CHAPTER 42 Malignant disease of the uterus
Introduction
Worldwide, approximately 200,000 women are diagnosed with endometrial cancer each year (Ferlay et al 2004). Of these, more than two-thirds arise in developed countries where age-standardized incidence is four times higher than in developing countries (Ferlay et al 2007). Endometrial cancer is now the most common gynaecological malignancy in Western Europe and North America, with an annual incidence of 81,500 women in the European Union and 40,100 women in North America. In the UK, the incidence of endometrial cancer increased by 29.3% between 1993 and 2005, with an incidence of 6891 women in 2005 (Office for National Statistics 2008). This increase is attributable to an increase in obesity, tamoxifen use for breast cancer and increased life expectancy (Reich 1992, Kitchener 2008). Given the anticipated increase in obesity, it is likely that this trend will continue.
Endometrial cancer has traditionally been believed to have a relatively good outcome. Whilst commonly believed to be a curable cancer in contrast to other gynaecological malignancies, the overall 5-year survival rate is disappointing considering that 75% of patients present with early (stage I) disease. Relative survival at both 1 and 5 years after diagnosis has risen steadily from 85% and 72%, respectively, in the late 1980s to 88% and 76% in the late 1990s (Cooper et al 2008). However, there is a significant gap in survival between the most affluent and the most deprived groups of approximately 4% based on data from England and Wales, with survival lagging 2–6% behind Europe (Sant et al 2003, Cooper et al 2008). This deprivation gap may be best explained by comorbidities such as obesity, hypertension and cardiovascular disease, which are more common in deprived communities (Kitchener 2008). In addition, survival in advanced-stage endometrial cancer and poor prognostic types of endometrial cancer continues to be poor.
With the implementation of improving outcomes guidance in endometrial cancer, women with higher grade and poor prognostic types of endometrial cancer should be managed in gynaecological oncology centres, thus centralizing resources and experience, and facilitating trials that investigate new therapies for these patients (Department of Health 1999).
Epidemiology
Most studies focus on the epidemiology of endometrioid cancers, as the epidemiology for non-endometrioid histotypes is less clear (Box 42.1).
Obesity
Body mass index (BMI) plays an important role with a strong linear increase in risk with increasing BMI. There is a six-fold increase in women with a BMI greater than 40 kg/m2 compared with those with a normal BMI (20–24 kg/m2), whereas women with a BMI under 20 kg/m2 have half the risk (Lindemann et al 2008). The fact that obesity increases risk has been attributed to changes in concentrations of endogenous hormones in obese women. Oestrogens produced in adipose tissue have a direct mitogenic effect on endometrial cells. In postmenopausal women, oestrogens derived from peripheral adipose tissue are the primary source of endogenous oestrogen, and the rate of production is related to the size of the adipose depots. In younger premenopausal women, obesity can also be associated with anovulatory cycles, resulting in the absence of counterbalancing progesterone. Oligo- and amenorrhoeic women with polycystic ovary syndrome can develop endometrial hyperplasia and later carcinoma. There is a weight-related increase in insulin and insulin-like growth factor-I, both of which are endometrial growth factors. Cytokines produced in fat tissue (leptin and adiponectin) may play a direct role in endometrial carcinogenesis, as well as transcription factors that can modulate both cellular lipid metabolism and tumorigenesis. There is also a strongly increased risk of endometrial cancer in diabetics, possibly because of shared underlying mechanisms.
Tamoxifen
Tamoxifen improves the survival of postmenopausal women with hormone-receptor-positive breast cancer. Breast and endometrial cancer share several risk factors including age of menopause, unopposed oestrogen use and obesity, and breast cancer patients have an increased rate of endometrial cancer regardless of endocrine therapy. The risk of endometrial cancer rises with both the use of higher doses and increasing duration of tamoxifen use. Treatment beyond 5 years increases the risk at least four fold. However, the absolute risk of endometrial cancer is still very small in tamoxifen-treated women (Early Breast Cancer Trialists’ Collaborative Group 1998). The use of aromatase inhibitors for postoperative adjuvant therapy instead of tamoxifen significantly reduces the risk of adverse gynaecological events (Duffy et al 2009). Switching from tamoxifen to an aromatase inhibitor may reduce the risk of endometrial cancer, but needs to be balanced against the side-effects and costs of therapy.
Hereditary non-polyposis colorectal cancer
Hereditary non-polyposis colorectal cancer (HNPCC) is one of the most common cancer family syndromes. The estimated lifetime risk of developing endometrial cancer in women carrying these mutations is approximately 42–60%. HNPCC results from germline mutations in DNA mismatch repair genes and is autosomal dominant in inheritance (Froggatt et al 1996). As well as an increased risk of malignancies in the colon, cancers of the endometrium, ovary, biliary tract, stomach, small intestine and renal tract occur more commonly in these women (Box 42.2). Endometrial cancer is the most common and usually occurs in the premenopausal period. The majority of mismatch repair gene defects involve the genes MSH2 and MLH1, with defects in PMS1 and PMS2 being less common (Papadopoulos and Lindblom 1997). A good family history will help to identify women who may carry these mutations. If such a mutation is found or if the woman is thought to be at high risk, there may be a role for prophylactic hysterectomy and bilateral salpingo-oophorectomy. Screening may be offered to those who do not wish to have surgery but with the caveat that there is no evidence that it is effective. There may also be a role for prophylactic progesterone in these women, and studies are currently investigating the role of the Mirena™ intrauterine system for prophylaxis.
Box 42.2 Lifetime risk of malignancy in women with HNPCC
| Colorectal | 30–50% |
| Endometrial | 40–60% |
| Ovarian | 9–12% |
| Gastric | 2–7% |
| Biliary | 13–15% |
| Urinary | 4–5% |
Hormones
The combined oral contraceptive pill has a large protective effect that lasts for at least 20 years after cessation. Older hormone replacement therapy (HRT) regimens that utilized unopposed oestrogen in women with an intact uterus increase the relative risk of endometrial carcinoma approximately six fold after 5 years of use (Weiderpass et al 1999). It is strongly recommended that women with intact uteri should not be started on regimes containing oestrogen alone, and should be offered endometrial protection by regimes with either 10–12 days of cyclical progestogens or continuous combined regimens (Lethaby et al 2004).
Pathology
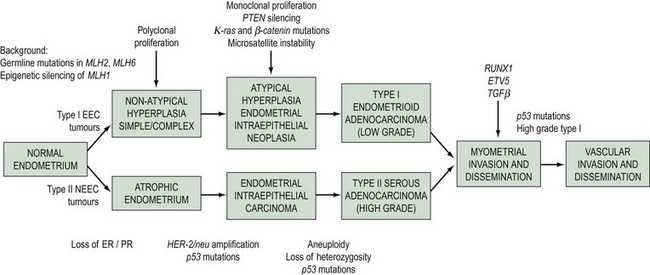
There is a growing consensus that endometrial cancers can be classified into two types based on clinical and molecular characteristics. Type I endometrial cancers (80%) are oestrogen dependent, usually arise in a background of endometrial hyperplasia, have well to moderately differentiated endometrioid histology, tend to be biologically indolent and have a good prognosis (Bokhman 1983). Type II cancers are not oestrogen driven, have a higher grade, occur in a background of atrophic endometrium and have a poorer prognosis (Bokhman 1983). Uterine papillary serous carcinomas and clear cell carcinomas are considered to be type II endometrial cancers. These cancers, and uterine serous papillary cancer in particular, are characterized by early metastasis, resistance to therapy and a high mortality rate. Within the type II cancer histological subtypes, Creasman et al (2004) found 5-year survival of 81% for surgically staged stage I clear cell cancer and 72% for uterine papillary serous cancer. This is in comparison with over 95% for well to moderately differentiated stage IA endometrioid cancers. Although unusual cancers only account for 10% of endometrial cancers, they account for more than 50% of recurrences and deaths (Acharya et al 2005).
The morphological and clinical differences are paralleled by genetic distinctions (Hecht and Mutter 2006). Type I cancers are associated with mutations in the K-ras2 oncogene, loss of the PTEN tumour suppressor gene, defects in DNA mismatch repair and near-diploid karyotype, whereas type II cancers are associated with mutations in TP 53 and ERBB-2 expression, and most are non-diploid. A dualistic model of endometrial tumorigenesis has been proposed which could explain the very different clinical outcomes in these groups of patients (Table 42.1, Figure 42.1). An understanding of the molecular heterogeneity of various histological types of endometrial cancer has the potential to lead to better individualization of treatment in the future (Maxwell et al 2005).
Table 42.1 Clinicopathological characteristics and genetic abnormalities in type I and type II endometrial carcinomas
| Characteristic | Type I | Type II |
|---|---|---|
| Unopposed oestrogen | Yes | No |
| Background endometrium | Hyperplastic | Atrophic |
| Morphology | Endometrioid | Serous, clear cell |
| Microsatellite instability | 20–40% | 0–5% |
| p53 mutations | 10–20% | 90% |
| β-Catenin mutations | 31–47% | 0–3% |
| K-ras mutations | 15–30% | 0–5% |
| PTEN inactivation | 35–50% | 10% |
| HER-2/neu | No information | 18–80% |
Source: From Doll A, Abal M, Rigau M, Monge M, Gonzalez M, Demajo S, et al. Novel molecular profiles of endometrial cancer-new light through old windows. J Steroid Biochem Mol Biol 2008;108(3–5):221–9.
Histopathology
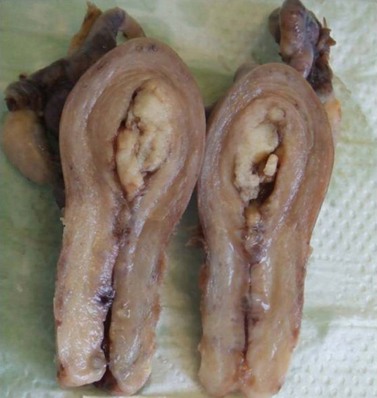
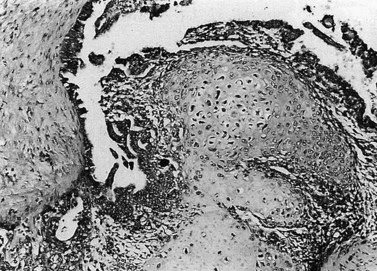
Endometrial carcinoma is usually seen as a raised, rough, perhaps papillary area occupying at least half of the endometrium. It often arises in the fundal region of the uterus; the internal os is rarely involved early in the disease (Figure 42.2). Myometrial invasion may be obvious to the naked eye. There seems to be no correlation between the degree of exophytic growth of the tumour within the uterine cavity and the presence of myometrial invasion.
There are several different histological subtypes of endometrial carcinoma (Table 42.2).
| Endometrioid (usual) carcinoma (includes glandular and villoglandular patterns) |
| Secretory |
| Ciliated cell |
| With squamous differentiation (includes adenoacanthoma and adenosquamous carcinoma) |
| Special variant carcinomas |
| Papillary serous adenocarcinoma |
| Clear cell carcinoma |
| Mucinous carcinoma |
| Pure squamous cell carcinoma |
| Mixed |
| Undifferentiated |
| Carcinosarcoma |
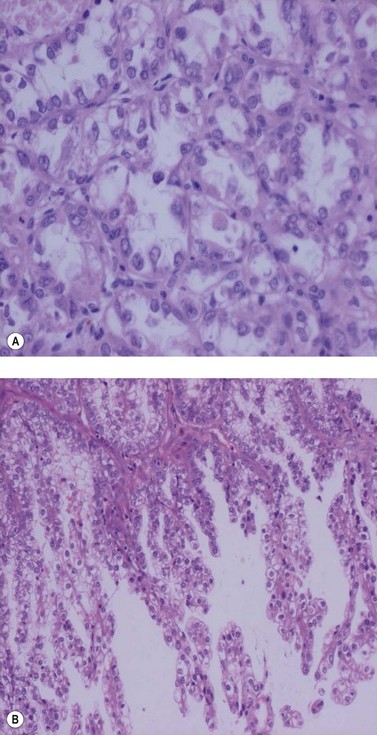
Endometrioid adenocarcinoma
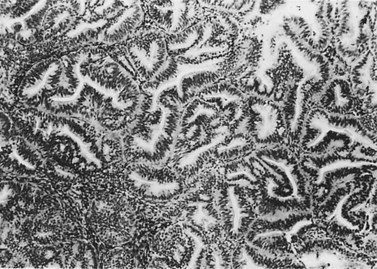
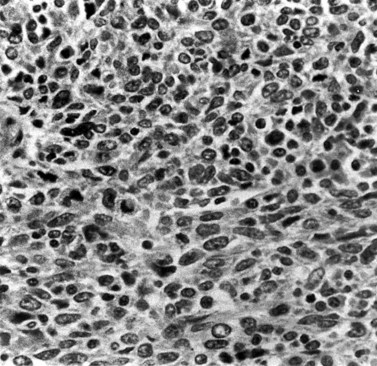
The most common endometrial carcinoma is referred to as endometrioid adenocarcinoma, the glandular pattern of which generally resembles that of normal proliferative-phase endometrium, although sometimes showing extreme complexity of the glands and cribriform pattern (Figure 42.3). Multilayering of the epithelial cells is nearly always seen. The secretory and ciliated cell variants are rare and well differentiated by definition. Secretory adenocarcinoma is a well-differentiated adenocarcinoma characterized by neoplastic glands with a subnuclear vacuolization resembling normal 17-day secretory endometrium. Mucinous adenocarcinoma is a low-grade neoplasm in which most of the cells contain prominent intracytoplasmic mucin, and should be distinguished from otherwise typical endometrioid carcinomas with abundant extracellular mucin but no intracellular mucin.
Endometrial adenocarcinoma with squamous metaplasia (adenoacanthoma)
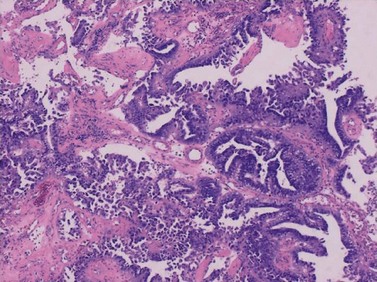
Up to 25% of endometrioid-pattern endometrial adenocarcinomas contain areas of squamous metaplasia. Those tumours in which the squamous component is morphologically benign are commonly termed ‘adenoacanthomas’. The squamous change is seen as islands of typical squamous epithelium, very often situated within the gland lumina or as surface or diffuse squamous metaplasia (Figure 42.4).
Papillary serous and clear cell adenocarcinomas
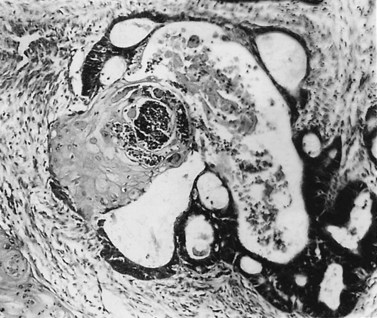
These are less common histological subtypes, both associated with a poor prognosis. Clear cell carcinoma is composed of large, clear cells containing glycogen, often forming papillary structures lined by ‘hobnail’ cells (Figure 42.5). Papillary serous carcinoma closely resembles ovarian papillary serous carcinoma, featuring severe atypia, a complex papillary pattern, high mitotic index, prominent myometrial invasion and psammoma bodies (Figure 42.6). Most patients are postmenopausal.
Histopathological reporting
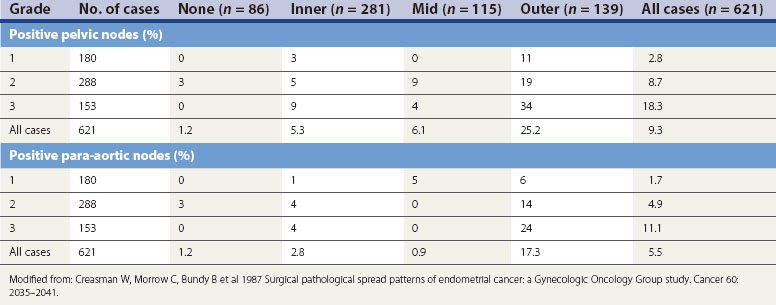
It is essential not only to recognize specific subtypes and to grade carcinomas accurately, but also to record the extent of myometrial invasion. The maximum depth of myometrial invasion, overall thickness of the myometrium, and minimum distance of the tumour from the serosal surface should be recorded. The presence of lymphovascular invasion should be noted. The cervical stroma, fallopian tubes and ovaries should all be examined carefully for tumour. Histopathological examination of the omentum would be valuable in uterine papillary serous carcinoma and clear cell carcinoma. The number of lymph nodes removed, the number of lymph nodes positive for malignancy, extracapsular spread and the extent of lymphatic spread (e.g. node replaced with malignancy or not) should also be reported. There is no convincing evidence for routine ultrastaging or special immunohistochemical analysis of surgically removed lymph nodes (Figure 42.7).
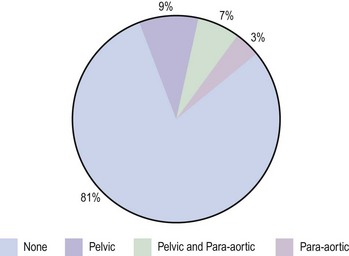
Figure 42.7 Lymph node involvement in clinical stage I endometrial carcinoma
Source: Ayhan et al 1994 Surgical pathological spread patterns of endometrial cancer: A Gynecologic Oncology Group Study. Cancer 60: 2035–2041.
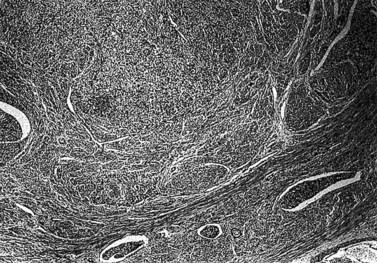
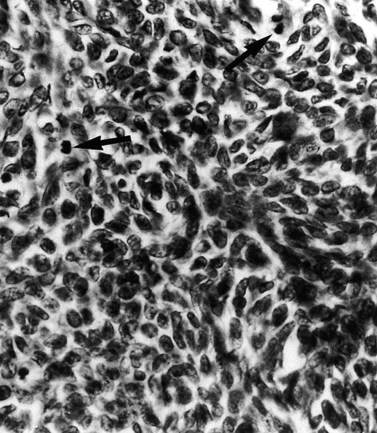
The national minimum dataset published by the Royal College of Pathologists provides a useful tool for both gynaecologists and histopathologists. Serous carcinoma, clear cell carcinoma, and squamous and undifferentiated carcinoma are not graded, these tumours being highly malignant neoplasia. Sarcomas are also highly malignant neoplasms (Figures 42.8–42.11). The architectural grade, nuclear grade and FIGO grade of tumours can be used to separate patients into groups with significantly different rates of progression of disease and relative survival.
Endometrial hyperplasia
Simple glandular hyperplasia is no longer considered to be a premalignant condition, but atypical hyperplasia is an ominous finding. Approximately 10–20% of all cases with atypia will have an underlying carcinoma present, and invasive cancer will develop later in up to 50% of cases. These figures are higher in the case of complex atypical hyperplasia than simple atypical hyperplasia (Silverberg 2000). Women with hyperplasia without cytological atypia may be treated with cyclical or continuous progestogens for 6 months and then undergo rebiopsy. Women with lesions showing cytological atypia who have finished childbearing should be offered a hysterectomy. The management of younger women with atypical hyperplasia is more difficult. Hyperplasia is discussed further in Chapter 38.
Spread of Disease
The current FIGO staging criteria are shown in Box 42.3.
Box 42.3
FIGO surgical staging of endometrial carcinoma
| Stage Ia | Tumor confined to the corpus uteri |
| IAa | No or less than half myometrial invasion |
| IBa | Invasion equal to or more than half of the myometrium |
| Stage IIa | Tumor invades cervical stroma, but does not extend beyond the uterusb |
| Stage IIIa | Local and/or regional spread of the tumor |
| IIIAa | Tumor invades the serosa of the corpus uteri and/or adnexaec |
| IIIBa | Vaginal and/or parametrial involvementc |
| IIICa | Metastases to pelvic and/or para-aortic lymph nodesc |
| IIIC1a | Positive pelvic nodes |
| IIIC2a | Positive para-aortic lymph nodes with or without positive pelvic lymph nodes |
| Stage IVa | Tumor invades bladder and/or bowel mucosa, and/or distant metastases |
| IVAa | Tumor invasion of bladder and/or bowel mucosa |
| IVBa | Distant metastases, including intra-abdominal metastases and/or inguinal lymph nodes |
b Endocervical glandular involvement only should be considered as Stage I and no longer as Stage II.
c Positive cytology has to be reported separately without changing the stage.
Source: FIGO Committee on Gynecologic Oncology 2009 Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. International Journal of Gynecology and Obstetrics 105: 103–104.
FIGO Staging
Depth of myometrial penetration is a more important guide to the likelihood of nodal metastases than tumour grade (Table 42.3; Creasman et al 1987). Serous papillary and clear cell cancers have the highest risk of spread to para-aortic nodes. Only 10% of cases subsequently proven to have nodal spread had clinically enlarged nodes but, on the other hand, enlarged lymph nodes are not necessarily involved with metastatic disease. Serous papillary carcinoma of the endometrium, histologically similar to serous papillary cancer of the ovary, has a very poor prognosis and a pattern of spread akin to ovarian malignancy.
Positive peritoneal washings are present in approximately 12–15% of all cases of endometrial cancer, with half of these having no histological evidence of extrauterine spread. The Gynaecological Cancer Intergroup have proposed changes to the FIGO staging which have been incorporated into the revised FIGO staging for endometrial cancer (Box 42.3). They recommend that the grading of tumours is added to stage I tumours as this carries a direct clinical relevance. They also recommend removing the upgrading to stage IIIC on the basis of positive peritoneal washings, as these are very likely due to handling at the time of surgery and do not have independent prognostic significance; and that metastasis to positive inguinal lymph nodes is considered stage IIIC rather than stage IV. Although not part of FIGO staging, some centres perform a serum CA125 in women with endometrial cancer as reports suggest that an elevated serum CA125 at diagnosis indicates a high risk of subsequent recurrence but is less effective at predicting myometrial invasion than ultrasound.
Diagnosis of Endometrial Cancer
Presenting symptoms
Investigations and Diagnosis of Endometrial Cancer
Patients with suspicious symptoms should initially be investigated with a transvaginal ultrasound to investigate the endometrial cavity. Transvaginal ultrasound is useful in the investigation of women with postmenopausal bleeding because it helps to identify those at higher risk of endometrial cancer who require further investigation, and is also an effective means of excluding endometrial cancer. Transvaginal ultrasound measurement of endometrial thickness of less than 5 mm can be a good diagnostic index (Scottish Intercollegiate Guidelines Network 2002). The pooled sensitivity and specificity of endovaginal ultrasound in the detection of endometrial cancer if a cut-off of 5 mm was used were 96% [95% confidence interval (CI) 94–98%] and 61% (95% CI 59–63%), respectively. Sensitivity decreased and specificity increased with increasing thresholds. Thin (<5 mm) endometrial measurement on endovaginal ultrasound can exclude endometrial disease in the majority of postmenopausal women with vaginal bleeding, regardless of hormone replacement use (Smith-Bindman et al 1998).
The mean endometrial thickness in women on sequential HRT with postmenopausal bleeding is greater than that in women with postmenopausal bleeding who are not on sequential HRT. Thus, an abnormal endometrial thickness in women with postmenopausal bleeding who are not using sequential HRT represents a greater probability of endometrial disease than in women taking HRT (Gupta et al 2002). Women presenting with postmenopausal bleeding and taking tamoxifen have a higher probability of malignancy (substantially >10%). In these cases, the ultrasound image is more difficult to interpret. Therefore, it is advisable to sample the endometrium initially and examine the cavity hysteroscopically.
Treatment
Surgery for early-stage endometrial cancer
In patients with high-risk histology features on preoperative biopsy, particularly uterine papillary serous cancer, the upper abdomen, omentum and liver should be assessed as these cancers metastasize to the upper abdomen like ovarian cancer. Surgery for carcinosarcoma can be challenging as the uterus is frequently fixed and pelvic spread is common. Removal of a large piece of infracolic omentum is recommended in women with uterine papillary serous cancer and clear cell cancer, since microscopic disease in the omentum may be present. In approximately 5% of cases, positive para-aortic nodes can be identified in the absence of involved pelvic lymph nodes. The incidence of nodal positivity is summarized in Table 42.3.
Role of lymphadenectomy
Two large independent surgical randomized controlled trials found that routine pelvic and/or para-aortic lymphadenectomy confer no therapeutic benefit. The Italian randomized controlled trial and the Medical Research Council’s ASTEC (A Study in the Treatment of Endometrial Cancer) trial differed in the extent of lymphadenectomy and use of postoperative radiotherapy (Benedetti Panici et al 2008, Kitchener et al 2009). However, lymphadenectomy may still play a role in accurate surgical staging and tailoring of adjuvant therapy. In the authors’ institution, regional lymphadenectomy is performed for patients estimated to have a risk of nodal metastasis greater than 10%; this includes stage IC, grade 3, unusual histology subtypes of patients. A pelvic lymphadenectomy is performed in patients with a preoperative biopsy showing grade 2–3 endometrial cancer and myometrial invasion greater than 50% on MRI (presumed stage IC, grade 2–3). Para-aortic lymphadenectomy is reserved for clinically enlarged lymph nodes at MRI or laparotomy. However, practice differs among centres in the UK and elsewhere regarding the extent of lymphadenectomy.
Laparoscopic surgery for endometrial cancer
Laparoscopic surgery for primary management of endometrial cancer has been successfully reported, with several case series reporting satisfactory outcomes for both survival and morbidity (O’Hanlan et al 2004, 2005, Ghezzi et al 2006, Barakat et al 2007, Istre et al 2007). A meta-analysis of laparoscopic surgery for endometrial cancer establishing its feasibility and efficacy has been published (Lin et al 2008). Three techniques exist: laparoscopically assisted vaginal hysterectomy and laparoscopic hysterectomy where the uterine arteries are divided laparoscopically; and total laparoscopic hysterectomy (TLH) where the entire procedure is completed laparoscopically. In TLH for endometrial cancer, the entire hysterectomy procedure is performed laparoscopically, including division of the uterine vessels, the uterus is removed through the open vault of the vagina and the vagina is sutured laparoscopically. TLH may have technical advantages in the obese nulliparous population where vaginal access may be difficult and there is no vaginal descent (O’Hanlan et al 2004, Ramirez et al 2006).
A large randomized trial of laparoscopy vs laparotomy for surgical staging of endometrial cancer has been published in abstract form by the Gynecologic Oncology Group (GOG) (Walker et al 2006). The trial established the efficacy of laparoscopic surgical staging, with 74.2% of patients randomized to receive laparoscopy undergoing successful staging. Other benefits favouring the laparoscopic arm were improvement in quality of life, decreased hospital stay and fewer complications greater than grade 2. Survival results are pending at this time. However, the survival and morbidity associated with TLH compared with conventional open surgery have not been established in a randomized clinical trial, and this is being addressed in LACE (Janda et al 2006).
Non-surgical management of endometrial carcinoma
Radiotherapy
Stages IA and IB, grade 3 or high-risk histology
Poorly differentiated endometrioid adenocarcinoma, clear cell carcinoma and papillary serous carcinoma pose a higher risk of not only locoregional but also systemic recurrence. This is evident from their poorer relapse-free survival (RFS) and overall survival (OS) compared with grades 2 and 3 endometrioid adenocarcinomas (Creasman et al 2004). Five-year survival rates for papillary serous carcinoma, grade 3 endometrioid carcinoma and clear cell carcinoma are 72%, 76% and 80%, respectively. Almost half of those perceived to have early-stage uterine papillary serous carcinoma are found to have advanced-stage disease at surgery or in the definitive pathology. OS and progression-free survival (PFS) slip down to 80% and 68%, respectively, for stage I uterine papillary serous carcinoma. Recurrence rates were as high as 29% for stage IB–IC uterine papillary serous carcinoma (Havrilesky et al 2007a). While systemic management of this particular category is still evolving, radiotherapy has been in use for decades to at least reduce the risk of locoregional recurrence. Historically, this has been achieved mainly by external beam radiotherapy as this would encompass most pelvic lymph nodes including obturator, internal iliac, external iliac and common iliac, which are the main lymph node groups to which endometrial cancer would metastasize (Table 42.4).
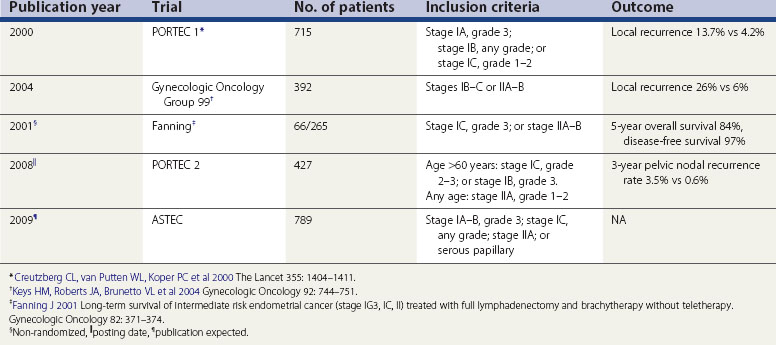
In the largest postoperative radiotherapy trial in endometrial cancer (PORTEC 1), 715 patients with FIGO stage IC, grades 2 and 3, stage IB, any grade and stage IA, grade 3 endometrioid carcinoma, serous papillary carcinoma and clear cell carcinoma were randomized to receive either external beam radiotherapy or no adjuvant radiotherapy following total abdominal hysterectomy, bilateral salpingo-oophorectomy and washings (Creutzberg et al 2000). Adjuvant radiotherapy reduced the risk of locoregional recurrence from 13.7% to 4.2%, which was statistically significant. The distant metastasis rate (7.9% vs 7%, respectively) and death due to distant metastases (6.4% vs 4.5%, respectively) at 5 years were similar in the radiotherapy and observation arms. There was no significant difference in survival between the treated and non-treated groups. Higher age of the patient (≥60 vs <60, hazard ratio 3.1, P = 0.02) and poor differentiation of the tumour (grade 3 vs grade 2, hazard ratio 4.9, P = 0.0008) were significant risk factors for cancer-related deaths. The risk of gastrointestinal toxicity was increased from 1% to 17% (P = 0.0001) in the treated group. PORTEC 1 registered 99 patients with FIGO stage IC, grade 3 endometrial carcinoma for analysis purposes only as they all received radiotherapy (Creutzberg et al 2004). Five-year distant metastasis rate was higher for FIGO stage IC, grade 3 compared with FIGO stage IC, grade 2 endometrial carcinoma (31% vs 20%, respectively) in patients treated with adjuvant radiotherapy. Five-year survival was 74% vs 58% for stages IB and IC, respectively.
Another study by the GOG looked at the value of adjuvant radiotherapy following definitive surgery that involved selective pelvic lymph node dissection (Keys et al 2004). Three hundred and ninety-two patients with intermediate risk of recurrence (FIGO stages IB, IC, IIA and IIB) who underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy, selective pelvic lymphadenectomy and selective para-aortic lymph node dissection were randomized to either adjuvant radiotherapy to the pelvis or no further adjuvant treatment. Based on GOG 33 trial, three factors were considered as risk factors: grade 2–3, involvement of outer third of myometrium and lymphovascular space invasion. Patients were considered to be at high–intermediate risk of recurrence if they were aged 70 years or more with one risk factor, 50 years or more with two risk factors, or any age with all three risk factors. It is interesting to note that one-third of stage I patients meet this criteria and that two-thirds of recurrences occur in this group. Risk of recurrence in patients at high–intermediate risk was reduced to 6% with radiotherapy compared with 26% when no adjuvant radiotherapy was given. At 4 years, there was a trend towards better survival with radiotherapy (92% vs 86%), although this was not statistically significant. The trial, however, was not powered to detect a survival advantage.
Two trials have evaluated the usefulness of brachytherapy without external beam radiotherapy, also labelled as ‘teletherapy’. The first of these studies was a prospective non-randomized study (Fanning 2001). Two hundred and sixty-five patients underwent complete surgical staging with full lymphadenectomy up to the duodenum, undertaken by one surgeon. Sixty-six patients were identified to have intermediate-risk disease on the basis of grade 3, involvement of outer myometrium (FIGO stage IC) or involvement of cervix (FIGO stage IIA–B), and all received adjuvant brachytherapy but no external beam radiotherapy. Five-year survival was reported to be 84% and PFS was 97%. Although one should avoid comparing the results of two different trials, it was noted with interest that this PFS was comparable with the PFS observed in GOG 99 trial for a similar group of patients who received teletherapy. The second pivotal randomized trial of adjuvant brachytherapy compared with external beam radiotherapy was planned on the basis of similar observations in phase II trials (PORTEC 2). Patients at high–intermediate risk (on the basis of PORTEC 1 results) were randomized to receive either external beam radiotherapy or brachytherapy. The primary endpoint was vaginal recurrence rate, and secondary endpoints were quality of life, OS and locoregional recurrence. The analysis was by intention to treat. A total of 427 patients were randomized into the two treatment groups. Three-year vaginal recurrence rate was 1.9% (P = 0.97 in both groups). Three-year pelvic nodal recurrence rate was 0.6% in the external beam radiotherapy group compared with 3.5% in the brachytherapy alone arm (P = 0.03). Three-year OS and RFS rates were 90.4% and 90.8% (P = 0.55) and 89.5 and 89.1 (P = 0.38), respectively. The distant recurrence rates were similar in both groups.
ASTEC is a large phase III, randomized trial which addressed two issues, namely the role of lymphadenectomy and adjuvant radiotherapy in early-stage endometrial cancer (Kitchener et al 2009). Although the results of the lymphadenectomy part of the trial have recently been published, the results of the radiotherapy part are not yet in the public domain.
Usually, a dose between 40 and 50 grays (Gy) is given in 20–28 fractions over 4–5.5 weeks using megavoltage (MV) photons of higher energy (typically between 8 and 15 MV). The side-effects of radiotherapy are described as early (usually temporary and appearing during treatment and up to 3 months after) or late (usually permanent and typically occurring more than 3 months after radiation therapy). Early side-effects include tiredness, diarrhoea, local soreness of skin/vagina, local hair loss and radiation cystitis. Delayed toxicity includes the risk of chronic proctitis or a weak bladder leading to poor control, urinary urgency and occasionally incontinence, but most importantly the risk of a fistula (involving bowel, vagina or urinary bladder) or narrowing of the bowel which may require colostomy. Obviously, this risk is directly proportional to the dose of radiotherapy and the amount of bowel in the radiation field. The risk is less than 5% over 5 years at the doses mentioned above (Milano et al 2007). Comorbidity, especially previous pelvic operations and inflammatory bowel disease, increase this risk significantly and therefore are relative contraindications to radiotherapy to the pelvis. Similarly, hereditary conditions including ataxia telangiectasia and xeroderma pigmentosum markedly increase radiation sensitivity, and are absolute contraindications to adjuvant radiotherapy.
Stages IC, IIA and IIB
Involvement of the outer half of the myometrium and cervical stroma are well known to increase the risk of local recurrence as well as regional lymph node spread. As many as one-third of patients with apparent stage IC, grade 2 tumours may have pelvic nodes involved, and one-quarter may have para-aortic nodes involved. There has been a definite role for radiotherapy in these stages following surgery to reduce that risk. The modality of radiotherapy, the dose and the extent of the area radiated have not been without controversy. While preliminary results of the largest phase III randomized trial of adjuvant radiotherapy in the UK do not show any advantage for external beam radiotherapy for stage IC disease, the fact that some centres were allowed to use brachytherapy in accordance with their local guidelines has made interpretation difficult. In fact, most radiation/clinical oncologists continue to offer radiotherapy to this group of patients, and were reluctant to recruit these patients into any trials of adjuvant radiotherapy or not. For these reasons, the PORTEC 1 trial excluded stage IC, grade 3 patients from randomization although they were registered in the trial to follow their progress (Creutzberg et al 2004).
With the adoption of pelvic lymphadenectomy, the use of external beam radiotherapy in early-stage cancer is being replaced by localized brachytherapy, eliminating the need for radiating a large pelvic volume including pelvic nodes. While the issue of pelvic node surgery in early endometrial cancer has been controversial and open to debate, patients who do undergo this type of surgery and who are node negative may be eligible for brachytherapy alone. The PORTEC 2 trial has shown no differences in vaginal vault recurrence rates between patients who had external beam radiotherapy or brachytherapy. However, the recent ASTEC publication discouraged the routine use of pelvic lymphadenectomy outside of a clinical trial (Kitchener et al 2009). This is unfortunate as it would lead to inadequate staging of endometrial cancer patients and hence deny adjuvant treatments to those with higher stage disease. Obviously, there are also significant implications of this in terms of acute and long-term toxicity of radiotherapy. There is a definite need for more evidence to finally resolve this issue. Detailed discussion on this subject is outwith the scope of this chapter.
Stage IVA
An attempt should be made to deliver a radical dose of radiotherapy in otherwise fit patients. This may include external beam radiotherapy as well as brachytherapy. The aim in the first place is to control the local disease, and hence the local symptoms, but a small number of patients may be salvaged. They may go on to live for many years (Churn and Jones 1999). For the majority, a palliative dose of radiation is given to help local symptoms.
Radiotherapy technique
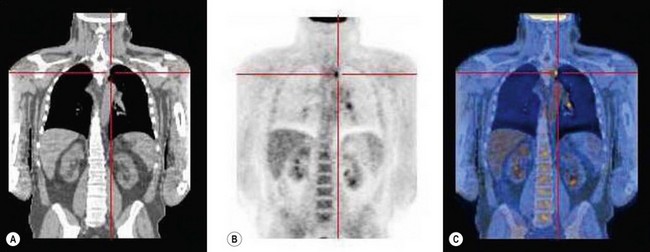
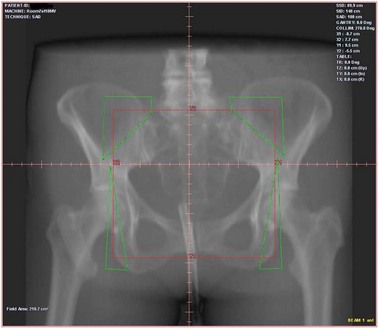
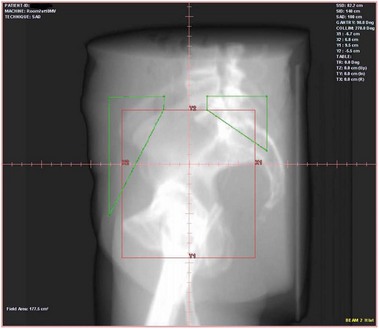
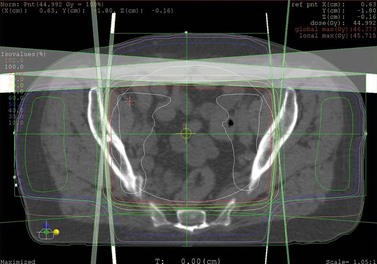
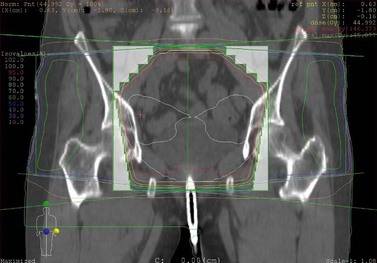
The quality of planning and delivery of radiotherapy has improved remarkably over the last few years in line with the improvements in computer and technological advances (Figure 42.12). The latest state-of-the-art multi-leaf-collimator-equipped linear accelerators with ability to deliver gated, conformal MV radiotherapy are in stark contrast with the old orthovoltage (KV) machines with limited planning techniques. For external beam radiotherapy, the patient first undergoes a planning scan which is usually a CT scan, although MR scans are likely to replace CT for this purpose. Work is also ongoing to explore the usefulness of PET-CT and PET-MRI in radiotherapy planning (Figure 42.13). After the planning CT, patients are marked with tattoos (usually pin head size) to ensure reproducibility of the treatment position during each fraction of radiotherapy. Clinical or radiation oncologists then draw the target volume, i.e. the area to be treated (Figures 42.14 and 42.15). Most patients are treated using a four-field plan (brick technique) to reduce the dose of radiation to normal tissues around the target volume (Figures 42.16 and 42.17). Once the physicist has planned the treatment and it is approved by the oncologist, treatment can be started. There are many quality assurance and safety nets at different stages to ensure safe radiation exposure in line with the local radiation protection laws. One treatment is given per day, Monday to Friday, with no treatment over the weekend. Patients can be treated over the weekend or even twice a day depending upon the availability of resources and staff. If treated twice a day, there has to be a minimum gap of 6 h between treatments to allow the normal tissue repair to take place. Patients are reviewed weekly to assess for any side-effects.
Where needed, brachytherapy is delivered using afterloading devices. In recent years, there has been a gradual move from low- (LDR) to medium-dose-rate (MDR) brachytherapy (dose rates <1200 cGy/min) and now to high-dose-rate (HDR) brachytherapy (dose rates >1200 cGy/min), meaning that the treatment can now be given in a few minutes rather than many hours. The results of HDR brachytherapy in cervical cancer confirm its efficacy at no significant additional toxicity (Lertsanguansinchai et al 2004). LDR and MDR brachytherapy require a single insertion, while HDR brachytherapy requires two to four treatments depending on the dose needed. Also, the total dose required is less for HDR systems.
Radiotherapy for recurrent disease
Proponents of radiotherapy claim that many of the recurrences are not localized, while advocates of no adjuvant radiotherapy for early disease stress that the majority of recurrences (although not all) are localized and therefore are salvageable, the so-called ‘Alabama approach’ (Barnes and Kilgore 2000). This approach, although not widely popular, is being adopted by some in view of no survival advantage of adjuvant radiotherapy and a definite, although small, risk of late serious toxicity of radiotherapy. Recurrent, localized disease at the vault can be treated with radiotherapy or surgery, and success rates are high (Creutzberg et al 2003). If radiotherapy is used, the doses required are high and the risk of toxicity is proportionally high. Nevertheless, any patient with localized recurrence should be viewed as potentially curable.
Chemotherapy
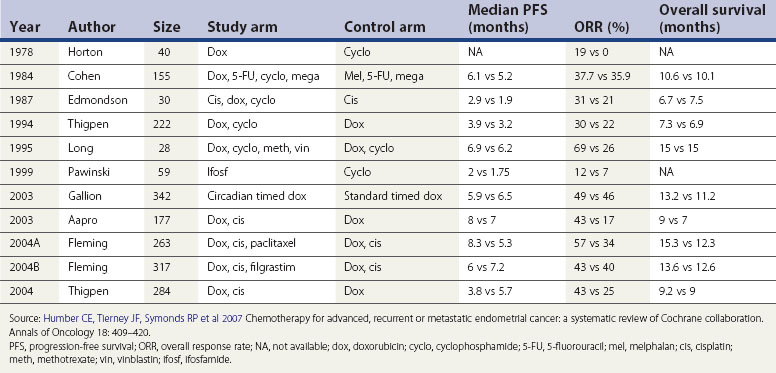
Early-stage localized disease has excellent survival after surgery and it would be difficult to demonstrate any benefit from adjuvant chemotherapy. In high-risk early-stage disease, the risk of systemic relapse is high and so is the 5-year disease-specific mortality rate. Papillary serous carcinoma, clear cell carcinoma and poorly differentiated endometrioid carcinoma infiltrating the outer half of the myometrium are particularly associated with poor OS (5-year OS 70–80%) (Murphy et al 2003). These high-risk scenarios may benefit from adjuvant chemotherapy. Until recently, there were no convincing phase III data to show a survival benefit for the use of chemotherapy; based on retrospective data, many oncologists were considering fitter patients for adjuvant chemotherapy, especially if they had serous carcinoma or even elements of clear cell carcinoma. Evidence is now gradually accumulating to support this approach. Most oncologists now agree that serous papillary carcinoma should be treated as ovarian carcinoma with adjuvant chemotherapy. A recently published systematic review (Table 42.5) evaluated data collected from 2288 patients recruited into 11 trials of chemotherapy for advanced disease.
A GOG study showed better OS of 15.3 vs 12.3 months, PFS of 8.3 vs 5.3 months and overall response rate of 57 vs 34% in favour of the addition of paclitaxel to the combination of doxorubicin and cisplatin, but at the cost of much higher toxicity (Fleming et al 2004). Combinations of an anthracycline and platinum are still probably the most commonly used regimes in endometrial carcinoma.
The Japanese GOG study showed that early-stage high-risk patients, including stages IC, grade 3, II and IIIA, had higher PFS (83.8% vs 66.2%, P = 0.024) and OS (89.7% vs 73.6%, P = 0.006) when treated with combination chemotherapy comprising cyclophosphamide, adriamycin and cisplatin compared with treatment with pelvic radiotherapy alone (Susumu et al 2008). A phase II feasibility study by the Radiation Therapy Oncology Group used concurrent chemotherapy (cisplatin 50 mg/m2 on days 1 and 28) during external beam radiotherapy of 45 Gy in 25 fractions followed by four cycles of adjuvant combination chemotherapy with cisplatin 50 mg/m2 and paclitaxel 175 mg/m2 four weekly (Greven et al 2006). Forty-six patients with grade 2 or 3 endometrial adenocarcinoma with FIGO stages between IC, IIB and IIIB were treated and followed-up to a median of 4.3 years. At 4 years, pelvic, regional and distant recurrence rates were observed to be 2%, 2% and 19%, respectively. Four-year DFS and OS were 81% and 85%, respectively. Grade 2 and 4 late toxicity was seen in 21% of cases and grade 1 and 2 in 57% of cases. The inferences drawn were that this was a feasible regime with acceptable toxicity showing excellent locoregional control, and should be tested in a randomized controlled trial. It has become the basis of the PORTEC 3 trial.
There seems to be a stronger view in favour of adjuvant chemotherapy in locally advanced disease as the risk of distant spread and resultant mortality is high. The response rates to different chemotherapeutic agents used alone range between 4% and 77%. A systematic review of chemotherapy in advanced endometrial carcinoma by Humber et al reported an improvement in OS to 15 months from 6.9 months for best supportive care (Humber et al 2007). Median PFS improved from 3.2 months to 8.3 months. Overall response rates ranged between 17% and 34% for single agents, but between 30% and 69% for combination chemotherapy. Unfortunately, randomized evidence is lacking but retrospective data have shown some improvement in RFS but not OS. A randomized trial of adjuvant chemotherapy with cisplatin and adriamycin has been completed by the European Organization for Research and Treatment of Cancer, and the results are encouraging (Aapro et al 2003). Many oncologists feel uncomfortable not giving adjuvant chemotherapy to this group of patients (FIGO stage IIIA onwards), and treat them with platinum +/− anthracycline/taxane.
Hormonal therapy
A proportion (11–25%) of endometrial carcinomas express ER and/or PR. Published data do not support the routine use of adjuvant hormones in early-stage disease. This is because of a high rate of recurrence following endocrine treatment in spite of very significant initial complete response rates (Ushijima et al 2007). However, in exceptional circumstances where, due to extreme comorbidities or patient choice, surgery or radiotherapy cannot be used, consideration may be given to the use of hormone treatment. For advanced endometrial carcinomas, especially those that are well differentiated, showing strong progesterone positivity and metastatic to the lungs, hormone therapy is a reasonable choice and should always be considered (Bouros et al 1996, Thigpen et al 1999). Long-term responses have been documented. The most commonly used agent is medroxyprogesterone in high doses such as 200 mg two to three times a day. Fluid retention and heart failure are recognized complications. Other hormonal agents such as aromatase inhibitors have been used with some efficacy (Rose et al 2000).
Uterine Sarcomas
The indications for adjuvant treatment are somewhat variable but mainly depend on the type of sarcoma, size and degree of surgical clearance. For small (≤5 cm) low-grade sarcomas with complete microscopic clearance, radiotherapy is unnecessary as the risk of local recurrence is low. Similarly, there is insufficient evidence to suggest that radiotherapy is beneficial in cases of endometrial stromal sarcomas. Tumours larger than 5 cm, of higher grade and malignant mixed Müllerian tumours, also known as ‘carcinosarcomas’, should be considered for adjuvant radiotherapy. Incomplete surgical clearance usually requires higher doses of radiotherapy. Previous history of radiotherapy to the pelvis is very important as this may be a causative factor in some pelvic sarcomas (Nakanishi et al 2001).
Since publication of the meta-analysis of adjuvant chemotherapy in soft tissue sarcoma and the small associated benefit, many oncologists have extrapolated the data to include uterine sarcomas of high grade, especially leiomyosarcomas (Sarcoma Meta-analysis Collaboration 2000). More recent updates have only shown a marginal benefit against significant toxicity (Pervaiz et al 2008). Doxorubicin and ifosfamide are given as a three-weekly cycle to a total of six. Common side-effects include nausea and vomiting, hair loss, cardiotoxicity, confusion and neutropenic sepsis. Another challenge is the management of malignant mixed Müllerian tumours. The prognosis of this aggressive subtype remains very poor, with 3-year OS of approximately 22%. The only randomized trial of cisplatin and ifosfamide that showed some benefit also revealed that the toxicity was quite significant (Sutton et al 2000, 2005). Although the use of adjuvant chemotherapy is gradually increasing, there is, as yet, no set standard. The relatively low incidence of these tumour types and frailty of many patients makes it difficult to plan randomized trials.
Prognosis
The aura of good prognosis surrounding endometrial cancer may just be a reflection of diagnosis and prompt treatment at an early stage. The prognosis for advanced-stage disease is quite poor, with the exception of metastatic disease responsive to progestogens. In this situation, years of control can sometimes be achieved with very significant improvement of symptoms. Although hormone-resistant disease may respond to chemotherapy, the responses are short-lived. Aggressive/high-risk histologies also carry a poorer prognosis (Table 42.6).
Table 42.6 Five-year overall survival by International Federation of Gynecologists and Obstetricians (FIGO) stage
| FIGO stage | Substage | 5-year overall survival (%) |
|---|---|---|
| I |
Source: Creasman WT, Carcinoma of the corpus uteri; Int J Gynecol Obstet 2003;83:79, with permission of Elsevier.
Stage IC poorly differentiated endometrioid, papillary serous and clear cell tumours have 5-year survival rates of 50–70% (Murphy et al 2003, Havrilesky et al 2007b). Uterine sarcomas are also aggressive histiotypes, but the prognosis ranges from approximately 30% OS for malignant mixed Müllerian tumours (Callister et al 2004) to 50–90% for endometrial stromal sarcomas (Chan et al 2008). Leiomyosarcomas probably fall in the middle, with a 5-year survival rate of 40% (Kahanpaa et al 1986) (Box 42.4).
Box 42.4 Staging system for uterine sarcomas
| Stage I | Sarcomas confined to the uterus |
| Stage II | Sarcomas involving the corpus and cervix |
| Stage III | Sarcomas spreading beyond the uterus but not outside the pelvis |
| Stage IV | Sarcomas spreading outside the pelvis or into the bladder or rectum |
Targeted Therapies
Amongst these, the most widely researched molecules are the epidermal growth factor receptor and the vascular endothelial growth factor (VEGF) receptor families. The growth and progression of tumours is unequivocally angiogenesis dependent. The main proangiogenic factor, namely VEGF, also known as ‘vascular permeability factor’, is a potent angiogenic cytokine that induces mitosis and also regulates the permeability of endothelial cells. VEGF is an endothelial-cell-specific growth factor and the principal regulator of angiogenesis under normal and pathological conditions in most organs. Increased levels of VEGF and angiogenic markers are associated with poor outcome in endometrioid endometrial cancer patients (Kamat et al 2007).
The human epidermal growth factor receptor-2 (HER-2 protein), which is also known as c-erbB-2 or neu, is a member of subclass 1 of the superfamily of receptor tyrosine kinases. HER-2 is an independent prognostic factor in endometrial cancer and an important oncogene in high grade and stage endometrial cancer (Morrison et al 2006). Overexpression of HER-2/neu was associated with an overall worse prognosis in uterine papillary serous cancer (Slomovitz et al 2004). Inhibition of the epidermal growth factor receptor in poor prognostic types of endometrial cancer may be of benefit. There is limited research into new therapies for endometrial cancer, despite the increasing incidence. Identification of new drugs and targeted therapies for poor prognostic types of endometrial cancer will benefit treatment at initial presentation and relapse.
Challenges and Future Directions
KEY POINTS
Aapro MS, van Wijk FH, Bolis G, et al. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC Gynaecological Cancer Group. Annals of Oncology. 2003;14:441-448.
Acharya S, Hensley ML, Montag AC, Fleming GF. Rare uterine cancers. The Lancet Oncology. 2005;6:961-971.
Barakat RR, Lev G, Hummer AJ, et al. Twelve-year experience in the management of endometrial cancer: a change in surgical and postoperative radiation approaches. Gynecologic Oncology. 2007;105:150-156.
Barnes MN, Kilgore LC. Complete surgical staging of early endometrial adenocarcinoma: optimizing patient outcomes. Seminars in Radiation Oncology. 2000;10:3-7.
Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. Journal of the National Cancer Institute. 2008;100:1707-1716.
Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecologic Oncology. 1983;15:10-17.
Bouros D, Papadakis K, Siafakas N, Fuller AFJr. Natural history of patients with pulmonary metastases from uterine cancer. Cancer. 1996;78:441-447.
Callister M, Ramondetta LM, Jhingran A, Burke TW, Eifel PJ. Malignant mixed Müllerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome. International Journal of Radiation Oncology, Biology, Physics. 2004;58:786-796.
Chan JK, Kawar NM, Shin JY, et al. Endometrial stromal sarcoma: a population-based analysis. British Journal of Cancer. 2008;99:1210-1215.
Churn M, Jones B. Primary radiotherapy for carcinoma of the endometrium using external beam radiotherapy and single line source brachytherapy. Clinical Oncology. 1999;11:255-262.
Cooper N, Quinn MJ, Rachet B, Mitry E, Coleman MP. Survival from cancer of the uterus in England and Wales up to 2001. British Journal of Cancer. 2008;99:S68-S69.
Creasman W, Morrow C, Bundy B, et al. Surgical pathological spread patterns of endometrial cancer: a Gynecologic Oncology Group study. Cancer. 1987;60:2035-2041.
Creasman WT, Kohler MF, Odicino F, Maisonneuve P, Boyle P. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecologic Oncology. 2004;95:593-596.
Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. The Lancet. 2000;355:1404-1411.
Creutzberg CL, van Putten WL, Koper PC, et al. Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecologic Oncology. 2003;89:201-209.
Creutzberg CL, van Putten WL, Warlam-Rodenhuis CC, et al. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. Journal of Clinical Oncology. 2004;22:1234-1241.
Department of Health. Guidance on Commissioning Cancer Services: Improving Outcomes in Gynaecological Cancers — the Manual. Department of Health, London http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4005385, 1999.
Doll A, Abal M, Rigau M, et al. Novel molecular profiles of endometrial cancer — new light through old windows. Journal of Steroid Biochemistry and Molecular Biology. 2008;108:221-229.
Duffy SR, Distler W, Howell A, Cuzick J, Baum M. A lower incidence of gynecologic adverse events and interventions with anastrozole than with tamoxifen in the ATAC trial. American Journal of Obstetrics and Gynecology. 2009;200:80 e1-7.
Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. The Lancet. 1998;351(9114):1451-1467.
Fanning J. Long-term survival of intermediate risk endometrial cancer (stage IG3, IC, II) treated with full lymphadenectomy and brachytherapy without teletherapy. Gynecologic Oncology. 2001;82:371-374.
Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002.Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No.5, Version 2.0. IARC Press, Lyon, 2004.
Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Annals of Oncology. 2007;18:581-592.
Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2004;22:2159-2166.
Froggatt NJ, Brassett C, Koch DJ, et al. Mutation screening of MSH2 and MLH1 mRNA in hereditary non-polyposis colon cancer syndrome. Journal of Medical Genetics. 1996;33:726-730.
Ghezzi F, Cromi A, Bergamini V, et al. Laparoscopic management of endometrial cancer in nonobese and obese women: a consecutive series. Journal of Minimally Invasive Gynecology. 2006;13:269-275.
Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecologic Oncology. 2006;103:155-159.
Gupta JK, Chien PF, Voit D, Clark TJ, Khan KS. Ultrasonographic endometrial thickness for diagnosing endometrial pathology in women with postmenopausal bleeding: a meta-analysis. Acta Obstetrica et Gynecologica Scandinavica. 2002;81:799-816.
Havrilesky LJ, Secord AA, Bae-Jump V, et al. Outcomes in surgical stage I uterine papillary serous carcinoma. Gynecologic Oncology. 2007;105:677-682.
Havrilesky LJ, Cragun JM, Calingaert B, et al. The prognostic significance of positive peritoneal cytology and adnexal/serosal metastasis in stage IIIA endometrial cancer. Gynecologic Oncology. 2007;104:401-405.
Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. Journal of Clinical Oncology. 2006;24:4783-4791.
Humber CE, Tierney JF, Symonds RP, et al. Chemotherapy for advanced, recurrent or metastatic endometrial cancer: a systematic review of Cochrane collaboration. Annals of Oncology. 2007;18:409-420.
Istre O, Langebrekke A, Qvigstad E. Changing hysterectomy technique from open abdominal to laparoscopic: new trend in Oslo, Norway. Journal of Minimally Invasive Gynecology. 2007;14:74-77.
Janda M, Gebski V, Forder P, Jackson D, Williams G, Obermair A. Total laparoscopic versus open surgery for stage I endometrial cancer: the LACE randomized controlled trial. Contemporary Clinical Trials. 2006;27:353-363.
Kahanpaa KV, Wahlstrom T, Grohn P, Heinonen E, Nieminen U, Widholm O. Sarcomas of the uterus: a clinicopathologic study of 119 patients. Obstetrics and Gynecology. 1986;67:417-424.
Kamat AA, Merritt WM, Coffey D, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clinical Cancer Research. 2007;13:7487-7495.
Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology. 2004;92:744-751.
Kitchener HC. Survival from endometrial cancer in England and Wales up to 2001. British Journal of Cancer. 2008;99(Suppl 1):S68-S69.
Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. The Lancet. 2009;373:125-136.
Lertsanguansinchai P, Lertbutsayanukul C, Shotelersuk K, et al. Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical carcinoma. International Journal of Radiation Oncology, Biology, Physics. 2004;59:1424-1431.
Lethaby A, Suckling J, Barlow D, Farquhar CM, Jepson RG, Roberts H 2004 Hormone replacement therapy in postmenopausal women: endometrial hyperplasia and irregular bleeding. Cochrane Database of Systematic Reviews 3: CD000402.
Lin F, Zhang QJ, Zheng FY, et al. Laparoscopically assisted versus open surgery for endometrial cancer — a meta-analysis of randomized controlled trials. International Journal of Gynecological Cancer. 2008;18:1315-1325.
Lindemann K, Vatten LJ, Ellstrom-Engh M, Eskild A. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. British Journal of Cancer. 2008;98:1582-1585.
Maxwell GL, Chandramouli GV, Dainty L, et al. Microarray analysis of endometrial carcinomas and mixed Müllerian tumors reveals distinct gene expression profiles associated with different histologic types of uterine cancer. Clinical Cancer Research. 2005;11:4056-4066.
Milano MT, Constine LS, Okunieff P. Normal tissue tolerance dose metrics for radiation therapy of major organs. Seminars in Radiation Oncology. 2007;17:131-140.
Morrison C, Zanagnolo V, Ramirez N, et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. Journal of Clinical Oncology. 2006;24:2376-2385.
Murphy KT, Rotmensch J, Yamada SD, Mundt AJ. Outcome and patterns of failure in pathologic stages I–IV clear-cell carcinoma of the endometrium: implications for adjuvant radiation therapy. International Journal of Radiation Oncology, Biology, Physics. 2003;55:1272-1276.
Nakanishi K, Yoshikawa H, Ueda T, et al. Postradiation sarcomas of the pelvis after treatment for uterine cervical cancer: review of the CT and MR findings of five cases. Skeletal Radiology. 2001;30:132-137.
Office for National Statistics. Cancer Statistics Registrations: Registrations of Cancer Diagnosed in 2005, England. Series MB1 no. 36. Office for National Statistics, London, 2008.
O’Hanlan KA, Huang GS, Lopez L, Garnier AC. Total laparoscopic hysterectomy for oncological indications with outcomes stratified by age. Gynecologic Oncology. 2004;95:196-203.
O’Hanlan KA, Huang GS, Garnier AC, et al. Total laparoscopic hysterectomy versus total abdominal hysterectomy: cohort review of patients with uterine neoplasia. Journal of the Society of Laparoendoscopic Surgeons. 2005;9:277-286.
Papadopoulos N, Lindblom A. Molecular basis of HNPCC: mutations of MMR genes. Human Mutatation. 1997;10:89-99.
Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573-581.
Ramirez PT, Slomovitz BM, Soliman PT, Coleman RL, Levenback C. Total laparoscopic radical hysterectomy and lymphadenectomy: the M.D. Anderson Cancer Center experience. Gynecologic Oncology. 2006;102:252-255.
Reich H. Laparoscopic hysterectomy. Surgical Laparoscopy and Endoscopy. 1992;2:85-88.
Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee RB. A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology. 2000;78:212-216.
Sant M, Aareleid T, Berrino F, et al. EUROCARE-3: survival of cancer patients diagnosed 1990–94 — results and commentary. Annals of Oncology. 2003;14(Suppl 5):61-118.
Sarcoma Meta-analysis Collaboration 2000 Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database of Systematic Reviews 2: CD001419.
Scottish Intercollegiate Guidelines Network. Investigation of Post-menopausal Bleeding. SIGN Publication No. 61. SIGN, Edinburgh, 2002.
Silverberg SG. Problems in the differential diagnosis of endometrial hyperplasia and carcinoma. Modern Pathology. 2000;13:309-327.
Slomovitz BM, Broaddus RR, Burke TW, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. Journal of Clinical Oncology. 2004;22:3126-3132.
Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. Journal of the American Medical Association. 1998;280:1510-1517.
Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecologic Oncology. 2008;108:226-233.
Sutton G, Brunetto VL, Kilgore L, et al. A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: a Gynecologic Oncology Group Study. Gynecologic Oncology. 2000;79:147-153.
Sutton G, Kauderer J, Carson LF, Lentz SS, Whitney CW, Gallion H. Adjuvant ifosfamide and cisplatin in patients with completely resected stage I or II carcinosarcomas (mixed mesodermal tumors) of the uterus: a Gynecologic Oncology Group study. Gynecologic Oncology. 2005;96:630-634.
Thigpen JT, Brady MF, Alvarez RD, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose–response study by the Gynecologic Oncology Group. Journal of Clinical Oncology. 1999;17:1736-1744.
Ushijima K, Yahata H, Yoshikawa H, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. Journal of Clinical Oncology. 2007;25:2798-2803.
Walker JL, Piedmote M, Spirtos N, et al. Surgical staging of uterine cancer: randomized phase III trial of laparoscopy vs laparotomy — a Gynecologic Oncology Group Study (GOG): preliminary results. Journal of Clinical Oncology 2006 ASCO Annual Meeting Proceedings Part I. 2006;24(Suppl 18):5010.
Weiderpass E, Adami HO, Baron JA, et al. Risk of endometrial cancer following estrogen replacement with and without progestins. Journal of the National Cancer Institute. 1999;91:1131-1137.