CHAPTER 122 Malignant Gliomas
Anaplastic Astrocytoma, Glioblastoma Multiforme, Gliosarcoma
Malignant astrocytomas, which include anaplastic astrocytoma (AA, World Health Organization [WHO] grade III) (Fig. 122-1, Table 122-1), glioblastoma multiforme (GBM, WHO grade IV) (Fig. 122-2, Tables 122-2 and 122-3), and gliosarcoma, are the most common malignant primary central nervous system (CNS) tumors in adults.1 Even with optimal treatment, median survival is only less than 2 years for patients with glioblastoma and 2 to 5 years for patients with anaplastic glioma.1–3 Malignant astrocytoma is characterized by its invasive and infiltrative nature, which makes curative resection unlikely.4 In the 1930s, Walter Dandy reported recurrence of contralateral gliomas even after hemispherectomy of the tumor-bearing hemisphere, thus illustrating how infiltrative these tumors are.5 The survival of these patients was still less than 2 years despite such aggressive resection.5 Advances have been made to steadily increase the survival of patients with these tumors. Four randomized, prospective, controlled multi-institutional trials have led to two new treatments that have received Food and Drug Administration approval for malignant gliomas.6–9 Brem and colleagues found that the use of carmustine-loaded biodegradable polymers improved survival in patients with recurrent malignant gliomas from 23 weeks to 31 weeks after revision resection (Fig. 122-3).6 Valtonen and associates found that the median time from surgery to death in patients with high-grade glioma was 58.1 weeks for those who received carmustine combined with a biodegradable polymer versus 39.9 weeks for the placebo group.8 Moreover, GBM patients treated with carmustine combined with a biodegradable polymer survived 53.3 weeks versus 39.9 weeks for the placebo group.8 In a multicenter long-term study by Westphal and coworkers, patients treated with Gliadel (carmustine with a biodegradable polymer) had a median survival of 13.8 months versus 11.6 months in placebo-treated patients.7 Stupp and coauthors reported a median survival of 14.6 months for patients with GBM after surgical resection, radiotherapy, and temozolomide chemotherapy.9 Advances in surgical adjuncts include intraoperative image-guided stereotaxis, functional magnetic resonance imaging (MRI),10,11 cortical mapping,12–14 and intraoperative MRI.15 Although these additions are aimed at assisting in increasing the extent of tumor resection, there is conflicting evidence whether the extent of resection is associated with improved survival of patients with high-grade glioma.16–21 This review focuses on AA, GBM, and gliosarcoma and discusses the epidemiology, molecular genetics, diagnosis, and surgical treatment, including the most recent data on the association between the extent of resection and survival of patients with these malignant lesions.
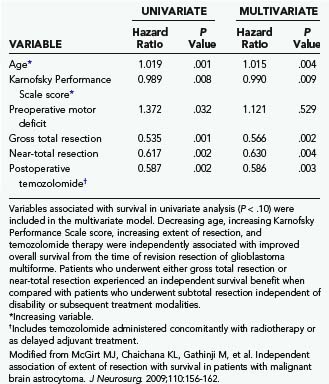
TABLE 122-1 Variables Associated with Overall Survival after Resection of World Health Organization Grade III Astrocytoma in Univariate and Multivariate Proportional Hazards Regression Analysis (Cox Model)
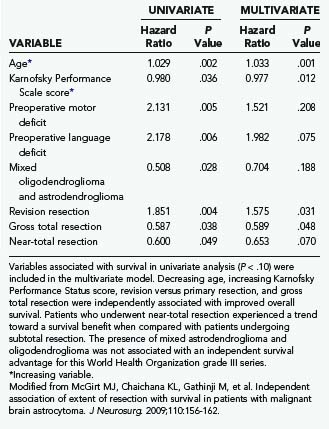
TABLE 122-2 Variables Associated with Overall Survival after Primary Resection of Glioblastoma Multiforme in Univariate and Multivariate Proportional Hazards Regression Analysis (Cox Model)
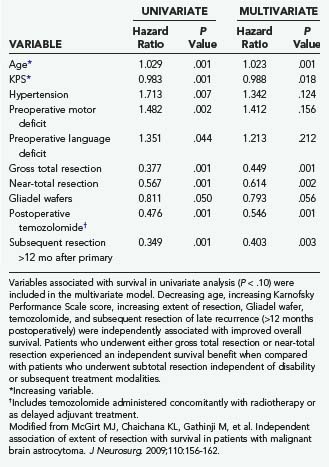
TABLE 122-3 Variables Associated with Overall Survival after Revision Resection of Glioblastoma Multiforme in Univariate and Multivariate Proportional Hazards Regression Analysis (Cox Model)
Epidemiology
Anaplastic Astrocytoma and Glioblastoma Multiforme
Malignant astrocytomas are the most common primary brain tumor in adults. Primary malignant CNS tumors account for about 2% of all cancers but cause a disproportionate rate of cancer-related morbidity and mortality.2,22 An estimated 43,800 new cases of benign and malignant brain tumors are diagnosed annually in the United States.22 Of these patients, approximately 12,760 will die.22 The incidence of brain tumors is 14.8 per 100,000 person-years, with approximately half being histologically malignant.22 Malignant CNS tumors are the leading cause of death from solid tumors in children and the third leading cause of cancer-related death in adolescents and adults aged 15 to 34 years.22 The average age at which AAs develop is approximately 40 years,23 as opposed to GBM, which has a mean age of 53 years with a peak incidence between the ages of 65 and 74.23,24 GBM is more common in men, with a male-to-female ratio of 1.5:1.25 There has been some controversy over a possible increase of approximately 1% to 2% per year in the incidence of brain tumors during the 1980s and 1990s,26,27 particularly in the elderly,28–30 as well as in children.31 Some studies indeed show a true increase in incidence rates independent of a greater ability to detect and diagnose brain tumors because of diagnostic improvements.2 This increase in the incidence of brain tumors may be due to the introduction of high-resolution neuroimaging, which has tremendously improved the clinical diagnosis of neurological diseases.32–34 Population-based incidence data from the Netherlands Cancer Registry have demonstrated a stable incidence of both childhood and adult gliomas.35 In adult gliomas, a significantly increasing incidence of high-grade astrocytoma was balanced by simultaneous decreases in low-grade astrocytoma, astrocytoma with unknown malignancy grade, and glioma of uncertain histology.35 Similar findings have been reported by Hoffman and coauthors, who analyzed data from six collaborating cancer registries of the Cancer Brain Tumor Registry of the United States (CBTRUS) from 1985 to 1999.36 Thus, these time trends may be explained at least in part by improvements in detection and diagnostic precision.
Gliosarcoma
Gliosarcomas represent between 2% and 8% of cases of GBM (WHO grade IV).37–41 The clinical findings and prognosis are similar in gliosarcoma and GBM.39 Meis and coworkers reported that there were no significant differences between gliosarcoma and GBM with regard to age, sex, pretreatment Karnofsky Performance Status (KPS) score, median survival, tumor location, and size.39 Most cases of gliosarcoma occur between the ages of 40 and 60, with a mean age of 53 years.39,40 However, gliosarcoma may also occur in children, and in rare cases infantile gliosarcoma has even been reported.42 Gliosarcoma occurs more frequently in males than females at a male-to-female ratio of 1.8:1.39,40
Clinical Manifestations
Anaplastic Astrocytoma and Glioblastoma Multiforme
AA and GBM often occur in the cerebral hemispheres.23 They can arise from low-grade astrocytoma (WHO grade II) but can also be diagnosed de novo at first biopsy without signs of a malignant precursor.23,43 AA has an innate tendency to progress to GBM. Like GBM, AA tends to recur locally, often at the margins of the tumor resection and even when gross total resection (GTR) has been performed.14 The symptoms and signs are relatively uniform but nonspecific in patients with GBM. Commonly, patients will have raised intracranial pressure, which may lead to headache, nausea and vomiting, blurred or double vision, and drowsiness. These signs and symptoms may be associated with extraocular palsies, objective papilledema, pupil abnormalities, or decreased level of consciousness. They are typically more prominent in the morning and improve over the course of the day. Relentless progressive headaches are a hallmark of the symptomatology of these tumors. Up to a third of patients with GBM have seizures. Neurological deficits are common and vary according to the location and extent of tumor infiltration. These deficits may be focal or global (altered cognition and change in personality). Although common in AA and GBM patients, neurological deficits from these lesions are often subtle and may go unrecognized until after the brain tumor is detected.
Gliosarcoma
The most common primary localization of gliosarcoma is in the temporal lobe, but these lesions also occur in the parietal, frontal, and occipital lobes (in decreasing order of frequency).30,39,41,44–47 Multifocal display of cerebral and cerebellar gliosarcomas has also been reported.48,49 GBM and gliosarcoma can spread into the cerebrospinal fluid pathways and invade the ventricles, cranial nerves, leptomeninges, and spinal cord.50,51 Gliosarcoma has a tendency toward peripheral brain localization and dural attachment.30,41,52–57 Jack and colleagues found that in contrast to AA and GBM, gliosarcoma has a marked tendency toward dural invasion and had a mixed dural and pial vascular supply in nearly half the cases.55 Despite similar features with glioblastoma, gliosarcoma has been found to metastasize much more frequently than glioblastoma, with extracranial metastases being reported in 15% to 30% of patients with gliosarcoma (Table 122-4).56–66 Metastases to visceral organs,56–60,62,63,65–70 as well as the spinal cord,51,61,65 have been reported. Metastatic tumor deposits usually contain both sarcomatous and glial cells, although there have been isolated reports of tumor deposits containing sarcomatous cells only.41,57,67
TABLE 122-4 Clinical Data on Representative Metastatic Gliosarcomas As Reported in the Recent Literature
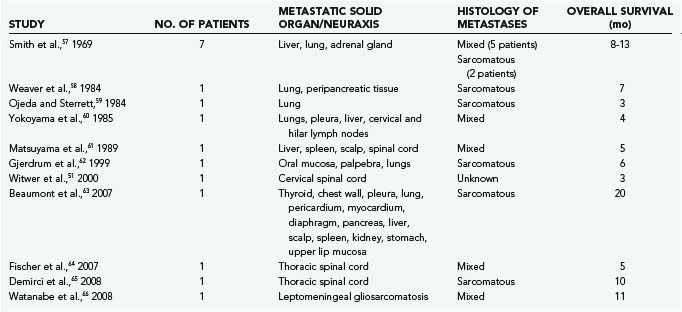
Gliosarcomas behave similarly to glioblastomas.38,39,41,44 Symptoms are largely determined by the location of the primary tumor and the presence of raised intracranial pressure.71 The most common symptoms of gliosarcoma are headache and hemiparesis, although nausea, seizures, and personality change may also occur. The most common signs of gliosarcoma are focal weakness, visual field defects, papilledema, and dysphasia.40,41,50
Histopathology
Anaplastic Astrocytoma and Glioblastoma Multiforme
Part of the reason that malignant gliomas are so lethal is that they grow by invasion, thus limiting the efficacy of surgery and other therapies (Figs. 122-4 and 122-5).72–74 Malignant glioma cells have been demonstrated to have great motility in both in vitro and in vivo rodent models.75–78 Increasing grade of glioma and exposure to growth factors, such as epidermal growth factor, increase the motility of the cells.79–82 Autopsy studies have shown that high-grade gliomas usually extend beyond a single carotid or vertebral artery distribution, often spread through the cerebrospinal fluid pathways, and may extend past the 2-cm margins demonstrated by computed tomography (CT) or MRI.73,83,84 Even after seemingly curative resection of these tumors, there are frequently microsatellites of tumor cells scattered throughout normal brain tissue that have the potential to continue proliferating and cause tumor recurrence in other areas of the brain.85 Infiltration of tumor into eloquent areas of the brain may limit the extent of tumor resection.86–88 As far back as 1928, Walter Dandy reported recurrence of contralateral gliomas even after hemispherectomy.5 Stereotactic biopsy samples obtained from brain regions distant from enhancing tumor, as delineated by MRI, show that areas with high signal intensity on T2-weighted images contain tumor cells.84 Biopsy of regions beyond any MRI signal abnormalities produces specimens that are histologically normal but nevertheless have been demonstrated to contain tumor cells with in vitro culture techniques.89 Thus, imaging techniques and histology have limited resolution in estimating the true extent of tumor cell invasion and thus inevitably underestimate the true extent of these tumors.
The current classification systems for malignant gliomas rely on the histologic characteristics of these lesions. The tumor size, nodal status, and metastasis (TNM) system that is used for many systemic cancers is not applicable to the grading of gliomas because these lesions rarely metastasize beyond the CNS. The utility of any particular glioma classification system has traditionally been validated by assessing how well it can predict length of survival. Cell lineage and thus the specific type of high-grade glioma can be determined with antibodies directed against specific cell markers. The WHO classification of brain tumors was first published in 197990 and then modified in 1993,91 2000,92 and 2007.93 Although imperfect, controversial, and without molecular basis, the current WHO system is the most widely used classification system for gliomas.91 Even though a wealth of genomic data have been added recently,57 morphology is still the “gold standard” of the WHO classification. The classification system consists of a four-tiered schema that includes pilocytic astrocytoma (WHO grade I), low-grade astrocytoma (WHO grade II), AA (WHO grade III), and GBM (WHO grade IV). WHO grade II tumors may have nuclear atypia. WHO grade III tumors display mitotic activity and nuclear atypia. WHO grade IV tumors show nuclear atypia, mitoses, and endothelial proliferation or necrosis.
Uncommon glioblastoma variants include gliosarcoma, which contains a prominent sarcomatous element; giant cell glioblastoma, which has multinucleated giant cells; small cell glioblastoma, which is associated with amplification of epidermal growth factor receptor (EGFR); and glioblastoma with oligodendroglial features, which may be associated with a better prognosis than standard glioblastomas.40,94
In addition to the WHO classification system, the MIB-1/Ki-67 labeling index is often included in the pathology reports of these tumors. The labeling index increases proportionally with tumor grade. AA (WHO grade III) typically has a labeling index in the range of 5% to 10%, although values vary considerably, even within different regions of the tumor itself.44,95–97 GBM (WHO grade IV) can also show extensive heterogeneity, with a mean labeling index generally between 10% and 20%.44,96,98,99
Molecular Biology
There has been tremendous progress in our understanding of the molecular pathogenesis and origin of malignant gliomas, especially with regard to cancer stem cells.100,101 Malignant transformation of gliomas results from the sequential accumulation of genetic mutations and deregulation of growth factor signaling pathways or failure of the cell cycle control mechanisms, or both.100–102 Significant advances have been made in elucidating the biology of gliomas. In 2008, Parsons and colleagues sequenced more than 20,000 protein-coding genes in 22 human GBM samples and found a new mutated gene, isocitrate dehydrogenase 1 (IDH1), shared by many patients with secondary GBM.57 This study illustrates the powerful ability that new and emerging research technologies have in enhancing our understanding of the biology of these tumors and discovery of new targets for future therapies.
Glioblastomas have traditionally been separated into two main subtypes, primary and secondary glioblastoma. These subtypes are morphologically indistinguishable and respond similarly to conventional therapy. However, they differ biologically and genetically and thus may respond differently to targeted molecular therapies.100,102 Primary glioblastomas are typically seen in patients older than 50 years and are characterized by EGFR amplification and mutations, loss of heterozygosity of chromosome 10q, deletion of the phosphatase and tensin homologue on chromosome 10 (PTEN), and deletion of chromosome p16. Secondary glioblastomas have transcriptional patterns and aberrations in DNA copy number that significantly differ from those of primary glioblastomas.100,102 These tumors occur in younger patients as low-grade astrocytoma or AA and then transform over a period of several years into glioblastoma. Secondary glioblastomas are much less common than primary glioblastomas and are characterized by mutations in the TP53 tumor suppressor gene,66 overexpression of platelet-derived growth factor receptor (PDGFR), abnormalities in the p16 and retinoblastoma (Rb) pathways, and loss of heterozygosity of chromosome 10q.100,103 It has recently been discovered that a specific mutation of IDH1 was present in 70% of WHO grades II and III astrocytomas and oligodendrogliomas, as well as in glioblastomas that developed from these lower grade lesions.57 Moreover, mutations of the oxidized nicotinamide adenine dinucleotide phosphate (NADP+)-dependent isocitrate dehydrogenases encoded by IDH1 and IDH2 occur in a majority of several types of malignant glioma, especially in secondary glioblastomas.57
The most common mutations involve tumor suppressor genes involved in cell cycle control, such as TP53 and PTEN. TP53 mutations occur more commonly in secondary GBM, whereas PTEN mutations occur more commonly in primary GBM.102,104,105 PTEN, a tumor suppressor gene that negatively regulates the phosphatidylinositol-3′-kinase (PI3K) pathway, is inactivated in 40% to 50% of patients with glioblastoma.100,106 Although PTEN mutations are frequently observed in GBM, these mutations are rare in lower grade astrocytic tumors.40 Compromised PTEN function may contribute to gliomagenesis through disrupted regulation of proliferation, stem cell self-renewal, angiogenesis, migration, invasion, and regulation of other tumor suppressor pathways such as TP53.107 TP53 plays a role in several cellular processes, including the cell cycle, response of cells to DNA damage, cell death, cell differentiation, and neovascularization.108 TP53 mutations are the first detectable genetic mutations in two thirds of precursor low-grade diffuse astrocytomas, and its frequency is similar to that of AAs and secondary glioblastomas derived thereof.66,109,110 TP53 mutations also occur in primary glioblastomas, but at a lower frequency (<30% of cases).110
Aberrations in the growth factor signaling pathways involving EGFR and PDGFR are also very prominent and play an important role in primary and secondary GBM.100 Amplification or mutation of EGFR occurs almost exclusively in primary GBM and is seen in approximately 40% to 50% of patients with these tumors. About half of tumors with EGFR amplification express a constitutively autophosphorylated variant of EGFR known as EGFRvIII that lacks the extracellular ligand-binding domain.94,100 This EGFRvIII variant has become an important therapeutic target for kinase inhibitors, immunotoxins, and peptide vaccines.100,111 Activating mutations in the extracellular domain of EGFR have also been identified.112 PDGFR signaling is very important in glial development.113 A PDGFR autocrine loop is frequently present in gliomas because the tumor cells express high levels of both ligand and receptor in this pathway, thereby ultimately stimulating proliferation of the tumor.100 Growth factor receptor signaling (i.e., PDGFR, EGFR) through intermediate signal transduction generators results in the activation of transcriptional programs for survival, proliferation, invasion, and angiogenesis. Common signal transduction pathways activated by these growth factors are the PI3K-Akt-mTOR (mammalian target of rapamycin) pathways, which are involved in cellular proliferation and inhibition of apoptosis, and the Ras-MAPK (mitogen-activated protein kinase) pathway, which is involved in cell cycle progression and proliferation.100 Many of these pathways lead to the upregulation of vascular endothelial growth factor (VEGF), which promotes angiogenesis.114,115
Role of Stem Cells in Pathogenesis and Resistance to Therapy
Although there has been great progress in characterizing the genetic mutations and signaling pathways involved in the development of malignant gliomas, the cellular origins of these tumors are unknown. The adult nervous system has been discovered to harbor neural stem cells that are capable of self-renewal, proliferation, and differentiation into distinctive mature cell types.116,117 There is now increasing evidence that these neural stem cells, or related progenitor cells, can be transformed into brain tumor stem cells and give rise to malignant gliomas by escaping the mechanisms that control proliferation, programmed differentiation, and apoptosis.118–122 These stem cells are identified by several immunocytochemical markers, such as CD133 (prominin 1) and nestin.106,118,121,123,124 Although stem cells are only a small subpopulation of cells within malignant gliomas, they appear to be critical for generating these tumors and maintaining their bulk.122,125 Glioma stem cells have been shown to produce VEGF and promote angiogenesis in the tumor microenvironment.126 Moreover, tumor stem cells have been found to require a vascular niche for optimal function.127 Thus, antiangiogenic therapy has potential for inhibiting the functioning of glioma stem cells.
Gliosarcomas
Gliosarcoma is a rare glioblastoma variant characterized by a biphasic tissue pattern with glial and mesenchymal components.37,40,58,128,129 The glial portion of gliosarcomas consists most commonly of astrocytes with nuclear atypia and mitotic figures.41 The sarcomatous region consists of neoplastic mesenchymal cells with associated reticulin formation.40 These cells are spindle shaped and demonstrate nuclear atypia, increased mitotic activity, and necrosis. Glial fibrillary acidic protein (GFAP) immunostaining is an important tool for distinguishing between gliosarcoma and other tumors such as glioblastoma or pure sarcoma.39 GFAP is present in glial regions but found in very low quantities in sarcomatous regions.47 Strong staining for vimentin, which is a marker for mesenchymal cells, occurs mostly in sarcomatous areas with scarce staining in glial regions.47 Likewise, there is no reticulin staining in glial regions, except around blood vessels, but sarcomatous regions are rich in reticulin.47
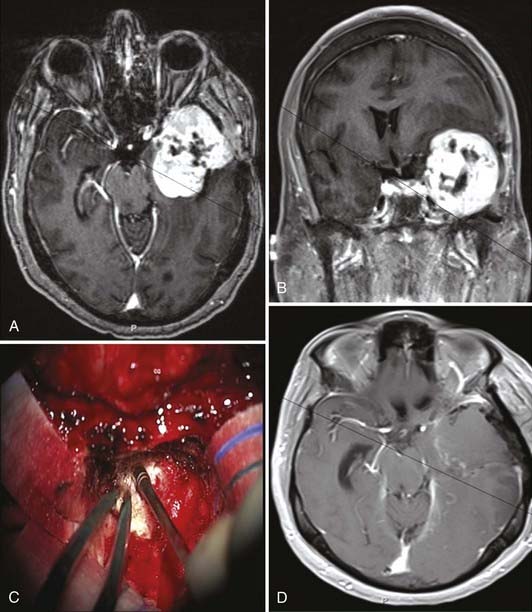
Gliosarcoma usually appears as a tough, well-circumscribed lobular mass often attached to the dura and at surgery may resemble a meningioma (Fig. 122-6).53,54 These tumors frequently contain areas of necrosis that are predominantly located in the poorly defined, soft astrocytic component.54,55 The sarcomatous component of these tumors is firm and well circumscribed, which often facilitates separation of it from adjacent brain tissue.54,55
Neuroimaging Studies
Anaplastic Astrocytoma and Glioblastoma Multiforme
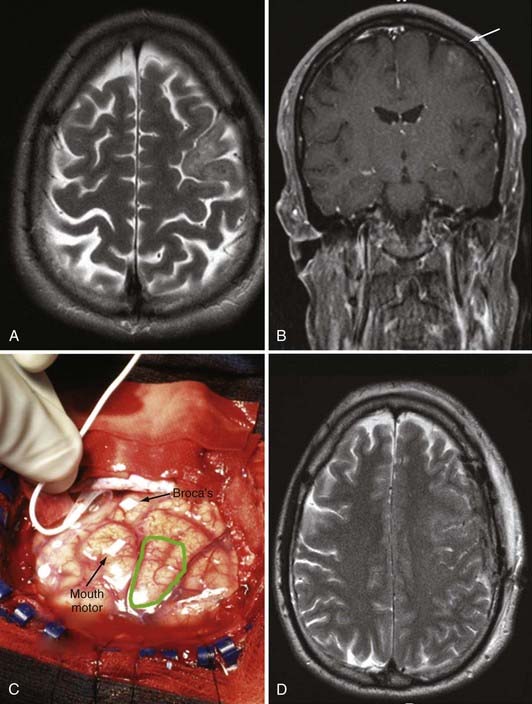
Although skull radiography, angiography, and CT were the major diagnostic modalities of brain imaging in the past, MRI is now the preferred study for brain tumors. CT is used in the acute environment as the first line of imaging to exclude hemorrhage or large areas of infarction in the brain. CT is particularly adept at delineating acute hemorrhage, as well as intratumoral calcification. However, once a mass lesion is suspected on non–contrast-enhanced CT, MRI is used to better characterize the mass because of its multiplanar capability and superior soft tissue contrast. Gadolinium contrast agent can enhance soft tissue contrast, further elucidate the boundaries of the tumor, and provide information about the integrity of the blood-brain barrier by detecting areas of blood-brain barrier breakdown. Standard T1- and T2-weighted MRI studies are able to detect brain tumors with high sensitivity with regard to size and localization. They are also able to detect mass effect, edema, hemorrhage, necrosis, and signs of increased intracranial pressure. High-grade glioma normally appears as an irregular hypodense lesion on T1-weighted MRI with various degrees of contrast enhancement and edema. The presence of ring-like enhancement surrounding irregularly shaped areas of presumed necrosis suggests glioblastoma (see Fig. 122-5A and B).130 However, AAs can appear as nonenhancing tumors, and even glioblastomas may initially be manifested as a nonenhancing lesions, especially in older patients (see Fig. 122-4A).131 Moreover, low-grade gliomas may occasionally demonstrate contrast enhancement.131 Functional MRI can be used to define the locations of functionally eloquent cortex, such as the motor cortex, Broca’s area, Wernicke’s area, and the visual cortex. It is important to note that in up to 40% of cases, MRI studies performed in the first month after radiotherapy may show increased enhancement.132 In 50% of these cases that show enhancement in the first month after radiotherapy, the increased enhancement reflects a transient increase in vessel permeability that is a result of radiotherapy, a phenomenon that improves with time and is designated “pseudoprogression.”132 Differentiating pseudoprogression from true progression of the cancer can be challenging initially, even with advanced imaging techniques. Although MRI is the current clinical gold standard and provides excellent structural detail, it has poor specificity in identifying viable recurrent tumors in brain treated with surgery/radiotherapy/chemotherapy.133
Magnetic resonance spectroscopy may be used to help differentiate tumors from stroke, old trauma, radionecrosis, infection, and multiple sclerosis. Fluorodeoxyglucose positron emission tomography (FDG-PET) is effective in demonstrating hypermetabolism in high-grade tumors. It is well established that FDG uptake has prognostic value.134,135 High FDG uptake in a previously known low-grade tumor establishes the diagnosis of anaplastic transformation.134,135 Thus, FDG-PET is also helpful in distinguishing tumor or tumor recurrence from radionecrosis. Amino acid and amino acid analogue PET tracers constitute another class of tumor-imaging agents that are particularly attractive for imaging brain tumors because of their high uptake in tumor tissue and low uptake in normal brain tissue, thus yielding greater tumor-to–normal tissue contrast.136,137 Superior diagnostic accuracy of F-dopa over FDG in evaluating recurrent low-grade and high-grade gliomas has been reported in the literature.138,139
Gliosarcomas
The appearance of gliosarcoma on CT is similar to that of infiltrating glioblastoma.54 Gliosarcomas most often appear as a well-demarcated hyperdense mass with heterogeneous or irregular ring enhancement (Fig. 122-6A and B).47,54,140,141 The majority of gliosarcomas are superficial with a dural base.47 Vasogenic edema is present in almost all cases of gliosarcoma, irrespective of tumor size.54,141 Central hypodensity, as a result of necrosis, is less common in gliosarcomas.55 Gliosarcomas with osteosarcomatous differentiation often demonstrate calcification, which appears intensely hyperdense.131 Typically, contrast-enhanced T1-weighted MRI of cerebral gliosarcomas demonstrates diffuse, inhomogeneous enhancement or irregular ring-like enhancement.30 Gliosarcomas tend to be well-defined lesions with either an inhomogeneous or cystic appearance and surrounding vasogenic edema.30 Dwyer’s group reported that in their series of six patients with gliosarcoma, all tumors were characterized as primarily intra-axial but abutting a dural surface.30 On T2-weighted imaging, all the tumors were of intermediate signal intensity with surrounding edema.30 The signal intensity of the gliosarcomas in the study was similar to gray matter but was hypointense relative to other glial neoplasms. On contrast-enhanced T1-weighted imaging, there was intense tumor enhancement, often with a ring-like appearance.30 The study concluded that gliosarcoma should be included in the differential diagnosis of any tumor that appears to be intra-axial but abutting a dural surface and is much less hypointense on T2-weighted images than other glial neoplasms.30
Prominent tumor uptake on single-photon emission computed tomography occurs in gliosarcoma, just as in glioblastomas.66 Uptake of thallium 201 by malignant glioma cells correlates directly with the level of proliferating cell nuclear antigen and the histologic grade of the tumor and is directly related to the amount of contrast enhancement on MRI.142 Both thallium 201 uptake and contrast enhancement on MRI depend on regional blood flow and breakdown of the blood-brain barrier; however, thallium 201 uptake also depends on tumor cell activity.142 Thus, thallium 201 scintigraphy may be an indicator of tumor activity and viability.
Management
General Medical Management
Much of the care of patients with malignant glioma involves general medical management of the signs and symptoms. When treating these patients, it is important to keep in mind that the most common problems include peritumoral edema, seizures, fatigue, venous thromboembolism, and cognitive dysfunction.30,143 Care must be taken when selecting antiepileptic drugs to prevent drug interactions. Many antiepileptic drugs, such as phenytoin and carbamazepine, induce hepatic cytochrome P-450 enzymes and thus may increase the metabolism of chemotherapeutic agents. For this reason, antiepileptic drugs that do not induce these enzymes, such as levetiracetam, are generally preferred.3 Whether patients who have never had a seizure should be treated with prophylactic antiepileptic drugs is controversial. The American Academy of Neurology issued practice guidelines indicating that there is no evidence that prophylactic antiepileptic drugs are beneficial in patients with brain tumors who have not had seizures and advises against the routine use of antiepileptic drugs in such patients.144
Peritumoral edema is frequently treated with corticosteroids such as dexamethasone. However, Cushing’s syndrome and corticosteroid myopathy may develop as a side effect in patients who require prolonged treatment with high doses of corticosteroids. Because corticosteroids can cause immunosuppression, brain tumor patients who receive corticosteroids are at increased risk for Pneumocystis jiroveci pneumonitis. Prophylactic antibiotic therapy has been considered in these patients,144 but a recent meta-analysis did not show a benefit from this approach.145 Corticosteroids may also produce long-term complications, including osteoporosis and compression fractures. Preventive measures such as vitamin D, calcium supplements, and bisphosphonates should be instituted. New therapies, including corticotropin-releasing factor, bevacizumab (VEGF monoclonal antibody), and VEGF receptor inhibitors, have been found to reduce peritumoral edema and may decrease the need for corticosteroids.143,146
There is also increased risk for venous thromboembolism from leg and pelvic veins in patients with malignant glioma, with a cumulative incidence of 20% to 30%.3,143,147 Unless these patients have a history of intracerebral hemorrhage or have other contraindications, it is generally safe to provide anticoagulation therapy for venous thromboembolism.3 The risk for intratumoral hemorrhage associated with anticoagulation therapy in patients with glioma who have venous thromboembolism is low.143,148 Low-molecular-weight heparin may be more effective and safer than warfarin.149 Conversely, inferior vena cava filters are associated with high complication rates.150
Patients with malignant gliomas often experience significant fatigue and may benefit from treatment with methylphenidate or modafinil.151 Methylphenidate may also help treat abulia. Donepezil and memantine may reduce memory loss.152 Depression is underdiagnosed in patients with malignant glioma, and antidepressants and psychiatric support should be taken into consideration for patients with brain tumors.153
Surgery
For many solid organ malignant tumors, GTR with clear surgical margins is associated with extended survival.105,106 However, the effect of extensive resection on prolonging survival in patients with malignant astrocytoma is less clear.16,21 At best, 99% resection (a 2-log kill) can be achieved. However, the remaining 1% is sufficient for these tumors to recur. Extensive resection of malignant astrocytomas is difficult because these tumors are frequently invasive and widely infiltrative and often involve eloquent areas.4
Several techniques have been developed that can improve the safety and efficacy of resection. Studies have shown that limiting the resection to grossly abnormal tissue is not enough to prevent functional deficits.154 Gliomas may invade brain tissue without impairing normal brain function, as has been demonstrated by large, asymptomatic brain tumors in the brainstem and thalamus that cannot be removed without incurring severe neurological deficits. Functional mapping techniques have enabled an increased extent of resection while avoiding injury to the functional cortex (i.e., language, motor, and sensory cortex). Moreover, intraoperative CT or MRI navigational systems have aided in the identification of tumor margins and anatomic landmarks. However, a significant limitation of these neuronavigational systems is that brain shifting during resection renders preoperative image-based planning inaccurate.
It remains unclear whether the extent of resection of high-grade gliomas is associated with improved survival.21,155,156 Intuitively, one would predict that extensive resection should prolong survival, as it has with other solid organ malignant tumors.105,106 Recurrence of malignant astrocytomas commonly occurs close to the tumor margin, where there is an increased tumor cell density at the periphery of the tumor and a sharp falloff in cell numbers as the distance from the resection cavity increases.157 Extensive resection theoretically decreases the number of remaining cells, renders the decreased tumor burden more responsive to adjuvant therapy, and potentially prolongs survival.16,20,158–160
Influence of Extent of Resection on Clinical Outcomes
To date, there have been four systematic reviews of the literature on the influence of the extent of resection on clinical outcomes.155,156,161,162 These reviews were limited in that they did not control for confounding variables, were often underpowered, included biopsies in their analyses, and frequently included studies conducted before 1990.155,156,161,162 In fact, these reviews stated that the lack of good scientific evidence precludes any definitive statement on the effect of extensive resection on survival of patients with malignant astrocytoma.155,156,161,162
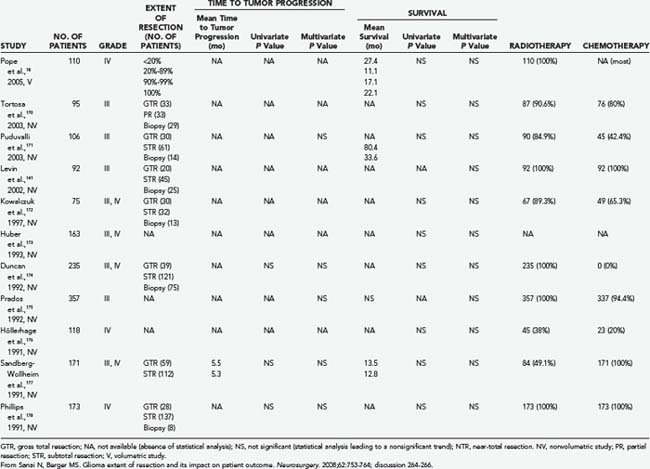
More recent studies have also been limited. Lacroix and colleagues found that after analyzing 416 consecutive patients with GBM, resections greater than 98% were significantly associated with improved survival.163 The limitation of this study was that 44% of the patients were previously treated at other institutions and adjuvant therapy was not included in the survival analysis, which may hinder accurate survival analysis.163 Buckner’s group2 and Laws and associates77 also concluded that patients who underwent surgical resection had longer survival times than did patients who underwent biopsy. It therefore remains unclear what effects GTR, near-total resection (NTR), and subtotal resection (STR) have on survival (see Tables 122-5 and 122-6 for summary2,18,19,52,141,160,163–178).
TABLE 122-5 Extent of Resection of High-Grade Glioma in Selected Volumetric and Nonvolumetric Studies in the Neurosurgical Literature: Positive Studies Indicating That More Extensive Tumor Resection Is Significantly Associated with Longer Life Expectancy
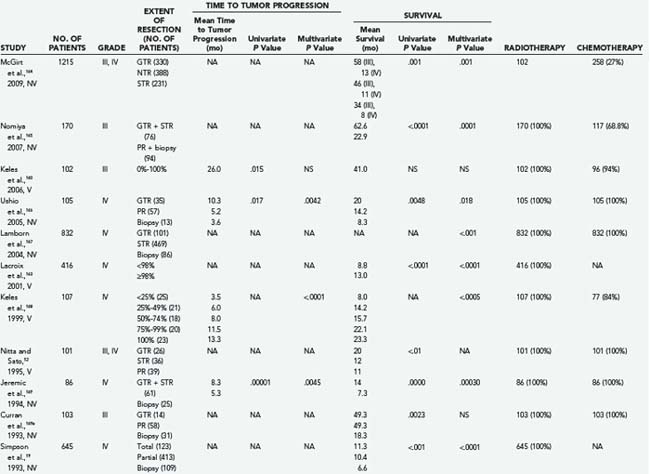
TABLE 122-6 Extent of Resection of High-Grade Glioma in Selected Volumetric and Nonvolumetric Studies in the Neurosurgical Literature: Negative Studies Indicating That More Extensive Tumor Resection Is Not Significantly Associated with Longer Life Expectancy
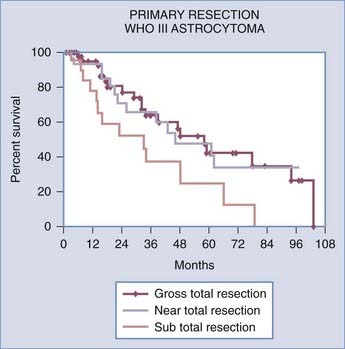
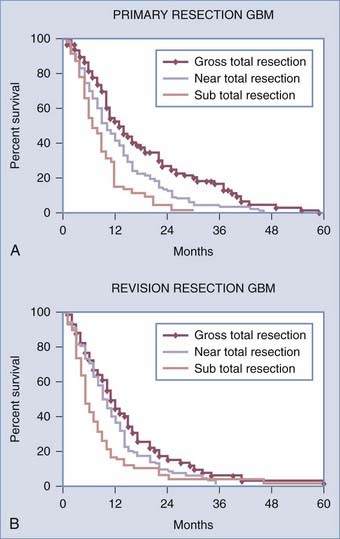
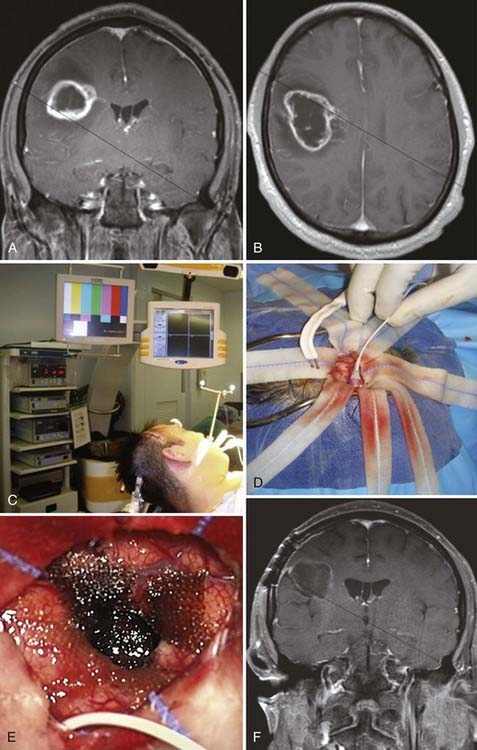
A recent retrospective study of more than 1000 patients by McGirt and coworkers adjusted for factors that may have an impact on survival and found that greater extent of resection is associated with prolonged survival, with both GTR and NTR independently being associated with prolonged survival.164 This was observed for both primary and revision resection of GBM (see Tables 122-2 and 122-3), independent of age, KPS score, subsequent resection, and the two adjuvant therapies proven to influence survival (Gliadel and temozolomide). Furthermore, longer survival was noted after GTR than after NTR for both primary and revision resection of GBM. Although GTR was associated with a further survival advantage over NTR in the surgical treatment of GBM, GTR and NTR demonstrated similar survival benefit when compared with STR in the treatment of AA (see Fig. 122-1). Nevertheless, residual nodular enhancement on postoperative MRI was associated with a 1-year survival disadvantage in patients undergoing primary resection of AA.
Extensive resection of malignant glioma may decrease the tumor burden to a point that makes adjuvant therapy (radiotherapy and chemotherapy) more effective. A lower tumor load has been shown to increase the efficacy of adjuvant chemotherapy and radiotherapy in killing the remaining cancer cells and increasing survival.94,179 Besides increasing survival, safe and extensive resection may be associated with other secondary benefits. Extensive resection may provide increased relief of symptoms and neurological improvement.180 One important consideration to take into account is that tumors amenable to GTR may also have been biologically more favorable to treatment.
Radiation Therapy and Chemotherapy for Anaplastic Astrocytoma and Glioblastoma Multiforme
Although great progress has been made in understanding the diverse mutations present in high-grade gliomas, the treatment approach is fairly uniform. A major advance in the treatment of high-grade gliomas occurred when Brem and associates found that the use of carmustine-loaded biodegradable polymers improved survival in patients with recurrent malignant glioma from 23 weeks to 31 weeks after revision resection (see Fig. 122-3).6 Valtonen and colleagues found that the median time from surgery to death in patients with high-grade glioma was 58.1 weeks in those who received carmustine-loaded biodegradable polymers versus 39.9 weeks in the placebo group.8 Moreover, GBM patients treated with carmustine polymer wafers survived 53.3 weeks as compared with 39.9 weeks for the placebo group.8 In a multicenter long-term study by Westphal and coworkers, patients treated with Gliadel-loaded biodegradable polymers had a median survival of 13.8 months versus 11.6 months for placebo-treated patients.7 Another major advance in the treatment of malignant glioma was reported by Stupp and colleagues, who demonstrated increased survival (median survival of 14.6 months) in patients with GBM after undergoing surgical resection along with radiotherapy and temozolomide (Temodar) chemotherapy.9 The Neuro-Oncology Surgical Outcomes Research Laboratory at The Johns Hopkins Hospital has demonstrated further improved outcomes with a mean survival of 20 months in patients with GBM when these treatment modalities (Gliadel, Temodar, and radiation therapy) were combined after surgical resection of tumor (Fig. 122-7).181 Although there are many approved and investigational drugs for the treatment of glioma, at this point the use of carmustine-loaded biodegradable wafers combined with Temodar and radiation therapy is the most effective treatment of high-grade gliomas.
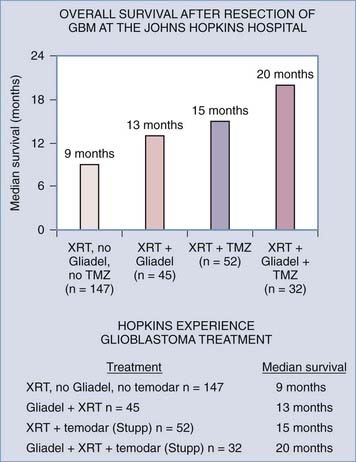
FIGURE 122-7 Assessment of multimodality treatment on survival of patients with glioblastoma multiforme at The Johns Hopkins Hospital, all of whom underwent surgical resection. The mean survival of patients treated with just radiation therapy after tumor resection was 9 months (n = 147 patients). Combination treatment with radiation therapy and intraoperative Gliadel placement increased mean patient survival to 13 months after surgical resection (n = 45). Treatment of patients with the radiation therapy and temozolomide9 further increased mean survival to 15 months (n = 52). Finally, multimodality treatment involving radiation therapy, temozolomide, and intraoperative placement of Gliadel resulted in the longest mean survival time of 20 months (n = 32).
(From Attenello FJ, Mukherjee D, Datoo G, et al. BCNU wafer in surgical treatment of glioma. Ann Surg Oncol. 2008;15:2887-2893.)
O6-Methylguanine-DNA methyltransferase (MGMT) is an important repair enzyme that contributes to resistance of tumors to alkylating agents such as carmustine or Temodar. MGMT promoter methylation silences the gene, thus decreasing DNA repair activity and increasing the susceptibility of tumor cells to carmustine or Temodar. In one study, glioblastoma patients with MGMT promoter methylation (45% of the total) who were treated with Temodar had a median survival of 21.7 months and a 2-year survival rate of 46%,182 whereas patients without MGMT promoter methylation who were treated with temozolomide had a significantly shorter median survival of just 12.7 months and a 2-year survival rate of 13.8%.182 Temodar is currently used for the treatment of glioblastomas regardless of MGMT promoter methylation status. Depletion of MGMT with a dose-dense temozolomide schedule and combinations of temozolomide with inhibitors of MGMT, such as O6-benzylguanine, are strategies that are undergoing current investigation.182–185
Gliosarcoma
Because gliosarcoma shares many features with glioblastoma, the two types of tumors are treated similarly—with surgery, radiotherapy, and chemotherapy.38,39,50 On resection, most gliosarcomas tend to be firm, well demarcated, and vascular.47 Thus, they can be excised to achieve gross macroscopic clearance.48 Unlike meningiomas, superficially located gliosarcomas are weakly adherent to the dura.54 Gliosarcomas with osteosarcomatous differentiation have a tougher consistency, which makes excision of them more difficult.70 Greater extent of resection may increase the survival of patients with gliosarcoma.
Radiotherapy should be offered to all patients with gliosarcoma because it improves long-term outcomes39,44 and increases survival by 8 to 15 weeks.41,44 In view of the fact that the clinical and prognostic features of primary gliosarcoma and glioblastoma are very similar, it is suggested that the chemotherapy protocols used for glioblastoma also be used for gliosarcoma.186 In a large retrospective study by Kozak’s group using the Surveillance Epidemiology and End Results (SEER) database, it was found that tumor excision, as opposed to biopsy only, and adjuvant radiotherapy may improve outcome.146 In phase II trials performed by Prados and colleagues, patients treated with the combination of erlotinib and temozolomide during and after radiotherapy had better survival than historical controls, with a median survival of 19.3 and 14.1 months, respectively.187 Because some of the studies have proposed that gliosarcoma might develop as a result of abnormalities of angiogenesis within a preexisting glioblastoma,41,55,58 chemotherapeutic agents that target abnormal angiogenesis, such as thalidomide, could be considered as a chemotherapeutic option.71 Such agents could potentially alter the natural history of gliosarcoma or prevent the development of gliosarcoma from preexisting glioblastoma. Among the newer treatment modalities is immunotherapy, in which therapeutic agents are directed against specific antigens. A suitable antigenic target could be vimentin because dense vimentin immunostaining is present in sarcomatous cells in gliosarcoma.188
Patient Outcome and Survival
The prognosis for patients with high-grade glioma is poor. Median survival is less than 2 years despite advances in surgical technology, radiotherapy, and chemotherapy9 and 2 to 5 years for patients with anaplastic glioma.1,3 Management typically consists of surgery followed by radiotherapy or chemotherapy, or both. Even after extensive treatment, residual tumor is inevitable and patients eventually succumb to this disease.11 Individual patient survival is heterogeneous, with some long-term survivors.189 Multiple prognostic factors have been proposed, including age, preoperative KPS score, tumor size, tumor location, and even marital status, among others (see Tables 122-1 to 122-3).76,163,164,190,191 Currently, age and KPS score are the most significant prognostic factors.164,167
The majority of large clinical studies have shown no significant difference in the outcome of patients with gliosarcoma and glioblastoma However, in a large retrospective study by Kozak’s group using the SEER database, gliosarcoma had a slightly worse prognosis than glioblastoma, which was statistically significant.146 The median length of survival of patients with gliosarcoma has been reported to range from 6 to 14.8 months.39,41,54,192,193 In the study of Morantz and colleagues, the average length of survival was 26 weeks after the onset of symptoms and 21 weeks after surgery.41 The survival rate of patients with gliosarcoma was 75% after 6 months and 19% after 1 year.41 Salvati and associates found that the median time to recurrence in gliosarcoma patients with predominantly gliomatous components was 53 weeks versus 62 weeks in patients with predominantly sarcomatous features.193
Future Directions
Just as carmustine-loaded biodegradable polymers significantly improved the survival of patients with recurrent malignant glioma from 23 to 31 weeks after revision resection7 and from 11.6 to 13.8 months after initial therapy,7 future therapies will further increase the survival of patients with high-grade glioma. Stronger understanding of the biology of these tumors has led to the development of several drugs against various disrupted cellular signals in these tumor cells. Advances are being made in angiogenesis therapy and vaccines. A very exciting recent finding in the field of glioma research is the discovery that neural and mesenchymal stem cells have a unique tropism for brain tumor cells.166,194 These stem cells have an ability to migrate across the blood-brain barrier into the tumor when administered intra-arterially.180,194 They have also been demonstrated to migrate from the contralateral hemisphere into the tumor.166,194 Several groups have shown that these stem cells can be genetically engineered to locally activate chemotherapeutic prodrugs around tumor cells,194 elicit an enhanced immune response against the tumor,195 and deliver viral therapy to attack the tumor cells.196,197 In the future, we may be able to implant biopolymer wafers or gels into the tumor resection cavity to locally release genetically engineered stem cells that can track down microsatellites of tumor cells and release tumoricidal substances/viruses, as well as elicit a powerful immune response against the tumor cells.198
Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group. Lancet. 1995;345:1008-1012.
Burger PC, Heinz ER, Shibata T, et al. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg. 1988;68:698-704.
Butowski N, Lamborn KR, Berger MS, et al. Historical controls for phase II surgically based trials requiring gross total resection of glioblastoma multiforme. J Neurooncol. 2007;85:87-94.
Chang SM, Parney IF, Huang W, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293:557-564.
Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol (Berl). 2007;114:443-458.
DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114-123.
Galanis E, Buckner JC, Dinapoli RP, et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. J Neurosurg. 1998;89:425-430.
Gallia GL, Tyler BM, Hann CL, et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009;8:386-393.
Hess KR. Extent of resection as a prognostic variable in the treatment of gliomas. J Neurooncol. 1999;42:227-231.
Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227-235.
McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156-162.
McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583-588.
Nazzaro JM, Neuwelt EA. The role of surgery in the management of supratentorial intermediate and high-grade astrocytomas in adults. J Neurosurg. 1990;73:331-344.
Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807-1812.
Quinones-Hinojosa A, Sanai N, Gonzalez-Perez O, et al. The human brain subventricular zone: stem cells in this niche and its organization. Neurosurg Clin N Am. 2007;18:15-20. vii
Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811-822.
Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044-1055.
Sneed PK, Gutin PH, Larson DA, et al. Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys. 1994;29:719-727.
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
Valtonen S, Timonen U, Toivanen P, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44-48.
Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425-436.
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492-507.
Westphal M, Ram Z, Riddle V, et al. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien). 2006;148:269-275.
Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765-773.
1 DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114-123.
2 Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853-860.
3 Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492-507.
4 Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol (Berl). 2007;114:443-458.
5 Dandy WE. Removal of right cerebral hemispheres for certain tumors with hemiplegia: preliminary report. JAMA. 1928;90:823-825.
6 Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group. Lancet. 1995;345:1008-1012.
7 Westphal M, Ram Z, Riddle V, et al. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien). 2006;148:269-275.
8 Valtonen S, Timonen U, Toivanen P, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44-48.
9 Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
10 Berman JI, Berger MS, Chung SW, et al. Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg. 2007;107:488-494.
11 Pirzkall A, Li X, Oh J, et al. 3D MRSI for resected high-grade gliomas before RT: tumor extent according to metabolic activity in relation to MRI. Int J Radiat Oncol Biol Phys. 2004;59:126-137.
12 Guggisberg AG, Honma SM, Findlay AM, et al. Mapping functional connectivity in patients with brain lesions. Ann Neurol. 2008;63:193-203.
13 Schiffbauer H, Berger MS, Ferrari P, et al. Preoperative magnetic source imaging for brain tumor surgery: a quantitative comparison with intraoperative sensory and motor mapping. J Neurosurg. 2002;97:1333-1342.
14 Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323-1329.
15 Muragaki Y, Iseki H, Maruyama T, et al. Usefulness of intraoperative magnetic resonance imaging for glioma surgery. Acta Neurochir Suppl. 2006;98:67-75.
16 Barker FG2nd, Prados MD, Chang SM, et al. Radiation response and survival time in patients with glioblastoma multiforme. J Neurosurg. 1996;84:442-448.
17 Dinapoli RP, Brown LD, Arusell RM, et al. Phase III comparative evaluation of PCNU and carmustine combined with radiation therapy for high-grade glioma. J Clin Oncol. 1993;11:1316-1321.
18 Pope WB, Sayre J, Perlina A, et al. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol. 2005;26:2466-2474.
19 Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239-244.
20 Vecht CJ, Avezaat CJ, van Putten WL, et al. The influence of the extent of surgery on the neurological function and survival in malignant glioma. A retrospective analysis in 243 patients. J Neurol Neurosurg Psychiatry. 1990;53:466-471.
21 Cai J, Wu Y, Mirua T, et al. Properties of a fetal multipotent neural stem cell (NEP cell). Dev Biol. 2002;251:221-240.
22 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66.
23 Keihus P, Davis R, Coons SW, et al. Anaplastic Astrocytoma. In: Keihues P, Cavenee WK, editors. Pathology and Genetics of Tumors of the Nervous System, World Health Organization Classification of Tumours. Lyon: IARC Press, 2000.
24 Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278-299.
25 Trouillas P, Menaud G, De The G, et al. [Epidemiological study of primary tumors of the neuraxis in the Rhone-Alps region. Quantitative data on the etiology and geographical distribution of 1670 tumors.]. Rev Neurol (Paris). 1975;131:691-708.
26 Muir CS, Storm HH, Polednak A. Brain and other nervous system tumours. Cancer Surv. 1994;19-20:369-392.
27 Preston-Martin S, Lewis S, Winkelmann R, et al. Descriptive epidemiology of primary cancer of the brain, cranial nerves, and cranial meninges in New Zealand, 1948-88. Cancer Causes Control. 1993;4:529-538.
28 Boyle P, Maisonneuve P, Saracci R, et al. Is the increased incidence of primary malignant brain tumors in the elderly real? J Natl Cancer Inst. 1990;82:1594-1596.
29 Greig NH, Ries LG, Yancik R, et al. Increasing annual incidence of primary malignant brain tumors in the elderly. J Natl Cancer Inst. 1990;82:1621-1624.
30 Shugg D, Allen BJ, Blizzard L, et al. Brain cancer incidence, mortality and case survival: observations from two Australian cancer registries. Int J Cancer. 1994;59:765-770.
31 Swensen AR, Bushhouse SA. Childhood cancer incidence and trends in Minnesota, 1988-1994. Minn Med. 1998;81:27-32.
32 Legler JM, Ries LA, Smith MA, et al. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91:1382-1390.
33 Modan B, Wagener DK, Feldman JJ, et al. Increased mortality from brain tumors: a combined outcome of diagnostic technology and change of attitude toward the elderly. Am J Epidemiol. 1992;135:1349-1357.
34 Christensen HC, Kosteljanetz M, Johansen C. Incidences of gliomas and meningiomas in Denmark, 1943 to 1997. Neurosurgery. 2003;52:1327-1333.
35 Houben MP, Aben KK, Teepen JL, et al. Stable incidence of childhood and adult glioma in The Netherlands, 1989-2003. Acta Oncol. 2006;45:272-279.
36 Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985-1999. Neuro Oncol. 2006;8:27-37.
37 Feigin IH, Gross SW. Sarcoma arising in glioblastoma of the brain. Am J Pathol. 1955;31:633-653.
38 Galanis E, Buckner JC, Dinapoli RP, et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. J Neurosurg. 1998;89:425-430.
39 Meis JM, Martz KL, Nelson JS. Mixed glioblastoma multiforme and sarcoma. A clinicopathologic study of 26 radiation therapy oncology group cases. Cancer. 1991;67:2342-2349.
40 Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109.
41 Morantz RA, Feigin I, Ransohoff J3rd. Clinical and pathological study of 24 cases of gliosarcoma. J Neurosurg. 1976;45:398-408.
42 Okami N, Kawamata T, Kubo O, et al. Infantile gliosarcoma: a case and a review of the literature. Childs Nerv Syst. 2002;18:351-355.
43 Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765-773.
44 Jaros E, Perry RH, Adam L, et al. Prognostic implications of p53 protein, epidermal growth factor receptor, and Ki-67 labelling in brain tumours. Br J Cancer. 1992;66:373-385.
45 Classen J, Hoffmann W, Kortmann RD, et al. Gliosarcoma—case report and review of the literature. Acta Oncol. 1997;36:771-774.
46 Boerman RH, Anderl K, Herath J, et al. The glial and mesenchymal elements of gliosarcomas share similar genetic alterations. J Neuropathol Exp Neurol. 1996;55:973-981.
47 Parekh HC, O’Donovan DG, Sharma RR, et al. Primary cerebral gliosarcoma: report of 17 cases. Br J Neurosurg. 1995;9:171-178.
48 Pakos EE, Goussia AC, Zina VP, et al. Multi-focal gliosarcoma: a case report and review of the literature. J Neurooncol. 2005;74:301-304.
49 Woltjer RL, Weil RJ, Moots PL, et al. Pathologic quiz case: cerebellar hemorrhage in an octogenarian. Gliosarcoma. Arch Pathol Lab Med. 2003;127:e345-e346.
50 Vecchio F, Giordano R, De Zanche L, et al. Intracranial sarcoma with reactive glioma: a clinicopathological case report. Eur Neurol. 1988;28:301-305.
51 Witwer BP, Salamat MS, Resnick DK. Gliosarcoma metastatic to the cervical spinal cord: case report and review of the literature. Surg Neurol. 2000;54:373-378.
52 Nitta T, Sato K. Prognostic implications of the extent of surgical resection in patients with intracranial malignant gliomas. Cancer. 1995;75:2727-2731.
53 Russell DS, Rubinstein LJ. Pathology of Tumors of the Nervous System. Baltimore: Williams & Wilkins; 1989. 794-797
54 Maiuri F, Stella L, Benvenuti D, et al. Cerebral gliosarcomas: correlation of computed tomographic findings, surgical aspect, pathological features, and prognosis. Neurosurgery. 1990;26:261-267.
55 Jack CRJr, Bhansali DT, Chason JL, et al. Angiographic features of gliosarcoma. AJNR Am J Neuroradiol. 1987;8:117-122.
56 Cerame MA, Guthikonda M, Kohli CM. Extraneural metastases in gliosarcoma: a case report and review of the literature. Neurosurgery. 1985;17:413-418.
57 Smith DR, Hardman JM, Earle KM. Contiguous glioblastoma multiforme and fibrosarcoma with extracranial metastasis. Cancer. 1969;24:270-276.
58 Weaver D, Vandenberg S, Park TS, et al. Selective peripancreatic sarcoma metastases from primary gliosarcoma. Case report. J Neurosurg. 1984;61:599-601.
59 Ojeda VJ, Sterrett GF. Cerebral gliosarcoma, pulmonary adenoid-cystic carcinoma, and pulmonary metastatic gliosarcoma: report of an untreated case. Pathology. 1984;16:217-221.
60 Yokoyama H, Ono H, Mori K, et al. Extracranial metastasis of glioblastoma with sarcomatous component. Surg Neurol. 1985;24:641-645.
61 Matsuyama J, Mori T, Hori S, et al. [Gliosarcoma with multiple extracranial metastases. Case report.]. Neurol Med Chir (Tokyo). 1989;29:938-943.
62 Gjerdrum LM, Bojsen-Moller M. October 1998—61 year old male with brain tumor and oral, lung, and palpebral masses. Brain Pathol. 1999;9:421-422.
63 Beaumont TL, Kupsky WJ, Barger GR, et al. Gliosarcoma with multiple extracranial metastases: case report and review of the literature. J Neurooncol. 2007;83:39-46.
64 Fischer S, Lee W, Aulisi E, et al. Gliosarcoma with intramedullary spinal metastases: a case report and review of the literature. J Clin Oncol. 2007;25:447-449.
65 Demirci S, Akalin T, Islekel S, et al. Multiple spinal metastases of cranial gliosarcoma: a case report and review of the literature. J Neurooncol. 2008;88:199-204.
66 Watanabe Y, Hotta T, Yoshioka H, et al. Primary diffuse leptomeningeal gliosarcomatosis. J Neurooncol. 2008;86:207-210.
67 Feigin I, Allen LB, Lipkin L, et al. The endothelial hyperplasia of the cerebral blood vessels with brain tumors, and its sarcomatous transformation. Cancer. 1958;11:264-277.
68 Garret R. Glioblastoma and fibrosarcoma of the brain with extracranial metastases. Cancer. 1958;11:888-894.
69 Ehrenreich T, Devlin JF. A complex of glioblastoma and spindle-cell sarcoma with pulmonary metastasis. AMA Arch Pathol. 1958;66:536-549.
70 Wharton SB, Whittle IR, Collie DA, et al. Gliosarcoma with areas of primitive neuroepithelial differentiation and extracranial metastasis. Clin Neuropathol. 2001;20:212-218.
71 Alatakis S, Stuckey S, Siu K, et al. Gliosarcoma with osteosarcomatous differentiation: review of radiological and pathological features. J Clin Neurosci. 2004;11:650-656.
72 Burger PC, Heinz ER, Shibata T, et al. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg. 1988;68:698-704.
73 Scherer HJ. The forms of growth in gliomas and their practical significance. Brain. 1940;63:1-35.
74 Scherer HJ. Cerebral astrocytomas and their derivatives. Am J Cancer. 1940;4:159-198.
75 Bernstein JJ, Goldberg WJ. Rapid migration of grafted cortical astrocytes from suspension grafts placed in host thoracic spinal cord. Brain Res. 1989;491:205-211.
76 Bernstein JJ, Goldberg WJ, Laws ERJr, et al. C6 glioma cell invasion and migration of rat brain after neural homografting: ultrastructure. Neurosurgery. 1990;26:622-628.
77 Bernstein JJ, Laws ERJr, Levine KV, et al. C6 glioma-astrocytoma cell and fetal astrocyte migration into artificial basement membrane: a permissive substrate for neural tumors but not fetal astrocytes. Neurosurgery. 1991;28:652-658.
78 Bernstein JJ, Goldberg WJ, Laws ERJr. Human malignant astrocytoma xenografts migrate in rat brain: a model for central nervous system cancer research. J Neurosci Res. 1989;22:134-143.
79 Chicoine MR, Silbergeld DL. The in vitro motility of human gliomas increases with increasing grade of malignancy. Cancer. 1995;75:2904-2909.
80 Chicoine MR, Madsen CL, Silbergeld DL. Modification of human glioma locomotion in vitro by cytokines EGF, bFGF, PDGFbb, NGF, and TNF alpha. Neurosurgery. 1995;36:1165-1170.
81 Westermark B, Magnusson A, Heldin CH. Effect of epidermal growth factor on membrane motility and cell locomotion in cultures of human clonal glioma cells. J Neurosci Res. 1982;8:491-507.
82 Lund-Johansen M, Bjerkvig R, Humphrey PA, et al. Effect of epidermal growth factor on glioma cell growth, migration, and invasion in vitro. Cancer Res. 1990;50:6039-6044.
83 Burger PC. Pathologic anatomy and CT correlations in the glioblastoma multiforme. Appl Neurophysiol. 1983;46:180-187.
84 Kelly PJ, Daumas-Duport C, Kispert DB, et al. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66:865-874.
85 Salazar OM, Rubin P. The spread of glioblastoma multiforme as a determining factor in the radiation treated volume. Int J Radiat Oncol Biol Phys. 1976;1:627-637.
86 Cedzich C, Taniguchi M, Schafer S, et al. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996;38:962-970.
87 Fadul C, Wood J, Thaler H, et al. Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology. 1988;38:1374-1379.
88 Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044-1055.
89 Silbergeld DL, Chicoine MR. Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg. 1997;86:525-531.
90 Zülch KJ. Histological Typing of Tumours of the Central Nervous System. Geneva: World Health Organization; 1979.
91 Kleihues P, Burger PC, Scheithauer BW. Histological typing of tumours of the central nervous system. In: World Health Organization International Histological Classification of Tumours. Heidelberg, Germany: Springer; 1993.
92 Kleihues P, Cavenee WK, World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Nervous System. Lyon, France: IARC Press; 2000.
93 Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC Press; 2007.
94 Pelloski CE, Ballman KV, Furth AF, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol. 2007;25:2288-2294.
95 Coons SW, Johnson PC. Regional heterogeneity in the proliferative activity of human gliomas as measured by the Ki-67 labeling index. J Neuropathol Exp Neurol. 1993;52:609-618.
96 Karamitopoulou E, Perentes E, Diamantis I, et al. Ki-67 immunoreactivity in human central nervous system tumors: a study with MIB 1 monoclonal antibody on archival material. Acta Neuropathol. 1994;87:47-54.
97 Raghavan R, Steart PV, Weller RO. Cell proliferation patterns in the diagnosis of astrocytomas, anaplastic astrocytomas and glioblastoma multiforme: a Ki-67 study. Neuropathol Appl Neurobiol. 1990;16:123-133.
98 Burger PC, Shibata T, Kleihues P. The use of the monoclonal antibody Ki-67 in the identification of proliferating cells: application to surgical neuropathology. Am J Surg Pathol. 1986;10:611-617.
99 Deckert M, Reifenberger G, Wechsler W. Determination of the proliferative potential of human brain tumors using the monoclonal antibody Ki-67. J Cancer Res Clin Oncol. 1989;115:179-188.
100 Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683-2710.
101 Lee da Y, Gutmann DH. Cancer stem cells and brain tumors: uprooting the bad seeds. Expert Rev Anticancer Ther. 2007;7:1581-1590.
102 Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445-1453.
103 Ueki K, Ono Y, Henson JW, et al. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996;56:150-153.
104 Maher EA, Brennan C, Wen PY, et al. Marked genomic differences characterize primary and secondary glioblastoma subtypes and identify two distinct molecular and clinical secondary glioblastoma entities. Cancer Res. 2006;66:11502-11513.
105 Tso CL, Freije WA, Day A, et al. Distinct transcription profiles of primary and secondary glioblastoma subgroups. Cancer Res. 2006;66:159-167.
106 Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356-362.
107 Endersby R, Baker SJ. PTEN signaling in brain: neuropathology and tumorigenesis. Oncogene. 2008;27:5416-5430.
108 Bogler O, Huang HJ, Kleihues P, et al. The p53 gene and its role in human brain tumors. Glia. 1995;15:308-327.
109 Watanabe K, Sato K, Biernat W, et al. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin Cancer Res. 1997;3:523-530.
110 Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892-6899.
111 Sathornsumetee S, Rich JN, Reardon DA. Diagnosis and treatment of high-grade astrocytoma. Neurol Clin. 2007;25:1111-1139. x
112 Lee JC, Vivanco I, Beroukhim R, et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006;3:e485.
113 Kesari S, Stiles CD. The bad seed: PDGF receptors link adult neural progenitors to glioma stem cells. Neuron. 2006;51:151-153.
114 Guo P, Hu B, Gu W, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083-1093.
115 Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610-622.
116 Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707-1710.
117 Quinones-Hinojosa A, Sanai N, Gonzalez-Perez O, et al. The human brain subventricular zone: stem cells in this niche and its organization. Neurosurg Clin N Am. 2007;18:15-20. vii
118 Assanah M, Lochhead R, Ogden A, et al. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor–expressing retroviruses. J Neurosci. 2006;26:6781-6790.
119 Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811-822.
120 Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832-846.
121 Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401.
122 Dirks PB. Brain tumor stem cells: bringing order to the chaos of brain cancer. J Clin Oncol. 2008;26:2916-2924.
123 Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010-4015.
124 Ma YH, Mentlein R, Knerlich F, et al. Expression of stem cell markers in human astrocytomas of different WHO grades. J Neurooncol. 2008;86:31-45.
125 Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425-436.
126 Bao S, Wu Q, Sathornsumetee S, et al. Stem cell–like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843-7848.
127 Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69-82.
128 Rubinstein LJ. The development of contiguous sarcomatous and gliomatous tissue in intracranial tumours. J Pathol Bacteriol. 1956;71:441-459.
129 Rubinstein LJ. Morphological problems of brain tumors with mixed cell population. Acta Neurochir (Wien). 1964;11(suppl 10):141.
130 Chen W, Silverman DH. Advances in evaluation of primary brain tumors. Semin Nucl Med. 2008;38:240-250.
131 Guerin C, Laterra J, Hruban RH, et al. The glucose transporter and blood-brain barrier of human brain tumors. Ann Neurol. 1990;28:758-765.
132 Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453-461.
133 Levivier M, Becerra A, De Witte O, et al. Radiation necrosis or recurrence. J Neurosurg. 1996;84:148-149.
134 Padma MV, Said S, Jacobs M, et al. Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol. 2003;64:227-237.
135 De Witte O, Levivier M, Violon P, et al. Prognostic value positron emission tomography with [18F]fluoro-2-deoxy-D-glucose in the low-grade glioma. Neurosurgery. 1996;39:470-476.
136 Ishiwata K, Kubota K, Murakami M, et al. Re-evaluation of amino acid PET studies: can the protein synthesis rates in brain and tumor tissues be measured in vivo? J Nucl Med. 1993;34:1936-1943.
137 Jager PL, Vaalburg W, Pruim J, et al. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med. 2001;42:432-445.
138 Chen W, Silverman DH, Delaloye S, et al. 18F-DOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47:904-911.
139 Schiepers C, Chen W, Cloughesy T, et al. 18F-FDOPA kinetics in brain tumors. J Nucl Med. 2007;48:1651-1661.
140 Hayashi K, Ohara N, Jeon HJ, et al. Gliosarcoma with features of chondroblastic osteosarcoma. Cancer. 1993;72:850-855.
141 Levin VA, Yung WK, Bruner J, et al. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int J Radiat Oncol Biol Phys. 2002;53:58-66.
142 Kinuya K, Ohashi M, Itoh S, et al. Differential diagnosis in patients with ring-like thallium-201 uptake in brain SPECT. Ann Nucl Med. 2002;16:417-421.
143 Wen PY, Schiff D, Kesari S, et al. Medical management of patients with brain tumors. J Neurooncol. 2006;80:313-332.
144 Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886-1893.
145 Green H, Paul M, Vidal L, et al. Prophylaxis of Pneumocystis pneumonia in immunocompromised non–HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:1052-1059.
146 Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83-95.
147 Gerber DE, Grossman SA, Streiff MB. Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol. 2006;24:1310-1318.
148 Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol. 1983;13:334-336.
149 Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153.
150 Levin JM, Schiff D, Loeffler JS, et al. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology. 1993;43:1111-1114.
151 Meyers CA, Weitzner MA, Valentine AD, et al. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J Clin Oncol. 1998;16:2522-2527.
152 Shaw EG, Rosdhal R, D’Agostino RBJr, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415-1420.
153 Litofsky NS, Farace E, Anderson FJr, et al. Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery. 2004;54:358-366.
154 Silbergeld DL, Ojemann JG, Miller JD. Brain tumors can invade normally functioning brain precluding resection. Epilepsia. 1994;35(suppl 8):15.
155 Hess KR. Extent of resection as a prognostic variable in the treatment of gliomas. J Neurooncol. 1999;42:227-231.
156 Nazzaro JM, Neuwelt EA. The role of surgery in the management of supratentorial intermediate and high-grade astrocytomas in adults. J Neurosurg. 1990;73:331-344.
157 Sneed PK, Gutin PH, Larson DA, et al. Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys. 1994;29:719-727.
158 Chang SM, Parney IF, Huang W, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293:557-564.
159 Butowski N, Lamborn KR, Berger MS, et al. Historical controls for phase II surgically based trials requiring gross total resection of glioblastoma multiforme. J Neurooncol. 2007;85:87-94.
160 Keles GE, Chang EF, Lamborn KR, et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006;105:34-40.
161 Grant R, Metcalfe SE. Biopsy versus resection for malignant glioma. Cochrane Database Syst Rev. 2, 2005. CD002034
162 Quigley MR, Maroon JC. The relationship between survival and the extent of the resection in patients with supratentorial malignant gliomas. Neurosurgery. 1991;29:385-388.
163 Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-198.
164 McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156-162.
165 Nomiya T, Nemoto K, Kumabe T, et al. Prognostic significance of surgery and radiation therapy in cases of anaplastic astrocytoma: retrospective analysis of 170 cases. J Neurosurg. 2007;106:575-581.
166 Ushio Y, Kochi M, Hamada J, et al. Effect of surgical removal on survival and quality of life in patients with supratentorial glioblastoma. Neurol Med Chir (Tokyo). 2005;45:454-460.
167 Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6:227-235.
168 Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52:371-379.
169 Jeremic B, Grujicic D, Antunovic V, et al. Influence of extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol. 1994;21:177-185.
169a Curran WJJr, Scott CB, Horton J, et al. Does extent of surgery influence outcome for astrocytoma with a typical or anaplastic foci (AAF)? A report from three Radiation Therapy Oncology Group (RTOG) trials. J Neurooncol. 1992;12:219-227.
170 Tortosa A, Vinolas N, Villa S, et al. Prognostic implication of clinical, radiologic, and pathologic features in patients with anaplastic gliomas. Cancer. 2003;97:1063-1071.
171 Puduvalli VK, Hashmi M, McAllister LD, et al. Anaplastic oligodendrogliomas: prognostic factors for tumor recurrence and survival. Oncology. 2003;65:259-266.
172 Kowalczuk A, Macdonald RL, Amidei C, et al. Quantitative imaging study of extent of surgical resection and prognosis of malignant astrocytomas. Neurosurgery. 1997;41:1028-1036.
173 Huber A, Beran H, Becherer A, et al. [Supratentorial glioma: analysis of clinical and temporal parameters in 163 cases.]. Neurochirurgia (Stuttg). 1993;36(6):189-193.
174 Duncan GG, Goodman GB, Ludgate CM, et al. The treatment of adult supratentorial high grade astrocytomas. J Neurooncol. 1992;13:63-72.
175 Prados MD, Gutin PH, Phillips TL, et al. Highly anaplastic astrocytoma: a review of 357 patients treated between 1977 and 1989. Int J Radiat Oncol Biol Phys. 1992;23:3-8.
176 Hollerhage HG, Zumkeller M, Becker M, et al. Influence of type and extent of surgery on early results and survival time in glioblastoma multiforme. Acta Neurochir (Wien). 1991;113:31-37.
177 Sandberg-Wollheim M, Malmstrom P, Stromblad LG, et al. A randomized study of chemotherapy with procarbazine, vincristine, and lomustine with and without radiation therapy for astrocytoma grades 3 and/or 4. Cancer. 1991;68:22-29.
178 Phillips TL, Levin VA, Ahn DK, et al. Evaluation of bromodeoxyuridine in glioblastoma multiforme: a Northern California Cancer Center Phase II study. Int J Radiat Oncol Biol Phys. 1991;21:709-714.
179 Friedman HS, Pluda J, Quinn JA, et al. Phase I trial of carmustine plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol. 2000;18:3522-3528.
180 Nakamizo A, Marini F, Amano T, et al. Human bone marrow–derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307-3318.
181 McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583-588.
182 Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003.
183 Quinn JA, Weingart JD, Brem H. Phase I trial of temozolomide plus O6-benzylguanine in the treatment of patients with recurrent or progressive cerebral anaplastic gliomas [abstract]. Prog Proc Am Soc Clin Oncol. 2003;22:103.
184 Buatti JM, Marcus RB, Mendenhall WM, et al. Accelerated hyperfractionated radiotherapy for malignant gliomas. Int J Radiat Oncol Biol Phys. 1996;34:785-792.
185 Quinn JA, Pluda J, Dolan ME, et al. Phase II trial of carmustine plus O(6)-benzylguanine for patients with nitrosourea-resistant recurrent or progressive malignant glioma. J Clin Oncol. 2002;20:2277-2283.
186 Jellinger K, Kothbauer P, Volc D, et al. Combination chemotherapy (COMP protocol) and radiotherapy of anaplastic supratentorial gliomas. Acta Neurochir (Wien). 1979;51:1-13.
187 Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27:579-584.
188 Salcman M. Experimental therapy for brain tumors. In: Kaye AH, Laws ER, editors. Brain Tumors. 2nd ed. London: Churchill Livingstone; 2001:408-409.
189 Dehdashti AR, Sharma S, Laperriere N, et al. Coincidence vs cause: cure in three glioblastoma patients treated with brachytherapy. Can J Neurol Sci. 2007;34:339-342.
190 Chang SM, Barker FG2nd. Marital status, treatment, and survival in patients with glioblastoma multiforme: a population based study. Cancer. 2005;104:1975-1984.
191 Chang SM, Parney IF, McDermott M, et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98:1175-1181.
192 Lutterbach J, Guttenberger R, Pagenstecher A. Gliosarcoma: a clinical study. Radiother Oncol. 2001;61:57-64.
193 Salvati M, Caroli E, Raco A, et al. Gliosarcomas: analysis of 11 cases do two subtypes exist? J Neurooncol. 2005;74:59-63.
194 Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846-12851.
195 Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447-450.
196 Gallia GL, Tyler BM, Hann CL, et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009;8:386-393.
197 Arnhold S, Hilgers M, Lenartz D, et al. Neural precursor cells as carriers for a gene therapeutical approach in tumor therapy. Cell Transplant. 2003;12:827-837.
198 Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20:1111-1117.