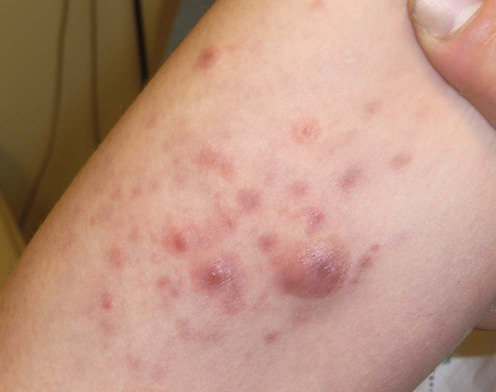
Lymphomatoid papulosis
Rachel S. Klein, Elisha Singer, Jacqueline M. Junkins-Hopkins, Carmela C. Vittorio, Alain H. Rook and Ellen J. Kim
Specific investigations
CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma.
Liu HL, Hoppe RT, Kohler S, Harvell JD, Reddy S, Kim YH. J Am Acad Dermatol 2003; 49: 1049–58.
The higher association with malignancy may represent selection bias.
First-line therapies
EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma.
Kempf W, Pfaltz K, Vermeer M, Cozzio A, Ortiz-Romero P, Bagot M, et al. Blood 2011; 118: 4024–35.
Third-line therapies




 Therapy not required
Therapy not required PUVA
PUVA Low-dose methotrexate
Low-dose methotrexate Topical corticosteroids
Topical corticosteroids Topical mechlorethamine (nitrogen mustard)
Topical mechlorethamine (nitrogen mustard) Topical carmustine
Topical carmustine Topical bexarotene
Topical bexarotene Oral bexarotene
Oral bexarotene Recombinant interferon
Recombinant interferon Excimer laser
Excimer laser Radiotherapy
Radiotherapy Topical methotrexate
Topical methotrexate Imiquimod cream
Imiquimod cream SGN-30
SGN-30
