Management of cerebral aneurysms
Aneurysm rupture
Nimodipine is the standard drug used to manage vasospasm because it improves collateral blood flow (Table 134-1); however, it does not relieve the vasospasm of the main vessel. Optimal management entails the use of hypertension, hydration, and hemodilution (triple-H therapy) to overcome the vasospasm, which usually lasts for up to 14 days.
Table 134-1
| Aneurysm Category | ||
| Management Aspect | Nonruptured | Ruptured |
| Monitoring | Standard | Standard plus ICP |
| Brain protection | No | Probable |
| Vasospasm | No | Most likely |
| Triple-H therapy | No | Yes |
| Surgical treatment | Elective | Emergent |
| Surgical treatment versus endovascular coil placement | Location-dependent | Location-dependent |
| Nimodipine | No | Yes |
| Outcomes | Good | Depends on Hunt-Hess classification and severity of bleed |
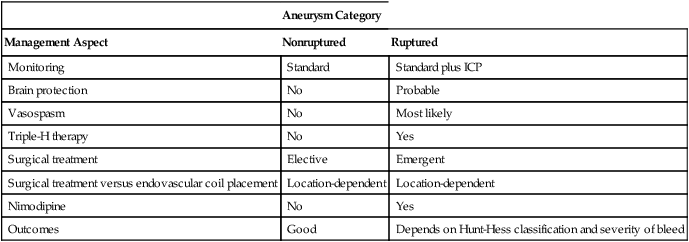
ICP, Intracranial pressure; triple-H therapy, use of hypertension, hydration, and hemodilution.
Anesthetic management
Aside from avoiding hyperglycemia and fever during periods when the brain is at risk for developing ischemic injury, definitive evidence for brain-protective interventions are devoid in the human literature (see Chapter 131). Despite this, many physicians use barbiturates or propofol to achieve burst suppression (4-6 bursts/min) during critical periods of aneurysm operations. The Intraoperative Hypothermia for Aneurysm Surgery Trial did not show any benefit of a mild temperature decrease (to 33° C) during operations. No other research results have been published on this topic recently.





