CHAPTER 47 Malignant disease of the breast
Introduction
Breast cancer is one of the most common malignancies afflicting women, and is the leading cause of cancer-related mortality (Hortobagyi et al 2005). Worldwide, more than 1.2 million women are diagnosed with breast cancer each year, affecting 10–12% of the female population and resulting in 500,000 deaths per year. In the UK in 2005, there were 45,947 new cases: 45,660 (over 99%) in women and 287 (<1%) in men, causing approximately 14,000 deaths per year (Information Statistics Division Online 2008, Office for National Statistics 2008, Northern Ireland Cancer Registry 2008, Welsh Cancer Intelligence and Surveillance Unit 2008). The lifetime risk of a women being diagnosed with breast cancer is one in nine (Health Statistics Quarterly 1999, Information Statistics Division Online 2008, Welsh Cancer Intelligence and Surveillance Unit 2008). Whilst it is impossible to predict who will develop breast cancer, it is possible to identify those women who are at increased risk for breast cancer and provide options for reducing the risk.
Trends in Breast Cancer Incidence and Prevalance
More than 80% of cases of breast cancer occur in women over 50 years of age, with the highest incidence in women aged 50–69 years. The incidence of breast cancer has been increasing for many years in economically developed countries. The UK age-standardized incidence of breast cancer per 100,000 women increased from 74 in 1975 to 123 in 2005 (Coleman 2000). The introduction of a national screening programme in the UK in 1988 led to a transient increase in breast cancer incidence in women aged 50–64 years, as early undiagnosed cancers were detected. This increase lasted for the first 4–7 years of the programme. However, an underlying increase in incidence predating screening continues today and is particularly evident in older age groups. Mortality rates for breast cancer have fallen in many developed countries since 1990, having been previously stable/increasing for several decades (Beral et al 1995, Peto et al 2000, Jatoi and Miller 2003). This reduction has been attributed to earlier detection following the implementation of breast screening, decreased use of exogenous hormones and the use of adjuvant therapies such as tamoxifen (Berry et al 2005).
As the incidence of breast cancer is high and the 5-year survival rate is approximately 80%, many women are alive who have been diagnosed with breast cancer. An estimated 550,000 women are alive in the UK who have received a diagnosis of breast cancer (Maddams et al 2008).
Risk Factors
Age
Age is the single most important risk factor for the development of breast cancer (Colditz and Rosner 2000). The incidence of breast cancer increases with age, doubling every 10 years to the menopause, and then the rate of increase slows, levelling to a plateau after 80 years (Anderson et al 2005). This creates a point of inflection in the age-specific incidence curve known as ‘Clemmensen’s hook’ (Clemmensen 1948). The incidence of oestrogen-receptor-alpha (ERα)-negative tumours rises rapidly to 50 years and then flattens out/decreases, whereas the incidence of ERα-positive tumours is similar up to the age of 50 years but then continues to increase, albeit at a slower pace. As a result, ERα-negative tumours tend to occur earlier in life and ERα-positive tumours are more common in older women. The peak age of onset for these two tumour phenotypes is 50 years and 70 years, respectively.
Ethnicity changes the effect of age on breast cancer risk. African-American women under 50 years of age have a higher age-specific incidence of breast cancer than their Caucasian counterparts (Vogel 1998). However, the incidence of breast cancer is higher in Caucasian women after 50 years of age. The difference in age-adjusted breast cancer mortality rates between African-American and Caucasian women in the 1980s was probably due to the greater incidence of ERα-negative tumours in African-American women, who will not have benefited from the introduction of adjuvant hormonal therapy (Jatoi et al 2005).
Geographical variation
Worldwide, more than 1 million women are diagnosed with breast cancer every year, accounting for 10% of all cancer cases and 23% of all female cancer cases (Ferlay et al 2004). Incidence rates for breast cancer are six times greater in the Western world than in underdeveloped countries. Approximately 430,000 new cases of breast cancer occur each year in Europe and an estimated 212,920 in the USA. The lowest rates in Europe are found in Romania and Latvia, and the highest rates are found in Northern and Western Europe. The incidence of breast cancer in American Hispanic women is 40–50% that of non-Hispanic White women.
Migrants from low-risk countries to high-risk countries have been shown to acquire the risk of the ‘host nation’ within two generations (Tominaga 1985, Ziegler et al 1993). Japanese migrants to the USA acquire an increased breast cancer risk compared with the population in Japan, and there is increasing evidence that the earlier in life a woman takes up residence in a ‘high-risk’ country, the higher the risk of breast cancer compared with the country of origin (Shimizu et al 1991, Ziegler et al 1993).
Ovulatory cycles
Events in a woman’s life that alter her lifetime ovulatory cycles appear to correlate with the risk of breast cancer. Women who start menstruating early or have a late menopause have a 30–50% increase in risk of developing breast cancer. Similarly, late menarche and an early menopause lead to an equivalent reduction in breast cancer risk. The risk of developing breast cancer is doubled in those women who have a natural menopause after 55 years of age compared with those who experience the menopause before 45 years of age. A menopause induced before 40 years of age reduces the risk of breast cancer by almost two-thirds (Hankinson et al 2004).
Age at first pregnancy
Late age at first birth and nulliparity increase the lifetime risk of breast cancer. Nulliparity has been a well-known risk factor for developing breast cancer since Ramizzini described horrendis mammarium canceris in Catholic nuns (Ramazzini 1713).The risk of breast cancer in women who have their first child after 30 years of age is double that of women who have their first child before 20 years of age. The group at highest risk are those women who have their first child after 35 years of age. These women have a greater risk than nulliparous women. An early age of birth of a second child confers a reduced risk. The protective effects of an early full-term pregnancy have been observed in a number of ethnic groups and geographic locations, suggesting that the parity-induced protection results from biological changes in the breast rather than environmental factors.
Benign breast disease
Prospective and retrospective studies have shown a relative risk of breast cancer of 1.5–1.6 for women with benign breast disease compared with the general population (Hartmann et al 2005). This increased risk has been shown to persist for 25 years after biopsy. The relative risk of proliferative changes with atypia was 4.24 compared with a relative risk of 1.88 for those without atypia and 1.27 for non-proliferative lesions. The age at diagnosis of the benign breast disease appears to modify the risks related to the histological appearance of benign breast disease. The presence of atypia in women under 45 years of age conveys twice the risk observed among women over 55 years of age. The Breast Cancer Detection and Demonstration Project showed that the risk of breast cancer among premenopausal women with atypia was elevated by a factor of 12.0, compared with 3.3 for postmenopausal women with aytpia (London et al 1992, Dupont et al 1993). An increase in breast cancers has been demonstrated in the same breast during the first 5 years of follow-up following a diagnosis of benign breast disease, particularly in women with atypia.
Ionizing radiation exposure
The understanding of radiation-related breast cancer in women derives from epidemiological studies of patients exposed to diagnostic or therapeutic medical radiation (mantle irradiation for lymphoma) and of the Japanese atomic bomb survivors (United Nations Scientific Committee on the Effects of Atomic Radiation 2000). The breast tissue of young women is highly sensitive to the carcinogenic action of ionizing radiation. Only the bone marrow and the infant thyroid gland are more sensitive to the cancer-causing effects of radiation. Ionizing radiation is an established breast cancer risk factor and this risk has been shown to increase linearly with dose. Age at exposure directly affects radiation-related breast cancer risk, with the greatest risk seen in women exposed before 20 years of age and a significantly reduced risk seen for postmenopausal women. Periods of enhanced cell proliferation, namely in utero, puberty and pregnancy, have been proposed to represent windows of increased susceptibility for mammary carcinogenesis (Ronckers et al 2005).
Oral contraceptive pill
Use of the oral contraceptive pill slightly increases the risk of breast cancer in current and recent users (1–4 years following cessation) with relative risks of 1.24 and 1.16, respectively (Collaborative Group on Hormonal Factors in Breast Cancer 2002). This risk diminishes after discontinuing use and returns to normal after 10 years.
These estimates are based on the Collaborative Group on Hormonal Factors in Breast Cancer study; a collaborative meta-analysis of 54 studies in 25 countries with data on over 50,000 women with breast cancer (Anonymous 1996). Cancers diagnosed in women who have used the oral contraceptive pill tend to be less clinically advanced than those detected in women who have never used it. Users of the oral contraceptive pill are generally younger women whose breast cancer risk is comparatively low, so the small excess in current users will result in a relatively small number of additional cases. Other findings of the study were:
A large case–controlled study of 4575 women aged 35–64 years showed that current or former use of the oral contraceptive pill was not associated with a significantly increased risk of breast cancer (Marchbanks et al 2002).
Hormone replacement therapy
Hormone replacement therapy (HRT) use increases the risk of breast cancer and reduces the sensitivity of mammography. The risk of breast cancer for current or recent users of HRT increases by 2.3% per year of use. For women who have used HRT for at least 5 years (average 11 years), the increased risk was 35% (Anonymous 1997a). The effect is substantially greater for oestrogen–progesterone combinations than for oestrogen-only HRT. Risk increases with duration of use; the risk for current users of oestrogen–progesterone combinations for more than 10 years was 2.31 compared with 1.74 for 1–4 years of use. Risk decreases with cessation of use; past users (>5 years following cessation) have a similar risk to those who have never taken HRT. One recent study reported that current users of combined HRT had a 2.7-fold elevated risk of lobular cancer and a 3.3-fold risk of ductal cancer. The risk of lobular cancer was only raised in women who had used HRT for 3 years or more.
In the UK over the past 10 years, it is estimated that 20,000 extra breast cancer cases have occurred among women aged 50–64 years as a result of HRT use, and three-quarters (15,000) of these additional breast cancers are due to the use of oestrogen–progesterone HRT. HRT is used by over 20 million women in Western countries for the alleviation of perimenopausal and postmenopausal symptoms, and is an important source of exogenous oestrogen and, in some cases, progesterone exposure. A recent review concluded that the excess incidence of breast cancer, stroke and pulmonary embolism in postmenopausal women who use HRT for 5 years was greater than the reduction in incidence of colorectal cancer and hip fracture (Beral et al 2002). The risks and benefits for treating menopausal symptoms should be evaluated on an individual basis.
A decrease in the incidence of breast cancer among postmenopausal women in the UK may be linked to a decrease in the use of HRT. Researchers calculated that between 1999 and 2005, the risk of the disease fell by 14% in women in their 50s, representing 1400 fewer cases in 2005, and 3300 fewer over the period (Parkin 2009). They suggest that the decrease in breast cancer since 1999 in women aged 50–59 years and since 2003 in women aged 60–64 years is a consequence of the reduced use of HRT. The use of HRT began to fall after the Million Women Study in the UK and the Women’s Health Initiative Study in the USA showed that HRT could increase the risk of breast cancer. In women aged 45–69 years, the use of HRT increased from 1992 to reach a peak of 25% in 2000–2001, before falling to approximately half that by 2006.
Hormone therapy after breast cancer is becoming an increasingly relevant problem as more women survive breast cancer. The HABITS (Hormonal Replacement Therapy After Breast Cancer — Is It Safe?) trial was designed to confirm the efficacy of HRT given to women after treatment for breast cancer (Holmberg and Anderson 2004). The trial confirmed an unacceptably high risk in women allocated to receive HRT compared with those receiving best symptomatic treatment, and as a result was terminated. Analysis of women in the HABITS trial after a median follow-up of 4 years showed that 17.6% in the HRT treatment arm had developed breast cancer recurrence or a new breast cancer, compared with 7.7% in the control arm. The estimated 5-year cumulative rate for disease recurrence was 22.2% in the HRT arm and 9.5% in the control arm.
Endogenous hormones
Higher levels of endogenous hormones have been hypothesized to increase breast cancer risk. A pooled analysis of nine prospective cohort studies found a statistically significant increased risk of breast cancer in postmenopausal women with higher levels of sex hormones (Key et al 2002). The risk for women whose oestradiol levels were in the top quintile was approximately twice that compared with women whose oestradiol levels were in the bottom quintile. Evidence in premenopausal women was inconclusive.
Diet
A high-fat diet has been positively associated with breast cancer in a number of animal and case–control studies. Pooled analysis of cohort studies found no significant association between fat intake and breast cancer risk, whilst a meta-analysis found an association between higher total and saturated fat intake and an increased risk of breast cancer. A recent prospective study confirmed a significant association between saturated fat and breast cancer risk (Bingham et al 2003).
Alcohol intake
A significant association between alcohol intake and breast cancer has been found, with an increased risk of 7% for each 10 g alcohol/day (Hamajima et al 2002, Baan et al 2007). Approximately 4% of breast cancers in women from developed countries may be attributable to alcohol. In a recent large cohort of middle-aged women in the UK, a population with low-to-moderate alcohol consumption [10 g alcohol (one drink)/day] has shown a statistically increased risk of breast cancer; they also showed an increase in cancers of the oral cavity, pharynx, oesophagus, larynx, rectum and liver (Allen et al 2009). They calculated that the increased risk accounted for an additional 11 breast cancers per 1000 women up to 75 years of age. Although alcohol and tobacco are closely related social habits, there is no direct association between tobacco and breast cancer.
Height
Taller women have an increased risk of breast cancer (Hunter and Willett 1993). A pooled analysis estimated that the relative risk for women over 1.75 m tall compared with women under 1.6 m tall was 1.22 for all women and 1.28 for postmenopausal women (van den Brandt et al 2000).
Physical activity
A report from the International Agency for Research on Cancer concluded that physical activity has a preventive effect on breast cancer. This may be an indirect effect as exercise lowers BMI, or a direct effect on hormonal and growth factor levels. This varies between studies, with one showing a 30–40% reduction in the risk of breast cancer with a few hours of vigorous activity per week versus none (Monninkhof et al 2007).
Mammographic density
There is extensive evidence that mammographic density is a risk factor for breast cancer, independent of other risk factors, and is associated with large relative and attributable risks for the disease. Women with increasingly dense breasts have two to six times the risk of breast cancer compared with women with less dense breasts (Boyd et al 1995). Parity, menopause and other risk factors only explain 20–30% of the variance in mammographic density (Boyd et al 2002). Early studies of mother–daughter sets and small twin studies suggested that genetic factors might explain a proportion of the variation of breast tissue patterns within a given population (Boyd et al 2002, Stone et al 2006). The genetic factors that influence mammographic density may also explain variations in breast tissue involution.
Previous history of breast cancer
If a woman has had breast cancer previously, the risk of developing a second primary breast cancer is two to six times greater compared with the the risk of the general population of developing a primary breast cancer (Chen and Thompson 1999).
Family history
A family history of breast cancer, particularly in first-degree relatives, is a well-known risk factor for the development of breast cancer. The past few years have seen a significant increase in knowledge of inherited breast cancer. Approximately 80% of breast cancers are sporadic, and the other 20% are familial, occurring within the context of a positive family history. Twin studies have shown that inherited factors may account for up to 25–30% of all breast cancers (Lalloo et al 2003). These cancers are the result of a combination of genetic and environmental factors that cause acquired genetic mutations over time (Vogel and Bevers 2003). A woman with one first-degree relative has approximately double the risk of breast cancer of a woman with no family history of the disease. The risk is greater if two (or more) relatives are affected. Multiple primary cancers in one individual or related early-onset cancers in a family pedigree are highly suggestive of a predisposing gene.
BRCA1 and BRCA2 account for the largest proportion of familial breast cancer cases. It is thought that 20–25% of familial breast cancer cases are due to mutations or genomic rearrangements within these genes. The frequency of these mutations is rare, occurring in 0.1–0.5% of the general population, compared with 2% in Ashkenazi Jews (Rebbeck et al 2002). The breast cancer risk attributable to BRCA1 and BRCA2 mutations in Ashkenazi Jews is as high as 15–30% (FitzGerald et al 1996, Abeliovich et al 1997, Struewing et al 1997, Metcalfe et al 2004). A recent analysis of 22 studies showed that carrying a deleterious BRCA1 or BRCA2 mutation confers an estimated lifetime risk for developing breast cancer of 65% and 45%, respectively (Antoniou et al 2003). By the age of 40 years, carrying a deleterious BRCA1 mutation confers a 20% chance of developing breast cancer, and the risk increases with age (Kauff et al 2002). Mutations in BRCA1 are strongly associated with ovarian and fallopian tube cancers (Antoniou et al 2003). The risk of ovarian carcinoma for BRCA1 carriers is 17%, 39% and 54% at 40, 70 and 80 years, respectively (Antoniou et al 2003). Penetrance of BRCA1 with respect to ovarian, fallopian and breast cancer is greater than that for BRCA2.
Tumours associated with BRCA1 carriers are more frequently grade 3, and ERα-negative and ovarian carcinomas, which occur in BRCA1/2 families, are mostly non-mucinous epithelial cancers (Lakhani et al 2002). No single technique is able to detect all mutations, and even by sequencing the entire gene, the detection rate is only 85% (Evans et al 2003). Once a mutation has been identified in a family, definitive genetic testing can inform women more accurately of their risks and give them an informed choice of their options. Mutational analysis of an unaffected individual is problematic without checking an affected relative. Identification of a mutation will confirm an increased risk; however, the absence of a mutation does not exclude the possibility of a refractory mutation of another gene. Where there is not a dominant family history or a BRCA1/2 mutation is not identified, risk estimation gives a 1.5–3 fold relative risk with a family history of a single first-degree relative affected (Newman et al 1988, Claus et al 1994).
Quantitative Risk Assessment
The Gail model
The Gail model quantifies a woman’s lifetime risk of developing breast cancer by incorporating patient age, age at menarche, age at first birth/nulliparity, number of breast biopsies, ethnic origin and history of breast cancer in first-degree relatives (Gail et al 1989, Costantino et al 1999). It does not, however, consider the ages of first-degree relatives with breast cancer, and overlooks a family history of bilateral breast disease, second-degree relatives with breast cancer and a family history of ovarian cancer. Also, the model does not account for a personal history of atypical hyperplasia and lobular carcinoma in situ, previous radiation therapy to the chest for the treatment of Hodgkin’s lymphoma, or recent migration from a region of low breast cancer risk (e.g. rural China) to a high-risk region. The Gail model remains the most frequently used in defining eligibility for risk reduction trials.
The Claus model
The Claus model provides breast cancer risk estimates based on which relatives were diagnosed with breast cancer and at what age their diagnosis was made. Initially, only data from mothers and sisters were included, but second-degree relatives were included subsequently (Claus et al 1994).
BRCAPRO
BRCAPRO was developed by statisticians at Duke University to calculate age-specific probabilities of developing breast and ovarian cancer, based on the probability that the individual carries a mutation on one of the BRCA genes (Berry et al 1997). The model uses the observed incidences of breast and ovarian cancer among BRCA gene mutation carriers and non-carriers to calculate the probability that a given individual is a mutation carrier based on his/her family history.
The Tyrer–Cuzick model
The Tyrer–Cuzick model identifies the risk of breast cancer in unaffected women by taking into account their probability of carrying genetic risk factors, namely a BRCA1/BRCA2 mutation; a notional common, low-penetrance dominant susceptibility allele that stands for all other genetic risk factors; and a number of other individual factors known to influence risk, such as age at menopause and menarche, weight, height, age, use of HRT, previous benign breast biopsies and parity (Tyrer et al 2004).
Future developments
The Breast Cancer Prevention Collaborative Group has advised that the following risk factors should be further examined by multivariate analysis in future studies: mammographic density, plasma hormone levels, bone density and fracture history, history of weight gain, BMI and hip–waist ratio (Santen et al 2007). It is thought that the addition of these risk factors will lead to a more accurate quantitative risk assessment model for use in future breast cancer prevention trials. Whilst breast cancer risk assessment using mathematical models based on epidemiological data is valid, no one model integrates benign breast disease, oestrogen exposure and family history in a comprehensive fashion. Therefore, it is important to use a variety of models in a specialized risk assessment clinic.
Screening, Genetic Testing and Risk Reduction
Breast cancer family history clinics are well established in the UK, and are run by medical oncologists, clinical geneticists, nurse specialists and breast surgeons in a multidisciplinary approach with close involvement of radiologists and psychologists (Evans et al 1994, 1996). After a risk assessment, women are divided into three risk groups: average, moderate and high risk. Patients should undergo pretest counselling to ensure understanding of the implications of a positive test. This should also include the risks and benefits of early cancer detection and the prevention modalities available. Counselling following testing should be available to help patients to cope with their test results and review prevention modalities. Once advised of their risk, women deciding to pursue risk reduction therapy can be presented with their management options.
Surgical management
Prophylactic/risk-reducing mastectomy (RRM) is an option for breast cancer risk reduction in high-risk women. The significant psychological and physical burden associated with RRM is reserved for those women whose lifetime risk of developing breast cancer is high. High-risk women are classified as those with a lifetime risk above 25%. This risk is the equivalent of having one first-degree relative with breast cancer diagnosed below the age of 50 years, or three affected relatives (first-degree) diagnosed under the age of 60 years. The efficacy of RRM is controversial and depends on the amount of residual tissue following the procedure. A retrospective study of 639 women with a family history of breast cancer suggests that RRM is associated with a 90% reduction in risk (Hartmann et al 1999). One small prospective study investigating the efficacy of prophylactic mastectomy in BRCA1/2 carriers with a mean follow-up of 3 years showed that those women undergoing mastectomy had a significant reduction in the incidence of breast cancer (0 of 76 women) compared with the surveillance group (eight of 63 women) (Meijers-Heijboer et al 2001).
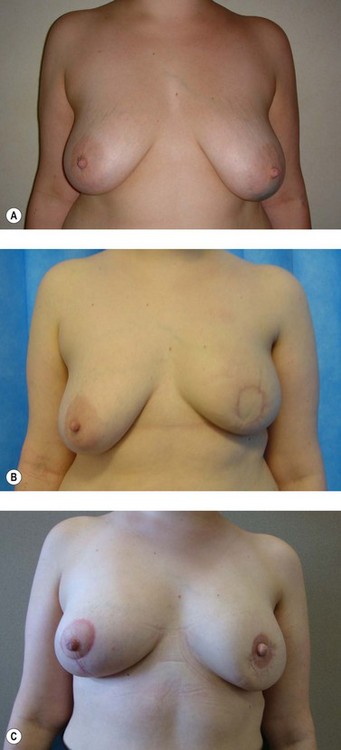
The use of RRM is increasing, with women often choosing to have breast reconstruction surgery either immediately or delayed. The timing of the reconstruction is controversial, with immediate reconstruction appropriate in the majority of women. It is now felt that the psychological benefit of emerging from a mastectomy with a breast mound far outweighs the need for a waiting period (Kronowitz 2007). The process from first consultation to surgery takes 6–12 months; this delay deliberately allows women enough time for the decision-making process.
Bilateral prophylactic oophorectomy
Studies have shown a significant reduction in the incidence of breast cancer in women with BRCA1/2 mutations that have undergone prophylactic bilateral oophorectomy (Rebbeck et al 2002). Before undergoing the procedure, the patient should take into account how long she wishes to maintain her fertility, and should receive counselling about the risks and benefits of prophylactic oophorectomy. Opinion is divided on the use of HRT following prophylactic oophorectomy, with some centres routinely recommending HRT for all patients up to 50 years of age; the decision to use oestrogens should be based on a consideration of symptoms affecting future health and quality of life.
Chemoprevention
Chemoprevention provides a non-invasive option for breast cancer risk reduction for many high-risk women. Tamoxifen, a selective oestrogen receptor modulator (SERM) well known for its antioestrogenic effects in breast tissue, was the first agent used in breast cancer risk reduction after it was found to reduce the incidence of all breast cancers by 38% and ER-positive tumours by 48% (Cuzick et al 2003).
Pathology
In-situ carcinoma
Ductal carcinoma in situ
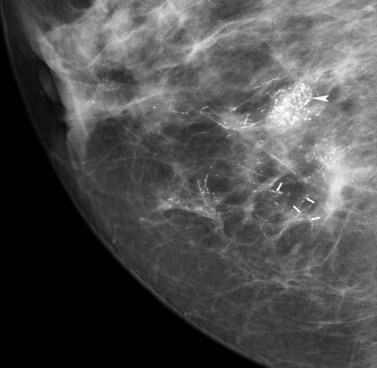
Breast screening has resulted in a marked increase in the detection of ductal carcinoma in situ (DCIS), accounting for 25–30% of all screen-detected tumours (Schwartz et al 2000). Over 90% of DCIS is impalpable, asymptomatic and often detected by screening as microcalcifications (Figure 47.1). The remaining 10% are symptomatic, presenting with nipple discharge, a palpable mass or Paget’s disease of the breast. When diagnosed clinically, DCIS is often extensive or associated with a concurrent invasive tumour. Postmortem studies have shown prevalence of DCIS from 0.2% to 14% (Bartow et al 1987, Nielsen et al 1987). Risk factors for DCIS include older age at first childbirth, nulliparity and a family history of breast cancer (Raktovich 2000). Retrospective studies of cases of low-grade DCIS misdiagnosed as benign showed that 20 years after local excision, approximately 33% had developed invasive disease (Page et al 1995). DCIS is classified into two major subtypes according to the presence/absence of comedo necrosis (atypical cells with abundant luminal necrosis that fill at least one duct) (Silverstein et al 1996). A system of low, intermediate and high nuclear grade is used to classify DCIS.
Most cases of DCIS are unicentric, with only 1% showing multicentric disease (Holland et al 1990b).
Multicentric tumour is defined as separate foci of tumour found in more than one breast quadrant or more than 5 cm from the primary tumour. Multifocal tumour is defined as more than one tumour foci in the same quadrant (Anonymous 1998c).
The spread of DCIS locally is along the branching ducts that form the glandular breast, and explains why most DCIS recurrences occur at or near the site of the initial tumour (Holland et al 1998). Micro-invasion is uncommon in DCIS, but confers a 2% risk of lymph node metastasis (van Dongen et al 1989). Studies have shown that poorly differentiated, high-grade comedo DCIS has low ER expression, high rates of cell proliferation, and overexpression of c-erbB2 (HER-2/neu) and epidermal growth factor receptor (EGFR) (Millis et al 1996). Low-grade lesions have high ER expression, lower rates of cell proliferation and rarely express HER-2 (Millis et al 1996, Boland et al 2002). Small, localized areas of DCIS (<4 cm) should be treated with breast-conserving surgery (BCS) with or without radiotherapy, and larger lesions need to be treated with mastectomy (Schwartz et al 2000).
The incidence of macroscopic nodal involvement in DCIS is less than 1%; however, nodal micrometastases have been reported in 5–14% of patients (Schuh et al 1986, Kitchen et al 2000). Two small, single-institution studies have reported a 3.1% rate of sentinel lymph node involvement in 223 patients with pure DCIS. Sentinel lymph node biopsy (SNB) for DCIS is not currently indicated but is under investigation, and may have a role in mastectomy for DCIS (Intra et al 2003). SNB should only be considered in those patients with a higher likelihood of underlying, undetected invasive disease (i.e. younger patients, DCIS >4 cm, high-grade DCIS and patients undergoing mastectomy). Twenty-five percent of cases recur within 8 years of BCS alone, 50% of which will present with invasive disease (Fisher et al 1999, Julien et al 2000, Ottesen et al 2000, Chan et al 2001); the recurrence rate for DCIS following mastectomy is 1% (Silverstein et al 1995). The key factor for recurrence is a clear margin at the time of surgery, and poor prognostic indicators include younger age at diagnosis (<40 years), poorly differentiated/high-grade tumours, presence of comedo necrosis, ER negativity and HER-2 positivity (Yen et al 2005).
The NSABP-B17, EORTC 10853 and UK/ANZ DCIS trials evaluated the value of radiotherapy following BCS for DCIS (Fisher et al 1999, Julien et al 2000, Houghton et al 2003). All of the trials reported a significant reduction in ipsilateral recurrence following radiotherapy. The EORTC trial showed a reduction in recurrence of DCIS from 8% to 5%, and a reduction in invasive recurrence from 8% to 4% at 5 years.
Lobular in-situ neoplasia
Lobular in-situ neoplasia (LISN; formerly known as lobular carcinoma in situ) is not itself a premalignant lesion but is a high-risk marker of invasive cancer. It is often an incidental finding during breast biopsy, and accounts for 0.5% of symptomatic and 1% of screen-detected tumours. Patients with LISN are younger, premenopausal with bilateral and multicentric disease of lower grade. Almost all patients express ER (Akashi-Tanaka et al 2000). If LISN is detected at core biopsy, the area should be subjected to excision biopsy to confirm the diagnosis and exclude an invasive focus. The NSABP P-1 prevention trial reported a 56% risk reduction of developing subsequent invasive cancer with tamoxifen in patients with a history of LISN (Dunn and Ford 2000).
Invasive carcinoma
Invasive lobular carcinoma
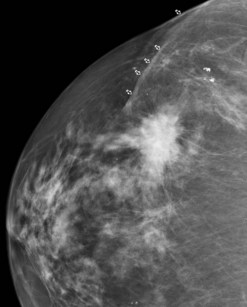
Invasive lobular carcinoma (ILC) is the second most common type of invasive breast cancer accounting for 8–14% of all invasive breast cancers (Arpino et al 2004). ILC clinically presents as a poorly defined thickening of the breast rather than a dominant mass. This makes the extent of the disease difficult to estimate on clinical examination, and difficult to visualize on mammography. Ultrasonography is more sensitive than mammography in detecting ILC, but may significantly underestimate the size of the lesions (Pritt et al 2004, Selinko et al 2004). Magnetic resonance imaging (MRI) is more accurate than either mammography or ultrasonography in defining the extent of the disease but is less widely available (Weinstein et al 2001). Due to the infiltrative growth pattern, there is a higher incidence of resection margin involvement than for IDC and a higher conversion rate to mastectomy (Yeatman et al 1995). ILC has a higher incidence of being bilateral, multifocal and multicentric than IDC (Figure 47.2) (Ashikari et al 1973). Axillary lymph nodes in ILC may remain impalpable even when extensively involved (Grube et al 2002). A large study from the National Cancer Database to examine treatment and outcomes in patients with ILC showed a similar 5-year survival rate between patients with ILC and IDC, and between those ILC patients receiving breast-conserving therapy and those receiving mastectomy (Winchester et al 1998).
Paget’s disease of the breast
Sir James Paget (1874) described ‘an eczematous change in the skin of the nipple preceding an underlying mammary cancer’, now known as Paget’s disease (Paget 1874). More than 95% of women with Paget’s disease of the nipple have an underlying malignancy, although 50% are clinically and mammographically undetectable (Vielh et al 1993). Paget’s disease accounts for 0.7–4.9% of all breast malignancies, and misdiagnosis as eczema and treatment with topical steroids explains an average 10–12-month delay in diagnosis (Kister and Haagensen 1970, Lagios et al 1984, Chaudary et al 1986, Dixon et al 1991, Kothari et al 2002). The first symptoms include burning, itching and change in sensation of the nipple–areola complex; raised skin lesions follow this, with a clear demarcation from the surrounding skin. Rarely, nipple deformity and retraction may occur if there is tethering from an underlying malignancy. Characteristically, it starts on the nipple and spreads to the areola and then surrounding skin, and in the later stages may present with ulceration and bleeding. Differential diagnoses include chronic eczema, benign papilloma of the nipple, basal cell carcinoma, Bowen’s disease and malignant melanoma (Jamali et al 1996).
A full-thickness punch biopsy of the nipple should be performed to confirm the diagnosis (Rosen 1996). Mammography, ultrasonography and MRI can be used to detect an underlying mass, as well as assessing the contralateral breast. Paget’s disease often presents with multicentric disease (75%); therefore, mastectomy is advocated as the procedure of choice (Kothari et al 2002). However, several small studies of BCS and radiotherapy for Paget’s disease have shown low rates of recurrence (Fourquet et al 1987, Dixon et al 1991, Kollmorgen et al 1998, Bijker et al 2001, Fu et al 2001, Marshall et al 2003). Attempts to preserve uninvolved areas of the nipple are associated with high rates of local recurrence (Stockdale et al 1989, Fu et al 2001). Staging by SNB or axillary dissection is required for all patients with invasive disease.
Inflammatory breast cancer
Inflammatory breast cancer (IBC) was first coined by Lee and Tannenbaum at the Memorial Hospital, New York in 1924 to describe an aggressive form of breast cancer with an incidence of 1–6% (Lee and Tannenbaum 1924, Levine et al 1985). A higher incidence is reported in African-Americans than in Caucasians and other ethnic groups (10.1%, 6.2% and 5.1%, respectively). Women with IBC often present at a younger age than women with non-IBC (NIBC) (Chang et al 1998, Anderson et al 2003, Charafe-Jauffret et al 2004). The 10-year survival rate of patients with IBC is 26.7%, compared with 44.8% for those with NIBC (Low et al 2002). A recent review of 635 patients at the M.D. Anderson, Texas (IBC, n = 214; stage III NIBC, n = 421) demonstrated a significantly reduced progression-free survival for IBC compared with NIBC (24 months and 35 months, respectively), and of overall survival for IBC compared with NIBC (42 months and 60 months, respectively).
The criteria for diagnosis was described by Haagensen including erythema, oedema involving more than two-thirds of the breast, tenderness, induration, warmth, enlargement, peau d’orange and diffuseness of the tumour on palpation (Haagensen 1971). These symptoms progress rapidly. At diagnosis, most patients (55–85%) have axillary lymph node involvement and up to 30% have distant metastases (Jaiyesimi et al 1992, Hance et al 2005). Pathology shows extensive lymphovascular invasion by tumour emboli, involving the superficial dermal plexus of vessels in the papillary and high reticular dermis (Taylor and Meltzer 1938). Primary IBC is the development of carcinoma and skin changes in a previously healthy breast, and secondary IBC is the development of inflammatory IBC in a breast that has had a previous malignancy or changes caused by irradiation.
One treatment protocol from the M.D. Anderson advises initial treatment with neoadjuvant sequential taxane (paclitaxel/docetaxel) and an anthracycline-based regimen (5-fluorouracil + epirubicin + cyclophosphamide) (Cristofanilli et al 2002). Patients who show a partial or complete response undergo surgery (mastectomy and axillary dissection) followed by adjuvant radiotherapy and hormonal therapy (if ERα-positive). For those patients who do not respond, radiotherapy to the breast and axilla is recommended, followed by surgery, if feasible, and adjuvant hormonal therapy (if ERα-positive) (Singletary 2008). SNB is not recommended in patients with IBC as the identification rate is only 70% and the false-negative rate is 40% (Stearns et al 2002). Currently, most practitioners recommend a delayed reconstruction after completion of therapy. Most local recurrences occur in the mastectomy flap.
A number of studies have reported a higher incidence of c-erbB2 (HER-2) in patients with IBC (Turpin et al 2002, Parton et al 2004). One small study (n = 22, IBC = 9, NIBC = 13) combining docetaxel with trastuzumab as part of the primary systemic therapy observed a complete response in 40% of patients (van Pelt et al 2003). Lapatinib (reversible inhibitor of c-erbB1 and c-erbB2) has shown partial responses in women with IBC who have been extensively pretreated (Burris et al 2005, Spector et al 2006). The combination of lapatinib with paclitaxel in a cohort of 21 chemotherapy-naïve IBC patients overexpressing HER-2 showed a clinical response rate of 95% (Cristofanilli et al 2006).
Other breast malignancies
Primary cutaneous melanoma of the skin of the breast is very rare, accounting for 0.28% of all cases of melanoma (Ariel and Caron 1972). It is more commonly found as a result of a distant metastasis from a primary elsewhere. A full history and physical examination for the presence of other melanomas should be performed. A core or surgical biopsy of the thickest portion of the melanoma will determine the depth of invasion. Immunostaining for HMB-45 and S100 enables differentiation of melanoma from other cutaneous masses. Staging is with computed tomography (CT) or positron emission tomography (PET), and lactate dehydrogenase levels should be measured. Wide local excision is recommended with margins dependent upon the depth of invasion, and SNB performed at the time of excision. Complete axillary dissection should be performed for those patients with clinically suspicious lymphadenopathy and those with melanomas larger than 4 mm (Essner et al 2002). Adjuvant therapy is similar to that for melanoma elsewhere in the body.
Radiation-induced primary angiosarcoma of the breast, first reported in 1981, is extremely rare (Maddox and Evans 1981). Approximately 60 cases have been reported, although the incidence is rising (Rao et al 2003). It presents as violet, blue, red or black skin nodules in a multifocal pattern, and is diagnosed by a punch, incisional or excisional biopsy (Fineberg and Rosen 1994). Fine needle aspiration, mammography and ultrasonography are often not helpful. Factor VIII-related antigen is positive in most angiosarcomas, distinguishing it from other sarcomas (Stokkel and Peterse 1992). Staging is based on the size, grade and depth of the tumour, with grade being the most important prognostic indicator. Most angiosarcomas are high grade and are treated with mastectomy (Fineberg and Rosen 1994). Axillary staging is not required, as most sarcomas do not metastasize to regional lymph nodes. The recurrence rate is 40% and prognosis is poor.
Primary breast lymphoma (PBL) is defined as a lymphoma localized to the breast and its draining basins, arising from lymphoid tissue in the breast (Smith et al 1987, Kuper-Hommel et al 2003). It accounts for 0.14% of all breast malignancies and 0.65% of all non-Hodgkin’s lymphomas, with the most common type being diffuse large B-cell lymphoma (Ha et al 1998, Kuper-Hommel et al 2003). PBL arises most commonly in the seventh decade as a painless, enlarged rubbery mass. Mammographic and ultrasound findings are non-specific, and PBL is categorized according to the Ann Arbor classification of lymphomas. Diagnosis is by core biopsy, and the patient is staged by CT of the neck, chest, abdomen and pelvis, and bone marrow biopsy. Surgery with adjuvant radiotherapy is no longer advocated due to the high incidence of local and disseminated recurrence (Kuper-Hommel et al 2003). Treatment of PBL is now with chemotherapy, followed by targeted radiotherapy, with a 2-year survival rate of 63% (Ha et al 1998, Brogi and Harris 1999).
Metastasis to the breast
This is uncommon and accounts for 0.5–6.6% of breast malignancies, with the contralateral breast being the most common site of metastatic malignancy (Bohman et al 1982, Paulus and Libshitz 1982, Amichetti et al 1990). Other malignancies that metastasize to the breast include lymphoma, melanoma, rhabdomyosarcoma and small cell lung cancer (Bartella et al 2003). These lesions are indistinguishable from primary breast cancers by examination and imaging. Core biopsy is preferable as immunohistochemistry plays an important role in diagnosis and prognosis is poor, with a 1-year survival rate of 20% (Bartella et al 2003).
Presentation
Breast cancers may present symptomatically or through screening.
Breast screening
Breast screening aims to reduce mortality through early detection. A number of trials carried out between the 1960s and 1980s showed that population screening by mammography can be expected to reduce breast cancer mortality by 25% (Olsen and Gotzsche 2001, Tabar et al 2001, Anonymous 2002b, Duffy et al 2002, Nystrom et al 2002). The benefit of screening is greatest in women aged 55–70 years (Anonymous 2002b, Nystrom et al 2002). There is no mortality benefit of screening women under 40 years of age, and the mortality benefit of those aged between 40 and 55 years is 20% (Anonymous 2002b, Nystrom et al 2002). Breast screening has been introduced in many countries over the past 20 years. In some countries, screening is advised in all women over 40 years of age; however, in countries providing population-based screening, women over 50 years of age are targeted.
The UK National Breast Screening Programme (NHSBSP) was set up by the Department of Health in 1988 in response to the recommendations of a working group, chaired by Professor Sir Patrick Forrest, which had been set up to consider whether or not to implement a population screening programme in the UK. The report ‘Breast Cancer Screening’ was published in 1986, and became known as ‘The Forrest Report’ (Forrest 1986). The NHSBSP was the first of its kind in the world. It began inviting women for screening in 1988, and national coverage was achieved by the mid 1990s. It provides screening by invitation, free at the point of delivery to all women between 50 and 70 years of age (initially 50–64 years, age range increased in 2004). Women over 70 years of age can attend but are not invited, and more than 70% of the invited population are required to attend in order to obtain an overall mortality benefit. The screening method is two-view mammography (cranio-caudal and medio-lateral oblique views) every 3 years. Approximately 5% of women screened are recalled for further assessment of a problem identified at screening. Three-quarters of women recalled simply require further imaging (ultrasound and/or mammography) and clinical assessment before being reassured and discharged. The remaining 25% will undergo a needle biopsy procedure in order to diagnose six cancers per 1000 women screened. Despite advances in needle biopsy techniques, 0.25% of all women screened will require an open surgical biopsy. Mammography can be expected to detect breast cancer 2 years before it becomes clinically apparent, and the frequency of mammography is determined by the lead time of breast cancer. Mammographic screening intervals, based on average growth time of breast cancer to age, should be yearly for women between 40 and 50 years of age, every 2 years in women between 50 and 60 years of age, and every 3 years thereafter. However, the Breast Screening Frequency trial did not show a significant benefit for women aged 50–64 years screened yearly compared with those screened every 3 years (Anonymous 2002a). One-third of breast cancers will present in the interval between screens, called ‘interval cancers’, and half of these will present in the third year after screening.
Screening high-risk young women
A national study evaluating mammographic screening for young women with a family history of breast cancer is being conducted (FH01 study), comparing screening in women aged 40–44 years with a moderate risk with a control arm; this study is currently unreported. Mammography has a higher predictive value in young women at high risk compared with age-matched controls, but is not sensitive, particularly in women with BRCA1 mutations (Chang et al 1999, Brekelmans et al 2001, Goffin et al 2001, Tilanus-Linthorst et al 2002, Hamilton et al 2004, Robson 2004, Warner et al 2004). Women with BRCA1 rarely present with associated DCIS, and the mammographic features are therefore usually of a mass lesion with no microcalcifications and no architectural distortion. These often present as interval cancers. BRCA2 cancers present similarly to sporadic cancers, and are more likely to be detected by mammography. Ultrasonographic features in BRCA1 cancers are often indeterminate or benign.
MRI is the most sensitive imaging modality in young women (Kriege et al 2004, Robson 2004). Genetically predisposed women commonly develop breast cancer when young and when dense breast tissue reduces the sensitivity of mammography. The Magnetic Resonance Imaging for Breast Screening (MARIBS) study compared the performance of contrast-enhanced MRI with mammography in this group of women (Leach et al 2005). It confirmed that contrast-enhanced MRI was significantly more sensitive than mammography in cancer detection for the entire cohort, but especially in the subgroup of BRCA1 carriers. Specificity for both procedures was acceptable. In spite of a high proportion of grade 3 tumours, the tumours were small and few women were node positive. These findings confirm those from the Netherlands six-centre MRI Screening (MRISC) study and a single-centre study from Toronto (Warner et al 2004, Kriege et al 2007). These reports support a policy of annual screening combining contrast-enhanced MRI and mammography, which would detect most tumours. These studies show evidence of effective small cancer detection, but do not have sufficient power to show whether mortality is reduced, for which there is no current evidence. Age for starting screening must be based on age rather than age of affected relatives. For women at moderate risk, screening should be started at 40 years of age, and for those at high risk, screening may be started at 30–35 years of age. High risk can be described as more than 8% by 50 years or more than 25% for lifetime. It is important that these women be followed-up in a specialist centre.
Diagnosis
Mammography
X-ray mammography has been the basis of breast imaging for more than 30 years. The sensitivity of mammography for breast cancer is dependent on age, as the denser the breast tissue, the less effective it is in detection (Figure 47.3). Breast tissue is more dense in younger women and whilst the sensitivity of mammography for breast cancer in women over 60 years of age approaches 95%, detection rates are less than 50% in women under 40 years of age (Kolb et al 2002). Mammography uses ionizing radiation and should only be used where a clinical benefit is likely. The benefits of mammography in women over 40 years of age is likely to outweigh the oncogenic effects of repeated exposure. There is rarely an indication for performing mammography in women under 35 years of age unless there is a strong clinical suspicion of malignancy. The false-negative rate for mammography has been reported to be between 10% and 30%. Although many cancers are mammographically occult, especially in dense breasts, a large number of these are visible retrospectively. These oversights can be reduced by double reading, improving sensitivity by up to 15% (Skaane et al 2007). Computer-aided design (CAD) programmes are available to assist radiologists to detect potentially suspicious abnormalities.
Ultrasound
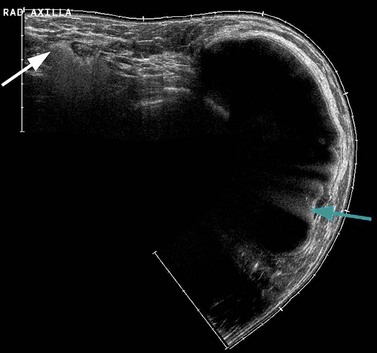
High-frequency ultrasound (>10 MHz) is a highly effective diagnostic tool in the investigation of focal breast symptoms (Wilson and Teh 1998). Ultrasound does not use ionizing radiation and has a very high sensitivity for breast pathology (Lister et al 1998). High-resolution ultrasonography can easily distinguish between most solid and cystic lesions, and can differentiate between benign and malignant lesions with a high degree of accuracy (Figure 47.4). It is the technique of choice for further investigation of focal symptomatic breast abnormalities for women under 35 years of age, and is used in conjunction with mammography in those over 35 years of age. Ultrasound is being used increasingly to assess the axilla in women with breast cancer. Those nodes with an abnormal morphology can be accurately sampled by fine needle aspiration.
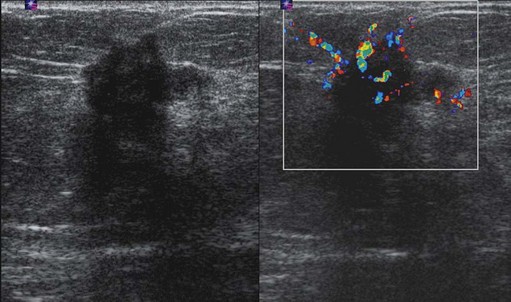
Figure 47.4 Invasive ductal carcinoma grade II ultrasound. This is the same tumour as shown in Figure 47.3. On the greyscale image, there is an irregular mass of reduced echogenicity which has posterior shadowing. The colour Doppler image shows surround vascularity which has a radial distribution feeding the cancer. Slight overlying skin thickening can be seen on the greyscale image.
Elastography ultrasound is a new adjunct to conventional B-mode ultrasound imaging, and uses the differences in tissue deformation to create an image of relative tissue stiffness (Wakeham et al 2006). Objects in the breast of different stiffness will displace different amounts, creating an image of relative stiffness (Svensson and Amiras 2006). Research into elasticity ultrasound has been ongoing for over 20 years, but it is only more recently with advances in computing power that commercial development of the potential clinical applications has become possible. A recent study has shown that benign lesions have a smaller size image on strain ultrasound than B-mode imaging, whilst malignant lesions usually have a larger strain image (Hall et al 2003). Malignant lesions mainly appear as stiff throughout, and strain imaging can reveal lesions that are occult on B-mode imaging, allowing accurate diagnostic biopsy. It may be that knowledge of the typical strain appearances may obviate the need to fine needle aspirate/biopsy certain benign lesions.
Magnetic resonance imaging
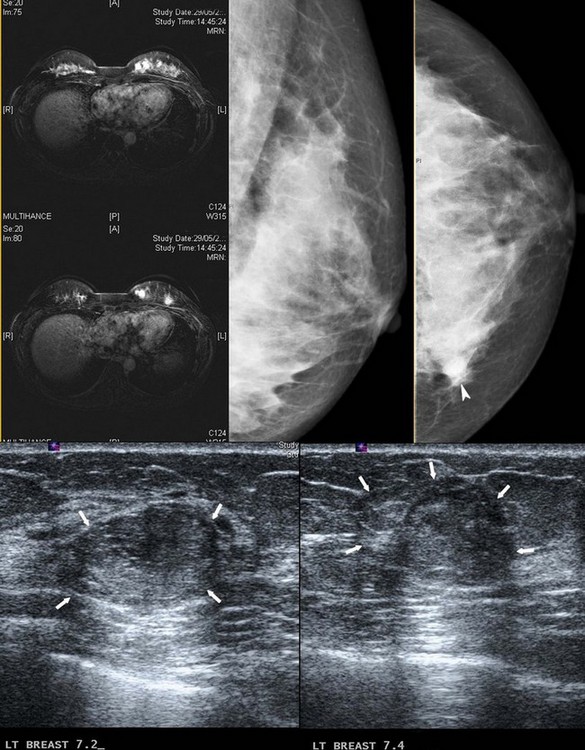
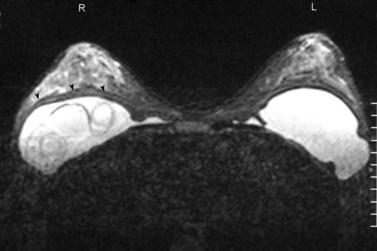
A Memorial Sloan-Kettering Cancer Center study of patients with invasive lobular cancer showed that MRI identified 27% more sites of cancer in the ipsilateral breast. Of these, 42% had DCIS and 58% had an infiltrating cancer (Quan et al 2003). Of the 62 women studied, multifocal cancer was present in 20%, multicentric cancer in 4%, and multifocal and multicentric cancer in 3%. Therefore, approximately one-quarter of additional ipsilateral cancers occur within the same quadrant. A recent multi-institutional study of women recently diagnosed with breast cancer showed that 3.1% had a cancer detected by MRI in an apparently uninvolved contralateral breast (Lehman et al 2007). MRI has been shown to accurately distinguish between scarring and tumour recurrence, provided that the scan is performed more than 18 months after surgery. The value of MRI screening in high-risk patients has been discussed previously. MRI is used to evaluate the integrity of saline and silicone implants (Figure 47.5), to discern extracapsular rupture from intracapsular rupture, and to identify silicone within the breast parenchyma (Holmich et al 2005). It is superior to ultrasonography (Figure 47.6) in detecting implant ruptures in patients with both double and single lumen implants (Di Benedetto et al 2008).
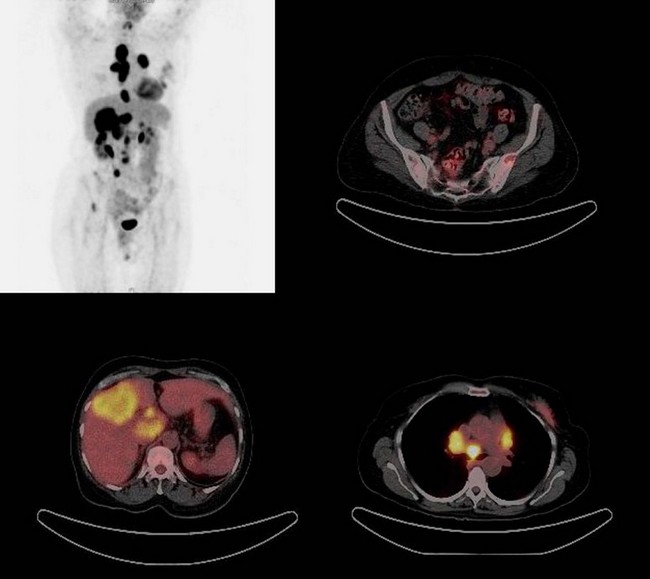
Positron emission tomography
Most breast malignancies have greater metabolism and concentrate 18F-fluorodeoxyglucose (FDG), the agent most frequently used in clinical PET. PET and PET/CT the latter of which combines anatomical and physiological imaging are increasingly used in oncology (Figure 47.7). However, it is widely agreed that FDG-PET does not have a role in the detection of primary breast cancer.
FDG-PET and FDG-PET/CT can improve staging by detecting otherwise occult disease, particularly in the locoregional and mediastinal nodal basins (Eubank et al 2004, Eubank and Mankoff 2005). Eubank et al showed that FDG-PET detected more widespread disease changes, affecting treatment in up to 44% of patients thought to have locoregional recurrence. Skeletal metastases account for 90% of all metastatic lesions, as well as being the most common site of initial metastatic involvement. A number of studies have shown that FDG-PET is superior to bone scintigraphy in detecting intramedullary and lytic lesions, but often fails to demonstrate blastic lesions, which are readily detected by bone scintigraphy (Cook et al 1998, Moon et al 1998, Kim et al 2001, Even-Sapir et al 2004, Isasi et al 2005, Nakai et al 2005, Tatsumi et al 2006).
Recently, a study of FDG-PET/CT evaluating asymptomatic breast cancer patients with rising tumour markers demonstrated 90% sensitivity for diagnosing recurrent tumour, affecting the clinical management in 51% of patients (Radan et al 2006). A number of studies have demonstrated the accuracy of FDG-PET in depicting response to treatment (Wahl et al 1993, Schelling et al 2000, Smith et al 2000, Chen et al 2004, Mankoff and Eubank 2006, Rousseau et al 2006). More recently, a decline in FDG uptake of more than 50% indicated a good response to treatment in the metastatic setting (Gennari et al 2000). One study demonstrated a change in FDG uptake after one cycle of chemotherapy, and an absence of FDG uptake following completion of treatment predicted a better survival than in those patients with residual FDG-positive disease (Cachin et al 2006).
Image-Guided Biopsy
Needle core biopsy
Automated core biopsy (14 G, 22 mm) provides significantly better sensitivity, specificity and positive predictive value than FNAC (Teh et al 1998, Britton 1999, Britton and McCann 1999, Vargas et al 2000). Most units in the NHSBSP now perform automated core biopsy as the primary diagnostic tool. Ultrasound provides real-time visualization of the biopsy procedure, and confirmation of sampling. Between 80% and 90% of breast abnormalities can be visualized on ultrasound, with the rest being performed under stereotactic X-ray guidance. Needle core biopsy is highly accurate in determining the nature of most breast lesions, and women with benign lesions can avoid unnecessary surgery. For those with breast cancer, needle biopsy provides an accurate diagnosis enabling physicians to make informed management decisions. Needle biopsy provides histology and tumour grade. A non-operative diagnosis should be possible in more than 90% of invasive cancers and 85% of non-invasive cancers.
Vacuum-assisted mammotome
When there is diagnostic uncertainty, 8 G vacuum-assisted mammotome can be used to obtain larger tissue volumes (approximately 300 mg/core) (Heywang-Kobrunner et al 1998, Brem et al 2001, Parker et al 2001). This is very successful for improving the diagnostic accuracy of borderline breast lesions and at sites where it is difficult to biopsy using other techniques. Indications for use include: small mass lesions, architectural distortions, failed needle biopsy, small clusters of microcalcifications, excision of small benign lesions and diffuse non-specific lesions. Two other types of vacuum-assisted core biopsy systems include minimally invasive breast biopsy and automated tissue excision and collection (an MRI-guided vacuum-assisted breast biopsy system).
Advanced breast biopsy instrumentation
Advanced breast biopsy instrumentation (ABBI) is used for surgical stereotactic biopsy of non-palpable breast lesions under radiographic guidance. This biopsy was developed for both diagnosis and therapy. ABBI requires an incision of 5–20 mm, is restricted to use on a prone table and requires the insertion of a localizing T-wire. This vacuum-assisted biopsy obtains a large cylinder of tissue from the subcutaneous tissue down to and beyond the lesion with an electrocautery snare. ABBI removes a larger volume of tissue, theoretically increasing the diagnostic accuracy of the biopsy. Failure rates of up to 31.5% have been reported due to poor patient selection, technical problems with the T bar and failure to remove tissue. Complication rates of ABBI procedures requiring intervention are significantly higher than those of core biopsy (1.1% and 0.2%, respectively). Complete removal of small lesions does occur and positive margins of 19–100% have been reported (Leibman et al 1999, Matthews and Williams 1999). ABBI is more expensive than any other percutaneous needle biopsy technique.
Treatment Planning and Patient Communication
A preoperative search for occult metastases by bone scan and liver ultrasound does not yield useful information in patients with operable primary breast cancer (Bishop et al 1979). Preoperative chest X-ray and relevant blood tests (full blood count, liver function tests and routine biochemistry) are agreed by local protocol.
Surgery and Radiotherapy
Surgery
The breast
The aim of breast cancer surgery is to achieve long-term local disease control with the minimum of morbidity. The majority of women diagnosed with breast cancer will have small breast cancers, suitable for BCS. The aims of BCS are to produce an acceptable cosmetic appearance and lower psychological morbidity (improved body image, sexuality and esteem) compared with mastectomy. Two studies have shown equivalence in terms of outcome for BCS compared with mastectomy (Anonymous 1995, Morris et al 1997). Meta-analysis of five trials comparing BCS with mastectomy involving 3006 women found no significant difference in the risk of death at 10 years (Anonymous 1995). It is important to ensure that only appropriate patients are selected for BCS, with the aim of minimizing local recurrence whilst achieving a good cosmetic outcome. Single cancers less than 4 cm in size can usually be managed by BCS. Many units have a 3-cm cut-off and lying more than 2 cm from the nipple–areolar complex for considering BCS, but it is the balance between tumour size and breast volume which determines whether or not a patient is suitable for BCS. For patients with large tumours, neoadjuvant chemotherapy can be considered.
Patients with multiple tumours in the same breast should not be considered for BCS as they have a high incidence of recurrence so are best treated with mastectomy with or without reconstruction (Fisher et al 1986, Kurtz et al 1990). Patients with two tumours in close proximity can be candidates for BCS provided that all disease is excised with clear margins. The aim of wide local excision is to remove all invasive and in-situ carcinoma with a 1-cm macroscopic margin of normal surrounding tissue. Incomplete excision rates should be approximately 10–25% and the most common problem following surgery is poor cosmetic result.
Women having more than 10–20% of breast volume removed are likely to have a poor cosmetic result (Cochrane et al 2003). Factors influencing local recurrence include young age (<40 years), tumour grade, presence of an extensive in-situ component and lymphovascular invasion (Holland et al 1990a, Anonymous 1991). More than 80% of local recurrences will occur at the site of previous cancer. Local recurrence following BCS is usually best treated with mastectomy, although re-excision is possible should the recurrence occur more than 5 years after initial treatment, as these are classified as a second primary cancer rather than recurrent disease. Local recurrence after 5 years is associated with a much better prognosis than recurrence within 5 years. The recently reported UK Standardisation of Breast Radiotherapy Trial (START) has demonstrated low rates of recurrence (3.5% at 5 years) following BCS (Dewar et al 2007). Local recurrence rates for invasive cancer after BCS should be less than 5% at 5 years with a target of less than 3% at 5 years.
The axilla
Management of the axilla is a subject of great debate. Surgical treatment of the axilla is two-fold, firstly in disease control and secondly in staging. Surgical staging of the axilla is by axillary lymph node dissection (ALND) for the majority of women, with data regarding nodal status being the most powerful variable in the prognosis for primary breast cancer. Disease-free interval and overall survival are directly related to the number of axillary nodes that contain metastases (Fisher et al 1984, Carter et al 1989). ALND provides the most accurate qualitative and quantitative assessment of the axilla, with the probability of lymph node involvement related to the size of the primary tumour. In small tumours with a size of approximately 10 mm, the risk of nodal metastases is 10% (Baxter et al 1996, Kollias et al 1999). Recurrence rates of 3–5% at 5 years have been reported following ALND, and it has been suggested that the axillary node recurrence rate should be less than 5% with a target of less than 3% (Fowble et al 1989, Siegel et al 1990, Halverson et al 1993, Forrest et al 1995).
Routine histological examination of dissected axillary lymph nodes may be inadequate in identifying the presence of small metastatic tumour deposits or ‘micrometastases’. This has been described as a small cluster of cells measuring 0.2–2 mm. Studies have shown that it is possible to identify micrometastases in 50% of ‘negative’ lymph node cases using serial axillary sections, immunohistochemistry and polymerase chain reaction to detect mRNA transcripts (Trojani et al 1987, Hainsworth et al 1993, Mann et al 2000). Recent studies have shown that cases initially deemed to be ‘node negative’ but subsequently shown to have micrometastases are associated with a worse outcome (Huvos et al 1971, Fisher et al 1978, Rosen et al 1981, Friedman et al 1988, Anonymous 1990, Clayton and Hopkins 1993, McGuckin et al 1996, Dowlatshahi et al 1997).
Significant morbidity is associated with axillary dissection, including seroma, wound infection, reduced shoulder mobility, motor nerve damage (median pectoral nerve, long thoracic nerve of Bell, thoracodorsal nerve), numbness and paraesthesia (80%), and lymphoedema (Siegel et al 1990, Shaw and Rumball 1990, Hladiuk et al 1992, Lin et al 1993). Lymphoedema incidence has been reported to be between 10% and 25%, with hypertension and BMI being risk factors. A 40% incidence of lymphoedema has been reported in those patients undergoing ALND and radiotherapy to the axilla.
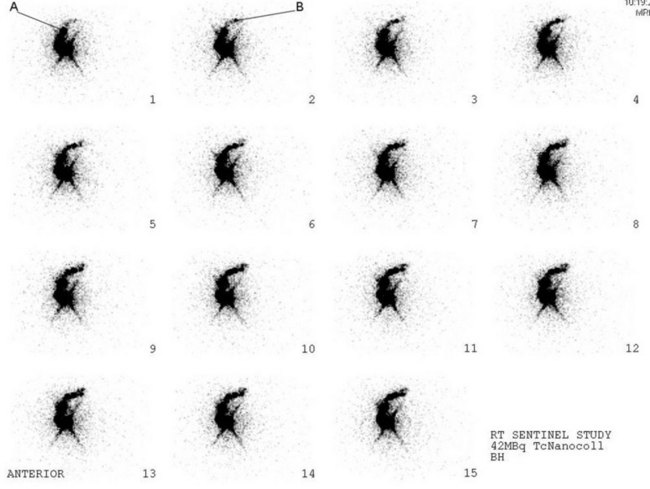
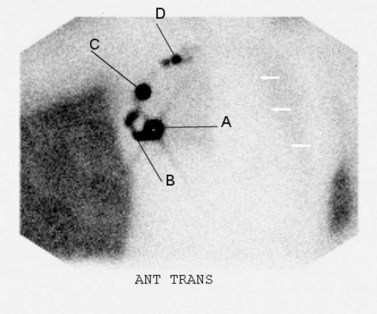
Cabanas first proposed the concept of SNB for penile cancer in 1977, followed by Morton for melanoma in 1992 (Cabanas 1977, Cochran et al 1992, Morton 1997). Krag et al first published the use of SNB in breast cancer in 1993 (Krag et al 1993). SNB is based on the knowledge that for any given tumour-bearing site, there is orderly progression of lymph drainage to a draining lymph node (sentinel node). It relies on the premise that skip metastases do not occur, and that the absence of tumour of the sentinel lymph node implies that the entire lymphatic bed is clear of metastatic disease. The aim of SNB is to provide accurate axillary staging by use of a minimally invasive surgical procedure and significantly reduced morbidity. The sentinel lymph nodes are identified using a radioisotope (Figures 47.8 and 47.9), injected on the day of surgery, and a coloured patent blue dye injected peritumorally or interdermally in the peri- and subareolar region 5–10 min before surgery with the breast gently massaged; this leads to an identification rate of 97% (Albertini et al 1996, Veronesi et al 1997, Cody 1999). Intraoperative identification of the sentinel lymph node is confirmed by a hand-held gamma probe, and visually by blue staining of the node. Risk factors of the procedure include allergic and anaphylactic reactions to the blue dye (Mullan et al 2001). Exposure to clinical staff from the radioisotope is negligible.
One disadvantage of SNB is that women with a positive sentinel node will require a second operation to clear the axilla. Intraoperative frozen section and imprint cytology have been used to assess the status of the sentinel nodes; however, neither procedure has proved acceptable. A small trial evaluating intraoperative reverse-transcriptase polymerase chain reaction (assay time 20–30 min) for epithelial markers mammaglobin and cytokeratin 19 has shown sensitivity of 96%, specificity of 100% and a positive predictive value of 100% for sentinel node detection (Cutress et al 2008). In most studies, the number of sentinel lymph nodes identified ranged from 1.5 to 4.0, and recent studies have shown that, in a small number of cases, the sentinel lymph node was beyond the first two lymph nodes removed (McCarter et al 2001, Zervos et al 2001, Kennedy et al 2003). McCarter et al claimed that at least three nodes are required to identify 99% of node-positive patients, and the false-negative rate is significantly higher (16.5%) when only one lymph node is removed (McCarter et al 2001). Goyal has shown that 99.6% of metastases from node-positive tumours are found within the first four nodes, suggesting that between two and four nodes should be removed for optimum staging of the axilla (Goyal et al 2005).
Multifocality and multicentricity, once thought to be a relative contraindication to SNB, now appears to be feasible and accurate with an acceptably low false-negative rate, and can be used in patients presenting with a clinically negative axilla (Kumar et al 2003, Goyal et al 2004). Currently, there are no randomized controlled trials addressing the feasibility, accuracy or timing of SNB in neoadjuvant chemotherapy. A meta-analysis assessing the reliability of SNB in patients following neoadjuvant chemotherapy reported sensitivity of 88% (Xing et al 2006). Some centres advocate SNB at diagnosis, prior to initiation of neoadjuvant chemotherapy.
The increase in BCS for early breast cancer has resulted in a 10-year risk of ipsilateral recurrent breast cancer of 10–20% (Fisher et al 1989a, van Dongen et al 2000). This has resulted in an increasing population with ipsilateral tumour recurrence with prior axillary surgery, who were traditionally treated with salvage mastectomy and axillary node dissection. However, emerging data indicate that there may be a role for SNB in identifying aberrant lymphatic drainage patterns in recurrent breast cancer (Intra et al 2005, Roumen et al 2006, Taback et al 2006). The incidence of axillary disease in patients with DCIS (6–9%) is greater in a subset of patients with microinvasion, younger patients, DCIS (>4 cm) and those with high-grade disease (Silverstein et al 1991, Cox et al 2000, Pendas et al 2000, Leonard and Swain 2004, Yen et al 2005). Current guidelines from the American Society of Clinical Oncology (ASCO) only recommend SNB in those patients undergoing mastectomy for DCIS, as an incidental invasive component is seen in 20% of these patients (Meyer et al 1999, Lyman et al 2005).
Internal mammary nodes (IMN) have been shown to have prognostic significance (Lacour et al 1983). IMN have been successfully biopsied in 60–85% of cases, with a prevalence of metastases in 5–27% of cases, resulting in upstaging and/or a change in management in 2–8% of patients. Whilst not standard practice, sentinel biopsy of IMN requires further investigation to determine efficacy and safety.
SNB is a new procedure, so long-term follow-up for axillary recurrence will be the ultimate measurement of its success. To date, the rate of axillary recurrence is lower than anticipated given the average false-negative rate of 5–6%. Non-operative staging of the axilla remains elusive. Clinical examination is unreliable, with sensitivity varying from 30% to 75% and specificity approaching 50%. Ultrasound in combination with FNAC can identify 40–45% of patients with involved nodes. PET has shown some promising early results, with sensitivity of 75% and specificity of 90%. Some Italian centres are using PET to assess the need for SNB in low-risk, clinically node-negative patients with a low probability of nodal involvement (Greco et al 2001).
Radiotherapy/radiation therapy
Breast radiotherapy
Multiple randomized trials have shown that radiotherapy substantially reduces the risk of local recurrence following BCS. Adjuvant radiotherapy aims to reduce the risk of locoregional recurrence and improve overall survival. Radiotherapy is not routinely given after mastectomy but tends to be reserved for those with large (>4 cm), high-grade tumours, direct invasion of the skin or pectoral fascia, and extensive nodal involvement who are at high risk of local recurrence. The 1995 Early Breast Cancer Trialists’ Collaborative Group confirmed that locoregional recurrence was reduced by 66% with postmastectomy radiotherapy (Anonymous 1995). This trial confirmed a reduced risk of breast cancer with radiotherapy, but an increased risk of death from other causes (cardiac deaths). Recent randomized trials of women treated with more modern radiotherapy have confirmed that postmastectomy radiotherapy will prevent one death for every 11 women treated, and one locoregional recurrence for every three to five women treated (Ragaz et al 1997, Overgaard et al 1999).
It is important to define the group of patients in whom radiotherapy is required and the group in whom it is not. One recent trial showed no increase in survival on addition of radiotherapy to women over 70 years of age being treated with tamoxifen who were ERα positive with no nodal involvement and tumours less than 2 cm in size (Hughes et al 2004, Bentzen et al 2008).
Hypofractionation radiotherapy aims to deliver an increased dose of radiotherapy over a reduced time frame. The START trial has demonstrated equivalent recurrence rates at 5 years for patients with early-stage breast cancer. The Faster Radiotherapy for Breast Cancer Patients (FAST) trial is currently comparing larger doses (5.7–6 Gy) of radiotherapy given once weekly for 5 weeks with the standard daily 2-Gy treatment (Yarnold et al 2004).
Endocrine Therapy
Breast cancer was the first malignancy for which systemic therapy was developed. The link between hormones and breast cancer growth and development has been recognized for more than a century. In 1896, George Beatson reported that removal of the ovaries from premenopausal women with advanced breast cancer produced a dramatic decrease in tumour size and improved the patient’s prognosis (Beatson 1896). In 1900, Stanley Boyd at Charing Cross Hospital accumulated the national case reports of oophorectomy, and noted that only one-third of patients responded to ovarian ablation and responses lasted, on average, for 1–2 years (Boyd 1900).
Discovery that oestrogenic hormones were produced in the ovary prompted the search for therapeutic antagonists to reduce the incidence of breast cancer in individuals predisposed to the disease by their sensitivity to oestrogenic hormones (Allen and Doisy 1923). Subsequent laboratory, epidemiological and clinical studies have established that oestrogens stimulate the growth of breast cancers (Lacassagne 1936). Oestrogen actions are mediated by two oestrogen receptors, ERα and ERβ, which are members of the nuclear receptor superfamily of transcription factors that regulate the expression of responsive genes upon binding to their cognate ligand (Chawla et al 2001). Whilst the importance of ERβ in breast cancer is at present unresolved, ERα is expressed in the majority of breast cancers and its presence correlates with response to endocrine therapies (Fuqua et al 2003). Thus, current clinical practice involves determination of the ERα status of the malignant cells, followed by adjuvant treatment of ERα-positive cases with endocrine agents that inhibit ERα activity.
Inhibition of ERα activity is achieved with two main strategies: using antioestrogens, primarily tamoxifen, that bind to ERα to inhibit its activity; or by blocking oestrogen synthesis, such that ERα is not activated. Peripheral oestrogen synthesis is the main source of oestrogens in postmenopausal women, and drugs that reduce peripheral oestrogen synthesis, by blocking the activity of the aromatase enzyme, are being used as second- and third-line agents in hormone-sensitive disease once resistance to tamoxifen has developed (Johnston and Dowsett 2003).
Tamoxifen
Tamoxifen, a non-steroidal antioestrogen, is currently the most common form of endocrine therapy for women with ERα-positive breast cancer. Tamoxifen is a selective estrogen receptor modulator (SERM) which has partial agonist activity in some tissues, including bone, lipids and endometrium, but antagonist activity in breast tissue. The antitumour effect of tamoxifen is mediated via competitive inhibition of oestrogen binding to ERs. In women with ERα-positive breast cancer, 5 years of tamoxifen results in a 41% relative risk reduction of recurrence and a 34% relative risk reduction of death (Anonymous 2005). The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 trial and the Scottish Adjuvant Tamoxifen trial demonstrated no benefit in taking tamoxifen for more than 5 years; in contrast, the Eastern Cooperative Oncology Group (ECOG) trial studying indefinite tamoxifen therapy in node-positive patients showed a statistically significant improvement in disease-free survival with extended tamoxifen (Tormey et al 1996, Fisher et al 2001, Stewart et al 2001). The adjuvant Tamoxifen Treatment — Offer More? (ATTOM) and Adjuvant Tamoxifen — Longer against Shorter (ATLAS) trials examining the efficacy of long-term tamoxifen had consistent findings, reporting increased disease-free survival but no overall survival advantage for long-term tamoxifen.
Tamoxifen is generally well tolerated, with the most common side-effects being hot flushes (50% of women), vaginal discharge and irregular menses (Fisher et al 1989b, 1996, Love et al 1991). These side-effects occur more often in pre-/perimenopausal women. The most important side-effect of tamoxifen is an increased risk of endometrial cancer, which equates to 80 extra cases per 10,000 women treated with tamoxifen at 10 years. The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) analysing data from 37,000 women demonstrated a two-fold increase in endometrial cancer in women taking tamoxifen for 1–2 years and a four-fold increase in those taking it for 5 years (Anonymous 1998b). Tamoxifen is also associated with an increase in endometrial polyps, ovarian cysts and endometrial hyperplasia (Kedar et al 1994). The overall reduction of risk in developing breast cancer outweighs the risk of developing endometrial cancer. In postmenopausal women, tamoxifen increases bone mineral density of the axial skeleton; however, in premenopausal women, there may be a decrease in bone density (Love et al 1992, Kristensen et al 1994, Powles et al 1996). Tamoxifen has been shown to reduce total cholesterol and low-density lipoproteins, which explains the reduction in cardiovascular deaths in those taking tamoxifen (Rutqvist and Mattsson 1993, Costantino et al 1997).
Aromatase inhibitors
Aromatase inhibitors (AIs) significantly reduce oestrogen production in postmenopausal women by inhibiting aromatase, a cytochrome P-450 enzyme found in adipose tissue, liver, muscle, the brain and breast cancer tissue (Miller 2003b). In premenopausal women, AIs are ineffective as they produce an increase in gonadotrophin secretion, which results in reduced feedback of oestrogen on the pituitary and hypothalamus. AIs present an alternative to tamoxifen for antagonizing oestrogenic effects on the breast in postmenopausal women. In the 1990s, third-generation AIs were developed which included two subgroups: a steroidal analogue exemethstane, which binds irreversibly to aromatase and is an enzyme inactivator (type 1 inhibitor); and non-steroidal inhibitors that bind reversibly to the haem group of the enzyme (type 2 inhibitor), of which the main agents are anastrozole and letrozole.
Two randomized trials compared initial adjuvant therapy with tamoxifen with an AI. The Arimidex, Tamoxifen Alone or Combination (ATAC) trial showed a significantly improved disease-free survival, time to recurrence and incidence of contralateral breast cancer for arimidex compared with tamoxifen (median follow-up 68 months) (Baum et al 2002, 2003, Howell et al 2005). There were significantly fewer disease recurrences in the arimidex group, but no difference was seen in overall survival. Similar results were seen in the Breast International Group (BIG) 1-98 study comparing letrozole with tamoxifen upfront (Thurlimann et al 2005). In both the ATAC and BIG 1-98 studies, more fractures, fewer thromboembolic events and fewer endometrial cancers were seen in those taking an AI compared with tamoxifen.
A number of trials have looked at sequential endocrine treatment (tamoxifen → AI) including Arimidex, Tamoxifem Alone or together (ATAC), Mayo Clinic-17 (MA-17), the Intergroup Exemethstane Study (IES), Arimidex-Nolvadex-95 (ARNO-95) and Austrian Breast and Colorectal Cancer Study Group (ABCSG) (Goss et al 2003, 2005, Coombes et al 2004, Boccardo et al 2005, Howell et al 2005, Jakesz et al 2005). The IES showed a 24% improvement in disease-free survival in women taking tamoxifen for 2–3 years followed by exemethstane compared with 5 years of tamoxifen (Coombes et al 2004). This trial demonstrated an improvement in overall survival in the sequential group, which was significant in the node-positive patients. These trials clearly demonstrate an improvement in disease-free survival, and ASCO advises that AIs should be part of the adjuvant treatment of early-stage breast cancer in postmenopausal women.
AIs have a different side-effect profile compared with tamoxifen; these include hot flushes, vaginal dryness, musculoskeletal pain and headache. AIs are not associated with an increase in thromboembolic disease or endometrial carcinoma, but are associated with an increase in bone fractures and osteoporosis (BIG 1-98 and ATAC trials). Bone monitoring, using dual energy X-ray absorptiometry scanning, should be available for patients taking AIs. All women that start an AI should be advised about calcium and vitamin D supplementation, smoking cessation and the importance of exercise (Hillner et al 2003). It is advised that bone mineral density should be obtained at baseline and monitored annually for all women taking an AI. The Zometa/Femara Adjuvant Synergy trial suggests that treatment with a bisphosphonate at initiation of an AI may prevent a decline in bone health (Brufsky et al 2005).
Faslodex (Fulvestrant or ICI 182,780)
Faslodex is a pure steroidal antioestrogen that binds with 100 times greater affinity to ERs than tamoxifen (Wakeling and Bowler 1992, Rajah et al 1996). It is licensed for use in postmenopausal women with hormone-receptor-positive advanced breast cancer failing on prior antioestrogen therapy. It has a distinct mechanism of action, differing from that of tamoxifen or the AIs, which may help to delay the development of resistance.
Ovarian ablation
There are many methods for suppressing ovarian function in premenopausal women, including surgical oophorectomy, radiation-induced ovarian ablation and gonadotrophin-releasing hormone (GnRH) agonists. GnRH agonists are increasingly being used to achieve ovarian suppression. They overstimulate and subsequently downregulate GnRH receptors. Their effect is to produce an initial rise in luteinizing hormone and follicle-stimulating hormone in the first 7–10 days of treatment, followed by a decrease after 14–21 days that leads to postmenopausal oestrogen and progesterone levels (Williams et al 1986, Cockshott 2000). After 7.3 years of follow-up, the Zoladex Early Breast Cancer Research Association (ZEBRA), randomizing 1640 lymph node-negative, premenopausal ER-positive women, demonstrated no difference in disease-free survival, overall survival or quality of life between the GnRH agonist goserelin (Zoladex) and cyclophosphamide, methotrexate and 5-fluorouracil (CMF) chemotherapy (Jonat et al 2002, Kaufmann et al 2003). A number of trials are investigating the role of GnRH agonists in adjuvant treatment of premenopausal, early-stage breast cancer — Suppression of Ovarian Function Trial (SOFT), Tamoxifen and Exemethstane Trial (TEXT), and the Premenopausal Endocrine Responsive Chemotherapy (PERCHE) trial.
Raloxifene
Raloxifene, initially studied as an osteoporotic drug, is a second-generation SERM that has oestrogen antagonist properties on the breast and endometrium, and oestrogenic effects on lipid metabolism, bone and blood clotting. The Multiple Outcomes of Raloxifene Evaluation (MORE) trial confirmed a significant reduction in vertebral fractures in patients taking raloxifene, and as a secondary outcome reduced the incidence of breast cancer in ER-positive patients by 90% but had no effect on ERα-negative patients (Cummings et al 2002). The prospective, randomized controlled Study of Tamoxifen and Raloxifene (STAR) showed no significant difference in the incidence of invasive breast cancer in either group after 3.2 years; however, fewer uterine cancers were reported in the raloxifene group (not statistically significant) (Vogel 2009).
Targeted Therapies
Targeting the epidermal growth factor receptor
The human EGFR-2 (HER-2)/neu (c-erbB-2) gene is localized to chromosome 17q and encodes a family of four transmembrane tyrosine kinase receptor proteins which are members of the EGFR or HER family (Ross et al 2003). These receptor tyrosine kinases provide a binding site for various ligands or proteins, which in turn activate downstream signalling pathways which are essential for cell proliferation and survival. Overexpression of the HER-2 protein occurs in 20–25% of breast cancers and is associated with a worse prognosis (disease-free survival and overall survival) (King et al 1985a, Slamon et al 1987, 1989). The most commonly used test for determining HER-2 protein overexpression is an immunohistochemical (IHC) assay scored from 0 to +3, with 0/+1 representing negative, +2 weakly positive and +3 positive. HER-2 can be measured quantitatively by fluorescence in-situ hybridization (FISH), which provides a direct measure of gene amplification (Perez et al 2002, Hicks and Tubbs 2005). FISH is currently the gold standard for evaluating HER-2 overexpression, and provides the best correlation with clinical response to trastuzumab. IHC3+ correlates well with FISH positivity, but only 20–24% of IHC2+ tumours are also FISH positive (Owens et al 2004).
A new method, chromogenic in-situ hybridization (CISH), has been approved by the US Food and Drug Administration for determining HER-2 status, as an alternative to FISH. CISH has several advantages over FISH in that it produces cheaper and permanent staining, allowing samples to be archived indefinitely (FISH signals are labile and fade over time). CISH is based on bright field microscopy and does not require an expensive fluorescence microscope with multiband pass filters. It allows concurrent analysis of morphological features of the tumours/cells and gene copy numbers, making the identification of components of interest easier. Tumour heterogeneity can be readily identified at low magnification (Tanner et al 2000, Zhao et al 2002, Arnould et al 2003).
Trastuzumab (Herceptin), a monoclonal antibody that targets HER-2, achieves tumour regression in some patients with HER-2-positive metastatic breast cancer (mBC). Patients with mBC taking trastuzumab in combination with chemotherapy have a longer time to progression (7.4 vs 4.6 months), a higher objective response rate (50% vs 32%), and a longer survival (25.2% vs 20.3%) than chemotherapy alone (Slamon et al 2001). A number of trials have been conducted investigating the role of trastuzumab in the adjuvant setting for HER-2-positive breast cancer. The Herceptin Adjuvant (HERA) trial compared 1–2 years of adjuvant trastuzumab with observation in HER-2-positive women who had completed adjuvant or neoadjuvant chemotherapy, surgery and/or radiotherapy (Piccart-Gebhart et al 2005). Analysis revealed a significant improvement in disease-free survival and overall survival in those taking trastuzumab, and this has become the standard of care for patients with HER-2-positive breast cancer.
Clinical benefit and response rates depend on the intensity of HER-2 overexpression (2+ or 3+), with response rates of 35% seen in grade 3+ but only minimal benefit seen in grade 2+ (Vogel et al 2002). Trastuzumab is well tolerated with side-effects including hypersensitivity and cardiotoxicity; the incidence of class III/IV congestive heart failure ranges from 0.4% to 3.8% (Adamo et al 2007, Anonymous 2008, Perez 2008).
Pertuzumab is a monoclonal antibody that prevents the formation of heterodimers between HER-2 and other members of the HER family (Nahta et al 2004). Pertuzumab localizes to a different domain of the HER-2 protein than trastuzumab. A synergistic interaction between pertuzumab and trastuzumab is being explored.
Dual tyrosine kinase inhibition
Lapatinib is active in refractory mBC patients and as a first-line metastatic treatment, with potential benefit in patients with brain metastases. Efficacy of lapatinib has been demonstrated in combination with capecitabine in patients with refractory erbB-2-overexpressing breast cancer (Geyer et al 2006). Lapatinib appears to have either very low or no incidence of cardiotoxicity. The most frequently reported adverse events include nausea, fatigue, itching, rash, diarrhoea, acne and dry skin. There are a number of ongoing phase III trials evaluating the role of lapatinib in the adjuvant setting, including the Tykerb Evaluation After Chemotherapy (TEACH) trial (Moy and Goss 2006).
Targeting the vascular endothelial growth factor pathway
Metastasis of all solid tumours requires angiogenesis (formation of new blood vessels). A number of agents have been developed that target the vascular endothelial growth factor (VEGF) pathway. Breast cancers that overexpress VEGF have a worse disease-free survival and overall survival. Bevacizumab (Avastin) is a human monoclonal antibody that recognizes all isoforms of VEGF-A. In the metastatic setting, bevacizumab has been shown to significantly increase response rates and progression-free survival when given in combination with paclitaxel (E2100 and Avastin and Docetaxel (AVADO) trials) (Miller 2003a, Miles et al 2008, Baar et al 2009). The Ribbon 1 and Ribbon 2 studies are currently investigating the combination of bevacizumab with a number of standard chemotherapy regimens for mBC.
Other targeted therapies
Other targeted therapies currently being evaluated include sunitinib (SU11248), an oral tyrosine kinase inhibitor which blocks a number of signalling pathways including VEGF receptor, platelet-derived growth factor receptor, kit and flt-3 (Miller and Burstein 2005). Rapamycin is an antibiotic that has demonstrated antitumour activity through cell cycle arrest resulting from inhibition of mammalian target of rapamycin (mTOR). Temsirolismus (CCI-779), an mTOR inhibitor, inhibited the proliferation of breast cancer cell lines in preclinical studies but has not entered routine clinical use (Yu et al 2001, Campbell et al 2004). Overexpression of insulin-like growth factor-1 receptor (IGF-1R) is associated with a poor prognosis and resistance to a number of therapies, including hormonal therapy and trastuzumab (Nahta et al 2005). A number of therapies are targeting IGF-1R, including the small molecule tyrosine kinase inhibitor (BMS-536924; Bristol-Myers Squibb) and monoclonal antibodies (CP-751,871; Pfizer).
Adjuvant Chemotherapy
Combination chemotherapy has a more prolonged benefit than single-agent chemotherapy, and is usually given in six cycles over a period of 4–6 months. The first adjuvant chemotherapy for breast cancer (CMF) was developed in the late 1970s, and was the mainstay of treatment for many years, significantly reducing risk of recurrence and death (Bonadonna et al 1976, 1985, Anonymous 1998a). CMF had a significant effect regardless of ER status, tamoxifen use and nodal status, but was influenced by age and menopausal status. Recent meta-analyses have confirmed the benefit of combination chemotherapy over monochemotherapy, with a greater benefit seen in younger patients (Anonymous 2005). For women under 50 years of age, chemotherapy significantly improved 10-year survival by over 10% for those with node-positive disease (53% vs 42%) and by 6% for those with node-negative disease (78% vs 71%). The National Cancer Institute of Canada MA.5 study confirmed the superiority of anthracyclines [six cycles of 5-fluorouracil, epirubicin, cyclophosphamide (FEC)] over CMF, and now anthracycline-based chemotherapy regimens are the standard of care (Levine et al 1998). The potential importance of taxanes (either paclitaxel or docetaxel) as adjuvant chemotherapy is emphasized by a large number of trials (Cancer and Leukemia Group (CALGB) 9344, National Surgical Adjuvant Breast and Bowel Project (NSABP) B-28, Breast Cancer International Research Group (BCIRG) and PACS01) (Henderson et al 2003, Mamounas et al 2003, Martin et al 2003). This body of evidence now supports the use of taxanes in high-risk women in the adjuvant setting (Goldhirsch et al 2005).
Neoadjuvant Medical Therapy
Neoadjuvant endocrine therapy
The use of primary tamoxifen in older women as an alternative to surgery has demonstrated short-term tumour regression but unsatisfactory long-term local control (Cheung et al 2000). Higher response rates have been seen in older women treated with neoadjuvant anastrozole, letrozole and exemethstane compared with tamoxifen. Letrozole is currently the only AI licensed for use in the neoadjuvant setting.
Neoadjuvant chemotherapy
Neoadjuvant chemotherapy has significant activity in early breast cancer, with overall objective response rates of 70–90%, which is higher than response rates seen in patients with metastatic disease. Complete remission rates of approximately 20% are seen in patients receiving neoadjuvant chemotherapy (Smith and Lipton 2001).
Triple-Negative Breast Cancers
These are defined as the 10–15% of breast cancers (an increasing percentage of which occur in premenopausal African and African-American women) which do not express the ER, progesterone receptor (PgR) or erb-B2 receptor (Slamon et al 1989, Konecny et al 2003, Carey et al 2006, Dawood et al 2009). This disease is resistant to existing targeted treatments and hormonal therapies, and associated with a high risk of local and systemic relapse (van de Rijn et al 2002, Abd El-Rehim et al 2004, 2005, Foulkes et al 2004, Nielsen et al 2004). These tumours are often poorly differentiated, the majority of which are in the basal group of breast cancers. This basal group stains positively for the basal cell cytokeratins 5/6 and 17, has an increased proliferative rate, develops central necrosis, and does not stain for ERα, PgR or HER-2 receptors (Livasy et al 2006).
Limited clinical data suggest that these cancers are chemosensitive, although it is unclear which regimen provides the best response rates. Triple-negative breast cancers often overexpress EGFR, and as a result may be targeted by EGFR-directed antibodies such as cetuximab or inhibitors of receptor phosphorylation such as gefitinib and erlotinib (Mendelsohn and Baselga 2006). The efficacy of cetuximab alone or in combination with carboplatin is being investigated in the metastatic setting. Other therapies such as imatinib, lapatinib, dasatinib, poly (ADP-ribose) polymerase inhibitors, survivin inhibitors and pertuzumab are being evaluated in the treatment of this subgroup of breast cancers (Agus et al 2005, Burris et al 2005, Modi et al 2005).
Prognostic Factors
Adjuvant systemic therapy is an important component of breast cancer treatment, aiming to extend disease-free survival and overall survival. When considering which patients are suitable for treatment, systemic risk is assessed based on a number of prognostic factors (Box 47.1).
Box 47.1 Prognostic factors in breast cancer
Axillary lymph node status
Involvement of local and regional lymph nodes is one of the most important prognostic factors in breast cancer (Galea et al 1992). The 10-year survival reduces from 75% to 25–30% in women with nodal involvement compared with women with no nodal involvement, and the greater the number of nodes, the worse the prognosis (Neville et al 1992). Metastatic involvement to a higher axillary level, particularly those in the apex, carries a worse prognosis (Bedwani et al 1981, Rosen and Groshen 1990).
Tumour size
Smaller tumours (<15 mm) have been shown to have a ‘better prognosis’ than larger tumours (>15 mm), with a risk of axillary lymph nodal involvement of 40% compared with 12–20%, respectively (Carter et al 1989, Henson et al 1991, O’Dwyer 1991, Galea et al 1992, Neville et al 1992).
Differentiation
Juan Hassiman was the first to suggest a correlation between the microscopic appearance of tumours and their degree of malignancy in the 19th century (Carstens et al 1985). Certain types of invasive breast cancer (tubular, mucinous, cribriform, medullary and lobular) carry a more favourable prognosis than invasive cancer of no special type (Carstens et al 1985, Eichhorn 2004, Sullivan et al 2005, Gatti et al 2006, Tan et al 2008, Contreras and Sattar 2009). Bloom and Richardson (1957) developed a numerical grading system assigned by pathologists to invasive breast cancers. It is the most common type of cancer grading system currently used, based on three morphological features of invasive breast cancers: (1) the degree of tumour tubule formation (percentage cancer composed of tubular structures); (2) the mitotic activity of the tumour (rate of cell division); and (3) the nuclear pleomorphism of tumour cells (nuclear grade, change in cell size and uniformity). Each of these features is assigned a score ranging from 1 to 3. The scores are then added together for a final score ranging from 3 to 9. This value is then used to grade the tumour as follows: 3–5, grade 1 tumour (well differentiated); 6–7, grade 2 tumour (moderately differentiated); and 8–9, grade 3 tumour (poorly differentiated).
Lymphovascular invasion
The presence of lymphovascular invasion closely correlates with local and regional lymph node involvement (Ejlertsen et al 2009).
ER /PgR status
A response to endocrine therapy is seen in 50–60% of patients with ERα-positive tumours, compared with a minimal response seen in ERα-negative patients (Horwitz and McGuire 1975, Hunt 2001). In patients with ERα-/PgR-positive tumours, the response rate increases to 78%.
Histopathology of BRCA1 mutations
Cancers associated with BRCA1 mutations have a significantly higher mitotic rate, are less likely to be ERα-/PgR-positive, more aneuploid and have greater lymphocytic infiltration than sporadic cancers (Marcus et al 1996, Anonymous 1997b, Karp et al 1997, Armes et al 1998, Robson et al 1998).
Expression of growth factor receptors
Expression of EGFR is inversely correlated with ERα positivity, and therefore may be associated with endocrine resistance (Nicholson et al 1994). Slamon et al (1987) showed that HER-2 gene amplification independently predicted poorer overall survival and disease-free survival in a multivariate analysis in node-positive patients. Larger tumour size and higher grade (poor prognostic factors) have been also correlated with HER-2 overexpression (Rilke et al 1991, Pietras et al 1995).
NPI = pathological tumour size (cm) × 0.2 + lymph node stage (1,2,3) + histological grade (1,2,3)
Genomics and Proteomics
Genomics (study of the human genome) and proteomics (analysis of the protein component of the gene) are two branches of molecular biology that will have a major role in understanding, diagnosing and potentially providing therapeutic targets in breast cancer. Gene expression cDNA arrays in the landmark study by Perou et al identified five distinct gene expression patterns — two ERα-positive groups referred to as luminal subtypes (A and B) as they share features with luminal epithelial cells arising from the inner layer of the duct lining, another group that overexpresses HER-2, and a normal breast gene expression group characterized by elevated expression of basal epithelial cell genes and reduced expression of basal epithelial cell genes (Perou et al 2000). The final group was a basal-like subgroup which shares features with normal basal epithelial cells (ERα, PgR and HER-2 negative). cDNA arrays have also distinguished cancers associated with BRCA1/BRCA2 mutations, ERα expression and prognostic subgroups in node-negative breast cancer. In the future, it is envisaged that gene expression profiles may guide decisions on the choice of adjuvant therapy for individual patients. Proteomics has already assumed a role in the monitoring of response and prediction of both resistance and relapse in patients treated with novel targeted therapies (van de Vijver et al 2002, McClelland and Gullick 2003).
Micrometastases in Breast Cancer
Disseminated tumour cells (DTCs) in the bone marrow of breast cancer patients are an independent predictor of poor prognosis. A number of studies have detected DTCs in 13–42% of patients without overt distant metastases (stage M0) (Schlimok et al 1987, Cote et al 1991, Harbeck et al 1994, Diel et al 1996, Molino et al 1997, Mansi et al 1999, Braun et al 2000b, Gebauer et al 2001, Gerber et al 2001, Wiedswang et al 2003, Braun et al 2005). The prognostic relevance of detecting DTCs has an impact on breast-cancer-specific survival, disease-free survival, distant disease-free survival and overall survival. In one multivariate analysis, bone marrow status was the most important independent factor for disease-free and overall survival. Several studies have shown that the presence of DTCs in bone marrow following adjuvant therapy is a predictor of poor prognosis (Braun et al 2000a, Wiedswang et al 2004, Janni et al 2005). Bone marrow analysis of high-risk patients (more than three involved axillary nodes), before and after receiving adjuvant taxane or anthracycline therapy, demonstrated that the presence of tumour cells was associated with an extremely poor prognosis and a heterogeneous response to treatment (Braun et al 2000a).
Circulating tumour cells (CTCs) are defined as micrometastases detected in peripheral blood. CTCs have been detected in 10–60% of primary breast cancer patients with no signs of overt metastases, depending on the detection technique used (Kraeft et al 2000, Witzig et al 2002). Several studies have used CTCs as a tool to assist breast cancer diagnosis (Reinholz et al 2005, Chen et al 2006). The majority of studies reporting on the prognostic significance of CTCs in patients with mBC use the Cell-Search (Veridex LLC) system. The presence of more than five CTCs per 7.5 ml of whole blood in patients with mBC before a new treatment was an independent predictor of overall survival and progression-free survival (Cristofanilli et al 2004). The presence of CTCs was also shown to be a better surrogate endpoint than current radiological imaging for assessing response to treatment (Budd et al 2006). The Southwest Oncology Group has launched a phase III trial (NCT00382018) evaluating a change in chemotherapy regimen in those mBC patients with elevated CTCs following the first follow-up assessment.
Gene Expression Signatures
Gene expression signatures as predictors of recurrence
In the top-down approach, a gene expression signature is derived by seeking profiles that are correlated with clinical outcome without a previous biological assumption (Loi et al 2005).
The 70-gene prognostic signature (MammaPrint)
The Microarray In Node-negative Disease may Avoid Chemotherapy Trial (MINDACT) (EORTC 10041/BIG 03/04) is evaluating the gene expression signature MammaPrint. This trial is setting out to see whether it may be possible to distinguish between patients with a high risk of developing metastatic disease and patients who could be spared chemotherapy as their distant metastases risk is low so adjuvant chemotherapy would offer minimal benefit (Cardoso et al 2008). The trial is enrolling node-negative breast cancer patients who will have their risk assessed through both traditional clinicopathological factors (using Adjuvant Online; http://www.cancerscreening.nhs.uk/breastscreen/index.html) and MammaPrint. If the main hypothesis of the MINDACT is validated, it will be the first study to provide level I evidence for a decrease in the indications of adjuvant chemotherapy.
Oncotype DX
Several signatures have been generated to predict prognosis in patients treated with endocrine therapy. Paik et al, in collaboration with Genomic Health Inc., developed a recurrence score based on 21 genes that appeared to accurately predict the likelihood of distant recurrence in tamoxifen-treated patients with node-negative, ER-positive breast cancer (Paik et al 2004). The process of clinical validation to achieve level I evidence is ongoing for Oncotype DX in the clinical trial TAILORx [Trial Assigning IndividuaLized Options for Treatment (Rx)]. This trial will evaluate whether women with node-negative, ERα-positive breast cancer need chemotherapy based on their recurrence score. The US Intergroup launched the trial in May 2006 and is expected to accrue over 10,000 patients, of which 29% will be low risk, 44% intermediate risk and 27% high risk.
Wound-response signature
Chang et al derived a wound-response signature with prognostic potential by examining the processes involved in wound healing and then drawing similarities with the oncogenic process (Chang et al 2004).
Invasiveness gene signature
Lui et al generated a 186-gene ‘invasiveness’ signature (IGS) by using gene expression profiling of tumorigenic breast cancer cells compared with normal breast epithelium (Liu et al 2007). The IGS is significantly associated with overall and metastases-free survival independent of established pathological and clinical variables.
Metastatic Breast Cancer
Although survival from breast cancer has been improving, approximately 40% of women with early-stage breast cancer will develop metastatic disease. Median survival time following relapse is 18–24 months, but many live for several years and a few for a decade or more. The treatment of mBC patients is to prolong life, maintain a good quality of life and relieve symptoms. The goals for treatment are dependent upon a number of variables including patient-related factors such as comorbidities and age, and tumour related factors including hormone receptor status, tumour grade and site of metastasis. The recommended radiological tests routinely employed in the staging work-up for mBC include bone scintigraphy and contrast-enhanced CT of the chest, abdomen and pelvis (Figures 47.10 and 47.11). Treatment options include endocrine therapy with tamoxifen, AI, GnRH analogues and chemotherapy. Approximately 50% of patients achieve tumour regression with duration of less than 1 year.
Chemotherapy in metastatic breast cancer
Studies have shown that 24–30% of women with node-negative breast cancer and 50–60% of those with node-positive disease at diagnosis will relapse (Honig 1996). Approximately 6–10% of patients will present with de-novo metastatic disease at diagnosis (Honig 1996, Cardoso et al 2002). The median survival for women presenting with de-novo mBC is 2–4 years, although an increased survival is seen in a small percentage of patients (Falkson et al 1995, Greenberg et al 1996, Honig 1996). Despite the many new options for the treatment of mBC, mortality rates have only shown a small decline from 1930 to 1998 (1.6% annually from 1989 to 1995, and 3.4% more recently) (Jemal et al 2002).
The chemotherapy regimen selected will depend on previous adjuvant therapies received, and is chosen not just on the basis of efficacy, but also with the aim of minimizing toxicity. Anthracycline-based chemotherapy is well established as the first-line option for women with mBC, when this has not been used previously in the adjuvant setting. Both paclitaxel and docetaxel have been evaluated in mBC after anthracycline use. Recent meta-analysis has shown a significantly increased overall survival, time to progression and response rates in taxane-containing chemotherapy regimens compared with non-taxane-containing regimens. Combination of the orally bioavailable prodrug capecitabine with docetaxel has shown greater response rates, time to disease progression and overall survival compared with docetaxel alone. Other agents include the semisynthetic vinca alkaloid vinorelbine, gemcitabine and the platinum salts cisplatin and carboplatin. There has been some evidence to suggest that sequential single agents may be equally effective in terms of survival, with reduced toxicity compared with combination chemotherapy. There appears to be little survival benefit in giving chemotherapy for more than 6 months. There is an underlying assumption that improvements in overall response rates translate into long-term survival benefit (Greenberg et al 1996, Fossati et al 1998, Pierga et al 2001, Bruzzi et al 2005).
Trastuzumab in metastatic breast cancer
Trastuzumab is effective in those women with mBC overexpressing HER-2 (only IHC3+ or FISH-positive patients benefit). Slamon et al (2001) were the first to show that addition of trastuzumab to chemotherapy in patients with HER-2-positive mBC was associated with a longer time to progression (7.4 months vs 4.6 months), higher response rate (50% vs 32%) and longer survival (25.1 vs 20.3 months). Combining trastuzumab with docetaxel has shown significantly greater response rates (61% vs 36%) and overall survival (24.1% vs 13.2 months) compared with single-agent docetaxel (Marty et al 2005). The duration of treatment with trastuzumab is unknown; however, this is being addressed by a large randomized study.
Other agents used in metastatic breast cancer
Epithilones are macrolide antibiotics isolated from the myxobacterium Sorangium cellulosum, which promote microtubule stabilization via a similar mechanism to that of the taxanes (Bollag et al 1995, Wartmann and Altmann 2002). Epithilones are associated with a lower susceptibility to drug resistance and greater efficacy in taxane-resistant tumours. Recently, the epithilone ixabepilone (Ixempra, Bristol-Myers Squibb) has been approved for use by the US Food and Drug Administration after having shown activity in chemotherapy-sensitive and chemotherapy-resistant tumour models, and synergistic activity with other chemotherapeutic and targeted agents. Randomized trials in patients with bony metastatic disease have shown that bisphosphonates can significantly reduce skeletal-related events, including pathological fractures, spinal cord compression, hypercalcaemia and the need for intervention with radiotherapy by 30–50%. They have also been shown to reduce bony pain, but have not shown any improvement in survival (Paterson et al 1993, van Holten-Verzantvoort et al 1993, Lipton et al 2000, Rosen et al 2001). The dual tyrosine kinase inhibitor lapatinib has shown significant efficacy in refractory metastatic HER-2-positive breast cancer patients (previously treated with trastuzumab) and as a first-line metastatic treatment, with potential benefit in patients with brain metastases.
Preservation of Fertility in Breast Cancer Patients
Breast cancer is the most common cancer affecting women of reproductive age, and approximately 13% of all breast cancer diagnoses are made in women aged under 45 years (Gloeckler Ries et al 2003). Many breast cancer patients may not have completed their family planning, and may wish to have children after their breast cancer treatment. Options for fertility preserving should be discussed before starting treatment in some patients. Standard regimens of radiation therapy for breast cancer do not affect the ovaries significantly; however, internal scatter radiation can reach the pelvis and ovaries. As a result, in-vitro fertilization (IVF) and egg harvesting should not be performed during treatment and pregnancy should be prevented (Arnon et al 2001, Falcone et al 2004, Jeruss and Woodruff 2009). Indirect evidence supports a delay of antioestrogen treatment to allow for pregnancy after surgery and radiotherapy are completed (Gradishar and Hellmund 2002, Goss 2007). As a result, the threat to fertility for a patient not receiving chemotherapy, whose baseline fertility is within the normal range, is small. Breast cancer treatments are well known to affect menstrual status and fertility among premenopausal women diagnosed with breast cancer (Lobo 2005). Amenorrhoea following systemic breast cancer treatment may result in loss of fertility, onset of menopausal symptoms, problems with sexual functioning, and exposure to the long-term health risks of an early menopause.
Risk of chemotherapy-related amenorrhoea and menopause
The risk of chemotherapy-related amenorrhoea depends on the patient’s age, the type of chemotherapeutic agents used and the total dose administered. Older women have a higher incidence of complete ovarian failure and permanent infertility compared with younger women (Minton and Munster 2002, Maltaris et al 2007). This higher incidence is explained by the greater primordial follicle reserve (ovarian reserve) in younger women, which decreases with age. The degree of damage will determine whether the amenorrhoea is permanent or temporary; should no oocytes remain viable, menses may cease altogether and menopause will follow. Menstrual function may resume months or occasionally years after treatment; however, the majority of women who remain amenorrhoeic 1 year after treatment will not regain ovarian function. Assessment of the ovarian reserve in patients treated with chemotherapy includes serum measurements of anti-Müllerian hormone, follicle-stimulating hormone, inhibin B and oestrogen levels (Lutchman Singh et al 2007, van Beek et al 2007). An ultrasound-guided antral follicle count can also be useful for assessing ovarian reserve (Lutchman Singh et al 2007).
Chemotherapy can cause ovarian damage by a number of mechanisms. Dividing cells are vulnerable to most cytotoxic agents; however, alkylating agents such as cyclophosphamide present the greatest potential for ovarian failure among all chemotherapeutic agents (Meirow 1999). Alkylating agents are not cell cycle specific and may affect cells not actively dividing, including the pregranulosa cells of the primordial follicles and oocytes. The higher the cumulative dose of cyclophosamide, the greater the observed incidence of menopause. It has been reported that with the classic CMF regimen, the incidence of amenorrhoea was 61% in women under 40 years of age and 95% in women over 40 years of age (Goldhirsch et al 1990). The regimen of six cycles of FEC induces menopause in 60% of patients (Venturini et al 2005). A prospective cohort study evaluating 595 women aged 25–40 years treated for early breast cancer with a number of different chemotherapy regimens showed that menstrual cycles were more likely to persist in women treated with regimens containing less cumulative cyclophosamide (Petrek et al 2006). Those patients on a CMF regimen were more likely than women on other regimens to bleed within the first month of chemotherapy, but were less likely to commence menses 1 year later. The study also showed that tamoxifen accounted for a 15% decrease in menstrual cycling at 1 and 2 years, regardless of chemotherapy regimen. Most premenopausal women will continue to menstruate whilst taking tamoxifen, although their menses may become irregular. One study has shown no effect of trastuzumab on fertility (Abusief et al 2006).
Strategies to preserve fertility in women with breast cancer
The ASCO and UK National Health Service guidelines recommend that the implications of oncological treatment for fertility should be discussed with patients. If sufficient time exists before a woman is about to start chemotherapy or radiotherapy, she may undergo IVF to cryopreserve embryos or an embryo (Falcone et al 2004, Roberts and Oktay 2005, Agarwal and Chang 2007, West et al 2007). This technique requires a delay in cancer treatment for up to 1 month, which may not be an option for some patients. Cryopreservation remains the most effective approach to preserve fertility; however, post-thaw survival rates of embryos are in the range of 35–90%, and implantation rates are between 8% and 30%. If multiple embryos are available, cumulative pregnancy rates can be more than 60% (Seli and Tangir 2005). Delivery rates per embryo transfer using cryopreserved embryos are in the range of 18–20%. This approach requires IVF and a male partner.
Oocyte banking is more difficult than embryo cryopreservation, as oocyte cooling and exposure to cryopreservation agents affects the cytoskeleton and causes hardening of the zona pellucida. The success is dependent upon the number of eggs harvested (<10 oocytes = very low chance of pregnancy). The overall livebirth rate per preserved oocyte is approximately 3%, which is much lower than that for IVF using fresh oocytes (Gosden 2005). Several studies are evaluating the use of GnRH analogues to preserve ovarian function during cytotoxic treatment. Research in a population of older women of reproductive age has suggested that addition of a GnRH analogue to treatment may increase a woman’s likelihood of remaining premenopausal after chemotherapy; however, the reproductive outcome was poor (Fox et al 2003).
It has been suggested that IVF could accelerate the disease process in women with ERα-positive breast cancer by increasing the oestrogen concentration. In one study of 100 patients, ovarian stimulation with concurrent administration of an AI or tamoxifen demonstrated no increase in recurrence and no effect on the IVF cycle (Oktay et al 2005, Azim et al 2008). Another technique whereby immature oocytes are collected and matured in vitro, avoiding ovarian stimulation, has been used in women with polycystic ovary syndrome (Lee 2007, Reinblatt and Buckett 2008). Oocyte donation is a well-established form of assisted reproduction, with 20% of replaced embryos resulting in a live birth. The harvesting of ovarian tissue is less established; this involves the removal of the ovarian cortex by laparoscopy, which is cryopreserved for later reimplantation. Only small numbers of women have had thawed ovarian tissue reimplanted, and only five live births have resulted to date (Donnez et al 2004, Meirow et al 2005, Demeestere et al 2007, Andersen et al 2008). The incidence of occult ovarian cancer in BRCA1/BRCA2 carriers is low, but the potential for development of ovarian cancer in the transplanted tissue makes it a poor option in this subgroup (Colgan et al 2001).
Pregnancy-Associated Breast Cancer
Pregnancy-associated breast cancer (PBAC) is defined as breast cancer diagnosed during pregnancy or lactation or 1 year post partum (Petrek 1991). After cervical cancer, breast cancer is the second most common malignancy in pregnant women (Antonelli et al 1996). The diagnosis is often delayed due to physiological and anatomical changes in the breast, and a low index of suspicion of breast cancer in these patients. The average age of patients with PABC is 32–38 years. The estimated incidence of PBAC is 0.2–3.8% of all breast cancers, and it is reported to occur in one in 3000–10,000 pregnancies (Anderson 1979, Gemignani et al 1999). Women are three times more likely to have a family history of breast cancer than age-matched, non-pregnant/non-lactating women (Ishida et al 1992). Carriers of the BRCA1/BRCA2 mutation who have had a full-term pregnancy are more likely to develop breast cancer before 40 years of age than nulliparous women with the BRCA1/BRCA2 mutation (Jernstrom et al 1999).
PABC often presents as a painless mass, although clinical examination of the breast may be increasingly difficult because of increased nodularity (King et al 1985b, Ribeiro et al 1986). During pregnancy, oestrogen, progesterone, prolactin and chorionic gonadotrophin rise, and the breasts undergo marked ductal and lobular proliferation with blood flow increasing by 180% and weight doubling (Scott-Conner and Schorr 1995). A study of 63 women with PABC showed that less than 20% were diagnosed prior to delivery and the median size of the tumour was 3.5 cm. Sixty-two percent of patients were found to have nodal involvement compared with 39% of matched non-pregnant controls (Petrek 1991). PABC is more likely to present with larger tumours, vascular invasion and distant metastases (Guinee et al 1994).
Between 70% and 80% of breast biopsies performed during pregnancy are benign (Woo et al 2003). A dominant mass presenting during pregnancy must be formally investigated by ‘triple assessment’. Mammography during pregnancy is not advised, and ultrasound can identify cystic lesions and help to characterize solid masses (Liberman et al 1994, Ahn et al 2003). Bone scans for metastatic disease are generally not recommended, and MRI can be used to evaluate liver and bony metastases (Baker et al 1987). PABC tends to present with ERα-/PgR-negative tumours, and appears to have a worse 5-year survival in women presenting with nodal involvement compared with non-pregnant matched controls (47% vs 59%) (Petrek 1991, Bonnier et al 1997). There appears to be an increased relative risk of dying from breast cancer if it develops within 4 years of giving birth, compared with age-matched women who have never been pregnant and who develop breast cancer (Duncan et al 1986, Guinee et al 1994).
Chemotherapy is contraindicated in the first trimester because of an increased risk of spontaneous abortion and a teratogenic risk of 14–19%, falling to 1.4% by the second trimester (Ebert et al 1997). Chemotherapy can cross the placenta and, if given up to 15 weeks of gestation, has been shown to interfere with cell differentiation leading to permanent organ malformation. Administration of FEC chemotherapy during the second and third trimesters in a study of 24 women showed no congenital malformations, although chemotherapy should be stopped 3 weeks before delivery (Berry et al 1999, Keleher et al 2002). Women with node-positive PABC are advised to start chemotherapy after the first trimester. Tamoxifen is associated with an increased risk of congenital malformations and spontaneous abortion, and therefore endocrine therapy is not recommended during pregnancy (Isaacs et al 2001).
The use of radiotherapy in women with PABC poses a particular dilemma. During the first trimester, the fetus is outside the radiation field of the chest wall but is still exposed to scatter radiation; however, during the later stages of pregnancy, the fetus lies outside the pelvis and is closer to the radiation field, but will have completed organogenesis and the effects of radiation will be reduced (Saunders 2001). There have been anecdotal reports of fetal malformations as well as normal pregnancy outcomes in pregnant women receiving radiotherapy for breast cancer. Therefore, the teratogenic effects of radiotherapy need to be weighed up against the improvement in disease-free survival.
Breast Reconstruction after Surgery for Breast Cancer
Further surgery may involve nipple–areola reconstruction and, in some cases, surgery to the contralateral breast to achieve symmetry. The process of breast reconstruction requires highly motivated surgical staff and patients, as many stages are involved. Skin-sparing mastectomy has significantly improved aesthetic outcomes with breast reconstruction. It allows mastectomy, with preservation of the breast skin and inframammary fold, with breast tissue excised through small skin incisions (Cunnick and Mokbel 2004). This technique produces excellent cosmetic results, particularly when combined with immediate reconstruction.
Breast reconstruction can be performed immediately at the time of mastectomy, or delayed following adjuvant therapy. Immediate reconstruction is advantageous as it results in reduced cost (single operation and hospital stay), superior cosmetic result (surgeon works with good-quality skin that is unscarred and not suffering from the effects of radiotherapy) and reduced psychological morbidity (Kroll et al 1995, Khoo et al 1998, Al-Ghazal et al 2000). The disadvantages of immediate reconstruction include limited time for patient decision making, increased operative time and the detrimental effect that chemotherapy and radiotherapy can have on some types of reconstruction (Kronowitz and Robb 2004). There are no significant differences in survival between immediate and delayed reconstruction. Delayed reconstruction allows the patient unlimited time for decision making, avoids adjuvant therapy delay and excludes the detrimental effects of adjuvant therapy. The mastectomy flaps, however, may be thin, scarred and contracted, resulting in a less pleasant aesthetic appearance. There are a number of contraindications to breast reconstruction including non-resectable chest wall disease, progressive systemic disease, patients with significant comorbidities and those felt to be unsuitable psychologically (Box 47.2 and Figure 47.12).
Box 47.2 Oncoplastic breast reconstructive techniques