Chapter 117 Macular Hole
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Introduction
Macular hole (MH) is a round full-thickness opening in the foveal center. In most cases it is idiopathic, i.e., due to abnormal vitreofoveal traction. The role of the vitreous cortex in the pathogenesis of macular hole became better understood with the biomicroscopic observations of JD Gass.1 The advent of optical coherence tomography (OCT), which showed the partially detached posterior hyaloid, clarified the understanding of MH formation. Spectral domain OCT (SD-OCT) subsequently provided more detailed views of the initial foveal changes induced by vitreofoveal traction. Macular holes have been known about since the 19th century. However, they only aroused renewed interest after Kelly and Wendel2 had shown that pars plana vitrectomy (PPV) combined with vitreous cortex detachment and fluid–gas exchange could close MH in a significant proportion of cases, although it was assumed that the retina would be unable to heal. The success rate of MH surgery gradually increased progressively and this surgery is now one of the most successful vitreoretinal surgeries.
History
Macular hole was first described in 1869 by Knapp,3 of a traumatic case. Noyes4 (1871) gave a detailed ophthalmoscopic description of a traumatic case, and Ogilvie (1900) was the first to use the term “hole at the macula.”5 Early in the 20th century, Kuhnt6 (1900) and Coats7 (1907) suggested that the origin of MH was degenerative, although Zeeman8 (1912) and Lister9 (1924) attributed it to a vitreoretinal tractional mechanism. The modern history of MH started with J.D. Gass who proposed a staging system ranging from impending to full-thickness MH, on the basis of his biomicroscopic observations.10–12 Kelly and Wendel2 performed the first successful surgery of MH, and Hee and Puliafito13 were the first to describe the stages of MH on OCT scans.14,15
Epidemiology and risk factors for idiopathic full-thickness macular holes
Prevalence
The prevalence of MH reported in the literature varies greatly. It was 3.3 per 1000 in the Baltimore Eye Study;16 0.2 per 1000 in the Blue Mountains Study;17 0.9 per 1000 in the Beijing study,18 and 1.7 per 1000 in a study in Southern India.19 In the Beaver Dam study, the prevalence was 2.9 per 1000 as cited by McCannel et al.20 The incidence of MH was studied in a county in Minnesota (USA) and was found to occur in 7.8 persons per 100 000 per year, with a female-to-male ratio of 3.3 : 1. MH was bilateral in 11.7% of patients.20
Incidence in the fellow eye
The data on the incidence of bilateral MH vary considerably from 5% to 16%. In a retrospective study of 84 cases followed-up for 39 months on average, Akiba and colleagues21 (1992) found that 16.6% of patients had an MH in the fellow eye, and that the proportion rose to 36.8% when that eye presented with a foveal cyst or a central yellow spot. However, no MH occurred in the fellow eye when the vitreous was detached.21 In a retrospective study by Lewis et al.,22 the incidence was 13% in 4 years; it was 15.6% at 5 years in a prospective study of 144 patients, by Ezra and colleagues;23 and 7.6% at 6 years in another prospective study of 122 patients.24 In 2004, Chan et al.25 proposed the concept of Stage zero (0) MH to designate fellow eyes with a normal macular profile on OCT but a posterior hyaloid still attached to the foveal center. In their prospective study of 94 fellow eyes, MH only occurred in 4.5% of cases without visible vitreofoveal adhesion and in 42% of cases with a Stage 0 MH. However Chan et al.’s study was limited by the use of OCT1, which at this time did not allowed sufficiently accurate assessment of vitreofoveal adhesion. In 2011, Takahashi et al.26 selected 42 fellow eyes of 176 patients with MH, examined with OCT3, and presented with persistent vitreofoveal adhesion and early Stage 1 intrafoveal lesions. MH occurred in 11.9% of these eyes during the 5-year follow-up.
Pathogenesis, from posterior vitreous detachment to impending macular hole
History of theories on the pathogenesis of macular hole
Vitreomacular traction
Vitreomacular traction has long been suspected to be a cause of MH formation, but until the advent of OCT it was difficult to visualize the vitreofoveal interface routinely. However various indications of the role of the vitreous had been given in the literature. Thus, in 1952 Grignolo provided histologic evidence of strong vitreomacular adherence to the fovea.30 In particular cases, biomicroscopic observations strengthened the hypothesis that anteroposterior traction of vitreous fibers on the fovea played a role in the formation of MH.31–36 The biomicroscopic observation of opercula also strengthened the possibility that anteroposterior traction is the cause of MH.35,37
Foveal cyst
The existence of a foveal cyst in the fellow eye of an eye with MH was shown histologically by Kornzweig in 195038 and by Frangieh et al. in 1981.14 Also in 1981, Bronstein et al.,39 and in 1982, McDonnell et al.40 analyzed a series including fellow eyes and eyes with MH. They concluded that idiopathic macular cysts and holes were part of the same disorder and described the role of the vitreous body in their formation. On the basis of scanning laser ophthalmoscope observations, Kishi et al.41 deduced, in 1995, that foveal cyst formation due to vitreous traction was the first step in MH formation. In 1998, Folk et al.,42 who used laser slit-lamp photography, were able to obtain a direct image of the foveal cysts which they considered to be a pre-hole condition. This was confirmed in the same year by Hee and Puliafito13 using optical coherence tomography (OCT), which showed, for the first time, the vitreous cortex attached on the roof of a foveal cyst. Foveal cyst appeared then to be the initial change in the foveal structure that predisposes to the risk of progression to MH.43,44
Contraction of the premacular vitreous cortex
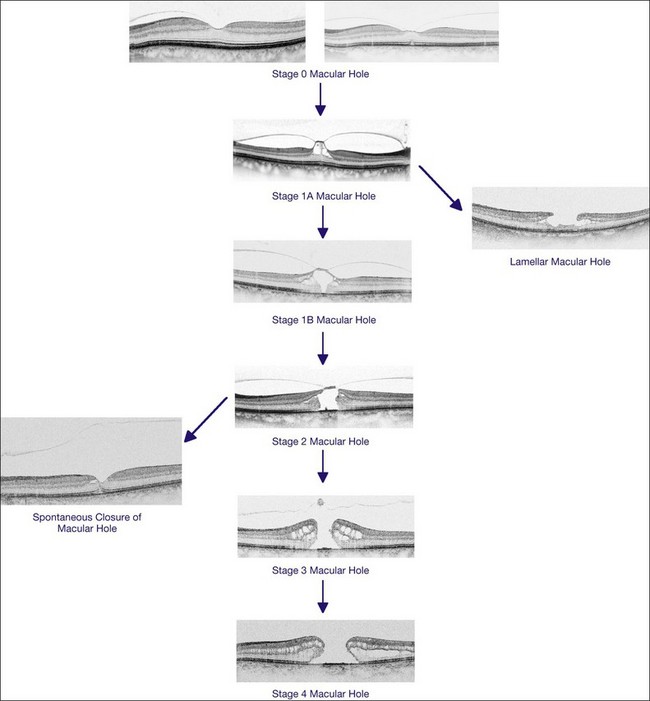
In 1988, Gass revised the biomicroscopic description of macular holes and proposed a new interpretation of the role of the vitreous in the pathogenesis of MH. He also proposed a system of MH staging, from impending to full-thickness MH. In his initial description in 1988, Gass postulated that the tangential contraction of the prefoveal “posterior hyaloid membrane” resulted in the detachment of the central photoreceptors and then in the opening of the fovea. The process of MH formation was divided into four stages which are still used today, despite the changes in their interpretation due to OCT findings. Finally, Gass proposed the use of a surgical approach design to prevent impending MH from evolving into full-thickness MH, by peeling off or dissecting the posterior hyaloid.1
Update on the pathogenesis of macular hole based on SD-OCT
For the first time, OCT has shown routinely the status of the posterior hyaloid or vitreous cortex, making it possible to combine in a single explanation, anteroposterior vitreous traction, the role of the vitreous cortex, and the formation of a foveal cyst as pre-hole condition. SD-OCT has now refined the description of the initial stages of impending MH, by showing discrete changes in the foveal tissue before the occurrence of a foveal cyst.26,45–47 These changes substantiate the concept of Stage 0 MH proposed by Chan et al. in 2004.25
Early stages of posterior vitreous detachment
How does PVD start in normal individuals?
The process of posterior vitreous detachment (PVD) is not yet clear, but its understanding has progressed thanks to the images obtained with OCT of the posterior hyaloid in normal eyes, and in fellow eyes of eyes with MH. It was usually thought that PVD occurred suddenly after a long period of vitreous gel liquefaction, intravitreal lacunae formation, and posterior hyaloid erosion by one of these lacunae, thus suddenly giving the liquefied vitreous access to the preretinal space.48–51 However, contrary to this belief, both OCT studies52 and ultrasonographic observations53 have established that in normal individuals, the process of PVD starts gradually at the posterior pole, around the fovea, and occurs relatively early in life, long before detachment of the Weiss ring.54
The attachments of the posterior hyaloid to the foveal center and optic disc are the last to be released. The reason for posterior hyaloid detachment from the retina remains unclear. It might be due to the action of anteroposterior forces that follow the direction of the collagen fibers of the vitreous body. An alternative explanation, which takes into account the presence of the precortical vitreous pocket,55–57 might be the passage of liquefied vitreous, behind the thinned posterior hyaloid which, combined with ocular movements, would extend its detachment. Both mechanisms might lead to the creation of oblique tractional forces on the foveal floor.
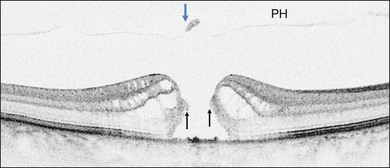
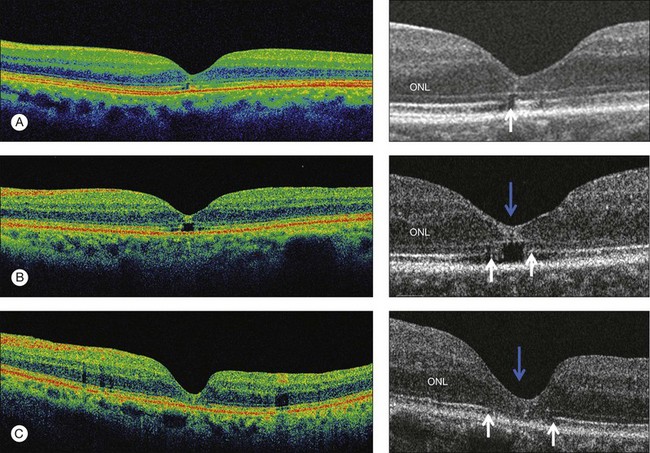
Early changes in foveal tissue (Stage 0 macular hole)
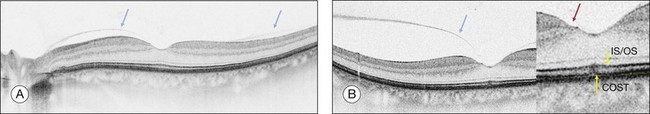
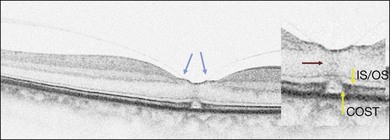
On the basis of the observation that in patients with MH, OCT of the fellow eye often showed partial vitreous detachment around the fovea,13,43,44 Chan et al.,25 who studied the risk of the fellow eye developing MH, proposed to call cases with partial perifoveal vitreous detachment but no foveal cyst, “Stage 0 MH” (Fig. 117.1 and see Fig. 117.2, available online). Today, in such cases, SD-OCT shows, even in asymptomatic eyes, slight changes in the foveolar structure long before the occurrence of the early foveal cyst that characterizes Stage 1 MH or impending hole. When this happens, it is possible to detect slight focal elevation of the inner curvature of the foveal center due to the traction exerted by the posterior hyaloid.46 Minor changes in the cone outer segment tips (COST, or Verhoeff membrane58), line at the foveal center,47 or subtle changes in the reflectivity of the center of the foveola, along an anteroposterior axis extending from the internal limiting membrane (ILM) to the inner segment/outer segment (IS/OS) junction line, may also be detected by high-definition SD-OCT scans. At this stage, complete normalization of the fovea may occur after vitreofoveal separation.
Impending macular hole
Stage 1A
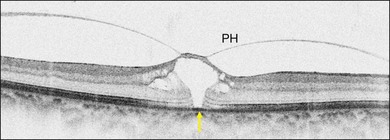
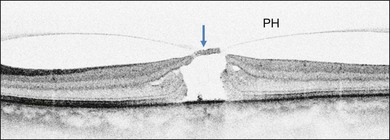
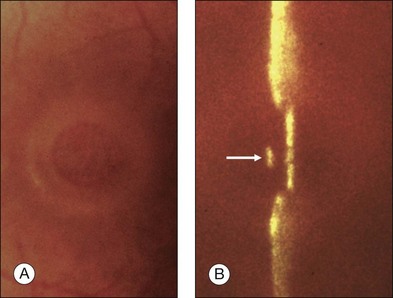
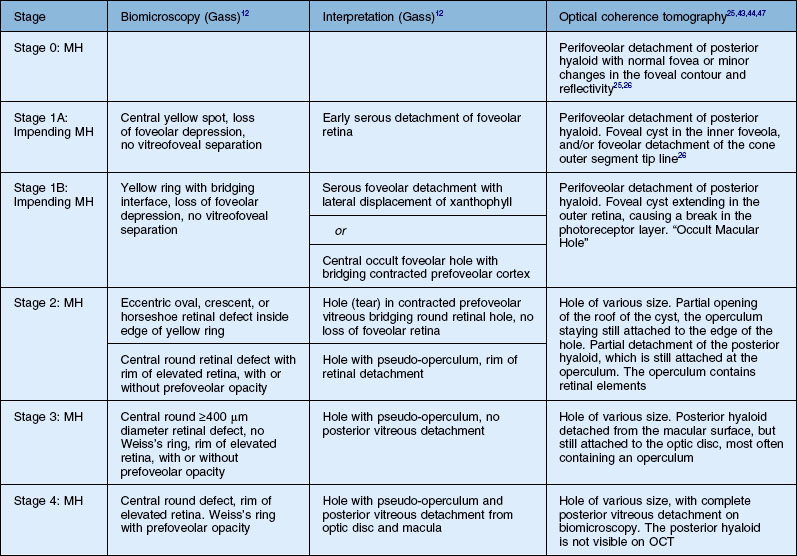
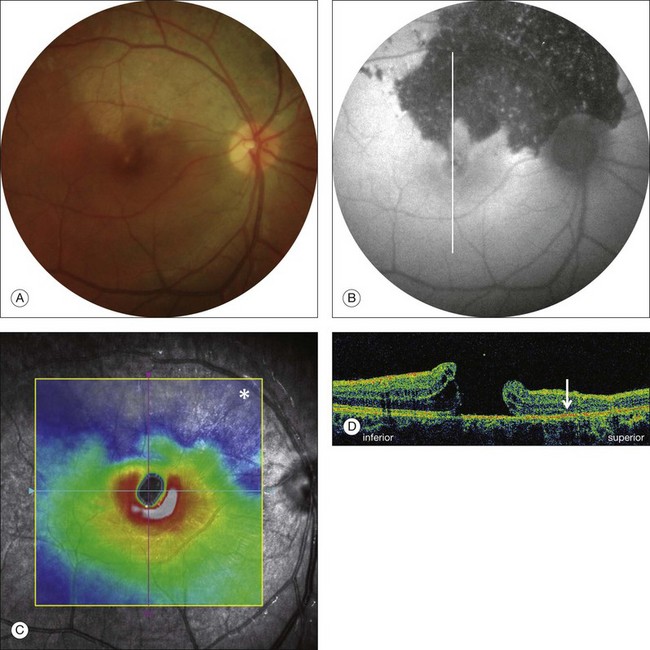
Stage 1A (Fig. 117.3 and see Fig. 117.4, available online) was defined by Gass as a central yellow spot and loss of the foveal depression associated with no vitreofoveal separation. The yellow spot was thought to be due to early serous detachment of the foveolar retina.12
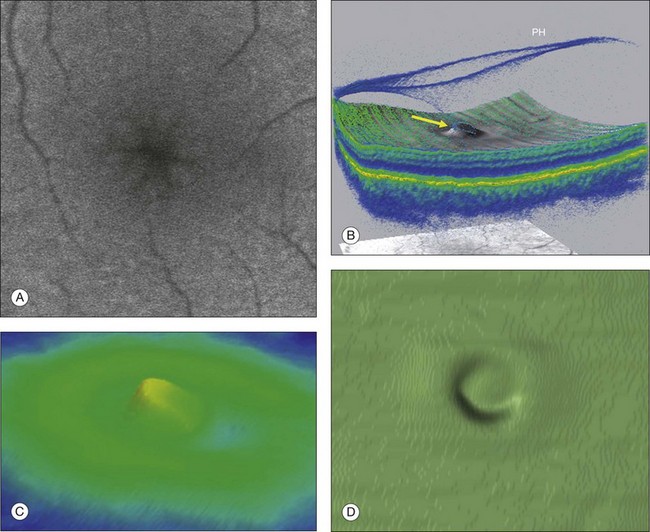
Fig. 117.4 Stage 1A impending macular hole (same case as in Fig. 117.3). (A) Autofluorescence photograph of the macula showing that the clear spaces of the cystic spaces encroach on the xanthophyll. (B) Reconstruction of the volume of the vitreoretinal interface showing the cone of the posterior hyaloid (PH) adherent to the top of the foveal pit (arrow). (C,D) Three-dimensional views of the foveal surface showing the elevation of the roof of the foveal cyst into the foveal pit.
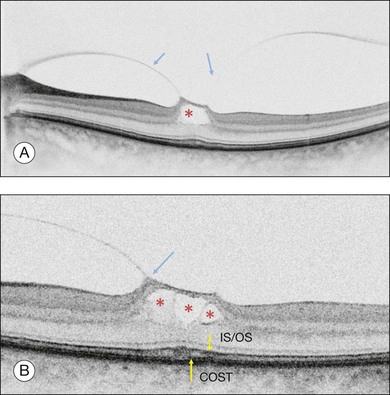
OCT subsequently showed that Stage 1A was in fact characterized by an inner foveal cyst. The foveal floor is elevated by the traction exerted by the posterior hyaloid which is detached around the foveola but still attached at its center.43,44 Some dissociated Henle fibers are often visible inside the cyst but the retinal outer layers may be intact.26,44 However, SD-OCT has shown that in some cases the photoreceptor IS/OS layer may be elevated or even disrupted at the foveal center. Moreover, Takahashi et al.47 showed that the yellow spot might be due to the foveolar detachment of the cone outer segment tip line, even before the development of inner splits or cysts.
Stage 1B
Stage 1B (Fig. 117.5) was also called an occult hole by Gass,12 who characterized this stage as the combination of a yellow ring in the fovea, the absence of vitreofoveal separation and the loss of the foveal depression. These features were interpreted as centrifugal displacement of the foveolar retina, with the contracted pre-foveolar cortex continuing to bridge the hole. OCT has shown that the posterior hyaloid is detached from the macular surface except at the foveal center, and that the inner foveal cyst characterizing Stage 1A is completed by disruption of the outer retina up to the retinal pigment epithelium (RPE).26,43,44,47,59 In that sense, Stage 1B impending MH is a true occult MH. The yellow ring is due to the edematous border of the disrupted outer retina.44
Clinical and imaging features of full-thickness macular hole
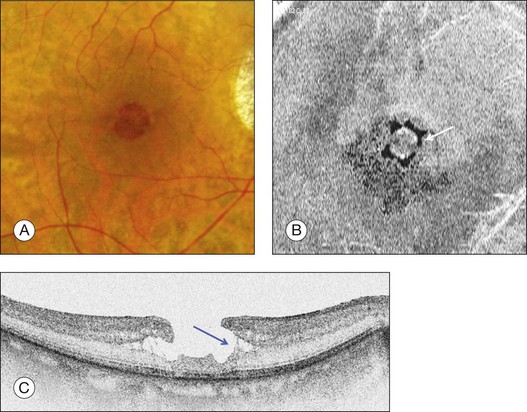
Stage 2 macular hole
Stage 2 MH (Fig. 117.6) was described as an eccentric oval, crescent or horseshoe-shaped retinal defect inside the edge of the yellow ring.12,60 This was interpreted as a tear in the contracted pre-foveolar vitreous tissue bridging the round retinal hole, with no loss of foveolar retina.12 OCT however, has shown that the incompletely detached operculum is pulled in an oblique direction by the incompletely detached posterior hyaloid.13,43 Moreover, recent observations by Takahashi and colleagues showed that neuronal elements formed a constitutive part of the operculum at least in some cases.45,47 This observation corroborates the histologic findings of Ezra, which showed the presence of cones constituents in two-thirds of the opercula he examined.61
Stage 3 macular hole
According to Gass’s definition, the Stage 3 MH (Fig. 117.7 and see Fig. 117.8, available online) is a central round retinal defect more than 400 µm in diameter, with a rim of elevated retina, with or without pre-foveolar pseudo-operculum and without a Weiss’s ring. In fact, OCT has shown that whereas the vitreous is not detached from the optic disc in these cases, it is completely detached from the retinal surface over the posterior pole and is not connected to the hole edge. Moreover, precise measurement of the hole diameter on OCT scans has shown that the size of Stage 3 MH varies greatly. Although the diameter of Stage 2 holes generally tends to be smaller than that of Stage 3 holes, large Stage 2 and small Stage 3 holes are in fact not uncommon.47,62
Macular hole and epiretinal membrane
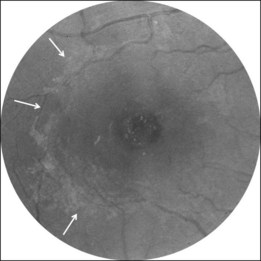
A noncontractile epiretinal membrane (ERM) (Fig. 117.9) may cover the macular surface around the hole. It is especially well visualized on blue reflectance photographs.63 The frequency of the formation of an ERM increases with the stage, duration, and size of the hole.63–65 However, this does not affect the surgical prognosis once the membrane has been peeled off during surgery.
OCT classification of macular hole
OCT has now become an essential complement to biomicroscopy for the diagnosis of macular diseases and especially macular holes, and the Gass’s classification now needs to be revised according to the OCT features (Fig. 117.10). However, the classification in four stages is still useful, although the interpretation of each stage should be updated. Diagnosis of Stage 1, i.e., impending MH, is helpful in detecting eyes at risk of full-thickness MH. It should also be useful to add a Stage 0 to the present classification. At this stage, the posterior hyaloid is still attached to the foveal center, sometimes resulting in minor changes in the contour of the foveal pit. The special feature of Stage 2 is that the posterior hyaloid is still attached to the hole edge and might constitute the target of vitreolysis drugs as a first-line nonsurgical treatment. Stages 2 and 3 have in common the fact that the vitreous is not detached from the optic disc, and in both stages, detachment of the vitreous is one of the goals of surgery in these cases. By Stage 4, the vitreous is already detached from the retina and optic disc pit and surgery is easier. The diameter of the hole, which is widely accepted as a prognostic factor of the surgical success rate, is weakly correlated with the anatomic stage of the hole. Lastly, the present classification does not take into account the possible presence of an epiretinal membrane around the hole, which is more frequent in Stages 3 and 4 (Table 117.1).
Differential diagnosis
Lamellar macular hole
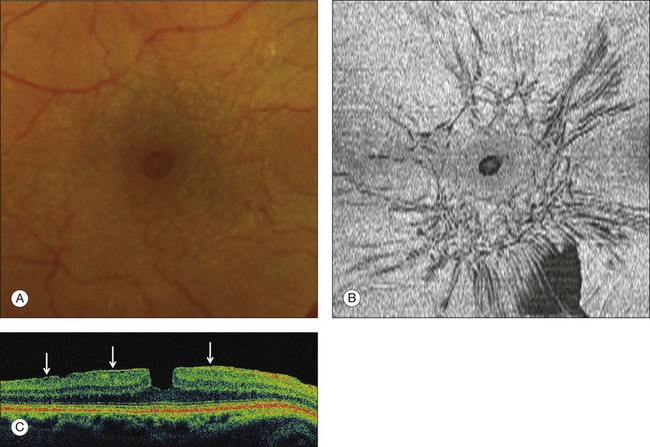
The term “lamellar macular hole” (LMH) was coined by Gass in 1975, to characterize a macular lesion resulting from the opening of the central cyst of a cystoid macular edema. The term “lamellar hole” was used to describe both the end stage of a cystoid macular edema14 and the aborted process of formation of a macular hole.44,66 With the advent of high resolution or spectral domain OCT, the thinning of the foveal center due to the contraction of an epiretinal membrane was also referred to as a lamellar macular hole.67
Histology of lamellar MH
Frangieh et al.14 described the histology of 17 cases of LMH of various etiologies. The cases were characterized by thinning of the foveal tissue leaving the RPE and photoreceptor layers intact, but causing partial loss of the inner nuclear layer. A cleft between the inner and outer retina was present at the edges of the lamellar hole, as well as cystic changes. In some cases, remnants of vitreous bands adhered to the lamellar hole edge. An ERM was found in several cases around the lamellar hole edge14,68 and was thought to be the cause of certain lamellar holes.68,69
Biomicroscopy
On biomicroscopy, LMHs differ from FTMHs because they are rarely round but rather bi- or tri-lobulated. Their center is reddish like that of FTMH, but their edge is thin whereas the FTMH edge is thick and elevated. The Watzke test, performed with a thin slit, is negative, but in small FTMH, the slit may appear as continuous.70 However, when the aiming beam of a laser is reduced to its minimum intensity and projected onto the center of an LMH, it is usually perceived by the subject, unlike that which occurs with a FTMH.71
Optical coherence tomography
Lamellar MH, i.e., defects in the inner fovea due to the avulsion of the roof of a foveal cyst (either tractional or due to cystoid macular edema), is characterized on OCT (Fig. 117.11) by irregular thinning of the foveal floor, a cleavage between the inner and outer retina at the lamellar hole edge, and the absence of a contractile epiretinal membrane.66 Using high-resolution OCT, Witkin et al.67 also described cases combining foveal thickening due to ERM contraction, with stretching of the foveal edge resulting in the thinning of the foveal floor. These cases may in fact represent a type of macular pseudohole induced by both centripetal and centrifugal contraction of the ERM between several eccentric epicenters.
Macular pseudoholes
The term “macular pseudohole” was coined by Allen and Gass72 in 1976, to designate a roundish centrofoveal image seen on biomicroscopy, which was due to the centripetal contraction of an epiretinal membrane. This contraction induces the verticalization of the edge of the foveal pit. Vision may remain relatively good and there are no microscotomas.
Other round foveolar images
Microholes
The term “microhole” was coined by Cairns73 in 1988, long before the use of OCT, for dark-reddish lesions in the center of the fovea ranging from 50 to 150 µm in diameter. None of these microholes turned into idiopathic FTMH. Retrospectively, it is today difficult to define the type of case, which at the time could have been described as a microhole, and what its OCT image would have been. Another series of cases presenting with a reddish foveal spot was examined with OCT1 by Douglas et al.74 who concluded that neither a hole nor an intraretinal break was disclosed by OCT. A better indication was given by Zambarakji et al.75 who studied cases very similar to those described by Cairns, and found, with OCT3, a break in the photoreceptor layer at the foveal center. What have been called “microholes” might therefore not be FTMH but in some cases, spontaneously closed FTMH.62 However, other diseases may also cause similar breaks in the centrofoveal inner segment/outer segment (IS/OS) line. Disruption of this line may be transient, as in multiple evanescent white dot syndrome,76 or either persist as a sequelae in solar retinopathy,77 or after exposition to drugs such as “poppers”.78
Non-idiopathic (secondary) MH
Orbital trauma and high myopia
These MH (Fig. 117.13, available online) usually occur in children and young male adults, due to accidents at work or in the home, or during ball games.79,80 They are due to sudden axial compression of the eye resulting in equatorial expansion and retinal rupture of the fovea. The hole may be combined with other fundus lesions such as choroidal or Bruch’s membrane disruption, commotio retinae, sclopetaria, or peripheral breaks. The visual prognosis depends not only on the closure of the hole but also on the topography of the other lesions. If a choroidal disruption passes through the fovea, or if the post-contusive RPE atrophy includes the foveal center, the visual prognosis will be poor. Unlike that which occurs in idiopathic MH, the vitreous is not detached at all from the posterior pole. Surgery is usually successful, but a traumatic MH may also sometimes close spontaneously within the first weeks of its occurrence.81–83
High myopia
MHs are one of the complications of high myopia, together with posterior staphyloma and choroidal atrophy. Their pathogenesis may be different from that of non-myopic eyes because the vitreous cortex often remains adherent to the retinal surface. Some of these MHs occur after a progressive decrease in vision due to the worsening of foveoschisis.84 Others may be asymptomatic and may only be disclosed by OCT examination of the fundus.85 Despite progress in vitreous cortex and ILM removal, the anatomic and visual postoperative prognoses for MH in high myopia remains lower than for idiopathic MH (see Chapter 113, High myopia and the vitreoretinal complications).86,87
Surgery for macular hole
Introduction
Kelly and Wendel first initiated successful surgery for MH and reported their results for 52 cases in 1991.2 Their success rate was 58% with a technique combining extensive vitrectomy, detachment of the posterior vitreous cortex, peeling of any epiretinal membrane around the hole, thorough fluid–gas exchange and postoperative facedown positioning. Two years later in a larger series of 170 eyes, the closure rate reached 73%.103 The technique of posterior vitreous cortex removal had benefited from the attempt made by de Bustros to prevent surgically the progression of impending hole to full thickness hole.104
How do vitrectomy and gas work to close the hole?
Releasing vitreous traction on the hole edge
For a long time, it was thought that the main role of vitrectomy was to release the vitreous traction on the hole edge. OCT modified this view by showing that the vitreous only exerts traction on the hole edge in Stage 2 holes via the undetached operculum, but not in Stages 3 and 4 holes. The role of vitreous traction release has been shown in certain cases of spontaneous MH closure62 and after vitreoretinal separation provoked by vitreolysis.105 However, in the majority of cases, i.e. Stages 3 and 4 MH, vitrectomy in itself exerts no action on the hole edge but is only useful in making room for gas or allowing maneuvers such as ERM or ILM peeling.
The role of gas in macular hole closure has been extensively debated. The most likely role is that the gas bubble acts first by dehydrating the hole edge and then by preventing fluid currents from hampering the healing process.106 However, even gas tamponade is not always essential for hole closure since for small macular hole (i.e., <400 µm), the mere release of vitreous attachment by vitreolysis may result in up to 60% of hole closure,105 although gas tamponade raises the success rate for small holes to ≥95%. For large holes, an effective gas tamponade is mandatory to obtain a success rate of over 90%.
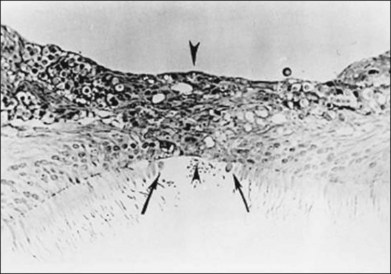
The healing process, histology, animal models, and early OCT
It has been shown, both in man and in animal models, that the closure of macular holes is due to the proliferation of glial cells, which close the break caused by vitreous traction on the foveal center (Fig. 117.14). Histopathologic studies of four eyes with surgically closed macular holes all showed that the hole was sealed by the proliferation of Müller cells, but that a residual defect persisted in the photoreceptor layer, which measured 16–250 µm.107–109
A rabbit model of retinal hole closure was constructed, which enabled the steps involved to be followed histologically.110 By the 4th day after gas tamponade, glial cell proliferation had occurred at the hole edge, and by the 7th day, the hole had been closed by glial tissue.
Optical coherence tomography has also occasionally been performed through silicone oil111 or through a gas bubble.112,113 These OCT observations showed that on the day after surgery the hole edge had flattened and that the hole had become smaller or had closed. In some eyes, the hole seemed to have closed at day 3, and all holes were closed by healing tissue by day 7.
Principles and techniques of macular hole surgery
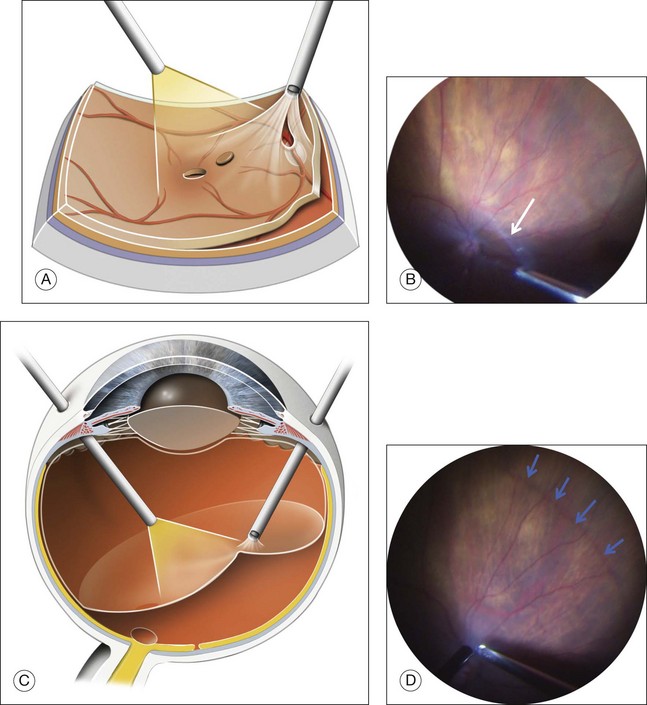
Posterior hyaloid detachment
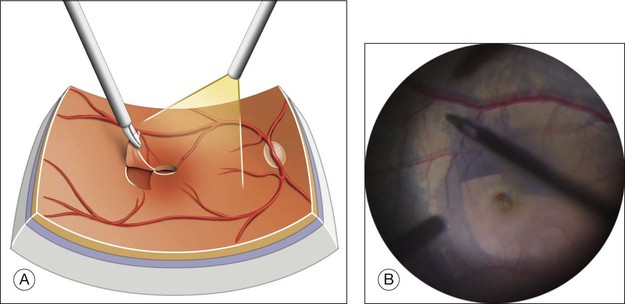
Detachment of the posterior hyaloid (PH) (Fig. 117.15; and see Video 11a, available online) is a crucial step in Stage 2 and 3 MH, in which, for Stage 2, the vitreous still adheres to the operculum and optic disc, and for Stage 3, to the optic disc only. The concept of detaching the posterior vitreous cortex after completing core vitrectomy was introduced in the late 1980s by the group headed by Ron Michels (S. de Brustos, pers comm). Histopathology showed that the substance which was separated from the retinal surface exactly corresponded to the vitreous cortex.114 Various tools have been used for this separation, including an aspirating cannula with a rigid or soft tip, a microvitreoretinal blade,115 or an aspirating forceps,116 and the vitreous probe. The 23G or 25G vitreous probes, whose aspirating port is closer to the tip of the probe, are now very effective for firm aspiration and detachment of the posterior hyaloid. Direct aspiration of the vitreous fibers attached to the Weiss ring indeed appears to be the most effective way of lifting the vitreous cortex en bloc and gradually extending its detachment to the equator in all the quadrants of the fundus. After completing core vitrectomy, it is also possible to inject diluted triamcinolone in front of the posterior pole, for clearer visualization of the vitreous cortex.
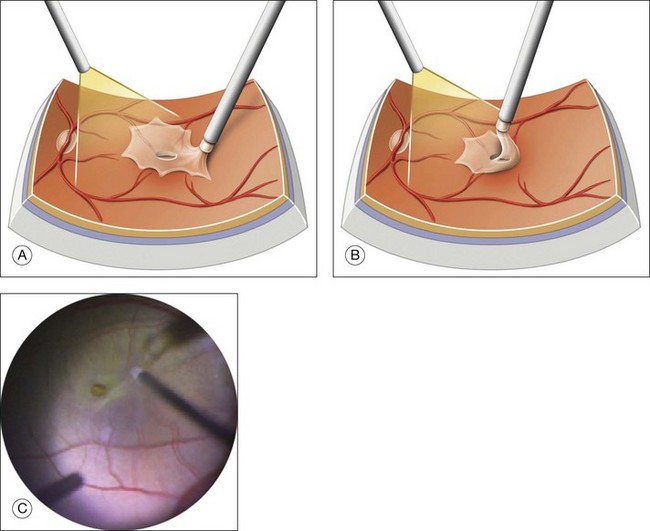
Epiretinal membrane peeling
When present, the epiretinal membrane around the hole should be peeled off.63,65 ERM are usually soft and friable. They can be removed by brushing the retinal surface, or with forceps (Fig. 117.16; see also Video 11b, available online). They adhere firmly to the thickness of the hole edge. If a dye that stains the ILM, such as Brilliant Blue, is used before removing the ERM, the ERM is not stained and appears as a “negative” at the surface at the ILM. The ERM can also be removed en bloc together with the ILM.
Internal limiting membrane peeling
ILM peeling (Fig. 117.17; see also Video 11c, available online), as a method of improving the MH closure rate was first described by Eckardt et al. in 1997.117 The technique has evolved and the use of dyes has greatly helped ILM visualization. End-gripping forceps are used to pinch and tear a flap of ILM without touching the optic nerve fibers. The ILM is then peeled off in a circular movement, thus creating a “maculorrhexis”.118 The optimal size of the area of peeling is still being debated.
Vital dyes
The use of dyes to stain the ERM and ILM, also known as chromovitrectomy119 has made the peeling of the retinal surface more precise, more complete and less traumatic. (See Chapter 127, Pharmacology at surgery.)
Indocyanine green and infracyanine
Indocyanine green (ICG) was introduced by Kadonosono et al.120 in 2000 for ILM staining, and is still widely used, despite concern in some quarters regarding its safety. ICG has a selective affinity for the ILM and was initially used at the concentration of 0.5%, but because of changes in the RPE and visual field defects, this concentration was reduced to 0.125% and even 0.05%, which makes the staining paler but still useful. Nevertheless, Engelbrecht et al. using an ICG concentration at 0.12% concentration, observed RPE changes in half their cases.121 A meta-analysis of ILM peeling in 837 cases showed worse functional results after the use of ICG at the higher concentration of 0.5%.122 The osmolarity of the ICG solution might have a harmful effect on the retina. Initially, an 0.5% ICG solution was used, which had an osmolarity of 250 mOsm. The use of a concentration of 0.05%, whose osmolarity is 290 mOsm, combined with a brief time of contact of no more than 30 seconds with the retina, seems to give no sign of RPE toxicity.123 Infracyanine, an iodine-free product, diluted in 5% glucose, results in an iso-osmolar solution, which might be safer for the retina.124 However infracyanine also enters the RPE cells which it may stain for months after surgery, and like ICG125 also stains the axons of the retinal ganglion cells.126,127
Finally, as exposure to light seems to increase the risk of damage to the retina, the light should be turned off during the short time of ICG contact with the retina.128 Opinion among vitreoretinal surgeons remains divided on the possible toxicity of ICG application to the retina. ICG should therefore only be used at the minimal concentrations of 0.025–0.05% (0.25–0.5 mg/mL) and for a very short time.
Trypan blue
Trypan blue (TB) stains the ERM well but the ILM less effectively. Nevertheless, it remains an alternative to ICG. To improve TB staining of the ILM, it must be used after fluid–gas exchange. Alternatively, it is easier to mix the dye isovolumetrically in 10% glucose, to create heavy TB, which falls onto the posterior pole and gives acceptable staining after a contact of 2 minutes.129 In most studies, Trypan blue gave no signs of toxicity for the RPE or for neuronal tissue.130
Brilliant Blue
Brilliant Blue (BB) has a selective affinity for the ILM and gives good staining in an iso-osmolar solution of 0.25 mg/mL (0.025%). The staining occurs after a brief contact with the dye injected onto the retinal surface. In animal experiments, the safety profile of BB was good,131 and it seems the best alternative to ICG.132,133
Type of gas to use in MH surgery
Various gases have been used for MH surgery, including C3F8, C2F6, SF6, and air. The rationale for preferring one gas to another is based on the expected duration of the gas bubble. If we assume that most MH close within 3–7 days of tamponade, a bubble large enough to insulate the macula from intraocular fluid during this period will be sufficient. This, for instance, is the case for a 17% C2F6–air mixture, provided the vitrectomy is large enough to create a large gas bubble. With this mixture, the gas bubble still covers the macula 1 week after surgery if the head is in the upright position, and still fills more than 70% of the vitreous cavity (Fig. 117.18). The essential characteristic of the gas mixture is not so much to persist for a long time in the eye as to decrease slowly in quantity during the first postoperative week. If the hole is small and a 3-day tamponade is assumed to be sufficient, 20% SF6–air, or even air alone, might be enough, but it may be preferable for the patient to remain facedown during that time.
Use of silicone oil in MH surgery
Silicone oil has been used to avoid the need for positioning in patients unable to maintain the facedown position, to allow air travel after surgery, or to ensure prolonged tamponade in case of failure of the initial surgery. In such cases, some authors stressed the advantages of the use of silicone oil, which gave good results.134–137 Others noted that the anatomic results were no better with silicone oil than with gas, or that the visual results were worse.138–140 Consequently, silicone oil should be used sparingly in MH surgery.141
Use of healing adjuvants
Soon after the onset of MH surgery was introduced, attempts were made to improve macular hole closure by applying healing adjuvants. Bovine transforming growth factor β (TGF-β) was used at first and gave favorable results in a randomized study,142 but these results were not reproduced with recombinant TGF-β.143 Autologous serum has not proved effective in improving MH closure,144 but autologous platelet concentrates successfully increased the closure rate of MH in a randomized study,145 although they are not used routinely.
Peroperative complications
Specific peroperative complications are rare. Those that do occur are usually due to vitreous detachment and ILM peeling. In Stages 2 and 3 MH, the posterior hyaloid has to be detached from the optic disc and the retina, which may result in retinal breaks at the equator or very occasionally, paravascular breaks.146,147 If extensive vitrectomy is accompanied by shaving of the vitreous base, intrabasal vitreous breaks may also occur, although today they are less frequent, thanks to the high cutting speed and improved fluidics of the new vitrectomy machines.148 Nevertheless, rates of 10–20% are currently reported for peripheral retinal breaks that occur during vitrectomy for MH.147 This highlights the imperative need to check the retinal periphery before performing fluid–gas exchange, in order to treat any peripheral breaks by laser or cryotherapy and avoid the risk of retinal detachment.147,149
Postoperative positioning
Although Kelly and Wendel considered that strict facedown positioning for at least a week was essential to the success of vitrectomy for MH,2 attempts to reduce or suppress the burden of positioning started as early as 1997 with a pilot study by Tornambe et al.150 The authors of this study postulated that the most important function of the gas bubble was to isolate the hole from vitreous fluid, and argued that if the bubble was large enough to cover the hole, successful closure would be possible regardless of the patient’s position. The same authors discussed the negative effect of the long duration of the hole on the success rate. It is clearer today that the indication for an alleviated position should take account of hole diameter151 and should be reserved for small holes.
Results of surgery
Anatomic results
As stated above, in the initial series of 52 cases published by Kelly and Wendel in 1991,2 the successful closure rate for MH surgery was 58%, but in the series of 170 eyes published a year later, the rate rose to 73%.103 However, in 1996, a randomized prospective study of vitrectomy for Stages 3 and 4 MH observation only achieved a success rate of 69% in the vitrectomy group,152 thus showing that at the time surgery for MH was still in the of learning curve phase. Today the closure rate is currently 85% or more.141,153
Results according to hole size
The concept that the success rate for MH surgery depends on hole diameter emerged in 2002 with the publications by Ip et al.154 and Ullrich et al.155 Ip and colleagues showed that the closure rate was 92% for MH <400 µm but only 56% for MH of ≥400 µm, as measured on OCT.154 Subsequent observations confirmed the importance of the size of the hole for its closure. In a series of MH, which had closed spontaneously, mean hole diameter at baseline was <200 µm.62 In a randomized study in which the MH closure rates were compared after intravitreal injection of ocriplasmin and after placebo, MH <250 µm had a closure rate of 58.3%.105 These findings highlighted the prognostic value of MH diameter for the efficacy of treatment and opened the way to the introduction of the idea of individual treatments tailored to the initial size of the hole.
Results according to ILM peeling
Since the publication by Eckardt et al.117 showing that the MH closure rate might improve if the ILM was peeled off, many publications, all of them retrospective, have reported closure rates of 87–100% in relatively large series of cases after peeling of the ILM.156–159 Some of these studies have also compared ILM peeling and non-peeling in large series of eyes, and give success rates between 77% and 89% without peeling compared with 92–97% with peeling.159–161 For instance, in a retrospective study, Kumagai obtained 92% MH closure after ILM peeling in 175 MH compared with 81% closure without ILM peeling in 417 MHs. An interesting observation by this author was that the initial success rate for holes measuring >400 µm was significantly less than that for holes measuring <400 µm.160 So far, however, only three prospective randomized studies of ILM peeling versus nonpeeling in MH surgery have been published. Success rates in the peeling groups ranged from 84% to 92%, compared with only 32–48% in the nonpeeling groups.162–164 These results for nonpeeling are far below the success rate of 58% initially obtained by Kelly and Wendel in 1991.2 The discrepancy between the results for the control groups in the above randomized studies and those of other retrospective studies seems to indicate that other conditions, in addition to ILM peeling, are necessary for MH closure.
On the other hand, it appears that the need for ILM peeling may depend on the size of the hole. In a retrospective study, Tadayoni et al.165 showed that ILM peeling had a positive effect on MH closure in a series of 84 eyes (100% MH closure with peeling, versus 83.3% without). However, this effect did not apply to MH <400 µm (100% closure in both group). For MHs >400 µm, the difference between the effects of peeling and non-peeling increased (100% closure with peeling versus 73.3% without). This tendency was confirmed by a multicenter randomized trial in which the results for ILM peeling were compared with those for non-peeling in a series of 80 MHs >400 µm, and in which the success rate for peeling was 94.9% compared with 73.2% without peeling.166
Results according to postoperative positioning
From the beginning of MH surgery, facedown positioning for at least 1 week has been considered of crucial importance for MH closure. However, as early as 1997, Tornambe and colleagues obtained a reasonable success rate of 79% for hole closure without any facedown positioning.150 Several other studies that only included a few patients variously combined phacoemulsification, ILM peeling, the use of air or gas mixtures, brief facedown positioning (1–3 days) or none, and obtained success rates of 87–90%. In a randomized prospective trial comparing facedown and seated positioning, facedown positioning was found to give a better closure rate. However, there was no difference between the rates for the two positions when MHs were <400 µm in diameter.153 Another prospective randomized study compared facedown positioning and an alleviated position for MH no larger than 400 µm in diameter, and found an equally high success rate of over 90% for both positions.151 It therefore appears that small holes at least do not require facedown positioning, provided that the gas bubble stays in contact with the macula until the healing process has started to close the hole. For this purpose, various gas mixtures can be used, as long as the volume of the gas bubble remains large enough for 3–7 days to make an effective tamponade of the macula in the upright position. Holes >600 µm may, on the contrary, require longer facedown positioning and a longer-acting gas mixture.
Visual outcome
Overall results
There are few recent prospective studies in which visual acuity has been recorded in a controlled procedure. Moreover, due to the high rate of postoperative cataract, VA measurement only gives pertinent information concerning the value of MH surgery once the cataract has been operated on, or after combined cataract and MH surgery. In a retrospective study of 74 eyes operated on successfully for an MH, Scott et al.167 found that after cataract surgery, median VA was 20/40 and that 58% of eyes had a VA of 20/40 or more. Among 99 patients with closed MH, 91% of whom were pseudophakic at the end of follow-up, Haritoglou et al.168 also found that VA improved from a median of 20/100 to 20/40. After MH closure, preferred retinal locus shifts from an eccentric location on the edge of the hole to a more central position, but the new location is not always in the middle of the hole. A relative or absolute microscotoma detected by microperimetry persists in some cases.70,169 Visual acuity improves mainly because of the improvement in fixation stability.170
Anatomic and visual correlation
Long duration and large size are MH characteristics, which were considered poor prognostic factors for MH surgery.154,155,171,172 Today, however this may not always be the case, because ILM peeling has improved the anatomic prognosis for large holes, which is nearly the same as for smaller ones, and even long-standing MH may benefit from surgery.173 The size of the hole nevertheless affects visual outcome because the closure of large holes may leave a larger glial scar than the closure of smaller ones (Fig. 117.19). In this connection, it has indeed been shown on OCT that the morphology and thickness of the foveal photoreceptor layer correlated with final visual acuity.174,175
Postoperative complications of surgery
Retinal detachment
Retinal detachment (RD) may complicate MH surgery, as it does for other vitrectomy procedures. At the beginning of MH surgery, an especially high proportion of RD (up to 14%) has been reported,176 mainly due to postoperative inferior breaks. These breaks have been attributed to the traction exerted by the gas bubble on the inferior vitreous in the upright position. In more recent publications, the rate of retinal detachment tended to decrease under 2%,151,177,178 probably due to a more efficient search for retinal breaks during surgery and to more thorough removal of the inferior peripheral vitreous. However, some other authors still find a higher rate of retinal detachment after MH surgery than after ERM surgery.149
Cataract
Cataract is a common complication of pars plana vitrectomy after the age of 60, which explains the high rate of its occurrence after MH surgery. The postoperative rate of pseudophakia at 3 or 5 years is commonly 85–98%.167,179 This observation led many surgeons to propose performing combined cataract and MH surgery. In a comparative retrospective study, Muselier et al.180 showed that visual results and complications were no different, whether the cataract had been removed after or during MH surgery, but that combined surgery shortened the period required for visual recovery.
Visual field defects
Inferotemporal sectorial visual field defects were first described in young patients who had undergone pars plana vitrectomy with fluid–gas exchange,181 and were attributed to retinal nerve fiber layer damage to the nasal portion of the optic nerve rim, probably due to traction during cortical vitreous peeling.182 Another hypothesis was that injury to the nerve fiber layer by dehydration during the fluid–air exchange might cause visual field defects.183 In a prospective study in 1997, visual field defects were observed in 23% of cases,184 but in another series published in 2001, this incidence was only 1%.185 In any case, sectorial field defects do not significantly affect the outcome of MH surgery.
Reopening of macular holes
Late reopening of MH after successful surgery, i.e., reopening of the hole months or years after its closure, has been attested on OCT, and has been found to occur in 5–7% of cases.186,187 The reason for reopening remains unclear, but in most cases, it might be the proliferation of a secondary ERM around the hole,65 rather than a side-effect of cataract surgery, as is sometimes suggested. Systematic ILM peeling seems to eliminate the risk of MH reopening.168,188 Reopening might be a complication specific to large MH,157 which are precisely those whose initial closure rate is improved by ILM peeling. Once an MH reopens, reoperation, with peeling of the ILM and any ERM, if this was not done during the first operation, usually results in hole closure and visual improvement. If ILM has already been removed, a tangential traction at the limit of ILM ablation should be looked for and removed. Otherwise, a simple fluid–gas exchange may be enough to close the hole again.
Vitreolysis as A nonsurgical treatment for macular holes
Ocriplasmin (ThromboGenics NV, Leuven, Belgium), is a recombinant containing the catalytic domain of plasmin and has the same catalytic properties as the human plasmin used for vitreolysis. It has been used in several clinical trials to create a posterior vitreous detachment.189 The authors of one phase 3 study compared the respective effects at 1 month of a single intravitreal injection of ocriplasmin and placebo, on the closure rate of Stage 2 MH <400 µm. The overall success rate for the ocriplasmin group was 40.6% compared with 10.6% in the placebo group. For MH with a diameter of ≤250 µm, the success rate rose to 58.3%, but for holes with a diameter of 250–400 µm, it was only 24.6%.105 In future, vitreolysis might therefore be part of the treatment of selected cases of small Stage 2 MH.
![]() Bonus images for this chapter can be found online at http://www.expertconsult.com
Bonus images for this chapter can be found online at http://www.expertconsult.com
Fig. 117.4Stage 1A impending macular hole (same case as in Fig. 117.3). (A) Autofluorescence photograph of the macula showing that the clear spaces of the cystic spaces encroach on the xanthophyll. (B) Reconstruction of the volume of the vitreoretinal interface showing the cone of the posterior hyaloid (PH) adherent to the top of the foveal pit (arrow). (C,D) Three-dimensional views of the foveal surface showing the elevation of the roof of the foveal cyst into the foveal pit.
Video 11Macular Hole. Alain Gaudric
a.Induction of Posterior Vitreous Detachment. Weiss’ ring and Posterior Hyaloid.
b.Ablation of an Epimacular Membrane around the Macular Hole.
1 Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629–639.
2 Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654–659.
3 Knapp H. Uber isolirte Zerreissungen der Aderhaut in Folge von Traumen auf dem Augapfel. Arch Augenheilkd. 1869;1:6–29.
4 Noyes HD. Detachment of the retina with laceration at the macula lutea. Trans Am Ophthalmol Soc. 1871;1:128–129.
5 Ogilvie FM. On one of the results of concussion injuries of the eye (“holes” at the macula). Trans Am Ophthamol Soc. 1900;20:202–229.
6 Kuhnt H. Ueber eine eigenthümliche Veränderung der Netzhaut ad maculam (Retinitis atrophicans sive rareficans centralis). Z Augenheilkd. 1900;3:105–112.
7 Coats G. The pathology of macular holes. Royal London Ophthalmol Hosp Rep. 1907;17:69–97.
8 Zeeman WPC. Uber Loch- und Cystenbildung der Fovea centralis. Graefe’s Arch Clin Exp Ophthalmol. 1912;80:259–269.
9 Lister W. Holes in the retina and their clinical significance. Br J Ophthalmol. 1924;8:1–20.
10 Gass JDM. Stereoscopic atlas of macular diseases: diagnosis and treatment, 3rd ed. St Louis: CV Mosby; 1987. p. 684–93
11 Gass JD, Joondeph BC. Observations concerning patients with suspected impending macular holes. Am J Ophthalmol. 1990;109:638–646.
12 Gass JD. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol. 1995;119:752–759.
13 Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology. 1995;102:748–756.
14 Frangieh GT, Green WR, Engel HM. A histopathologic study of macular cysts and holes. Retina. 1981;1:311–336.
15 Ho AC, Guyer DR, Fine SL. Macular hole. Surv Ophthalmol. 1998;42:393–416.
16 Rahmani B, Tielsch JM, Katz J, et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology. 1996;103:1721–1726.
17 Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104:1033–1040.
18 Wang S, Xu L, Jonas JB. Prevalence of full-thickness macular holes in urban and rural adult Chinese: the Beijing Eye Study. Am J Ophthalmol. 2006;141:589–591.
19 Sen P, Bhargava A, Vijaya L, et al. Prevalence of idiopathic macular hole in adult rural and urban south Indian population. Clin Experiment Ophthalmol. 2008;36:257–260.
20 McCannel CA, Ensminger JL, Diehl NN, et al. Population-based incidence of macular holes. Ophthalmology. 2009;116:1366–1369.
21 Akiba J, Kakehashi A, Arzabe CW, et al. Fellow eyes in idiopathic macular hole cases. Ophthalmic Surg. 1992;23:594–597.
22 Lewis ML, Cohen SM, Smiddy WE, et al. Bilaterality of idiopathic macular holes. Graefes Arch Clin Exp Ophthalmol. 1996;234:241–245.
23 Ezra E, Wells JA, Gray RH, et al. Incidence of idiopathic full-thickness macular holes in fellow eyes. A 5-year prospective natural history study. Ophthalmology. 1998;105:353–359.
24 Chew EY, Sperduto RD, Hiller R, et al. Clinical course of macular holes: the Eye Disease Case–Control Study. Arch Ophthalmol. 1999;117:242–246.
25 Chan A, Duker JS, Schuman JS, et al. Stage 0 macular holes: observations by optical coherence tomography. Ophthalmology. 2004;111:2027–2032.
26 Takahashi A, Yoshida A, Nagaoka T, et al. Macular hole formation in fellow eyes with a perifoveal posterior vitreous detachment of patients with a unilateral macular hole. Am J Ophthalmol. 2011;151:981–989.
27 The Eye Disease Case–Control Study Group. Risk factors for idiopathic macular holes. Am J Ophthalmol. 1994;118:754–761.
28 Evans JR, Schwartz SD, McHugh JD, et al. Systemic risk factors for idiopathic macular holes: a case–control study. Eye. 1998;12:256–259.
29 Shah SP, Bunce C, Johnston RL, et al. Are biometric parameters a risk factor for idiopathic macular hole formation? Results of a matched case–control series. Br J Ophthalmol. 2006;90:117–118.
30 Grignolo A. Fibrous components of the vitreous body. Arch Ophthalmol. 1952;47:760–774.
31 Schepens CL. Fundus changes caused by alterations of the vitreous body. Am J Ophthalmol. 1955;39:631–633.
32 Maumenee AE. Further advances in the study of the macula. Arch Ophthalmol. 1967;78:151–165.
33 Reese AB, Jones IS, Cooper WC. Macular changes secondary to vitreous traction. Am J Ophthalmol. 1967;64:544–549.
34 Avila MP, Jalkh AE, Murakami K, et al. Biomicroscopic study of the vitreous in macular breaks. Ophthalmology. 1983;90:1277–1283.
35 Kakehashi A, Schepens CL, Trempe CL. Vitreomacular observations. II. Data on the pathogenesis of idiopathic macular breaks. Graefes Arch Clin Exp Ophthalmol. 1996;234:425–433.
36 Schepens CL. Macular holes and PVD. Ophthalmology. 1996;103:349–350.
37 Akiba J, Quiroz MA, Trempe CL. Role of posterior vitreous detachment in idiopathic macular holes. Ophthalmology. 1990;97:1610–1613.
38 Kornzweig AL, Feldstein M. Studies of the eye in old age: II. Hole in the macula. A Clinico-pathologic Study. Am J Ophthalmol. 1950;33:243–247.
39 Bronstein MA, Trempe CL, Freeman H. Fellow eyes of eyes with Macular Holes. Am J Ophthalmol. 1981;92:757–761.
40 McDonnell PJ, Fine SL, Hillis AI. Clinical features of idiopathic macular cysts and holes. Am J Ophthalmol. 1982;93:777–786.
41 Kishi S, Kamei Y, Shimizu K. Tractional elevation of Henle’s fiber layer in idiopathic macular holes. Am J Ophthalmol. 1995;120:486–496.
42 Folk JC, Boldt HC, Keenum DG. Foveal cysts: a premacular hole condition associated with vitreous traction. Arch Ophthalmol. 1998;116:1177–1183.
43 Gaudric A, Haouchine B, Massin P, et al. Macular hole formation: new data provided by optical coherence tomography. Arch Ophthalmol. 1999;117:744–751.
44 Haouchine B, Massin P, Gaudric A. Foveal pseudocyst as the first step in macular hole formation: a prospective study by optical coherence tomography. Ophthalmology. 2001;108:15–22.
45 Takahashi A, Nagaoka T, Ishiko S, et al. Foveal anatomic changes in a progressing stage 1 macular hole documented by spectral-domain optical coherence tomography. Ophthalmology. 2010;117:806–810.
46 Kumagai K, Hangai M, Larson E, et al. Vitreoretinal interface and foveal deformation in asymptomatic fellow eyes of patients with unilateral macular holes. Ophthalmology. 2011;118:1638–1644.
47 Takahashi A, Nagaoka T, Yoshida A. Stage 1-A macular hole: a prospective spectral-domain optical coherence tomography study. Retina. 2011;31:127–147.
48 Jaffe NS. Complications of acute posterior vitreous detachment. Arch Ophthalmol. 1968;79:568–571.
49 Eisner G. posterior vitreous detachment. Klin Monatsbl Augenheilkd. 1989;194:389–392.
50 Sebag J. The vitreous:structure, function, and pathobiology. The vitreous: structure, function, and pathobiology. New York: Springer-Verlag; 1989. p. 80–95
51 Wilkinson CP, Rice TA. Michels retinal detachment, 2nd ed. St Louis: Mosby; 1990. p. 30–4
52 Uchino E, Uemura A, Ohba N. Initial stages of posterior vitreous detachment in healthy eyes of older persons evaluated by optical coherence tomography. Arch Ophthalmol. 2001;119:1475–1479.
53 Johnson MW, Van Newkirk MR, Meyer KA. Perifoveal vitreous detachment is the primary pathogenic event in idiopathic macular hole formation. Arch Ophthalmol. 2001;119:215–222.
54 Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149:371–382.
55 Worst JG. Cisternal systems of the fully developed vitreous body in the young adult. Trans Ophthalmol Soc U K. 1977;97:550–554.
56 Kishi S, Hagimura N, Shimizu K. The role of the premacular liquefied pocket and premacular vitreous cortex in idiopathic macular hole development. Am J Ophthalmol. 1996;122:622–628.
57 Spaide RF. Measurement of the posterior precortical vitreous pocket in fellow eyes with posterior vitreous detachment and macular holes. Retina. 2003;23:481–485.
58 Srinivasan VJ, Monson BK, Wojtkowski M, et al. Characterization of outer retinal morphology with high-speed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49:1571–1579.
59 Michalewski J, Michalewska Z, Dziegielewski K, et al. Evolution from macular pseudohole to lamellar macular hole – spectral domain OCT study. Graefes Arch Clin Exp Ophthalmol. 2011;249:175–178.
60 Johnson RN, Gass JD. Idiopathic macular holes. Observations, stages of formation, and implications for surgical intervention. Ophthalmology. 1988;95:917–924.
61 Ezra E, Fariss RN, Possin DE, et al. Immunocytochemical characterization of macular hole opercula. Arch Ophthalmol. 2001;119:223–231.
62 Privat E, Tadayoni R, Gaucher D, et al. Residual defect in the foveal photoreceptor layer detected by optical coherence tomography in eyes with spontaneously closed macular holes. Am J Ophthalmol. 2007;143:814–819.
63 Blain P, Paques M, Massin P, et al. Epiretinal membranes surrounding idiopathic macular holes. Retina. 1998;18:316–321.
64 Cheng L, Freeman WR, Ozerdem U, et al. Prevalence, correlates, and natural history of epiretinal membranes surrounding idiopathic macular holes. Vitrectomy for Macular Hole Study Group. Ophthalmology. 2000;107:853–859.
65 Cheng L, Azen SP, El-Bradey MH, et al. Effects of preoperative and postoperative epiretinal membranes on macular hole closure and visual restoration. Ophthalmology. 2002;109:1514–1520.
66 Haouchine B, Massin P, Tadayoni R, et al. Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am J Ophthalmol. 2004;138:732–739.
67 Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113:388–397.
68 Guyer DR, Green WR, de Bustros S, et al. Histopathologic features of idiopathic macular holes and cysts. Ophthalmology. 1990;97:1045–1051.
69 Johnson RN, McDonald HR, Lewis H, et al. Traumatic macular hole: observations, pathogenesis, and results of vitrectomy surgery. Ophthalmology. 2001;108:853–857.
70 Guez JE, Le Gargasson JF, Massin P, et al. Functional assessment of macular hole surgery by scanning laser ophthalmoscopy. Ophthalmology. 1998;105:694–699.
71 Martinez J, Smiddy WE, Kim J, et al. Differentiating macular holes from macular pseudoholes. Am J Ophthalmol. 1994;117:762–767.
72 Allen AW, Jr., Gass JD. Contraction of a perifoveal epiretinal membrane simulating a macular hole. Am J Ophthalmol. 1976;82:684–691.
73 Cairns JD, McCombe MF. Microholes of the fovea centralis. Aust N Z J Ophthalmol. 1988;16:75–79.
74 Douglas RS, Duncan J, Brucker A, et al. Foveal spot: a report of thirteen patients. Retina. 2003;23:348–353.
75 Zambarakji HJ, Schlottmann P, Tanner V, et al. Macular microholes: pathogenesis and natural history. Br J Ophthalmol. 2005;89:189–193.
76 Li D, Kishi S. Restored photoreceptor outer segment damage in multiple evanescent white dot syndrome. Ophthalmology. 2009;116:762–770.
77 Jain A, Desai RU, Charalel RA, et al. Solar retinopathy: comparison of optical coherence tomography (OCT) and fluorescein angiography (FA). Retina. 2009;29:1340–1345.
78 Vignal-Clermont C, Audo I, Sahel JA, et al. Poppers-associated retinal toxicity. N Engl J Med. 2010;363:1583–1585.
79 Shakin JL, Yannuzzi LA. Posterior segment manifestations of orbital trauma. Adv Ophthalmic Plast Reconstr Surg. 1987;6:115–135.
80 Atmaca LS, Yilmaz M. Changes in the fundus caused by blunt ocular trauma. Ann Ophthalmol. 1993;25:447–452.
81 Barreau E, Massin P, Paques M, et al. [Surgical treatment of post-traumatic macular holes]. J Fr Ophtalmol. 1997;20:423–429.
82 Kusaka S, Fujikado T, Ikeda T, et al. Spontaneous disappearance of traumatic macular holes in young patients. Am J Ophthalmol. 1997;123:837–839.
83 Parmar DN, Stanga PE, Reck AC, et al. Imaging of a traumatic macular hole with spontaneous closure. Retina. 1999;19:470–472.
84 Shimada N, Ohno-Matsui K, Yoshida T, et al. Progression from macular retinoschisis to retinal detachment in highly myopic eyes is associated with outer lamellar hole formation. Br J Ophthalmol. 2008;92:762–764.
85 Coppe AM, Ripandelli G, Parisi V, et al. Prevalence of asymptomatic macular holes in highly myopic eyes. Ophthalmology. 2005;112:2103–2109.
86 Patel SC, Loo RH, Thompson JT, et al. Macular hole surgery in high myopia. Ophthalmology. 2001;108:377–380.
87 Ikuno Y, Tano Y. Vitrectomy for macular holes associated with myopic foveoschisis. Am J Ophthalmol. 2006;141:774–776.
88 Sakarya Y, Sakarya R, Kara S, et al. Giant macular hole in Alport syndrome. Ophthalmic Genet. 2011;32:64.
89 Georgalas I, Markomichelakis N, Ladas I. Retinal detachment due to a macular hole in a patient with Behçet disease treated with vitrectomy and silicone oil tamponade. Eur J Ophthalmol. 2008;18:1023–1024.
90 Mehta M, Katsumi O, Tetsuka S, et al. Best’s macular dystrophy with a macular hole. Acta Ophthalmol (Copenh). 1991;69:131–134.
91 Albini TA, Lakhanpal RR, Foroozan R, et al. Macular hole in cat scratch disease. Am J Ophthalmol. 2005;140:149–151.
92 Chaudhry NA, Flynn HW, Jr., Smiddy WE, et al. Macular hole surgery in the presence of prominent macular drusen. Arch Ophthalmol. 2000;118:131–132.
93 Rajagopal J, Shetty SB, Kamath AG, et al. Macular hole following electrical shock injury. Can J Ophthalmol. 2010;45:187–188.
94 Kusaka S, Hayashi N, Ohji M, et al. Macular hole secondary to fungal endophthalmitis. Arch Ophthalmol. 2003;121:732–733.
95 Thach AB, Lopez PF, Snady-McCoy LC, et al. Accidental Nd:YAG laser injuries to the macula. Am J Ophthalmol. 1995;119:767–773.
96 Uemoto R, Mizuki N. Spontaneous closure of a macular hole caused by a ruptured retinal arterial macroaneurysm. Eur J Ophthalmol. 2008;18:462–465.
97 Jin ZB, Gan DK, Xu GZ, et al. Macular hole formation in patients with retinitis pigmentosa and prognosis of pars plana vitrectomy. Retina. 2008;28:610–614.
98 Sodi A, Bini A, Passerini I, et al. Occurrence of full-thickness macular hole complicating Stargardt disease with ABCR mutation. Eur J Ophthalmol. 2006;16:335–338.
99 Manschot WA, Von Winning CHOM. Hole in the macula due to syphilis. Am J Ophthalmol. 1953;36:261–262.
100 Lee SH, Park KH, Kim JH, et al. Secondary macular hole formation after vitrectomy. Retina. 2010;30:1072–1077.
101 Kobayashi I, Inoue M, Okada AA, et al. Vitreous surgery for macular hole in patients with Vogt-Koyanagi-Harada disease. Clin Experiment Ophthalmol. 2008;36:861–864.
102 Brasil OF, da Cunha AL, de Castro MB, et al. Macular hole secondary to X-linked juvenile retinoschisis. Ophthalmic Surg Lasers Imaging. 42, 2011. Online: e4–5
103 Wendel RT, Patel AC, Kelly NE, et al. Vitreous surgery for macular holes. Ophthalmology. 1993;100:1671–1676.
104 de Bustros S. Early stages of macular holes. To treat or not to treat. Arch Ophthalmol. 1990;108:1085–1086.
105 Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367(7):606–615.
106 Berger JW, Brucker AJ. The magnitude of the bubble buoyant pressure: implications for macular hole surgery. Retina. 1998;18:84–88.
107 Funata M, Wendel RT, de la Cruz Z, et al. Clinicopathologic study of bilateral macular holes treated with pars plana vitrectomy and gas tamponade. Retina. 1992;12:289–298.
108 Madreperla SA, Geiger GL, Funata M, et al. Clinicopathologic correlation of a macular hole treated by cortical vitreous peeling and gas tamponade. Ophthalmology. 1994;101:682–686.
109 Rosa RH, Jr., Glaser BM, de la Cruz Z, et al. Clinicopathologic correlation of an untreated macular hole and a macular hole treated by vitrectomy, transforming growth factor-beta 2, and gas tamponade. Am J Ophthalmol. 1996;122:853–863.
110 Yamana T, Kita M, Ozaki S, et al. The process of closure of experimental retinal holes in rabbit eyes. Graefes Arch Clin Exp Ophthalmol. 2000;238:81–87.
111 Jumper JM, Gallemore RP, McCuen BW, 2nd., et al. Features of macular hole closure in the early postoperative period using optical coherence tomography. Retina. 2000;20:232–237.
112 Sato H, Kawasaki R, Yamashita H. Observation of idiopathic full-thickness macular hole closure in early postoperative period as evaluated by optical coherence tomography. Am J Ophthalmol. 2003;136:185–187.
113 Masuyama K, Yamakiri K, Arimura N, et al. Posturing time after macular hole surgery modified by optical coherence tomography images: a pilot study. Am J Ophthalmol. 2009;147:481–488.
114 Smiddy WE, Michels RG, de Bustros S, et al. Histopathology of tissue removed during vitrectomy for impending idiopathic macular holes. Am J Ophthalmol. 1989;108:360–364.
115 Han DP, Abrams GW, Aaberg TM. Surgical excision of the attached posterior hyaloid. Arch Ophthalmol. 1988;106:998–1000.
116 Gaudric A, Massin P, Qinyuan C. An aspirating forceps to remove the posterior hyaloid in the surgery of full-thickness macular holes. Retina. 1996;16:261–263.
117 Eckardt C, Eckardt U, Groos S, et al. [Removal of the internal limiting membrane in macular holes. Clinical and morphological findings]. Ophthalmologe. 1997;94:545–551.
118 Park DW, Lee JH, Min WK. The use of internal limiting membrane maculorrhexis in treatment of idiopathic macular holes. Korean J Ophthalmol. 1998;12:92–97.
119 Rodrigues EB, Meyer CH, Mennel S, et al. Mechanisms of intravitreal toxicity OF indocyanine green dye: implications for chromovitrectomy. Retina. 2007;27:958–970.
120 Kadonosono K, Itoh N, Uchio E, et al. Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol. 2000;118:1116–1118.
121 Engelbrecht NE, Freeman J, Sternberg P, et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2002;133:89–94.
122 Rodrigues EB, Meyer CH. Meta-analysis of chromovitrectomy with indocyanine green in macular hole surgery. Ophthalmologica. 2008;222:123–129.
123 Stalmans P, Van Aken EH, Veckeneer M, et al. Toxic effect of indocyanine green on retinal pigment epithelium related to osmotic effects of the solvent. Am J Ophthalmol. 2002;134:282–285.
124 Haritoglou C, Gandorfer A, Gass CA, et al. Histology of the vitreoretinal interface after staining of the internal limiting membrane using glucose 5% diluted indocyanine and infracyanine green. Am J Ophthalmol. 2004;137:345–348.
125 Weinberger AW, Kirchhof B, Mazinani BE, et al. Persistent indocyanine green (ICG) fluorescence 6 weeks after intraocular ICG administration for macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2001;239:388–390.
126 Paques M, Genevois O, Regnier A, et al. Axon-tracing properties of indocyanine green. Arch Ophthalmol. 2003;121:367–370.
127 Tadayoni R, Paques M, Girmens JF, et al. Persistence of fundus fluorescence after use of indocyanine green for macular surgery. Ophthalmology. 2003;110:604–608.
128 Yip HK, Lai TY, So KF, et al. Retinal ganglion cells toxicity caused by photosensitising effects of intravitreal indocyanine green with illumination in rat eyes. Br J Ophthalmol. 2006;90:99–102.
129 Lesnik Oberstein SY, de Smet MD. Use of heavy Trypan blue in macular hole surgery. Eye. 2010;24:1177–1181.
130 Grisanti S, Szurman P, Tatar O, et al. Histopathological analysis in experimental macular surgery with trypan blue. Br J Ophthalmol. 2004;88:1206–1208.
131 Enaida H, Hisatomi T, Hata Y, et al. Brilliant blue G selectively stains the internal limiting membrane/brilliant blue G-assisted membrane peeling. Retina. 2006;26:631–636.
132 Rodrigues EB, Costa EF, Penha FM, et al. The use of vital dyes in ocular surgery. Surv Ophthalmol. 2009;54:576–617.
133 Farah ME, Maia M, Rodrigues EB. Dyes in ocular surgery: principles for use in chromovitrectomy. Am J Ophthalmol. 2009;148:332–340.
134 Goldbaum MH, McCuen BW, Hanneken AM, et al. Silicone oil tamponade to seal macular holes without position restrictions. Ophthalmology. 1998;105:2140–2148.
135 Lappas A, Foerster AM, Kirchhof B. Use of heavy silicone oil (Densiron-68) in the treatment of persistent macular holes. Acta Ophthalmologica. 2009;87:866–870.
136 Saeed MU, Heimann H, Wong D, et al. Heavy silicone oil tamponade after failed macular hole surgery with perfluoropropane (C3F8): a report of five cases. Graefes Arch Clin Exp Ophthalmol. 2009;247:707–709.
137 Schurmans A, Van Calster J, Stalmans P. Macular hole surgery with inner limiting membrane peeling, endodrainage, and heavy silicone oil tamponade. Am J Ophthalmol. 2009;147:495–500.
138 Karia N, Laidlaw A, West J, et al. Macular hole surgery using silicone oil tamponade. Br J Ophthalmol. 2001;85:1320–1323.
139 Lai JC, Stinnett SS, McCuen BW. Comparison of silicone oil versus gas tamponade in the treatment of idiopathic full-thickness macular hole. Ophthalmology. 2003;110:1170–1174.
140 Tafoya ME, Lambert HM, Vu L, et al. Visual outcomes of silicone oil versus gas tamponade for macular hole surgery. Semin Ophthalmol. 2003;18:127–131.
141 Gaudric A. Macula hole surgery: simple or complex? Am J Ophthalmol. 2009;147:381–383.
142 Glaser BM, Michels RG, Kuppermann BD, et al. Transforming growth factor-beta 2 for the treatment of full-thickness macular holes. A prospective randomized study. Ophthalmology. 1992;99:1162–1172.
143 Thompson JT, Smiddy WE, Williams GA, et al. Comparison of recombinant transforming growth factor-beta-2 and placebo as an adjunctive agent for macular hole surgery. Ophthalmology. 1998;105:700–706.
144 Ezra E, Gregor ZJ. Surgery for idiopathic full-thickness macular hole: two-year results of a randomized clinical trial comparing natural history, vitrectomy, and vitrectomy plus autologous serum: Morfields Macular Hole Study Group Report no. 1. Arch Ophthalmol. 2004;122:224–236.
145 Paques M, Chastang C, Mathis A, et al. Effect of autologous platelet concentrate in surgery for idiopathic macular hole: results of a multicenter, double-masked, randomized trial. Platelets in Macular Hole Surgery Group. Ophthalmology. 1999;106:932–938.
146 Carter JB, Michels RG, Glaser BM, et al. Iatrogenic retinal breaks complicating pars plana vitrectomy. Ophthalmology. 1990;97:848–854.
147 Chung SE, Kim KH, Kang SW. Retinal breaks associated with the induction of posterior vitreous detachment. Am J Ophthalmol. 2009;147:1012–1016.
148 Scartozzi R, Bessa AS, Gupta OP, et al. Intraoperative sclerotomy-related retinal breaks for macular surgery, 20- versus 25-gauge vitrectomy systems. Am J Ophthalmol. 2007;143:155–156.
149 Guillaubey A, Malvitte L, Lafontaine PO, et al. Incidence of retinal detachment after macular surgery: a retrospective study of 634 cases. Br J Ophthalmol. 2007;91:1327–1330.
150 Tornambe PE, Poliner LS, Grote K. Macular hole surgery without face-down positioning. A pilot study. Retina. 1997;17:179–185.
151 Tadayoni R, Vicaut E, Devin F, et al. A randomized controlled trial of alleviated positioning after small macular hole surgery. Ophthalmology. 2011;118:150–155.
152 Freeman WR, Azen SP, Kim JW, et al. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. The Vitrectomy for Treatment of Macular Hole Study Group. Arch Ophthalmol. 1997;115:11–21.
153 Guillaubey A, Malvitte L, Lafontaine PO, et al. Comparison of face-down and seated position after idiopathic macular hole surgery: a randomized clinical trial. Am J Ophthalmol. 2008;146:128–134.
154 Ip MS, Baker BJ, Duker JS, et al. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002;120:29–35.
155 Ullrich S, Haritoglou C, Gass C, et al. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002;86:390–393.
156 Rice T. Internal limiting membrane removal in surgery for full-thickness macular holes. In: Madreperla SA, McCuen BW. Macular hole: pathogenesis, diagnosis, and treatment. Boston: Butterworth Heinemann; 1999:125–146.
157 Brooks HLJ. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000;107:1939–1949.
158 Haritoglou C, Gass CA, Schaumberger M, et al. Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am J Ophthalmol. 2001;132:363–368.
159 Sheidow TG, Blinder KJ, Holekamp N, et al. Outcome results in macular hole surgery: an evaluation of internal limiting membrane peeling with and without indocyanine green. Ophthalmology. 2003;110:1697–1701.
160 Kumagai K, Furukawa M, Ogino N, et al. Vitreous surgery with and without internal limiting membrane peeling for macular hole repair. Retina. 2004;24:721–727.
161 Tognetto D, Grandin R, Sanguinetti G, et al. Internal limiting membrane removal during macular hole surgery: results of a multicenter retrospective study. Ophthalmology. 2006;113:1401–1410.
162 Kwok AK, Lai TY, Wong VW. Idiopathic macular hole surgery in Chinese patients: a randomised study to compare indocyanine green-assisted internal limiting membrane peeling with no internal limiting membrane peeling. Hong Kong Med J. 2005;11:259–266.
163 Christensen UC, Kroyer K, Sander B, et al. Value of internal limiting membrane peeling in surgery for idiopathic macular hole stage 2 and 3: a randomised clinical trial. Br J Ophthalmol. 2009;93:1005–1015.
164 Lois N, Burr J, Norrie J, et al. Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: a pragmatic randomized controlled trial. Invest Ophthalmol Vis Sci. 2011;52:1586–1592.
165 Tadayoni R, Gaudric A, Haouchine B, et al. Relationship between macular hole size and the potential benefit of internal limiting membrane peeling. Br J Ophthalmol. 2006;90:1239–1241.
166 Tadayoni R, Creuzot-Garcher C, Korobelnik J-F, et al. Internal Limiting Membrane Peeling for Large Macular Holes: A Randomized, Multicentric, and Controlled Clinical Trial. Invest. Ophthalmol Vis Sci. 2009;50:5206.
167 Scott IU, Moraczewski AL, Smiddy WE, et al. Long-term anatomic and visual acuity outcomes after initial anatomic success with macular hole surgery. Am J Ophthalmol. 2003;135:633–640.
168 Haritoglou C, Gass CA, Schaumberger M, et al. Long-term follow-up after macular hole surgery with internal limiting membrane peeling. Am J Ophthalmol. 2002;134:661–666.
169 Haritoglou C, Ehrt O, Gass CA, et al. Paracentral scotomata: a new finding after vitrectomy for idiopathic macular hole. Br J Ophthalmol. 2001;85:231–233.
170 Tarita-Nistor L, Gonzalez EG, Mandelcorn MS, et al. Fixation stability, fixation location, and visual acuity after successful macular hole surgery. Invest Ophthalmol Vis Sci. 2009;50:84–89.
171 Ryan EH, Jr., Gilbert HD. Results of surgical treatment of recent-onset full-thickness idiopathic macular holes. Arch Ophthalmol. 1994;112:1545–1553.
172 Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol. 2003;87:1015–1019.
173 Scott RA, Ezra E, West JF, et al. Visual and anatomical results of surgery for long standing macular holes. Br J Ophthalmol. 2000;84:150–153.
174 Villate N, Lee JE, Venkatraman A, et al. Photoreceptor layer features in eyes with closed macular holes: Optical coherence tomography findings and correlation with visual outcomes. Am J Ophthalmol. 2005;139:280–289.
175 Christensen UC, Kroyer K, Sander B, et al. Prognostic significance of delayed structural recovery after macular hole surgery. Ophthalmology. 2009;116:2430–2436.
176 Park SS, Marcus DM, Duker JS, et al. Posterior segment complications after vitrectomy for macular hole. Ophthalmology. 1995;102:775–781.
177 Tabandeh H, Chaudhry NA, Smiddy WE. Retinal detachment associated with macular hole surgery: characteristics, mechanism, and outcomes. Retina. 1999;19:281–286.
178 Rizzo S, Belting C, Genovesi-Ebert F, et al. Incidence of retinal detachment after small-incision, sutureless pars plana vitrectomy compared with conventional 20-gauge vitrectomy in macular hole and epiretinal membrane surgery. Retina. 2010;30:1065–1071.
179 Leonard RE, 2nd., Smiddy WE, Flynn HW, Jr., et al. Long-term visual outcomes in patients with successful macular hole surgery. Ophthalmology. 1997;104:1648–1652.
180 Muselier A, Dugas B, Burelle X, et al. Macular hole surgery and cataract extraction: combined versus consecutive surgery. Am J Ophthalmol. 2010;150:387–391.
181 Melberg NS, Thomas MA. Visual field loss after pars plana vitrectomy with air/fluid exchange. Am J Ophthalmol. 1995;120:386–388.
182 Ezra E, Arden GB, Riordan-Eva P, et al. Visual field loss following vitrectomy for stage 2 and 3 macular holes. Br J Ophthalmol. 1996;80:519–525.
183 Welch JC. Dehydration injury as a possible cause of visual field defect after pars plana vitrectomy for macular hole. Am J Ophthalmol. 1997;124:698–699.
184 Paques M, Massin P, Santiago PY, et al. Visual field loss after vitrectomy for full-thickness macular holes. Am J Ophthalmol. 1997;124:88–94.
185 Gass CA, Haritoglou C, Messmer EM, et al. Peripheral visual field defects after macular hole surgery: a complication with decreasing incidence. Br J Ophthalmol. 2001;85:549–551.
186 Paques M, Massin P, Santiago PY, et al. Late reopening of successfully treated macular holes. Br J Ophthalmol. 1997;81:658–662.
187 Christmas NJ, Smiddy WE, Flynn HW, Jr. Reopening of macular holes after initially successful repair. Ophthalmology. 1998;105:1835–1838.
188 Passemard M, Yakoubi Y, Muselier A, et al. Long-term outcome of idiopathic macular hole surgery. Am J Ophthalmol. 2010;149:120–126.
189 de Smet MD, Gandorfer A, Stalmans P, et al. Microplasmin intravitreal administration in patients with vitreomacular traction scheduled for vitrectomy: the MIVI I trial. Ophthalmology. 2009;116:1349–1355.