Chapter 120 Macular Translocation
Background and rationale
Preservation of the integrity of the foveal photoreceptors is vital to visual outcome in patients with macular disease, including diseases such as age-related macular degeneration (AMD), myopic degeneration, and pattern dystrophies. Many of these diseases initially affect subretinal layers, including the retinal pigment epithelium (RPE), Bruch’s membrane, and the choriocapillaris. These layers provide critical support for overlying outer retina, including the foveal photoreceptors.1 Ultimately, disruption of these tissues results in structural and functional deterioration of the photoreceptors with subsequent vision loss. Macular translocation surgery moves the fovea from over a severely diseased subretinal bed to a new location with healthier subretinal tissues to allow for improved function and ideally restoration of functional central vision.
Animal studies
Machemer and Steinhorst utilized a rabbit model to demonstrate the feasibility of purposeful retinal detachment with subretinal infusion via a transscleral approach.2 Electron microscopy confirmed preservation of photoreceptor nuclei and mitochondria in the inner segments with some disruption of the outer segments, suggesting this technique was reasonably atraumatic and may be well tolerated. In addition, successful retinal translocation around the axis of the optic nerve following 360° peripheral retinectomy was also demonstrated. Reattachment of the retina resulted in retinal folds due to the rigidity of the medullary wings. Additionally, maximal shaving of the vitreous base was found to be critical for creation of the retinectomy. Residual vitreous resulted in increased difficulty and less predictability during creation of the retinectomy.2
The rabbit model has also been used to demonstrate the utility of calcium- and magnesium-free infusate in reducing retinal adhesion without creating cellular toxicity.3,4 Collateral damage to photoreceptor outer segments was also reduced by using calcium- and magnesium-free solutions. Temporary electroretinogram (ERG) depression of b-wave amplitude was noted on postoperative day 1, but this was transient.3 Animal studies have also revealed that dark adaptation results in easier retinal detachment with reduced retinal damage. In model surgery, a red-free intraocular light source was used during the surgical procedure to prevent reversal of dark adaptation.4
Historical perspective and evolution of technique
In 1983, Lindsey first proposed translocation of the retina.5 Tiedeman et al. published their proposal for retinal translocation in 1985.6 Machemer published the first human surgical cases in 1993. All three subjects had AMD and were treated with 360° peripheral retinectomy and translocation (MTS360, MT360).7 The original MTS360 technique involved pars plana vitrectomy with transscleral injection of subretinal fluid with 360° retinectomy, removal of subretinal blood and choroidal neovascularization (CNV); partial fill with silicone oil; retinal translocation; complete silicone oil fill, and finally laser retinopexy. All surgeries were performed under general anesthesia. One of the original patients had significant improvement in central acuity (1/200–20/80).7
The original procedure has gone through multiple evolutionary iterations with major developments by Eckardt et al., Toth and Freedman, and Tano.8–10 Changes to technique have focused on facilitating retinal detachment, effectively translocating the macula, reducing complications (e.g., proliferative vitreoretinopathy, PVR), and decreasing the duration of surgery.8–18 Because retinal rotation results in significant cyclotorsion,8,9,12,14,17,19 extraocular muscle surgery to counter-rotate the globe is used routinely to manage the cyclotropia.8 Attempts at reducing the extent of the retinectomy (e.g., <360°) have not been widely adopted due to very high rates of PVR.10,11,20,21
Variations on MTS360 also included the development of limited macular translocation (LMT) which shifted the macula a shorter distance than with MTS360. De Juan developed a technique to shorten the sclera following detachment of the superotemporal retina across the macula. This resulted in redundant retina that allowed the foveal center to be relocated downward by gravity after surgery, by positioning the patient upright with a partially gas-filled eye. Because of the variable and limited distance of macular displacement, this procedure has decreased in use.22–24
Principles of foveal relocation
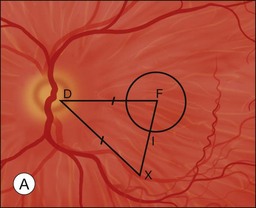
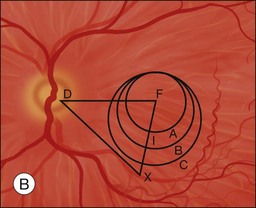
The primary goal of macular translocation surgery is to relocate the fovea to a new location of healthier subretinal tissues in order to preserve and maintain foveal function to maximize visual acuity. Thus the fovea should be relocated to an area outside that of the subfoveal lesion.1 The distance from the preoperative foveal center to the edge of the lesion is the minimum desired translocation. The distances of postoperative foveal displacement versus the minimum desired translocation have been found to be predictors of anatomical success following macular translocation.25
Characteristics of the underlying lesion (e.g., shape, size) determine the minimum desired translocation. Most surgeons move the retina further than the minimum distance, in order to have a reasonable margin between the lesion edge and the new foveal location. Lesion location is also critical to required translocation distance. Central larger lesions may require the same displacement distance as smaller eccentric lesions (Fig. 120.1). The postoperative foveal location in MTS360 is highly predictable, since the new foveal location is determined intraoperatively and the retina is reattached at the new location. In LMT, the postoperative foveal location is much less predictable because the fovea is not placed at a new position but rather settles to a new position after surgery. Because LMT results in a maximum translocation distance of 1500 µm, frequently with lesser displacement, and is generally only in a downward direction, it is frequently insufficient for treatment of subfoveal lesions that progress to surgical intervention.25–30 On the other hand, MTS360 is more versatile with an average foveal displacement of 3500 µm and the capacity to translocate the macula upward or downward.8–11
Preoperative considerations
Indications
Although clear guidelines for the indications for macular translocation are not established, there are several generally agreed upon principles. Anti-VEGF (vascular endothelial growth factor) therapy is clearly the first-line treatment for subfoveal neovascular disease.31,32 MTS360 surgery is only considered in patients with bilateral central vision loss due to the postoperative torsional diplopia. The surgical eye is typically the eye with better visual potential, more recent vision loss and greater preservation of retinal architecture. It may be considered in nonresponders to anti-VEGF treatment, eyes with extensive subretinal fibrosis, or some cases of massive subretinal hemorrhage, retinal pigment epithelial tears or submacular diseases that are not VEGF-driven.8,9,12,15,17,26,33–39 Although macular translocation has been reported in numerous diseases including neovascular AMD, non-neovascular AMD with geographic atrophy, myopic degeneration with CNV, ocular histoplasmosis with CNV, adult-onset foveomacular vitelliform dystrophy, punctate inner choroidopathy with subfoveal CNV, angioid streaks with CNV, North Carolina macular dystrophy, and central serous chorioretinopathy, there are cases where it has a lower likelihood of success.8,9,12,15,17,26,33,36–39 In cases of central geographic atrophy with AMD, the atrophy has often recurred in the new foveal location, and many would consider this a contraindication to surgery. Underlying ocular inflammatory diagnoses (e.g., punctate inner choroidopathy, ocular histoplasmosis) may also be associated with worse outcome.33 General inclusion and exclusion criteria for patients being assessed for MTS360 are outlined in Table 120.1.
Table 120.1 Inclusion and exclusion criteria for macular translocation with 360° peripheral retinectomy
* Duration of vision loss determined by patients’ reports of when they were unable to perform vision-related daily activities such as reading and driving.
Diagnostic testing
Preoperative evaluation for macular translocation may include fundus photography, fluorescein angiography, optical coherence tomography, fundus autofluorescence, fixation, and microperimetry. These tests may be useful to identify eyes that are poor candidates for MTS360 such as eyes with severe macular chorioretinal scarring, extensive neurosensory retinal atrophy, widespread RPE atrophy, CNV in the site of proposed translocation, retinal angiomatous lesions, chorioretinal anastomoses, or unexpected retinal vascular disease. Unfortunately, overall these tests, including fixation and microperimetry have been shown to be poor predictors of 1 year visual outcomes in patients who were considered eligible for MTS360.40
Optical coherence tomography (OCT) has revolutionized the diagnosis and management of numerous vitreoretinal diseases. The high-resolution view of the ultrastructure of the retina with spectral domain can be helpful in identifying outer retinal atrophy which may limit the utility of macular translocation. Histological evidence has shown significant photoreceptor atrophy overlying disciform scars.41 If outer retinal atrophy is present in the macula on OCT, patients are probably unlikely to realize improvement in visual function after macular translocation. Surprisingly, retinal thinning on time domain OCT before surgery was associated with good visual outcomes in one study.40 However, time domain OCT lacks the resolution of SDOCT imaging, therefore resolution was not sufficient to identify photoreceptor layer details. In some cases, perhaps the more compact retina was healthier than the edematous retina before MTS360. Additionally, OCT may be helpful in identifying other structural abnormalities that are important to preoperative planning (e.g., large intraretinal cysts, areas of adhesion).
Fluorescein and/or indocyanine green angiography are utilized to assess the extent, location, and activity of the CNV and/or submacular disease process. Additionally, preoperative fluorescein angiography can be helpful in identifying retinal angiomatous proliferation or chorioretinal anastomosis, which should be considered in surgical planning, as these can result in a retinal tear or significant intraoperative hemorrhage during the separation of the retina from the RPE and choroid.42,43 Additionally, the RPE integrity and perfusion status of the choroid and retina can be assessed. Fundus autofluorescence can also be useful for evaluation of the RPE health of the future subfoveal RPE bed. Hyper- or hypofluorescence in the area of the future foveal location should be considered before committing to surgery.
Surgical technique for macular translocation
MTS360
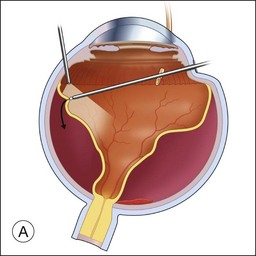
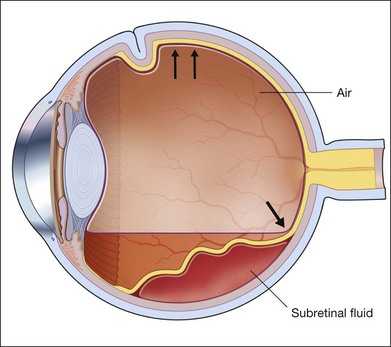
If a patient is phakic, cataract extraction and intraocular lens placement are performed simultaneously at the time of macular translocation. Complete pars plana vitrectomy with elevation of the posterior hyaloid, if attached, is the first step of MTS360. Careful, close shaving of the vitreous base with the vitreous cutter and 360° of scleral depression is performed. Retinal detachment is induced with subretinal fluid injection through a retinotomy that is typically located peripherally within the vitreous base. Posterior retinotomies have been associated with greater epiretinal membrane formation (Fig. 120.2A). A specialized cannula is utilized to infuse the subretinal fluid.8–11,14,15 A very small retinotomy is made inferonasally with the vitreous cutter at the ora serrata. The retinotomy is marked using endodiathermy. In order to limit back flow, a silicone-tipped cannula larger than the retinotomy is used to infuse subretinal fluid to detach the retina.9 If progression of retinal detachment does not occur, a fluid–air exchange is performed which facilitates subretinal fluid displacement from areas of detached retina to areas of attached retina. Although animal models have shown that calcium- and magnesium-free solutions may facilitate retinal detachment, prolonged use of these solutions in humans may result in higher postoperative keratopathy and the use of these solutions is not common.
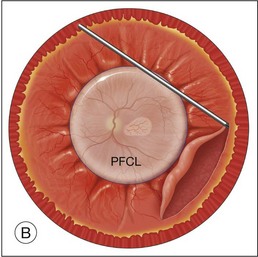
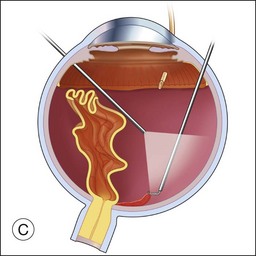
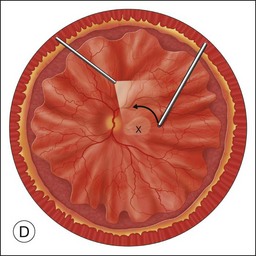
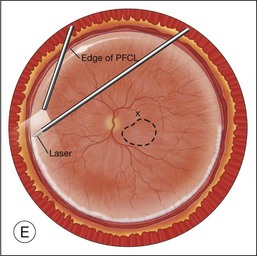
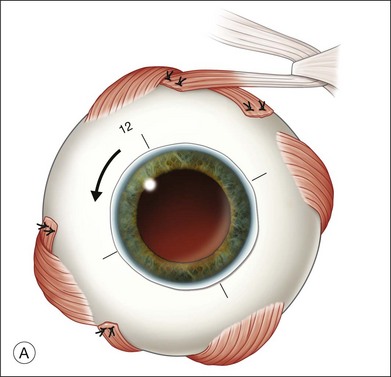
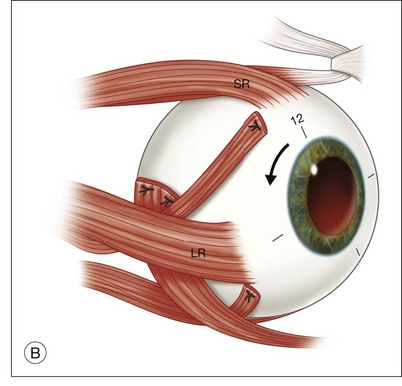
Once the retina is totally detached, the retina is cut at the ora serrata with the vitreous cutter (peripheral retinectomy)9–11 or retinal scissors (peripheral retinotomy) (Fig. 120.2B).8,10,11,15 Perfluorocarbon liquid (PFCL) is utilized over the posterior pole of the retina to stabilize the retina while performing the peripheral retinectomy. Once the retina is completely free from the ora serrata, the PFC is removed and the retina is reflected allowing removal of the subfoveal lesion, if present (Fig. 120.2C). The retina is then translocated into the new position. Most commonly, the retina is translocated superiorly (Fig. 120.2D). A small bubble of preretinal PFCL is utilized to stabilize the retina while the translocation is performed. Multiple instruments have been utilized for the retinal manipulation during translocation including retinal forceps8,10,11 and a silicone-tipped needle,14 although the diamond-dusted silicone-tipped needle is excellent for atraumatically snagging the retinal surface with very gentle pressure over an arcade, and then sliding the retina. The tip may be connected to a syringe containing perfluorocarbon liquid.9 Once appropriate displacement has been achieved (typically approximately 45° off the CNV bed, which equates to the center of the old CNV bed under the inferotemporal arcade), additional PFCL is added to reattach the retina in the manner of a giant retinal tear. Laser photocoagulation is then applied to the retinectomy margins under PFCL tamponade. A direct PFCL/silicone oil exchange is performed to avoid retinal slippage (Fig. 120.2E).
Limited macular translocation
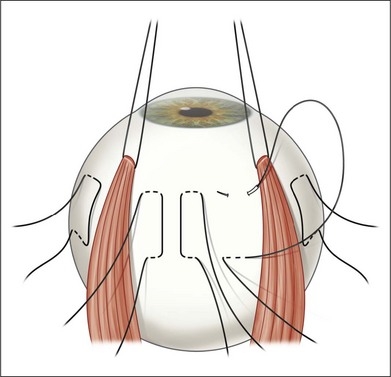
Rectus traction sutures are placed prior to vitrectomy under the lateral rectus and superior or inferior rectus muscles, depending on the planned location of translocation. Five to six horizontal mattress sutures, often 5–0 nylon, are preplaced before inducing a retinal detachment. The sutures are located along the equator, extending for 150–160°, centered in the superotemporal (or rarely inferotemporal quadrant as superior translocation is much more difficult to achieve) (Fig. 120.3). If inferior translocation is needed, the superotemporal quadrant is shortened by placing one suture nasal to the superior rectus, one suture inferior to the lateral rectus, and 3–4 sutures in the superotemporal quadrant. The sutures are left untied until after creation of the retinal detachment. Although this technique creates chorioscleral infolding, other techniques including chorioscleral outpouching have also been reported.44–46
Early postoperative management
Positioning
Positioning following MTS360 is based on the surgeon’s preferences. Approaches include face-down positioning or alternating side-to-side positioning thought to diminish pooling of factors for PVR in aqueous medium at the 6 o’clock position. With LMT, the patient is brought to an upright position (Fig. 120.4) for 24 hours to facilitate inferior translocation.
Extraocular muscle surgery following macular translocation
Given the extensive translocation that occurs during MTS360, often 30–45° in an upward direction, the amount of torsion exceeds the maximum amplitude of cyclofusion (typically around 15°).47 Translocation results in both horizontal and vertical strabismus. In order to alleviate the symptoms associated with excess cyclotorsion, extraocular muscle surgery is performed to strengthen the inferior oblique and weaken the superior oblique. Rectus muscle transposition is also often performed to increase excyclorotation.8,47 Two major techniques have been described. In contrast to MTS360, LMT does not typically require extraocular muscle surgery due to the limited magnitude of translocation.
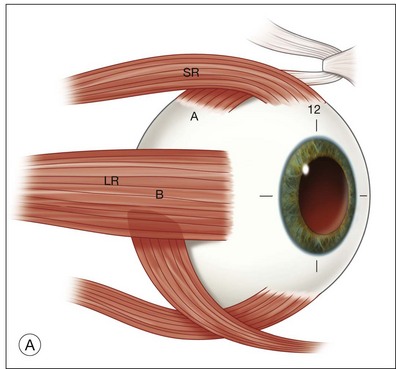
The Freedman technique involves performing the extraocular muscle surgery 8 weeks after the MTS360 procedure. Because of separate and thus shorter procedures, general anesthesia is not needed for the extraocular muscle surgery or the MTS360. The post-translocation amount of torsion determines the number of operated muscles. The inferior oblique is advanced to the temporal border of the superior rectus muscle with the anterior and posterior edges 13 and 15 mm posterior to the limbus, respectively, and a complete superior oblique tenotomy is performed. If an eye has between 20 and 35° of torsion, superior transposition of the lateral rectus muscle is also performed adjacent to the superior rectus, 7 mm from the limbus (Fig. 120.5). For the majority of cases, more than 35° of torsion is present and thus the medial rectus is also transposed to the nasal edge of the inferior rectus muscle.47
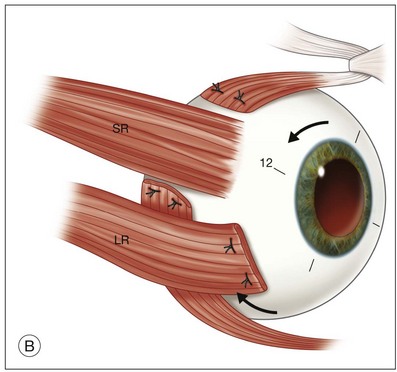
Simultaneous extraocular muscle surgery is performed during the initial MTS360 procedure when using the Eckardt technique.8 General anesthesia is typically utilized during the combined procedure. Strengthening of the inferior oblique is achieved by advancement or through a 12-mm tuck. The superior oblique is weakened by recession or tenotomy. Each rectus muscle is split by about one-quarter of the width of the entire muscle for a radial length of 15–17 mm. Each strip is passed under the remaining portion of the rectus, transposing them clockwise (right eye) or counter-clockwise (left eye) to the margins of the adjacent rectus muscle (windmill technique) producing excyclorotation (Fig. 120.6).8
Functional outcomes for macular translocation surgery
Neovascular AMD
Multiple reports have documented functional outcomes after MTS360 for neovascular AMD (Table 120.2).8–12,14–18,48–50 In addition, there has been a prospective randomized clinical trial of MTS360 versus photodynamic therapy with follow-up through 2 years. In that study, MTS360 produced superior vision recovery when compared with photodynamic therapy at both 1 and 2 years, with a gain in near acuity of 7 letters after MTS360 versus loss of 9.6 letters in the PDT group, and a gain of 0.3 letters in distance acuity after MTS360 versus loss of 12.6 letters in the PDT group.49,51 The subsequent success of antivascular endothelial growth factor inhibitors in the treatment of choroidal neovascularization (CNV) has diminished the need for macular translocation surgery and changed the context of these analyses.31,32
Table 120.2 Visual acuity results of macular translocation with 360° peripheral retinectomy for age-related macular degeneration
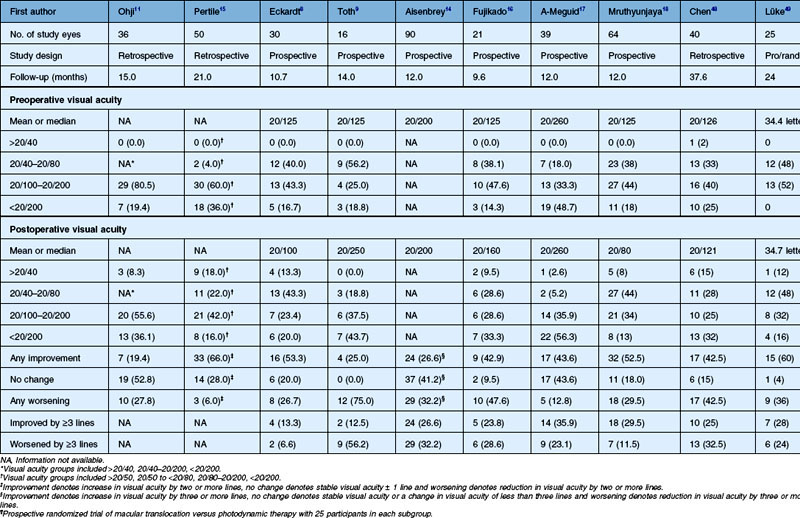
There have been several reviews of the data from clinical series after MTS360 that show benefit of treatment and document the potential complications of this surgery.8–12,14–18,48,49,52 Duration of follow-up ranged from 9.6 months to 38 months. Mean preoperative visual acuity ranged from 20/125 to 20/260. Mean postoperative visual acuity ranged from 20/80 to 20/260 and some patients achieved a distance visual acuity of 20/40 or better. The percentage of patients with postoperative improvement in distance visual acuity has ranged from 43% to 66%.8,15,18,48 Gains in visual acuity of three lines or more ranged from 13% to 36%, while the percentage of patients in each study who lost three or more lines of visual acuity after MTS360 ranged from 6.6% to 56.2% (Fig. 120.7).8–12,14–18,48,49
MTS360 has also been utilized following photodynamic therapy (PDT) and other non-surgical treatments. Prior to the anti-VEGF era, eyes treated with a single prior PDT session improved after MTS360 from mean visual acuity of 20/160 to postoperative mean acuity of 20/100, while eyes with more than one previous PDT session generally worsened from 20/200 before MTS360 to a mean postoperative visual acuity of 20/250.53 In the anti-VEGF era, a study of 43 patients with anti-VEGF therapy or PDT or both, sustained significant improvement in all measures of visual function at 1 year after MTS360 (mean gain of >9 letters).40 An additional study examined 38 consecutive patients in the anti-VEGF era and showed that MTS360 resulted in improved visual acuity, particular in those patients with submacular hemorrhage and CNV, secondary to pathologic myopia.54,55
Numerous studies have also reported changes in near visual acuity, with near acuity sometimes improving greater than distance acuity.13,18,49,51 Reported mean preoperative near visual acuity ranged from 20/100 to 20/160, with a significant improvement in mean near visual acuity to 20/63 and 20/55, postoperatively.8,18 MTS360 has been shown to have better improvement in near visual acuity when compared to PDT (+7 letters versus −9.6 letters).56 In several studies, over 20% of patients achieved postoperative near visual acuity 20/40 or better.8,18,40,57
The impact of MTS360 on other visual functions, including reading speed, contrast sensitivity, and color vision, have also been reported. Significant improvements in reading speed have been found following MTS360 with a mean increase of 26–45 words/minute.18,40,57,58 MTS360 has been shown to not only improve reading speed and fixation quality, but also to improve saccades during reading activities.59 Improved contrast sensitivity has also been documented following MTS360, while color vision has been minimally affected following MTS360.40,58
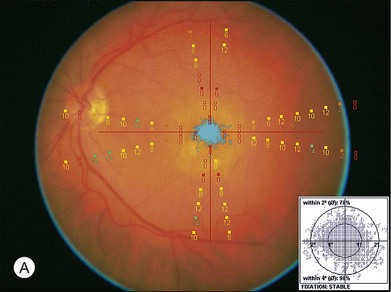
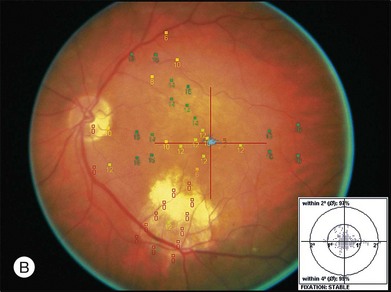
Goldman perimetry studies have shown peripheral field loss but overall good preservation of the visual field following MTS360.60 Microperimetry studies have shown recovery of central visual sensitivity following MTS360 (Fig. 120.8)40,59,61 and a new focal scotoma in the superotemporal field due to the translocated CNV bed.61 Reduction in photopic and scotopic electroretinograms have been documented following MTS360 for up to 5 months after surgery. The mechanism for ERG reduction is not clear, but may be related to length of surgery, composition of subretinal infusate, and duration of detachment.62–64 In addition to ERG, electro-oculogram findings have shown a decreased dark trough following MTS360, although the Arden ratio was within normal limits.65
Vision-related quality of life (QOL) has been found to significantly reduce in patients with bilateral severe AMD.66 Following MTS360, vision-related QOL has been reported to have significant improvement particularly in relation to distance acuity, near acuity, and reading speed. Near acuity was found to have the greatest impact in vision-related QOL. A one line change in near acuity resulted in a 13.7 point change in QOL score, compared with a 1.4–2.1 point change for a one line change in distance acuity or an increase in reading speed of 15 words/minute.66 Vision-related QOL showed significant improvement in vision-related subscores following MTS360 in a prospective randomized study.56 MTS360 showed significantly higher improvement in QOL scores for general vision, mental health, and dependency compared with treatment with PDT.56
Regarding LMT, one study reported that 48% of patients at 6 months gained two or more lines of visual acuity and at 1 year, 40% of patients had gained two or more lines. A total of 31% of patients lost two or more lines of visual acuity.67,68
Non-neovascular AMD
Macular translocation has also been utilized in geographic atrophy (GA) secondary to AMD. Multiple reports have discussed the use of macular translocation for GA.36,37,69,70 The first study published included eight eyes undergoing MTS360. Five of eight eyes had visual improvement, one remained stable and two eyes had worsening visual acuity.36 A more recent study included four eyes with GA, and in this study, the mean preoperative and postoperative visual acuity were unchanged.69 Recurrent RPE atrophy is a significant concern in patients treated with macular translocation and GA secondary to AMD.36,69,70
Non-AMD diagnoses
In addition to AMD, multiple other submacular diseases can result in bilateral vision loss. In cases of severe functional limitation, consideration may be given to macular translocation when other treatment modalities are not effective or a viable option (e.g., anti-VEGF therapy, photodynamic therapy, focal laser photocoagulation). Some of these conditions may not be VEGF-driven and may not respond to intravitreal therapy. Although limited in number, macular translocation has been utilized in many of these non-AMD conditions.33 One recent study examined 16 subjects treated with MTS360 for conditions other than AMD. Conditions included in this study were adult-onset foveomacular vitelliform dystrophy (AOFVD), myopic degeneration, ocular histoplasmosis, punctate inner choroidopathy (PIC), Best disease, angioid streaks, North Carolina macular dystrophy, and central serous.33 Of all of these conditions, myopic degeneration has been the most widely studied for macular translocation. A large study of MTS360 for myopic degeneration included 52 eyes with a final visual acuity of 20/40 or better in 39% of subjects.71 In general, eyes with myopic degeneration had smaller CNVs (e.g., 0.4–1.2 disc diameters) and required less retinal rotation than eyes with neovascular AMD. A study of 15 eyes undergoing MTS360 for myopic degeneration reported that 73% of eyes showed visual improvement, with only 10% of eyes losing vision.16,38,57 A smaller series of three eyes with myopic degeneration, showed a final visual acuity of 20/60 or better in 3/3 eyes, with 2/3 eyes improving ≥3 lines.33 LMT has also been utilized for treatment of myopic degeneration. A study of 11 eyes undergoing LMT revealed that 64% of eyes achieved a final visual acuity of 20/100 or better.72 Another study comparing LMT to submacular surgery and CNV removal in myopic degeneration reported a statistically significant greater improvement in visual acuity following LMT (+3.8 versus −0.07 lines), although these reports were prior to the anti-VEGF era.73
A case study of MTS360 for idiopathic CNV reported worsening visual acuity from 20/63 preoperatively, to 20/100 postoperatively.16 Comparing inflammatory preoperative diagnoses (e.g., presumed ocular histoplasmosis syndrome (POHS), PIC), with noninflammatory diagnoses (e.g., myopic degeneration, AOFVD, Best disease, North Carolina macular dystrophy, angioid streaks, and central serous), only 20% of eyes with a preoperative inflammatory diagnosis maintained a visual acuity of 20/100 or better, and the mean final visual acuity in those eyes was 20/209.33 Three of five (60%) eyes with inflammatory disease lost ≤3 lines of visual acuity, and the mean line change in visual acuity was a loss of 1.4 lines.33 In eyes with a noninflammatory diagnosis the mean final visual acuity was 20/83 with 73% of eyes maintaining a final visual acuity of ≥20/100. Five of 11 eyes maintained a final visual acuity of ≥20/50. The mean line change was +1.0 lines.33 Though this study was retrospective and small, this suggests that increased caution should be used prior to proceeding with MTS360 for inflammatory diagnoses. The most likely cause of worsening final visual acuity appeared to be an increase in postoperative complications for this group.33 Polypoidal choroidal vasculopathy (PCV) has also been treated with MTS360, with two reported cases showed visual improvement following MTS360.39
Postoperative surgical complications following macular translocation
Surgical complications following MTS360 for neovascular AMD are summarized in Table 120.3.8–12,14–18,48 Retinal detachment is among the most common complications with a prevalence of 7.8–42.8%. The higher rates of retinal detachment were reported in studies of early experience of surgeons with MTS360. Recurrence of CNV is also a common complication following MTS360. The prevalence of this complication ranges from 0.0% to 27.8%. Often due to the typical extrafoveal location of the CNV, thermal laser with or without anti-VEGF therapy can be employed. CME and epiretinal membrane (ERM) formation are also two frequently reported complications. CME has not been consistently reported, however studies have given a range of 0.0–44%. Many of these studies were prior to the widespread use of optical coherence tomography (OCT) and subclinical CME may go unrecognized. The studies reporting the highest rate of CME, used OCT to examine every patient postoperatively.18,51 ERM formation has been reported to occur in 6.6–28.2% of patients, postoperatively. However, the lack of uniform definition of ERM and the lack of widespread OCT use should lend caution to the interpretation of these numbers. Other complications such as macular hole, keratopathy, and hypotony tended to be less frequent.
Table 120.3 Surgical complications after macular translocation with 360° peripheral retinectomy for age-related macular degeneration
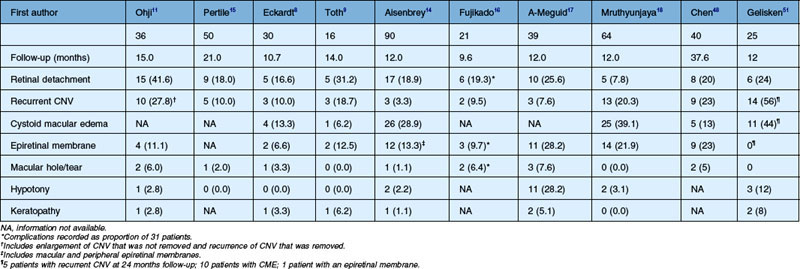
Complications following MTS360 for non-AMD diagnoses have also been reported. For myopic degeneration, MTS360 has been reported to have a retinal detachment rate of 34% and recurrent CNV rate of 6%.71 A small study examining MTS360 for multiple non-AMD conditions showed that eyes with an underlying inflammatory diagnosis had a significantly higher complication rate compared with non-inflammatory diagnoses.33 In that report, the overall complication rates were 50% for ERM (including mild ERM on OCT); 31% for CME; 13% for retinal detachment, and 13% for CNV. A total of 38% needed an office-based procedure due to a complication (e.g., subtenon injection, laser). Surgical intervention was required in 19% of eyes. In eyes with an underlying inflammatory diagnosis, 100% (5/5) of eyes required some type of intervention for a complication, compared with only 36% (4/11) of eyes with a noninflammatory diagnosis. In eyes with an underlying inflammatory diagnosis, the CNV recurrence rate was 40%.33
Given the extensive rotation associated with MTS360, binocular vision is often not recovered and extraocular muscle surgery is required. Almost all patients have a central scotoma in the fellow eye preoperatively, but their peripheral vision is well maintained. The findings regarding binocularity are variable. Using Bagolini glasses, five studies reported findings of postoperative binocular vision in 6.6–28.5% of eyes.8,10,11,14,17,74 However, one study reported a 0% rate of binocular function in patients.75
LMT has a similar spectrum of complications, including retinal detachment, frequently associated with proliferative vitreoretinopathy. A study of 153 eyes found a retinal detachment rate of 16%; retinal break of 8%; macular fold of 5%, and CNV at the retinotomy site of 1.3%. Torsional diplopia is a common issue, but unlike MTS360, extraocular muscle surgery is typically unnecessary.76
Retinal and RPE changes after macular translocation
A striking finding in patients with GA treated with macular translocation is the recurrence of the RPE atrophy in the new foveal bed which has now been reported in multiple studies.36,69,70 In one study, recurrent RPE changes were seen in 75% of eyes treated with MTS360.69 Similar findings of new RPE atrophy are quite rare in patients with neovascular AMD treated with MTS360.69 The precipitating factor for RPE atrophy is not clear. Given the rarity of similar findings in MTS360 for neovascular AMD, surgical trauma seems to be an unlikely cause. RPE atrophy may be the primary event, particularly if the increased metabolic activity of the overlying foveal photoreceptors results in increased stress to the fragile RPE. Alternatively, apoptotic photoreceptors may result in secondary RPE atrophy in the new foveal bed. Additional research is needed to further elucidate the mechanisms involved in this phenomenon.
The reports of recurrence of GA were in the era before more routine autofluorescence imaging. The fundus autofluorescence techniques are useful to monitor the health of the new RPE under the macula. In patients with neovascular AMD and with non-AMD diseases such as myopia, the RPE under the new macula demonstrates hyperautofluorescence that persists with little change over months to years.77 This finding is often compatible with good visual acuity but may represent stress of densely packed photoreceptors over non-foveal RPE. In addition, the RPE that was in the shadow of retinal vessels for the lifetime of the patient and is now exposed for imaging after translocation, shows decreased autofluorescence relative to adjacent RPE.78
Unintentional macular translocation following retinal detachment repair
In one recent study, patients with gas-filled eyes after vitrectomy for retinal reattachment were positioned upright for several minutes prior to face-down positioning.78 This upright positioning was carried out to determine if subtle shifts in the retina occurred inferiorly. Fundus autofluorescence found hyperfluorescent changes corresponding to the preoperative location of the retinal vessels, suggesting an inferior rotation of the retina in 63% (27/43) of eyes. In those eyes with hyperfluorescent changes, a synoptophore measurement revealed that 59% of eyes had excyclotorsion and 49% of eyes had a vertical deviation. Interestingly, none of these eyes had symptomatic diplopia. Risk factors associated with retinal translocation included macula off status and increased size of retinal detachment. The use of perfluorocarbon liquid had no association with risk of retinal rotation.78 Because it is often difficult to assure postoperative prone positioning without inadvertent upright positioning, placing the patient initially on their side with the temporal retina dependent in the early period after surgery may reduce the risk of inadvertent translocation.
Advantages of macular translocation and future directions
In the anti-VEGF era, the landscape for macular translocation has changed significantly. The excellent results achieved with anti-VEGF therapy for neovascular AMD have shifted primary treatment of AMD away from surgical intervention.31,32 As Zeimssen and Gelisken79 point out, “Generally, in the presence of highly effective anti-VEGF drugs, FMT can be discussed for second-line treatment, if the fellow eye has poor function and no additional risk factors of the affected eye are known…”
Numerous researchers note that MTS360 may well have a role in the management of bilateral submacular diseases, including AMD. This may be particularly true in cases with early subretinal fibrosis with preservation of the outer retina. If the fellow eye has lost significant visual acuity and anti-VEGF therapy or other modalities have been unsuccessful, consideration may be given to proceeding with macular translocation. Without surgical removal, progressive enlargement of CNV may occur resulting in enlargement of the area of photoreceptor damage.80,81 Central acuity may not recover even with arrest of CNV leakage, due to underlying fibrosis and loss of RPE. MTS360 may allow recovery of function through the translocation of the foveal photoreceptors off the scarred underlying tissues to an area with improved vitality.1 Cases of extensive subretinal hemorrhage may also be amenable to MTS360, as the procedure allows for removal of the subretinal blood during the translocation process.7
1 Toth CA, Machemer R. Macular translocation. In: Fine S, Berger J, Maguire M. Age-related macular degeneration. St Louis: Mosby; 1999:353–362.
2 Machemer R, Steinhorst UH. Retinal separation, retinotomy, and macular relocation: I Experimental studies in the rabbit eye. Graefes Arch Clin Exp Ophthalmol. 1993;231:629–634.
3 Fang XY, Hayashi A, Cekic O, et al. Effect of Ca2+-free and Mg2+-free BSS Plus solution on the retinal pigment epithelium and retina in rabbits. Am J Ophthalmol. 2001;131:481–488.
4 Faude F, Wendt S, Biedermann B, et al. Facilitation of artificial retinal detachment for macular translocation surgery tested in rabbit. Invest Ophthalmol Vis Sci. 2001;42:1328–1337.
5 Lindsey P, Finkelstein D, D’Anna S. Experimental retinal relocation. Invest Ophthalmol Vis Sci. 1983;24:S242.
6 Tiedeman JS, de Juan E, Jr., Machemer R, et al. Surgical rotation of the macula. Invest Ophthalmol Vis Sci. 1985;26:S59.
7 Machemer R, Steinhorst UH. Retinal separation, retinotomy, and macular relocation: II. A surgical approach for age-related macular degeneration? Graefes Arch Clin Exp Ophthalmol. 1993;231:635–641.
8 Eckardt C, Eckardt U, Conrad HG. Macular rotation with and without counter-rotation of the globe in patients with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1999;237:313–325.
9 Toth CA, Freedman SF. Macular translocation with 360-degree peripheral retinectomy impact of technique and surgical experience on visual outcomes. Retina. 2001;21:293–303.
10 Tano Y. Pathologic myopia: where are we now? Am J Ophthalmol. 2002;134:645–660.
11 Ohji M, Fujikado T, Kusaka S, et al. Comparison of three techniques of foveal translocation in patients with subfoveal choroidal neovascularization resulting from age-related macular degeneration. Am J Ophthalmol. 2001;132:888–896.
12 Wolf S, Lappas A, Weinberger AW, et al. Macular translocation for surgical management of subfoveal choroidal neovascularizations in patients with AMD: first results. Graefes Arch Clin Exp Ophthalmol. 1999;237:51–57.
13 Lai JC, Lapolice DJ, Stinnett SS, et al. Visual outcomes following macular translocation with 360-degree peripheral retinectomy. Arch Ophthalmol. 2002;120:1317–1324.
14 Aisenbrey S, Lafaut BA, Szurman P, et al. Macular translocation with 360 degrees retinotomy for exudative age-related macular degeneration. Arch Ophthalmol. 2002;120:451–459.
15 Pertile G, Claes C. Macular translocation with 360 degree retinotomy for management of age-related macular degeneration with subfoveal choroidal neovascularization. Am J Ophthalmol. 2002;134:560–565.
16 Fujikado T, Shimojyo H, Hosohata J, et al. Effect of simultaneous oblique muscle surgery in foveal translocation by 360 degrees retinotomy. Graefes Arch Clin Exp Ophthalmol. 2002;240:21–30.
17 Abdel-Meguid A, Lappas A, Hartmann K, et al. One year follow up of macular translocation with 360 degree retinotomy in patients with age related macular degeneration. Br J Ophthalmol. 2003;87:615–621.
18 Mruthyunjaya P, Stinnett SS, Toth CA. Change in visual function after macular translocation surgery with 360 degree peripheral retinectomy for neovascular age-related macular degeneration. Ophthalmology. 2004;111:1715–1724.
19 Seaber JH, Machemer R. Adaptation to monocular torsion after macular translocation. Graefes Arch Clin Exp Ophthalmol. 1997;235:76–81.
20 Ninomiya Y, Lewis JM, Hasegawa T, et al. Retinotomy and foveal translocation for surgical management of subfoveal choroidal neovascular membranes. Am J Ophthalmol. 1996;122:613–621.
21 Akduman L, Karavellas MP, MacDonald JC, et al. Macular translocation with retinotomy and retinal rotation for exudative age-related macular degeneration. Retina. 1999;19:418–423.
22 de Juan E, Jr., Loewenstein A, Bressler NM, et al. Translocation of the retina for management of subfoveal choroidal neovascularization II: a preliminary report in humans. Am J Ophthalmol. 1998;125:635–646.
23 Imai K, Loewenstein A, de Juan E, Jr. Translocation of the retina for management of subfoveal choroidal neovascularization I: experimental studies in the rabbit eye. Am J Ophthalmol. 1998;125:627–634.
24 Imai K, de Juan E, Jr. Experimental surgical macular relocation by scleral shortening. ARVO abstracts. Invest Ophthalmol Vis Sci. 1996;37:S116.
25 Au Eong KG, Pieramici DJ, Fujii GY, et al. Macular translocation: unifying concepts, terminology, and classification. Am J Ophthalmol. 2001;131:244–253.
26 Lewis H, Kaiser PK, Lewis S, et al. Macular translocation for subfoveal choroidal neovascularization in age-related macular degeneration: a prospective study. Am J Ophthalmol. 1999;128:135–146.
27 Pieramici DJ, De Juan E, Jr., Fujii GY, et al. Limited inferior macular translocation for the treatment of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Am J Ophthalmol. 2000;130:419–428.
28 Lewis H. Macular translocation with chorioscleral outfolding: a pilot clinical study. Am J Ophthalmol. 2001;132:156–163.
29 Deramo VA, Meyer CH, Toth CA. Successful macular translocation with temporary scleral infolding using absorbable suture. Retina. 2001;21:304–311.
30 Benner JD, Meyer CH, Shirkey BL, et al. Macular translocation with radial scleral outfolding: experimental studies and initial human results. Graefes Arch Clin Exp Ophthalmol. 2001;239:815–823.
31 Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444.
32 Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431.
33 Ehlers JP, Maldonado R, Sarin N, et al. Treatment of non-age-related macular degeneration submacular diseases with macular translocation surgery. Retina. 2011;31:1337–1346.
34 Polito A, Cereda M, Romanelli F, et al. Macular translocation with 360 degrees retinotomy for management of retinal pigment epithelial tear: long-term results. Br J Ophthalmol. 2011;95:74–78.
35 Steel DH, Sandhu SS. Submacular haemorrhages associated with neovascular age-related macular degeneration. Br J Ophthalmol. 2011;95:1051–1057.
36 Eckardt C, Eckardt U. Macular translocation in nonexudative age-related macular degeneration. Retina. 2002;22:786–794.
37 Cahill MT, Freedman SF, Toth CA. Macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch Ophthalmol. 2003;121:132–133.
38 Fujikado T, Ohji M, Kusaka S, et al. Visual function after foveal translocation with 360-degree retinotomy and simultaneous torsional muscle surgery in patients with myopic neovascular maculopathy. Am J Ophthalmol. 2001;131:101–110.
39 Terasaki H, Miyake Y, Suzuki T, et al. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002;86:321–327.
40 Mettu PS, Sarin N, Stinnett SS, et al. Recovery of the neurosensory retina after macular translocation surgery is independent of peroperative macular sensitivity in neovascular age-related macular degeneration. Retina. 2011;31:1637–1649.
41 Kim SY, Sadda S, Pearlman J, et al. Morphometric analysis of the macula in eyes with disciform age-related macular degeneration. Retina. 2002;22:471–477.
42 Lafaut BA, Aisenbrey S, Vanden Broecke C, et al. Clinicopathological correlation of deep retinal vascular anomalous complex in age related macular degeneration. Br J Ophthalmol. 2000;84:1269–1274.
43 Kuhn D, Meunier I, Soubrane G, et al. Imaging of chorioretinal anastomoses in vascularized retinal pigment epithelium detachments. Arch Ophthalmol. 1995;113:1392–1398.
44 Kamei M, Roth DB, Lewis H. Macular translocation with chorioscleral outfolding: an experimental study. Am J Ophthalmol. 2001;132:149–155.
45 Kamei M, Roth DB, Lewis H. Macular translocation using scleral clips to create an outpouching radial fold of the sclera, choroid and retinal pigment epithelium. ARVO abstracts. Invest Ophthalmol Vis Sci. 2000;41:S540.
46 Lewis H. Macular translocation with chorioscleral outfolding: a pilot clinical study. Am J Ophthalmol. 2001;132:156–163.
47 Freedman SF, Holgado S, Enyedi LB, et al. Management of ocular torsion and diplopia after macular translocation for age-related macular degeneration: prospective clinical study. Am J Ophthalmol. 2003;136:640–648.
48 Chen FK, Patel PJ, Uppal GS, et al. Long-term outcomes following full macular translocation surgery in neovascular age-related macular degeneration. Br J Ophthalmol. 2010;94:1337–1343.
49 Lüke M, Ziemssen F, Völker M, et al. Full macular translocation (FMT) versus photodynamic therapy (PDT) with verteporfin in the treatment of neovascular age-related macular degeneration: 2-year results of a prospective, controlled, randomised pilot trial (FMT-PDT). Graefes Arch Clin Exp Ophthalmol. 2009;247:745–754.
50 Skaf AR, Mahmoud T. Surgical treatment of age-related macular degeneration. Semin Ophthalmol. 2011;26:181–191.
51 Gelisken F, Voelker M, Schwabe R, et al. Full macular translocation versus photodynamic therapy with verteporfin in the treatment of neovascular age-related macular degeneration: 1-year results of a prospective, controlled, randomised pilot trial (FMT-PDT). Graefes Arch Clin Exp Ophthalmol. 2007;245:1085–1095.
52 Eandi CM, Giansanti F, Virgili G. Macular translocation for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 4, 2008. CD006928
53 Park CH, Toth CA. Macular translocation surgery with 360-degree peripheral retinectomy following ocular photodynamic therapy of choroidal neovascularization. Am J Ophthalmol. 2003;136:830–835.
54 Yamada Y, Miyamura N, Suzuma K, et al. Long-term follow-up of full macular translocation for choroidal neovascularization. Am J Ophthalmol. 2010;149:453–457.
55 Haverbeke GM, Claes C. Full macular translocation for choroidal neovascularization in the era of intravitreal pharmacological therapy. Retina. 2010;30:1739–1743.
56 Lüke M, Ziemssen F, Bartz-Schmidt KU, et al. Quality of life in a prospective, randomised pilot-trial of photodynamic therapy versus full macular translocation in treatment of neovascular age-related macular degeneration – a report of 1 year results. Graefes Arch Clin Exp Ophthalmol. 2007;245:1831–1836.
57 Fujikado T, Asonuma S, Ohji M, et al. Reading ability after macular translocation surgery with 360-degree retinotomy. Am J Ophthalmol. 2002;134:849–856.
58 Toth CA, Lapolice DJ, Banks AD, et al. Improvement in near visual function after macular translocation with 360-degree peripheral retinectomy. Graefe’s. Arch Clin Exp Ophthalmol. 2004;242:541–548.
59 Uppal G, Feely M, Crossland M, et al. Assessment of reading behaviour with an infrared eyetracker following 360 degree macular translocation for age related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:6486–6496.
60 Kubota A, Ohji M, Kusaka S, et al. Evaluation of the peripheral visual field after foveal translocation. Am J Ophthalmol. 2001;132:581–584.
61 Chieh JJ, Stinnett SS, Toth CA. Central and pericentral retinal sensitivity after macular translocation surgery. Retina. 2008;28:1522–1529.
62 Luke C, Aisenbrey S, Luke M, et al. Electrophysiological changes after 360 degrees retinotomy and macular translocation for subfoveal choroidal neovascularisation in age related macular degeneration. Br J Ophthalmol. 2001;85:928–932.
63 Terasaki H, Miyake Y, Suzuki T, et al. Change in full-field ERGs after macular translocation surgery with 360 degrees retinotomy. Invest Ophthalmol Vis Sci. 2002;43:452–457.
64 Sickel W. Retinal metabolism in dark and light. In: Fuortes MG, ed. Handbook of sensory physiology. Berlin: Springer; 1972:667–727.
65 Luke C, Alteheld N, Aisenbrey S, et al. Electro-oculographic findings after 360 degrees retinotomy and macular translocation for subfoveal choroidal neovascularisation in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2003;241:710–715.
66 Cahill MT, Stinnett SS, Banks AD, et al. Quality of life after macular translocation with 360-degree peripheral retinectomy for age-related macular degeneration. Ophthalmology. 2005;112:152–158.
67 Fujii GY, de Juan E, Jr., Pieramici DJ, et al. Inferior limited macular translocation for subfoveal choroidal neovascularization secondary to age-related macular degeneration: 1-year visual outcome and recurrence report. Am J Ophthalmol. 2002;134:69–74.
68 Pieramici DJ, De Juan E, Fujii GY, et al. Limited inferior macular translocation for the treatment of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Am J Ophthalmol. 2000;130:419–428.
69 Cahill MT, Mruthyunjaya P, Bowes Rickman C, et al. Recurrence of retinal pigment epithelial changes after macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch Ophthalmol. 2005;123:935–938.
70 Khurana RN, Fujii GY, Walsh AC, et al. Rapid recurrence of geographic atrophy after full macular translocation for nonexudative age-related macular degeneration. Ophthalmology. 2005;112:1586–1591.
71 Chan WM, Ohji M, Lai TY, et al. Choroidal neovascularisation in pathological myopia: an update in management. Br J Ophthalmol. 2005;89:1522–1528.
72 Fujii GY, Humayun MS, Pieramici DJ, et al. Initial experience of inferior limited macular translocation for subfoveal choroidal neovascularization resulting from causes other than age-related macular degeneration. Am J Ophthalmol. 2001;131:90–100.
73 Hamelin N, Glacet-Bernard A, Brindeau C, et al. Surgical treatment of subfoveal neovascularization in myopia: macular translocation vs surgical removal. Am J Ophthalmol. 2002;133:530–536.
74 Fricke J, Neugebauer A, Nobis H, et al. Counterrotation of the globe in macular translocation. Graefes Arch Clin Exp Ophthalmol. 2000;238:664–668.
75 Freedman SF, Holgado S, Enyedi LB, et al. Management of ocular torsion and diplopia after macular translocation for age-related macular degeneration: prospective clinical study. Am J Ophthalmol. 2003;136:640–648.
76 Fujii GY, Pieramici DJ, Humayun MS, et al. Complications associated with limited macular translocation. Am J Ophthalmol. 2000;130:751–762.
77 Sawa M, Gomi F, Ohji M, et al. Fundus autofluorescence after full macular translocation surgery for myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246:1087–1095.
78 Shiragami C, Shiraga F, Yamaji H, et al. Unintentional displacement of the retina after standard vitrectomy for rhegmatogenous retinal detachment. Ophthalmology. 2010;117:86–92.
79 Ziemssen F, Gelisken F. [Macular translocation – a therapeutic approach for neovascular macular degeneration in the era of anti-VEGF therapy?]. Klin Monbl Augenheilkd. 2009;226:31–37.
80 TAP. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials – TAP report. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Arch Ophthalmol. 1999;117:1329–1345.
81 Bressler NM. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials – TAP report 2. Arch Ophthalmol. 2001;119:198–207.