Chapter 197 Management of Adult Brachial Plexus Injuries
Brachial plexus injuries comprise approximately one third of all peripheral nerve injuries and are seen in just more than 1% of patients presenting to a trauma facility.1 They usually affect younger patients, with a median age of 34.2 Because of the association of such injuries with violent trauma and contact sports, males are affected more frequently than females. By the same line of reasoning, they are also often associated with injuries in other organ systems that are life-threatening. It was estimated that as many as 80% of patients with severe traumatic brachial plexopathy had multiple trauma to the head and skeletal system.3 Hence, there can be a delay in the diagnosis and treatment of brachial plexus injuries while the management of the other injuries are given priority.
The brachial plexus can be injured in several ways. The most common etiology is trauma, which can result in open or closed injuries. Among the closed injuries, the most common is caused by stretch or contusion, usually secondary to motor vehicular accidents involving motorcycles.4 Sports such as football, cycling, and skiing, among others, can also cause such injuries. Regardless of the setting, the mechanism is the same: the head and neck are usually forcefully pushed in one direction and the shoulder and arm in another. This results in severe stretching of the soft tissues, including the plexal elements and less frequently, the blood vessels.4 As for open injuries, the common etiologies are gunshot wounds and lacerations with knives or glass. Iatrogenic injuries may be open or closed. Nontraumatic causes of brachial plexus injuries include thoracic outlet syndrome and nerve sheath tumors, which cause injury by compression of plexal elements. In a survey of 1019 brachial plexus lesions by Kim et al.,2 the most common type of brachial plexus injury was due to stretch or contusion (50%), followed by thoracic outlet syndrome (16%) and nerve sheath tumors (16%). Gunshot wounds (12%) and lacerations (7%) complete the list. Because the majority of brachial plexus injuries are due to stretch/contusion, this chapter will focus on the diagnosis and management of such injuries.
Injury patterns can also be classified into supraclavicular and infraclavicular injuries. The supraclavicular plexus refers to the C5–T1 spinal nerves and the upper, middle, and lower trunks with their branches and divisions. On the other hand, the infraclavicular plexus refers to the cords and the nerves. In the series reported by Kim,2 72% of brachial plexus stretch injuries are supraclavicular, while 28% are infraclavicular. Of the supraclavicular injuries, C5–T1 palsy is the most frequent injury pattern, followed by C5–C7 then C5–C6. In terms of spontaneous recovery rate, C5–C6 has the best prognosis, with 30% of patients regaining significant function by 3 to 4 months. This compares favorably with C5–C7 palsy where approximately 16% recover spontaneously in the early months, and C5–T1 (pan-plexus palsies) with only 4% incidence of spontaneous recovery.4
Supraclavicular plexus injuries are more likely (52%) to come to surgery due to the severity of these injuries, with up to two thirds having avulsion of at least one spinal nerve.1 The infraclavicular lesions are less likely (17%) to be operated on, with half sustaining only neurapraxic injuries. Of the supraclavicular plexus injuries requiring surgery due to the lack of clinical recovery, two thirds will have at least some involvement of lower plexal (C8 and T1 spinal nerve) elements. The majority of these with pan-plexal involvement will have avulsed one or more spinal nerves, and exceedingly few will regain any useful function without intervention. On the contrary, when lower plexal elements are spared, and the primary injury thus less extensive, up to 25% may still make a good functional recovery of involved elements after neurolysis alone, without nerve repair.4
Surgical Anatomy
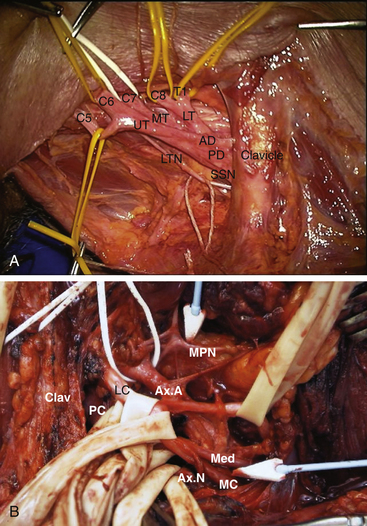
The brachial plexus originates at the level of the spine and usually includes the C5, C6, C7, C8, and Tl spinal nerves, the three trunks of the plexus, and their anterior and posterior divisions (Fig. 197-1A). Spinal nerves and trunks are supraclavicular, whereas divisions tend to lie behind the clavicle. Lateral, posterior, and medial cords are infraclavicular, as are their origins for the major nerves of the upper extremity (Fig. 197-1B).
Spinal Nerves
Each individual spinal nerve or root of the plexus originates as multiple sensory rootlets from the dorsal root entry zone of the posterolateral sector of the spinal cord and usually as one ventral or motor rootlet from the ventrolateral portion of the cord. The dorsal rootlets combine to form one dorsal root per spinal segment before entering the foramen. Within the foramen, the course of the roots varies between 10 and 16 mm. The dorsal root ganglion is located at an intraforaminal level and usually at its midpoint. Shortly distal to this, the anterior and posterior roots blend together to form the (mixed) spinal nerve. Posterior primary branches or rami then go to the paraspinal muscles and the much larger anterior ramus contributes to the brachial plexus.
Trunks
The upper and middle trunks are the most readily identified portions of the supraclavicular plexus. The upper trunk is usually adherent to and sometimes partially covered by the anterior scalene muscle. As one proceeds distally along the lateral edge of the trunk, the suprascapular nerve is encountered arising from the dorsolateral surface of the distal upper trunk, just as it forms anterior and posterior divisions. This trident-like structure, with suprascapular nerve, posterior and anterior divisions (from lateral to medial) is an excellent landmark for the termination of the upper trunk (Fig. 197-1A).
Divisions
Although each trunk has an anterior and a posterior division as outlined above, they can blend with other divisions before forming cords. Sometimes one or more divisions trade bundles of nerve fibers back and forth several times.5 In addition, the site at which cords begin distal to the clavicle can vary from patient to patient. Separating divisions in cases in which there has been a stretch injury, gunshot wound, or prior vascular dissection can be quite difficult. The surgeon works from trunks in a distal direction and cords in a proximal one to expose the divisions.
Cords
These are named, by convention, in relation to the axillary artery at the level of pectoralis minor. The lateral cord is usually superficial to the artery and is the first major neural element encountered after section of the pectoralis minor muscle as one begins dissection in the infra-clavicular region. It terminates in a contribution to the median nerve and an oblique takeoff running laterally to form the musculocutaneous nerve (Fig. 197-1B). The latter dives immediately between the two heads of the biceps muscle but usually gives off one or more coracobrachialis branches first.
The posterior cord is deep or posterior to axillary artery. Several subscapular branches (upper and lower) usually arise from the posterior cord and run inferiorly and obliquely. A relatively sizable branch, the thoracodorsal, runs from its posterior aspect almost directly posteriorly to supply the latissimus dorsi. The cord then divides into its two major branches, the axillary and the radial nerves. After coursing inferiorly and slightly laterally, the axillary nerve dives down to reach the quadrilateral space and eventually the deltoid muscle in the posterior arm. The major posterior cord outflow is the radial nerve, which runs inferiorly towards the humeral groove to wind around the humerus. A very important anatomic landmark is the medial relation between the radial nerve and the profundus branch of the axillary artery. This can be used to locate the proximal radial and differentiate it from the more lateral axillary nerve.6
Diagnosis
History and Physical Examination
A comprehensive physical examination begins with inspection. Typical positioning of the limb suggests involvement of the upper or lower plexus elements or both. For example, upper plexus palsy (Erb’s palsy) has the characteristic “waiter’s tip” position, a lower plexus palsy (Klumpke’s palsy) typically results in a “claw hand,” and a pan-plexus palsy usually results in a flail arm. One then proceeds to perform an assessment of the shoulders, neck, and high back from behind with the patient standing. One can readily spot asymmetry of the shoulder girdles, dropped shoulder, or laterally rotated scapula. In addition, the parascapular area is inspected for rhomboid atrophy, winging of the scapula, or atrophy of supraspinatus, infraspinatus, or deltoid muscles. Muscular atrophy can be a true neurogenic type secondary to muscle denervation, or at times from disuse. The mechanics of shoulder abduction and internal and external rotation of the upper arm should be viewed from behind, as can the response of latissimus dorsi to a deep cough.7 If there is a question of diaphragmatic paralysis, the chest can be percussed from behind, matching inspiratory tympani with that on expiration. Then, standing at the patient’s side, one can recheck internal and external rotation of the arm as well as adduction of the arm by the pectoralis and other muscles. Biceps/brachialis and brachioradialis can then be tested as elbow flexors and triceps as an elbow extensor. With the elbow fully extended, pronation and supination are tested, followed by wrist extension and flexion.
Patterns of Injury
On presentation, an attempt is made to localize the injury to the involved plexus elements based on the history and physical examination, supplemented by electrodiagnostic and imaging investigations. During assessment, functional loss of each element is graded as complete, incomplete or none. Generally, the clinical deficits with truncal, cord, and cord-to-nerve level injuries are relatively constant, the exception being the wrist and finger function that remain after combined middle and lower trunk damage. In addition, lower trunk loss sometimes involves more than hand intrinsic muscle and ulnar distribution sensory loss.7 C7 injuries can result in surprisingly few deficits, often only partial triceps weakness, as other spinal nerves carry input to the muscles supplied by this element. At the division level, injuries can have different patterns of loss, depending on which truncal outflows are involved and the proportion of anterior and posterior division loss. The typical motor and sensory involvement for each spinal nerve and truncal element are summarized in Table 197-1.
TABLE 197-1 Plexus Element Injured and Respective Distribution of Motor Deficit
| Structure Involved | Distribution of Loss |
|---|---|
| C5 | Supra- and infraspinatus, deltoid; rhomboids and serratus anterior also if injury is very proximal |
| C6 | Biceps, brachialis, brachioradialis, supinator |
| C7 | Triceps, pronator teres, some latissimus dorsi |
| C8 | Wrist and finger flexors, finger and thumb extensors, some hand intrinsics |
| T1 | Hand intrinsics |
| Upper trunk (C5–6) | Supra- and infraspinatus, deltoid, biceps and brachialis, brachioradialis, supinator |
| Middle trunk | Same as C7 |
| Lower trunk (C8–T1) | All hand intrinsics, some wrist and finger flexor and extensor loss |
Case Illustration: Diagnosis
A 56-year-old, right-handed man was involved in a motorcycle accident and dislocated his right shoulder joint. He noticed numbness of his radial three fingers and was unable to abduct his shoulder or flex his elbow. Initially he also had difficulty extending his wrist, fingers, and elbow. His shoulder dislocation was reduced, and he was evaluated by a neurosurgeon 6 weeks later. Atrophy of his deltoid and biceps muscles was evident. No contraction of his deltoid, supraspinatus, infraspinatus, biceps, or brachioradialis muscles could be appreciated, but his elbow, wrist, and finger extension had recovered to MRC grade 4. His wrist and finger flexors and hand intrinsic muscles functioned normally. Clinically, he presented with Erb’s palsy. Electrodiagnostic studies confirmed denervation of his biceps, deltoid, and supraspinatus muscles with reduced activation of triceps and pronator teres. Positive radial nerve sensory nerve action potentials (SNAPs) were recorded with stimulation at his insensate thumb, suggestive of a preganglionic lesion.
Diagnosis: Electrical and Imaging Studies
Preoperative Electrical Studies of Brachial Plexus Lesions
Special Electromyographic Studies
For supraclavicular palsies, the major question to be answered is how far proximal or medial the injury extends along the spinal nerves and roots. Special electromyographic (EMG) studies are of some help in this regard.8 Muscles that can be tested include the paraspinal, serratus anterior, and rhomboid muscles, since their innervation is very proximal. EMG studies of the other muscles supplied by the brachial plexus may help the clinician ascertain the location and extent of a plexus lesion, and may suggest the possibility of recovery even if there is no clinical evidence of this.
Sensory Nerve Action Potential Recording
Presence of a SNAP recorded at skin level along the dipole of a peripheral nerve such as ulnar, median, or radial, providing clinical loss is complete in the distribution of the nerve stimulated, suggests preganglionic injury of one or more dorsal roots.9 This means that one or more dorsal roots feeding the nerve have been injured between the dorsal root ganglion and the spinal cord. Injury at this site does not lead to Wallerian degeneration of the ganglion’s more distal afferent fibers, so the nerve is still capable of conducting electrical impulses even if there is no central connection.
Even though the presence of a SNAP in the distribution of a nerve with complete clinical loss strongly implies a preganglionic injury, the absence of a SNAP in the distribution of a nerve with plexus dorsal root input does not exclude preganglionic injury. If an injury is extensive enough, as frequently happens, it damages both the pre- and post-ganglionic segments of the spinal nerve. In these cases, the sensory fibers do degenerate, and no SNAP is recorded even though root-level damage extends close to the spinal cord.10 Moreover, electrical evidence of preganglionic injury to the dorsal root does not guarantee that the ventral root is damaged as severely and as closely to the spinal cord, although it does strongly suggest it.11
Imaging Studies of Brachial Plexus Injuries
Plain Radiographs
Special attention should be given to the clavicle, scapula, shoulder joint including the humeral bone, ribs, and the cervical spine, because stretch injuries of the brachial plexus have a high association with fracture and dislocation. Alternatively, there can be severe plexus injury without any history of shoulder dislocation or radiographic presence of a fracture.1 One cannot conclude that the fracture site localizes the level of the lesion; instead, plexus damage is often seen proximal to the fracture site.12
Fracture dislocations of the cervical spine are often associated with myelopathy but can also be associated with severe plexus stretch on one side or the other. If seen with either blunt or penetrating injuries, transverse process fractures augur poorly for the root or spinal nerve involved at that level. Fractures of the lamina of the vertebral column are more likely to be associated with myelopathy than those of the spinous processes, and vertebral body fractures bode poorly for reparability of roots at that level.7
Angiography and Venography
These studies are probably done more often than is needed in the management of plexus palsies.11 Even so, we cannot be too critical of their use, particularly if penetrating injuries involving upper arm, shoulder, or neck are present. Angiography is certainly indicated if there is absence of a radial or carotid pulse, an expanding mass in the area of the wound, or presence of a bruit or a thrill in the area of the wound. Less often, arteriography is indicated for vascular injury associated with stretch/contusion.6,13 Associated vascular injury, especially if it involves the subclavian artery, denotes a particularly severe stretch injury to the plexus.1 This is because the blunt or stretch injury involving vessels as well as the plexus has severely distracted the shoulder and arm, resulting in a lengthy and proximal neural injury. This makes the probability of direct repair of a significant portion of the plexus lesion less likely than in one without vascular injury, but it does not completely exclude repair as a possible treatment option (Table 197-2).
TABLE 197-2 Relative Contraindications for Exploration and Direct Repair of Adult Plexus Stretch Injuries
| Evidence of proximal spinal nerve injury at multiple levels, especially the upper levels; included is extensive paraspinal denervation by electromyogram, as well as rhomboid, serratus anterior, or diaphragmatic paralysis |
| Presence of sensory nerve action potentials elicited by stimulation of and recording from multiple peripheral nerves (median, radial and ulnar), whose sensory domain is anesthetic |
| Presence of pseudomeningoceles at multiple spinal nerve levels, especially the upper levels |
| Presence of extensive fractures of the cervical spine or serious cervical myelopathy |
| Injury limited to the C8–T1 spinal nerves or their outflows |
| Referral of the patient 1 year or more after injury |
| Cases in which there has been severe scapular or thoracic distraction, especially if there has been subclavian artery avulsion |
Myelography and CT Myelography
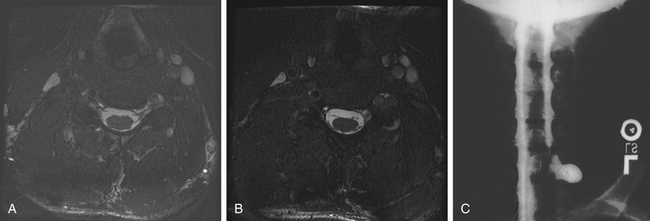
Imaging studies such as myelography, CT, and MRI determine the location and severity of brachial plexus injuries and aid in treatment planning. Standard myelography (Fig. 197-2) is used to detect nerve root avulsions, and the addition of CT scan has increased its positive predictive value to more than 95%.14 A traumatic pseudomeningocoele is a valuable sign of nerve root avulsion, although it is not pathognomonic. Nerve root avulsion may occur without a meningocoele, and conversely, a meningocoele may exist without nerve root avulsion15 (see Fig. 197-2). Absence of rootlets from the corresponding spinal level also indicates root avulsions, whereas thinning of one root suggests partial avulsion of that root.16
Magnetic Resonance Imaging with Contrast
Magnetic resonance imaging of the cervical spine and the brachial plexus is fast replacing myelography and CT myelography as the imaging of choice for brachial plexus stretch injuries, because of superb contrast resolution, multiplanar imaging capability, and noninvasive nature of the study. Although some authors15,16 still advocate myelography as the gold standard in imaging brachial plexus injuries, developments in MRI techniques, incorporating coronal and oblique slices of the neck and shoulder, and use of phase array surface coils, are improving the ability of this modality to image the brachial plexus.17–22 More recent studies from centers with dedicated protocols for brachial plexus imaging demonstrate greatly improved sensitivity of MRI for evaluating nerve root avulsions.20,23
Important MRI findings suggesting preganglionic injuries include nerve root avulsion (Fig. 197-2) and signal changes in the spinal cord, as well as abnormal enhancement of the intradural nerve roots and paraspinal muscles. Muscle enhancement suggests denervation injury, and may occur as early as 24 hours after a nerve is injured.15 Another MRI finding of an avulsed nerve root is the lack of low signal intensity in the fat of the neural foramen, best seen on T1 weighted images.24 MRI is also excellent in visualizing postganglionic brachial plexus injuries. Edema and fibrosis of the plexus can manifest as thickening, and stump neuromas postinjury can also be seen.15
Ultrasound
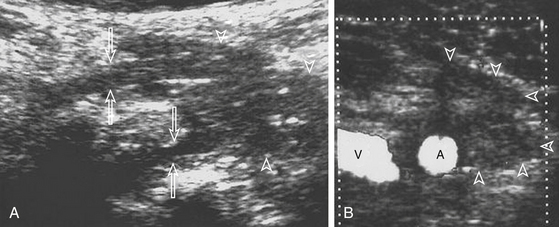
The successful use of ultrasound (US) in imaging brachial plexus25–27 and peripheral nerve anatomy28 reinforces the relevance of US study of brachial plexopathies. The advantages of sonography of the brachial plexus are its ability to directly visualize the nerve and provide fine details such as nerve disruption, detect the presence of scar tissue and its relation to the nerve, demarcate changes in nerve diameter (thickening) and texture (infiltration), and delineate anatomic relationships, especially to blood vessels. On US, visualization of vascularity does not require the injection of contrast medium. Sonography is easily tolerated by the patient, cost-effective, and has been successfully applied for surgical planning using skin markings before operation.
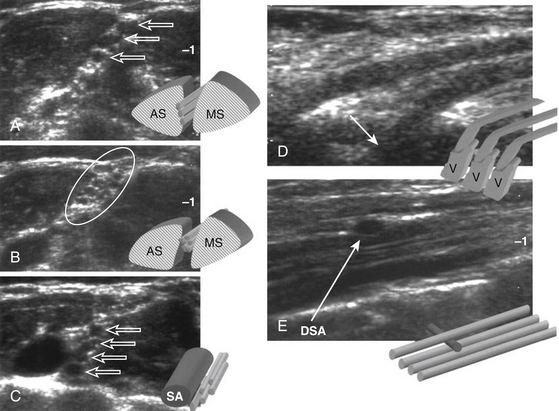
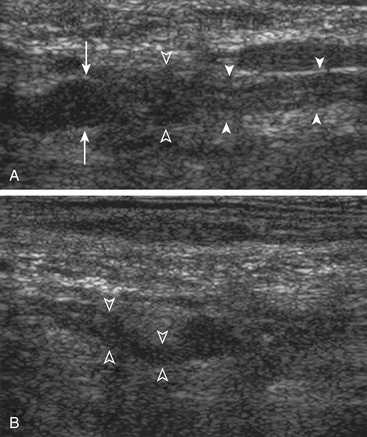
Ultrasound examinations are performed on conventional US units equipped with color Doppler capabilities. The nerves are initially localized at the level of the vertebral foramina and then followed longitudinally and transversely down to the axillary region. The identification of the normal nerve on US is based on the recognition of a hypoechoic structure tubular in shape in the longitudinal axis and round on transverse sections (Fig. 197-3). In brachial plexus injuries, three main types of ultrasound findings are noted: (1) disruption of nerve continuity (Fig. 197-4A) is associated with swelling of the proximal and distal stumps and a “wavy” course of the retracted distal segment (Fig. 197-4B); (2) scar tissue mass is irregular in shape and hypoechoic in texture (Fig. 197-5); and (3) segmental fusiform thickening of the trunk of the plexus represents a traumatic neuroma.
New Techniques
New techniques for imaging brachial plexus injuries include MR myelography, MR neurography, and Bezier surface reformation.15,29 MR myelography is used to evaluate nerve root avulsions but is less sensitive than CT myelography. MR neurography is used to visualize postganglionic nerve roots. Bezier surface reformation reformats images from CT myelographic volume data and demonstrates entire nerve roots on a single image.15 Most of these techniques have just recently been introduced as clinical applications.
Initial Management, Indications, and Timing of Surgery
Closed Injuries
At present, not all patients with severe stretch injury involving the brachial plexus can be meaningfully helped by an operation. This is especially so in complete avulsion cases, wherein direct repair is not feasible and nerves available for successful nerve transfer are very limited. It is best to evaluate the patient as early as possible. This includes grading any residual motor or sensory function in the extremity and obtaining a baseline set of electrodiagnostic studies at 3 or 4 weeks after injury. Appropriate imaging studies should be ordered. The patient is usually followed for about 3 to 4 months with periodic clinical and electrodiagnostic examinations. At the end of that period, the patient is operated on if there is no significant improvement in function and if there are not too may contraindications to exploration (Table 197-2). Consideration for surgical exploration includes the possibility of direct repair of the plexus at levels favorable for repair—that is, C5, C6, C7 and their more distal outflows, or lesions amenable to good outcomes with nerve transfers. The ideal time frame for surgery would be around 4 to 5 months postinjury. This would allow sufficient time for the plexus elements to recover. Even direct intraoperative recordings cannot exclude adequate regeneration in these elements, especially those with lengthy lesions in continuity, until 3 to 4 months from the time of injury. In addition, those cases capable of spontaneous recovery in the distribution of one or more elements may have them prematurely resected and replaced by grafts if the operation is done too early. In any case, maintenance of a good range of motion in all involved joints is of paramount importance while awaiting surgery.
Open Injuries/Lacerations
Open injuries are usually explored promptly because of concomitant injuries to other organs. Clean and sharp lacerations of the plexus elements are usually repaired acutely, within 72 hours, in the same sitting as the exploration. If the laceration is jagged, with the nerve ends contused and edematous, it is advisable to suture the ends of the injured nerve element to surrounding muscle to prevent it from retracting and shrinking. The definitive repair is delayed for 3 weeks in order to allow the longitudinal extent of the injury to be fully delineated, so that debridement of the nerve to healthy proximal and distal stumps can be performed prior to repair. If primary repair cannot be achieved, nerve grafts may be necessary.
Gunshot and Missile Injuries of the Brachial Plexus
Missile injuries to have brachial plexus have become more prevalent in recent years, accounting for 2.6% to 14% of all treated peripheral nerve injuries.30,31 Previously, they were thought to exhibit no spontaneous recovery with time, causing persistent pain and severe functional disability.30,32–34 Vrettos et al.34 observed that low-velocity civilian gunshot wounds are associated with a small shock wave and temporary cavitation, causing a transient neurologic deficit that may recover spontaneously. It is therefore recommended that civilian gunshot injuries to the plexus be observed for 3 to 4 months before surgical exploration is undertaken.32,34,35 Indications for surgery include absent or very poor return of function after 2 months or more, as well as severe pain. War-associated gunshot and violent explosion injuries often cause multiorgan injuries with a high prevalence of soft tissue damage including major vessels and peripheral nerves. The primary damage caused by an explosion or high-velocity missile is due to the pressure wave of the blast as it travels through air and causes contusion of the soft tissues. The secondary damage is caused by objects propelled outward by the explosion, which includes a variety of metallic objects such as shrapnel, nails, and bullets, as well as nonmetallic objects such as incidental material from the site of the explosion. Nerve elements are rarely injured by direct impact, but by shock waves and cavitation leading to compression and stretch injuries.
Gunshot wounds of the brachial plexus are associated with a high incidence of vascular injuries. Aside from transection of a major vessel, gunshot wounds can result in pseudoaneurysms or arteriovenous fistulas, which can compress the plexus and produce progressive loss of function and severe pain.35 Often, an associated vascular injury will warrant emergency repair, and the scarring and adhesions from this surgery may complicate the subsequent plexus exploration and repair.
Iatrogenic Plexus Injuries
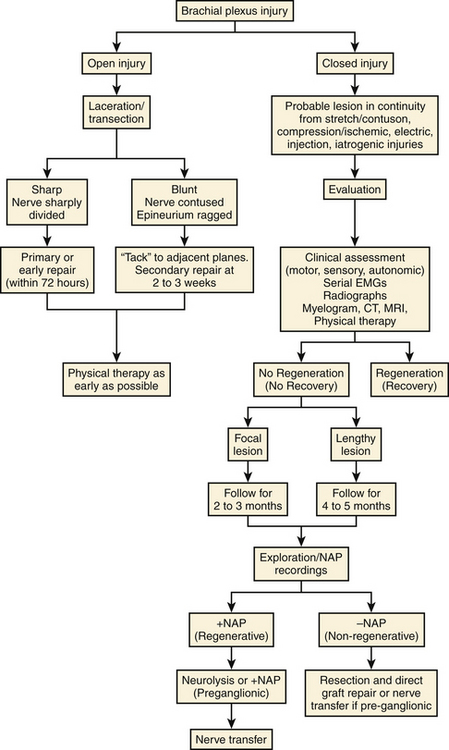
An algorithm for the management of brachial plexus injuries is outlined in Appendix 1 at the end of this chapter.
Pain
Brachial plexus injuries can be accompanied by pain, which may decrease in severity or disappear altogether in the first few months after injury. Pain is usually ameliorated by early institution of physical therapy, especially range of motion exercises, and the use of neuropathic pain medications such as gabapentin and amitriptyline. Brachial plexus exploration and external neurolysis appear to decrease the pain in some patients. For pain that is refractory to medical management and brachial plexus surgery, ablative procedures such as lesioning of the dorsal root entry zone may provide considerable relief.4
Goals of Surgery
The main objectives in treating severe adult brachial plexus injuries are to restore shoulder abduction, external rotation, elbow flexion, and forearm supination.36The combination of these movements will allow the patient to hold a food tray, to bring his hand up to his mouth, and to be able to push doors open while carrying items with the healthy arm. Restoration of sensation is a secondary objective, and primarily directed to the median nerve distribution. Unfortunately, intrinsic hand function is more difficult to restore, with poor outcomes when repairing or grafting to lower trunk elements; therefore, the limited available options should be directed towards the more attainable goals listed above. It is essential that the surgical strategy be planned thoughtfully to maximize the functional outcome. It is also vital that joint range of motion be preserved using physiotherapy, to prepare for the return of muscle function.
Types of Procedures
These can be grouped into five broad categories:
1. Neurolysis. This involves thorough exploration and dissection of the plexus elements in a circumferential, 360-degree fashion. No further interference is necessary when the nerve is found to be regenerating, as is shown by positive nerve action potentials across the injured nerve. This procedure is not intended to be therapeutic, except in cases where there is accompanying neuropathic pain.
2. Suture (end-to-end) repair. This is the ideal repair used in focal injuries, when the nerve ends can be reapproximated without tension.37 It is the goal during early exploration of sharp penetrating injuries (within 72 hours). Few surgeons advocate reimplantation of avulsed spinal rootlets.
3. Graft (direct) repair. A nerve graft is used to breach the gap between two unscarred nerve ends to provide a conduit for the regenerating axons. This is performed when NAP’s are negative across the injured nerve segment. Sural nerve or local expendable sensory nerves are harvested and used for long gaps. Some authors advocate use of commercially available tubular conduits for short gaps up to 3 cm in length;38,39 however, we prefer not to use tubes for large-diameter proximal nerve injuries as encountered with most plexal lesions.
4. Neurotization (nerve transfers). This involves the repair of a distal denervated nerve element using a proximal foreign nerve as the donor to reinnervate and restore function to a denervated end-organ. The donor nerve is brought as close as possible to the new end-organ, to shorten the regeneration distance. This allows for earlier reinnervation and potentially improved outcomes. Most nerve transfers can be accomplished without the use of interposition grafts, and this translates to one microsuture repair site, as compared to two. There is a correspondingly decreased likelihood of loss of axons (and/or their misdirection) with fewer repair sites.40
5. Salvage procedures. Tendon and free muscle transfers, occasionally with shoulder arthrodesis, can be performed with reasonable success.41,42 Unlike nerve repair and transfer techniques, timing of these procedures is less important, and is usually delayed until no further neural recovery is expected. Limb amputation is now rarely required. There is also an emerging field of bionics that may offer additional options in the future.
Operative Technique and Use of Intraoperative Electrophysiology
For supraclavicular and infraclavicular exploration, the patient is positioned supine with a roll under the ipsilateral shoulder and the head turned 30 degrees toward the opposite side. A large area extending from the mandible down to the lower chest, and laterally including the involved limb and the shoulder, should be prepped and draped. The ipsilateral leg should also be prepped in preparation for harvesting the sural nerve in case it is necessary to perform nerve grafts. For supraclavicular exploration, a transverse skin incision is made around 2 cm above and parallel to the clavicle, at the base of the posterior triangle of the neck (as in Fig. 197-6). Meanwhile, for infraclavicular exploration, a curvilinear hockey stick incision is made over the deltopectoral groove (Fig. 197-6).
After the overlying soft tissues have been dissected and the brachial plexus exposed, the elements are dissected in a 360-degree fashion to free them from the surrounding structures and any scars that may have resulted from the injury. Nerve action potentials (NAPs) are measured by stimulating the proximal spinal nerve and recording from distal trunks or divisions as well as cords. This is done to determine if the injured nerve element is regenerating. If the trace is flat (absent), then the injury is either postganglionic or pre- and post-ganglionic, and the proximal spinal nerve is sectioned until viable fascicular structure is visualized, for possible lead-out to grafts (Fig. 197-6A). If NAPs are positive but small in amplitude and relatively slow in conduction then the element is regenerating, and therefore is not sectioned. If the response has relatively high amplitude and is rapid in conduction, preganglionic injury with sensory fiber sparing is likely, unless there has been preoperative clinical and/or EMG evidence of sparing in its distribution, which suggests that it is an intact or partially injured element. When NAP recordings are positive, the element (usually spinal nerve) is not sectioned to look at the fascicular structure, unless visual inspection suggests the possibility of split repair, which is unusual in the stretch/contusion category.
Strategies for repair depend on intraoperative electrophysiology results and the level involved (Table 197-3). Only an external neurolysis is done if NAPs indicate regeneration. If NAPs are negative but normal fascicular structure is found on sectioning the nerve proximal to the injured segment, direct repair with grafts is done. In addition, neurotization or nerve transfers are also done to improve the outcome. Results with such combined repairs appear more favorable than in those with nerve grafts or neurotization alone, and several authors have advocated combined direct plexal graft repair with neurotization, as dictated by the individual findings for each case.2,43,44 For injuries that are clearly preganglionic, outright nerve transfers are performed.
TABLE 197-3 Repair Preferences for Supraclavicular Plexus Stretch Injuries
1. Neurolysis for elements where NAPs indicate regeneration (this is uncommon but does occur)
2. Direct repair if NAPs are negative, and yet on sectioning, fascicular structure is found proximally. Grafts are from proximal spinal nerves to divisions or cords. The following transfers may be added:
3. If all 5 plexus roots are avulsed, do the following transfers:
MRC, Medical Research Council; NAP, nerve action potential.
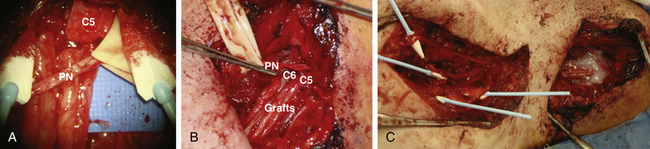
Case Illustration: Operative Treatment of Stretch Injury
The relatively well preserved C5 and C6 fascicular patterns, as well as positive evoked potentials from these nerves, were in favor of continuity of the ventral rootlets to these nerves. The plan was to bypass the neuroma in continuity with sural nerve grafts from the C5 proximal stumps to the axillary nerve and from the C6 proximal stump to the lateral cord (Fig. 197-6B and C). With the latter, we expect to reinnervate the musculocutaneous nerve as well as the lateral cord contribution to the median nerve. To reinnervate the suprascapular nerve, we elected to do a distal accessory nerve to suprascapular nerve transfer.46 With successful regeneration, this should restore function to the shoulder abductors (supraspinatus and deltoid) and elbow flexors. An additional option to increase the likelihood of restoration of elbow flexion would be to supplement the plexo-plexal graft repairs by transfer of the functional pectoral nerve to one half of the musculocutaneous nerve.4
Results
Supraclavicular Plexus
A majority (72%) of stretch injuries to the brachial plexus occur in the supraclavicular plexus, compared to 28% in the infraclavicular plexus.2 Unfortunately, the most common supraclavicular stretch injury pattern is C5–T1 (57%), followed by C5–C7 (20%), C5–C6 (15%), and C8–T1/C7–T1 (8%).2 Patients with C5–C6 and C5–C7 patterns of injury have the most favorable outcome after repair, especially in cases wherein there is no root avulsion and a graft repair would be sufficient. In the series based on material from Kline’s experience,4 43 of the 55 patients with C5–C6 palsy required graft repair, with the majority attaining useful function postoperatively. For example, 19 patients recovered to grade 3, 8 patients to grade 4, and 7 patients to grade 3 or 4. For patients with C5–C7 pattern of injury, majority required graft repair as well, with a good average outcome of grade 3 to 4. On the other hand, patients with flail arm (C5–T1) and injuries to the lower trunk exhibit poor results in general. For instance, only 35% of patients with flail arm recovered to an average postoperative grade of 3 or better after graft repair. Recovery, if present, was seen only in shoulder abduction, elbow flexion, and elbow extension; useful wrist or finger motion was seldom obtained.4 Generally, a higher percentage of patients show good recovery if the injury is limited to the upper trunk.7 Krishnan et al.45 reported even poorer results, with the average outcome of patients with C5–C7 injuries being MRC grade 3, and those with multiple root avulsions having a very poor outcome (MRC grade 0–2). This is why they performed secondary functional reconstructive procedures in 43% of their patients after the primary nerve reconstruction showed lack of improvement. Procedures that resulted in improved individual outcomes include the Steinler procedure, wrist extension restoration, claw hand correction, and free functional muscle flap transfer to the arm and forearm.45 In most cases, outcomes with direct repair of many stretch injuries were still less than optimal; thus, supplementation of direct repair with nerve transfers is recommended whenever possible.
For transfers, the use of intraplexus motor donors is preferred over extraplexus ones because the former have a greater number of axons. In addition, there is less need of postoperative central reeducation to achieve functional recovery.16,46 However, intraplexal donors may not always be available for transfer since they can be involved in the injury. A full and detailed discussion of all the possible nerve transfer options available, including their pros and cons, has been recently published.46 Some of the authors’ preferred nerve transfers include the spinal accessory nerve (SAN) to SSN and triceps branch or medial pectoral nerve to the axillary nerve for C5 palsy, and one of the medial pectoral nerve, intercostal nerves, or ulnar nerve fascicle to the musculocutaneous nerve (MCN) for C6 palsy.46
Spinal accessory nerve transfer to the SSN to restore shoulder abduction has mixed results, but more recent literature reports good functional recovery and stability of the shoulder especially when combined with axillary nerve reinnervation.16,46 The SAN can also be used for neurotization of the MCN to achieve elbow flexion, with MRC grade 3 or better outcomes achieved in 65% to 83% of patients.46 To regain shoulder abduction, the triceps branch can also be used as a donor for the axillary nerve, with promising results.47 The medial pectoral nerve and intercostal nerves can be used to reinnervate the MCN, resulting in a useful outcome (MRC grade 3 or better) in elbow flexion in 84% and 72% of patients, respectively.46 In this and other transfers, a direct end-to-end repair is highly preferable to using interposed grafts. Use of the ulnar nerve fascicle for transfer to the biceps branch is also known as the Oberlin transfer;48 the results are excellent, with 93% of patients recovering elbow flexion to MRC grade 4.49
Infraclavicular Plexus
Infraclavicular plexus injuries are less frequent than supraclavicular ones, but clinical loss can be just as severe. These cord and cord-to-nerve lesions have a higher incidence of associated injuries, such as vascular (axillary artery) and bony (shoulder joint, humerus) injuries. Unlike gunshot wounds involving plexus at an infraclavicular level, stretch injuries tend to be less focal and thus less likely to be restricted to one level of plexus elements. In other words, damage is seldom restricted to cords alone and more likely to extend from divisions to cords and to more distal nerves. If repair is necessary in such instances, results are not as good because of the length and severity of the lesion in continuity. Operations for these infraclavicular plexus injuries are technically more demanding than in the supraclavicular region because of the need to dissect and skeletonize large vessels which often have had prior repair. Results of suture and graft repair are relatively favorable for lateral and posterior cord and their outflows, as well as for medial cord to median nerve, but outcome of medial cord to ulnar nerve repair is poor.4
Complications
There is a significant risk of vascular injury if vascular exploration or repair was performed at an earlier date, so it is prudent to have a vascular surgeon available for these cases. The vessels that are at higher risk for injury are the vertebral artery (during foraminal dissection), the subclavian artery and vein (during proximal subclavicular dissection), and the circumflex humeral artery (during quadrangular space dissection).
Future Directions
The management of brachial plexus injuries has come a long way in the last 50 years. Better patient selection and early exploration, coupled with advances in nerve repair and microsurgery, and the increased use of nerve transfers, have improved the functional recovery of these patients. However, the outcomes we are able to achieve are still far from ideal, especially for patients with pan-plexus and lower trunk injuries. Future directions in brachial plexus injury management include advances in nerve rootlet reimplantation,50,51 implantation of peripheral nerve grafts into the spinal cord to induce regeneration of spinal motor neurons that can traverse the graft,16 use of nerve conduits to bridge gaps between injured nerves,14 and use of stem cells and growth factors that may augment nerve injury repair and accelerate nerve regeneration.16,51
Addas B.M.J., Midha R. Nerve transfers for severe nerve injury. Neurosurg Clin North Am. 2009;20:27-38.
Bhandari P.S., Sadhotra L.P., Bhargava P., et al. Current trends in the management of brachial plexus injuries. Indian J Neurotrauma. 2008;5:21-25.
Burkholder L.M., Houlden D.A., Midha R., et al. Neurogenic motor evoked potentials: role in brachial plexus surgery (case report). J Neurosurg. 2003;98:607-610.
Doi K., Otsuka K., Okamoto Y., et al. Cervical nerve root avulsion in brachial plexus injuries: magnetic resonance imaging classification and comparison with myelography and computerized tomography myelography. J Neurosurg. 2002;96:277-284.
Du R., Auguste K.I., Chin C.T., et al. Magnetic resonance neurography for the evaluation of peripheral nerve, brachial plexus, and nerve root disorders. J Neurosurg. 2010;112:362-371.
Gilliatt R. Physical injury to peripheral nerves: physiological and electrodiagnostic aspects. Mayo Clin Proc. 1981;56:361-370.
Gousheh J. The treatment of war injuries in the brachial plexus. J Hand Surg [Am]. 1995;20A:S68-S76.
Graif M., Martinoli C., Rochkind S., et al. Sonographic evaluation of brachial plexus pathology. Eur Radiol. 2004;14:193-200.
Kim D.H., Cho Y.J., Tiel R.L., et al. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg. 2003;98:1005-1016.
Kim D.H., Murovic J.A., Tiel R.L., et al. Penetrating injuries due to gunshot wounds involving the brachial plexus. Neurosurg Focus. 2004;16:E3.
Krishnan K.G., Martin K.D., Schackert G. Traumatic lesions of the brachial plexus: an analysis of outcomes in primary brachial plexus reconstruction and secondary functional arm reanimation. Neurosurgery. 2008;62:873-885.
Leechavenvongs S., Witoonchart K., Uerpairojkit C., et al. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part II: a report of 7 cases. J Hand Surg [Am]. 2003;28:633-638.
Malessy M., Thomeer R. Evaluation of intercostal to musculocutaneous nerve transfer in reconstructive brachial plexus surgery. J Neurosurg. 1988;88:266-271.
Midha R. Brachial plexus anatomy and pre-operative physiology. In: Kim D.H., Midha R., Murovic J.A., et al. Kline & Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. 2nd ed. New York: Elsevier Saunders; 2008:279-298.
Midha R. Stretch injuries to the brachial plexus. In: Kim D.H., Midha R., Murovic J.A., et al. Kline & Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. 2nd ed. New York: Elsevier Saunders; 2008:325-362.
Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16:E5.
Midha R. Epidemiology of brachial plexus injuries in a multitrauma population. Neurosurgery. 1997;40:1182-1189.
Oberlin C., Beal D., Leechavengvongs S., et al. Nerve transfer to biceps muscle using a part of the ulnar nerve for C5–C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg [Am]. 1994;19:232-237.
Rowshan K., Jones N.F., Gupta R. Current surgical techniques of peripheral nerve repair. Oper Tech Orthop. 2004;14:163-170.
Shin A.Y., Spinner R.J., Bishop A.T. Nerve transfers for brachial plexus injuries. Oper Tech Orthop. 2004;14:199-212.
Terzis J.K., Kostopoulos V.K. The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg. 2007;119:73e-92e.
Vrettos B.C., Rochkind S., Boome R.S. Low velocity gunshot wounds of the brachial plexus. J Hand Surg (Br). 1995;20:212-214.
Wilbourn A.J. Brachial plexus disorders. In: Dyck P.J., Thomas P.K. Peripheral neuropathy. 3rd ed. Philadelphia: WB Saunders; 1993:911-950.
Wu J.C., Huang W.C., Huang M.C., et al. A novel strategy for repairing preganglionic cervical root avulsion in brachial plexus injury by sural nerve grafting. J Neurosurg. 2009;110:775-785.
Yoshikawa T., Hayashi N., Yamamoto S., et al. Brachial plexus injury: clinical manifestations, conventional imaging findings, and the latest imaging techniques. Radiographics. 2006;26(suppl 1):S133-S134.
1. Midha R. Epidemiology of brachial plexus injuries in a multitrauma population. Neurosurgery. 1997;40:1182-1189.
2. Kim D.H., Cho Y.J., Tiel R.L., et al. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg. 2003;98:1005-1016.
3. Narakas A.O. Traumatic brachial plexus lesions. In: Dyck P.J., Thomas P.K., Lambert E.H., et al. Peripheral Neuropathy. Philadelphia: WB Saunders; 1984:1394.
4. Midha R., et al. Stretch injuries to the brachial plexus. In: Kim D.H., Midha R., Murovic J.A. Kline & Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. 2nd ed. New York: Elsevier Saunders; 2008:325-362.
5. Terzis J., Liberson W.T., Maragh H. Motorcycle brachial plexopathy. In: Terzis J., editor. Microreconstruction of Nerve Injuries. Philadelphia: WB Saunders; 1987:361-384.
6. Kline D.G., Hackett E.R., Happel L.H. Surgery for lesions of the brachial plexus. Arch Neurol. 1986;43:170-181.
7. Midha R. Brachial plexus anatomy and pre-operative physiology. In: Kim D.H., Midha R., Murovic J.A., et al. Kline & Hudson’s Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. 2nd ed. New York: Elsevier Saunders; 2008:279-298.
8. Yiannikas C., Shahani B.T., Young R.R. The investigation of traumatic lesions of the brachial plexus by electromyography and short latency somatosensory potentials evoked by stimulation of multiple peripheral nerves. J Neurol Neurosurg Psychiatry. 1983;46:1014-1022.
9. Gilliatt R. Physical injury to peripheral nerves: physiological and electrodiagnostic aspects. Mayo Clin Proc. 1981;56:361-370.
10. Burkholder L.M., Houlden D.A., Midha R., et al. Neurogenic motor evoked potentials: role in brachial plexus surgery (case report). J Neurosurg. 2003;98:607-610.
11. Wilbourn A.J. Brachial plexus disorders. In: Dyck P.J., Thomas P.K. Peripheral Neuropathy. 3rd ed. Philadelphia: WB Saunders; 1993:911-950.
12. Dolenc V. Diagnosis and treatment of lesions of the brachial plexus and adjacent structures. Clin Neurosurg. 1983;11:110-127.
13. McGillicuddy J.E. Clinical decision making in brachial plexus injuries. Neurosurg Clin North Am. 1991;2:137-150.
14. Bhandari P.S., Sadhotra L.P., Bhargava P., et al. Current trends in the management of brachial plexus injuries. Indian J Neurotrauma. 2008;5:21-25.
15. Yoshikawa T., Hayashi N., Yamamoto S., et al. Brachial plexus injury: clinical manifestations, conventional imaging findings, and the latest imaging techniques. Radiographics. 2006;26(suppl 1):S133-S134.
16. Terzis J.K., Kostopoulos V.K. The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg. 2007;119:73e-92e.
17. Kichari J.R., Hussain S.M., Den Hollander J.C., et al. MR imaging of the brachial plexus: current imaging sequences, normal findings, and findings in a spectrum of focal lesions with MR-pathologic correlation. Curr Probl Diagn Radiol. 2003;32:88-101.
18. Carvalho G.A., Nikkhah G., Matthies C., et al. Diagnosis of root avulsions in traumatic brachial plexus injuries: value of computerized tomography myelography and magnetic resonance imaging. J Neurosurg. 1997;86:69-76.
19. Aagaard B.D., Maravilla K.R., Kliot M. Magnetic resonance neurography: magnetic resonance imaging of peripheral nerves. Neuroimaging Clin North Am. 2001;11:131-146. viii
20. Doi K., Otsuka K., Okamoto Y., et al. Cervical nerve root avulsion in brachial plexus injuries: magnetic resonance imaging classification and comparison with myelography and computerized tomography myelography. J Neurosurg. 2002;96:277-284.
21. Gupta R.K., Mehta V.S., Banerji A.K., et al. MR evaluation of brachial plexus injuries. Neuroradiology. 1989;31:377-381.
22. Hayes C.E., Tsuruda J.S., Mathis C.M., et al. Brachial plexus: MR imaging with a dedicated phased array of surface coils. Radiology. 1997;203:286-289.
23. Grant G.A., Britz G.W., Goodkin R., et al. The utility of magnetic resonance imaging in evaluating peripheral nerve disorders. Muscle Nerve. 2002;25:314-331.
24. Hyodoh K., Hyodoh H., Akiba H., et al. Brachial plexus: normal anatomy and pathological conditions. Curr Probl Diagn Radiol. 2002;31:179-188.
25. Graif M., Martinoli C., Rochkind S., et al. Sonographic evaluation of brachial plexus pathology. Eur Radiol. 2004;14:193-200.
26. Sheppard D.J., Lyer R.B., Fenstermacher M.J. Brachial plexus: demonstration by US. Radiology. 1998;208:402-406.
27. Yang W.T., Chui P.T., Metreweli C. Anatomy of the normal brachial plexus revealed by sonography and the role of sonographic guidance in anesthesia of the brachial plexus. AJR Am J Roentgenol. 1998;171:1631-1636.
28. Martinoli C., Bianchi S., Derchi L.E. Ultrasonography of peripheral nerves. Semin Ultrasound CT MR. 2000;21:205-213.
29. Du R., Auguste K.I., Chin C.T., et al. Magnetic resonance neurography for the evaluation of peripheral nerve, brachial plexus, and nerve root disorders. J Neurosurg. 2010;112:362-371.
30. Gousheh J. The treatment of war injuries in the brachial plexus. J Hand Surg [Am]. 1995;20A:S68-S76.
31. Markovic D., Bajek G., Eskinja N. Peripheral nerve injuries in the 1991–1993 war in Croatia and Bosnia and Herzegovina. Croat Med J. 1995;36:108-113.
32. Klein S.R., Bongard F.S., White R.A. Neurovascular injuries of the thoracic outlet and axilla. Am J Surg. 1988;156:115-118.
33. Stewart M.P., Birch R. Penetrating missile injuries of the brachial plexus. J Bone Joint Surg Br. 2001;83:517-524.
34. Vrettos B.C., Rochkind S., Boome R.S. Low velocity gunshot wounds of the brachial plexus. J Hand Surg (Br). 1995;20:212-214.
35. Kim D.H., Murovic J.A., Tiel R.L., et al. Penetrating injuries due to gunshot wounds involving the brachial plexus. Neurosurg Focus. 2004;16:E3.
36. Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16:E5.
37. Rowshan K., Jones N.F., Gupta R. Current surgical techniques of peripheral nerve repair. Oper Tech Orthop. 2004;14:163-170.
38. De Ruiter G.C.W., Spinner R.J., Yaszemski M.J., et al. Nerve tubes for peripheral nerve repair. Neurosurg Clin North Am. 2009;20:91-105.
39. Myckatyn T.M., Mackinnon S.E. Surgical techniques of nerve grafting (standard/vascularized/allograft). Oper Tech Orthop. 2004;14:171-178.
40. Malessy M., Thomeer R. Evaluation of intercostal to musculocutaneous nerve transfer in reconstructive brachial plexus surgery. J Neurosurg. 1988;88:266-271.
41. Shin A.Y., Spinner R.J., Bishop A.T. Nerve transfers for brachial plexus injuries. Oper Tech Orthop. 2004;14:199-212.
42. Kozin S.H. Tendon transfers for radial and median nerve palsies. J Hand Ther. 2005;18:208-215.
43. Kline D.G., Hudson A.R. Coaptation of anterior rami of C-3 and C-4. J Neurosurg. 1991;75:667-668.
44. Yamada S., Peterson G.W., Soloniuk D.S., et al. Coaptation of the anterior rami of C-3 and C-4 to the upper trunk of the brachial plexus for cervical nerve root avulsion. J Neurosurg. 1991;74:171-177.
45. Krishnan K.G., Martin K.D., Schackert G. Traumatic lesions of the brachial plexus: an analysis of outcomes in primary brachial plexus reconstruction and secondary functional arm reanimation. Neurosurgery. 2008;62:873-885.
46. Addas B.M.J., Midha R. Nerve transfers for severe nerve injury. Neurosurg Clin North Am. 2009;20:27-38.
47. Leechavenvongs S., Witoonchart K., Uerpairojkit C., et al. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part II: a report of 7 cases. J Hand Surg [Am]. 2003;28:633-638.
48. Oberlin C., Beal D., Leechavengvongs S., et al. Nerve transfer to biceps muscle using a part of the ulnar nerve for C5–C6 avulsion of the brachial plexus: anatomical study and report of four cases. J Hand Surg [Am]. 1994;19:232-237.
49. Leechavengvongs S., Witoonchart K., Uerpairojkit C., et al. Nerve transfer to biceps muscle using a part of the ulnar nerve in brachial plexus injury (upper arm type): a report of 32 cases. J Hand Surg [Am]. 1998;23:711-716.
50. Htut M., Misra V.P., Anand P., et al. Motor recovery and the breathing arm after brachial plexus surgical repairs, including re-implantation of avulsed spinal roots into the spinal cord. J Hand Surg [Eur]. 2007;32:170-178.
51. Wu J.C., Huang W.C., Huang M.C., et al. A novel strategy for repairing preganglionic cervical root avulsion in brachial plexus injury by sural nerve grafting. J Neurosurg. 2009;110:775-785.