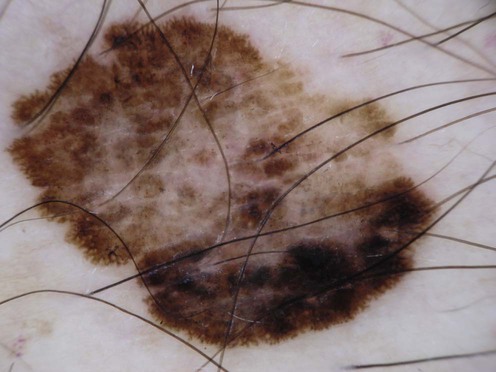
Malignant melanoma
Management strategy
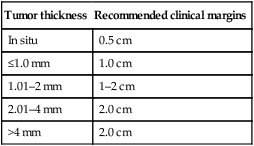
Staging of melanoma is crucial because it not only assigns patients into well-defined risk groups, it also aids in clinical decision-making as reviewed in the latest National Comprehensive Cancer Network (NCCN) clinical practice guidelines on melanoma. The goal of surgical management of primary cutaneous melanoma is to achieve negative histological margins to prevent recurrence and metastases. The current surgical margin guidelines from the NCCN are based on Breslow depth (Table 143.1). The standard treatment for stage IA melanoma is wide local excision. Total excision of primary melanoma with wide margins offers the best chance for cure. However, melanoma cells may extend non-contiguously for several millimeters beyond the visible lesion.
Table 143.1
Current melanoma excisional margin guidelines
| Tumor thickness | Recommended clinical margins |
| In situ | 0.5 cm |
| ≤1.0 mm | 1.0 cm |
| 1.01–2 mm | 1–2 cm |
| 2.01–4 mm | 2.0 cm |
| >4 mm | 2.0 cm |
Specific investigations
First-line therapies
Second-line therapies







 Surgical excision
Surgical excision Selective lymphadenectomy
Selective lymphadenectomy Elective lymphadenectomy
Elective lymphadenectomy Pegylated interferon alfa-2b
Pegylated interferon alfa-2b Paclitaxel and carboplatin
Paclitaxel and carboplatin Imiquimod
Imiquimod Interleukin-2
Interleukin-2 Isolated limb perfusion
Isolated limb perfusion Bcl-2 antisense and dacarbazine
Bcl-2 antisense and dacarbazine Dacarbazine
Dacarbazine Dacarbazine
Dacarbazine Vemurafenib
Vemurafenib Trametinib
Trametinib Imatinib
Imatinib Radiation therapy
Radiation therapy Mohs micrographic surgery
Mohs micrographic surgery