2000;8:E7.
38. el Masry W.S., Biyani A. Incidence, management, and outcome of post-traumatic syringomyelia. In memory of Mr Bernard Williams. J Neurol Neurosurg Psychiatry. 1996;60:141-146.
39. Haney A., Stiller J., Zelnik N., Goodwin L. Association of posttraumatic spinal arachnoid cyst and syringomyelia. J Comput Tomogr. 1985;9:137-140.
40. Klekamp J., Batzdorf U., Samii M., Bothe H.W. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86:233-240.
41. Kramer K.M., Levine A.M. Posttraumatic syringomyelia: a review of 21 cases. Clin Orthop Relat Res. 1997:190-199.
42. Perrouin-Verbe B., Lenne-Aurier K., Robert R., et al. Post-traumatic syringomyelia and post-traumatic spinal canal stenosis: a direct relationship: review of 75 patients with a spinal cord injury. Spinal Cord. 1998;36:137-143.
43. Rossier A.B., Foo D., Shillito J., Dyro F.M. Posttraumatic cervical syringomyelia. Incidence, clinical presentation, electrophysiological studies, syrinx protein and results of conservative and operative treatment. Brain. 1985;108(Pt 2):439-461.
44. Schurch B., Wichmann W., Rossier A.B. Post-traumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. J Neurol Neurosurg Psychiatry. 1996;60:61-67.
45. Sgouros S., Williams B. Management and outcome of posttraumatic syringomyelia. J Neurosurg. 1996;85:197-205.
46. Foster J.B. Neurology of syringomyelia. In: Batzdorf U., editor. Syringomyelia: Current Concepts in Diagnosis and Treatment. Baltimore: Williams and Wilkins; 1991:91-115.
47. Caplan L.R., Norohna A.B., Amico L.L. Syringomyelia and arachnoiditis. J Neurol Neurosurg Psychiatry. 1990;53:106-113.
48. Fehlings M.G., Bernstein M. Syringomyelia as a complication of tuberculous meningitis. Can J Neurol Sci. 1992;19:84-87.
49. Kakar A., Madan V.S., Prakash V. Syringomyelia—a complication of meningitis—case report. Spinal Cord. 1997;35:629-631.
50. Phanthumchinda K., Kaoropthum S. Syringomyelia associated with post-meningitic spinal arachnoiditis due to Candida tropicalis. Postgrad Med J. 1991;67:767-769.
51. Tabor E.N., Batzdorf U. Thoracic spinal Pantopaque cyst and associated syrinx resulting in progressive spastic paraparesis: case report. Neurosurgery. 1996;39:1040-1042.
52. Andrews B.T., Weinstein P.R., Rosenblum M.L., Barbaro N.M. Intradural arachnoid cysts of the spinal canal associated with intramedullary cysts. J Neurosurg. 1988;68:544-549.
53. Castillo M., Quencer R.M., Green B.A., Montalvo B.M. Syringomyelia as a consequence of compressive extramedullary lesions: postoperative clinical and radiological manifestations. AJR Am J Roentgenol. 1988;150:391-396.
54. Clifton A.G., Ginsberg L., Webb W.J., Valentine A.R. Idiopathic spinal arachnoid cyst and syringomyelia. Br J Radiol. 1987;60:1023-1025.
55. Hormigo A., Lobo-Antunes J., Bravo-Marques J.M., Marques M.S. Syringomyelia secondary to compression of the cervical spinal cord by an extramedullary lymphoma. Neurosurgery. 1990;27:834-836. discussion 836
56. Kaar G.F., N’Dow J.M., Bashir S.H. Cervical spondylotic myelopathy with syringomyelia. Br J Neurosurg. 1996;10:413-415.
57. Lucci B., Reverberi S., Greco G. Syringomyelia and syringomielic syndrome by cervical spondylosis. Report on three cases presenting with neurogenic osteoarthropathies. J Neurosurg Sci. 1981;25:169-172.
58. Mallucci C.L., Stacey R.J., Miles J.B., Williams B. Idiopathic syringomyelia and the importance of occult arachnoid webs, pouches and cysts. Br J Neurosurg. 1997;11:306-309.
59. Middleton T.H., Al-Mefty O., Harkey L.H., et al. Syringomyelia after decompressive laminectomy for cervical spondylosis. Surg Neurol. 1987;28:458-462.
60. Quencer R.M., el Gammal T., Cohen G. Syringomyelia associated with intradural extramedullary masses of the spinal canal. AJNR Am J Neuroradiol. 1986;7:143-148.
61. Wasserberg J., Marks P., Hardy D. Syringomyelia of the thoracic spinal cord associated with spinal meningiomas. Br J Neurosurg. 1987;1:485-488.
62. Yu Y.L., Moseley I.F. Syringomyelia and cervical spondylosis: a clinicoradiological investigation. Neuroradiology. 1987;29:143-151.
63. Dietemann J.L., Babin E., Wackenheim A., et al. Percutaneous puncture of spinal cysts in the diagnosis and therapy of syringomyelia and cystic tumors. Neuroradiology. 1982;24:59-63.
64. Levy R., Rosenblatt S., Russell E. Percutaneous drainage and serial magnetic resonance imaging in the diagnosis of symptomatic posttraumatic syringomyelia: case report and review of the literature. Neurosurgery. 1991;29:429-433. discussion 433-434
65. Anderson N.E., Willoughby E.W., Wrightson P. The natural history of syringomyelia. Clin Exp Neurol. 1986;22:71-80.
66. Boman K., Iivanainen M. Prognosis of syringomyelia. Acta Neurol Scand. 1967;43:61-68.
67. Williams B., Fahy G. A critical appraisal of “terminal ventriculostomy” for the treatment of syringomyelia. J Neurosurg. 1983;58:188-197.
68. Vinters H.V. Neuropathology of syringomyelia. In: Batzdorf U., editor. Syringomyelia: Current Concepts in Diagnosis and Treatment. Baltimore: Williams & Wilkins; 1991:35-58.
69. Boiardi A., Munari L., Silvani A., et al. Natural history and postsurgical outcome of syringomyelia. Ital J Neurol Sci. 1991;12:575-579.
70. Mariani C., Cislaghi M.G., Barbieri S., et al. The natural history and results of surgery in 50 cases of syringomyelia. J Neurol. 1991;238:433-438.
71. Van Calenbergh F., Hoorens G., Van den Bergh R. Syringomyelia: a retrospective study. Part II: diagnostic and therapeutic approach. Acta Neurol Belg. 1990;90:100-110.
72. Royo-Salvador M.B. A new surgical treatment for syringomyelia, scoliosis, Arnold-Chiari malformation, kinking of the brainstem, odontoid recess, idiopathic basilar impression and platybasia. Rev Neurol. 1997;25:523-530.
73. Milhorat T.H., Bolognese P.A., Nishikawa M., et al. Association of Chiari malformation type I and tethered cord syndrome: preliminary results of sectioning filum terminale. Surg Neurol. 2009;72:20-35.
74. Barkovich A.J., Wippold F.J., Sherman J.L., Citrin C.M. Significance of cerebellar tonsillar position on MR. AJNR Am J Neuroradiol. 1986;7:795-799.
75. Mikulis D.J., Diaz O., Egglin T.K., Sanchez R. Variance of the position of the cerebellar tonsils with age: preliminary report. Radiology. 1992;183:725-728.
76. Vernooij M.W., Ikram M.A., Tanghe H.L., et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821-1828.
77. Meadows J., Kraut M., Guarnieri M., et al. Asymptomatic Chiari type I malformations identified on magnetic resonance imaging. J Neurosurg. 2000;92:920-926.
78. Elster A.D., Chen M.Y. Chiari I malformations: clinical and radiologic reappraisal. Radiology. 1992;183:347-353.
79. Levy E.I., Heiss J.D., Kent M.S., et al. Spinal cord swelling preceding syrinx development. Case report. J Neurosurg. 2000;92:93-97.
80. Santoro A., Delfini R., Innocenzi G., et al. Spontaneous drainage of syringomyelia. Report of two cases. J Neurosurg. 1993;79:132-134.
81. Williams B. Spontaneous drainage in syringomyelia. J Neurosurg. 1994;80:949-950.
82. Holly L.T., Batzdorf U. Slitlike syrinx cavities: a persistent central canal. J Neurosurg. 2002;97:161-165.
83. Wetjen N.M., Heiss J.D., Oldfield E.H. Time course of syringomyelia resolution following decompression of Chiari malformation type I. J Neurosurg Pediatr. 2008;1:118-123.
84. Pillay P.K., Awad I.A., Little J.R., Hahn J.F. Surgical management of syringomyelia: a five year experience in the era of magnetic resonance imaging. Neurol Res. 1991;13:3-9.
85. Kempe L.G. Operative Neurosurgery. New York: Springer-Verlag; 1970.
86. Mazzola C.A., Fried A.H. Revision surgery for Chiari malformation decompression. Neurosurg Focus. 2003;15:E3.
87. Pare L.S., Batzdorf U. Syringomyelia persistence after Chiari decompression as a result of pseudomeningocele formation: implications for syrinx pathogenesis: report of three cases. Neurosurgery. 1998;43:945-948.
88. Sacco D., Scott R.M. Reoperation for Chiari malformations. Pediatr Neurosurg. 2003;39:171-178.
89. Tubbs R.S., Webb D.B., Oakes W.J. Persistent syringomyelia following pediatric Chiari I decompression: radiological and surgical findings. J Neurosurg. 2004;100:460-464.
90. Munshi I., Frim D., Stine-Reyes R., et al. Effects of posterior fossa decompression with and without duraplasty on Chiari malformation–associated hydromyelia. Neurosurgery. 2000;46:1384-1389. discussion 1389-1390
91. Cacciola F., Capozza M., Perrini P., et al. Syringopleural shunt as a rescue procedure in patients with syringomyelia refractory to restoration of cerebrospinal fluid flow. Neurosurgery. 2009;65:471-476. discussion 476
92. Sgouros S., Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1-10.
93. Levi A.D., Sonntag V.K. Management of posttraumatic syringomyelia using an expansile duraplasty. A case report. Spine. 1998;23:128-132.
94. Batzdorf U., Khoo L.T., McArthur D.L. Observations on spine deformity and syringomyelia. Neurosurgery. 2007;61:370-377. discussion 377-378
95. Milhorat T.H., Johnson W.D., Miller J.I. Syrinx shunt to posterior fossa cisterns (syringocisternostomy) for bypassing obstructions of upper cervical theca. J Neurosurg. 1992;77:871-874.
96. Krayenbühl H.H. Evaluation of the different surgical approaches in the treatment of syringomyelia. Clin Neurol Neurosurg. 1975;77:111-128.
97. Lee T.C., Gau Y.L., Lui C.C. Treatment of symptomatic syringomyelia with ventriculoperitoneal shunt: report of a case. J Formos Med Assoc. 1992;91:548-551.
98. Suzuki S., Chiba Y., Hidaka K., et al. [A new operative technique of posttraumatic syringomyelia: thecoperitoneal shunt]. No Shinkei Geka. 1998;26:541-546.
99. Vassilouthis J., Papandreou A., Anagnostaras S., Pappas J. Thecoperitoneal shunt for syringomyelia: report of three cases. Neurosurgery. 1993;33:324-327. discussion 327-328
100. Park T.S., Cail W.S., Broaddus W.C., Walker M.G. Lumboperitoneal shunt combined with myelotomy for treatment of syringohydromyelia. J Neurosurg. 1989;70:721-727.
101. Vanaclocha V., Saiz-Sapena N. Duraplasty with freeze-dried cadaveric dura versus occipital pericranium for Chiari type I malformation: comparative study. Acta Neurochir (Wien). 1997;139:112-119.
102. Vengsarkar U.S., Panchal V.G., Tripathi P.D., et al. Percutaneous thecoperitoneal shunt for syringomyelia. Report of three cases. J Neurosurg. 1991;74:827-831.
103. Johnston I., Jacobson E., Besser M. The acquired Chiari malformation and syringomyelia following spinal CSF drainage: a study of incidence and management. Acta Neurochir (Wien). 1998;140:417-427. discussion 427-428
104. Matsumoto T., Symon L. Surgical management of syringomyelia—current results. Surg Neurol. 1989;32:258-265.
105. Welch K., Shillito J., Strand R., et al. Chiari I “malformations”—an acquired disorder? J Neurosurg. 1981;55:604-609.
106. Barbaro N.M. Surgery for primarily spinal syringomyelia. In: Batzdorf U., editor. Syringomyelia: Current Concepts in Diagnosis and Treatment. Baltimore: Williams & Wilkins; 1991:183-198.
107. Batzdorf U., Klekamp J., Johnson J.P. A critical appraisal of syrinx cavity shunting procedures. J Neurosurg. 1998;89:382-388.
108. Takayasu M., Shibuya M., Kouketsu N., Suzuki Y. Rapid enlargement of a syringomyelia cavity following syringo-subarachnoid shunt: case report. Surg Neurol. 1996;45:366-369.
109. Wester K., Pedersen P.H., Krakenes J. Spinal cord damage caused by rotation of a T-drain in a patient with syringoperitoneal shunt. Surg Neurol. 1989;31:224-227.
110. Batzdorf U. Chiari I malformation with syringomyelia. Evaluation of surgical therapy by magnetic resonance imaging. J Neurosurg. 1988;68:726-730.
111. Fischbein N.J., Dillon W.P., Cobbs C., Weinstein P.R. The “presyrinx” state: a reversible myelopathic condition that may precede syringomyelia. AJNR Am J Neuroradiol. 1999;20:7-20.
112. Jinkins J.R., Reddy S., Leite C.C., et al. MR of parenchymal spinal cord signal change as a sign of active advancement in clinically progressive posttraumatic syringomyelia. AJNR Am J Neuroradiol. 1998;19:177-182.
113. Bidzinski J. Pathological findings in suboccipital decompression in 63 patients with syringomyelia. Acta Neurochir Suppl (Wien). 1988;43:26-28.
114. Hida K., Iwasaki Y., Koyanagi I., et al. Surgical indication and results of foramen magnum decompression versus syringosubarachnoid shunting for syringomyelia associated with Chiari I malformation. Neurosurgery. 1995;37:673-678. discussion 678-679
115. Vaquero J., Martinez R., Arias A. Syringomyelia-Chiari complex: magnetic resonance imaging and clinical evaluation of surgical treatment. J Neurosurg. 1990;73:64-68.