Lung ultrasound: The basics
Sonographic reverberations of the pleural line. These hyperechoic horizontal lines appear at regular intervals deep to the pleural line and between the rib shadows.
Also called a lung rocket, this hyperechoic vertical sonographic artifact arises from the inferior aspect of the pleural line and extends to the edge of the screen without fading. B-lines obscure A-lines and move with lung sliding.
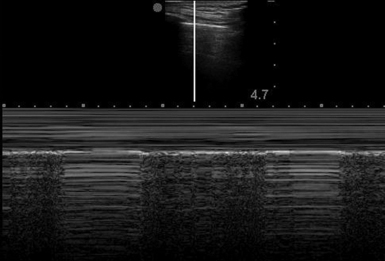
Also called the stratosphere sign, this is an M-mode pattern of uninterrupted horizontal lines only, indicative of a lack of normal aeration.
A general term for hyperechoic vertical sonographic artifacts that may be present during lung ultrasound. B-lines are a type of comet tail.
Dynamic and static air bronchogram:
Sonographic air bronchograms are tubular bright artifactual structures within a tissue, such as lung consolidation; they correspond to air bronchograms on radiographs. On M-mode, static bronchograms will produce straight lines, whereas dynamic bronchograms produce a sinusoidal pattern.
A term applied when the lung’s appearance on lung ultrasound resembles that of the liver.
A dynamic sign, this is the cyclic appearance of findings associated with aerated lung (lung sliding, A- or B- lines) creeping into and then out of view with each respiratory cycle.
Similar to lung sliding, this dynamic sign is perceived as shimmering of the pleural line in concert with the heartbeat and is commonly seen at the left anterior chest wall.
A dynamic sign perceived as shimmering of the pleural line in association with respiration.
This sign is an M-mode pattern of uninterrupted horizontal lines superficial to the pleural surface with a granular pattern deep to this level; it indicates a normal aeration pattern in an inflating and deflating lung.
See barcode sign.
General chest ultrasound (lung ultrasound and echocardiography) is a core element of the holistic approach to critical care ultrasound presented in Chapter 1. Lung ultrasound and echocardiography are illustrated in Chapters 19 to 24 and Chapters 26 to 34, respectively; moreover, features of hemodynamic monitoring in the intensive care unit (ICU) are presented in Chapters 36 to 40.
The normal lung
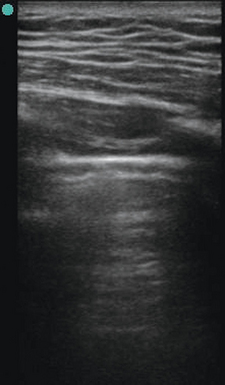
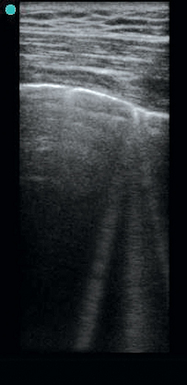
A low-frequency transducer, 3.5 to 5.0 MHz, is positioned in longitudinal orientation over the chest wall in an intercostal space with the probe marker held cranially by convention. Gain and depth settings are adjusted to focus attention at the space between the rib shadows. The pleural line (Figure 19-1), a bright hyperechoic horizontal linear structure, is visualized between the rib shadows. A dynamic sign perceived as shimmering of the parietal and visceral pleura in contact is referred to as lung sliding and is part of the normal aeration pattern. Confirmation of lung sliding can be pursued with the use of M-mode. Directing the ultrasound beam between the rib shadows to transect the pleural line allows analysis of motion over time in the single-dimensional interface at that location. In the presence of normal pleural interaction, the resulting screen after initiating M-mode will display a pattern referred to as the seashore sign (Figure 19-2). Horizontal lines superficial to the pleural surface indicate a lack of motion of the chest wall structures with respiration. Deep to this is a bright hyperechoic horizontal line (representing the pleural surface), and immediately deep to the pleural line is a granular pattern indicating a normal aeration pattern in an inflating and deflating lung.
Evaluation of the lung parenchyma is based on the presence of different artifact patterns, all of which emanate from the pleural line. The A-line (Figure 19-3) is a reverberation of the pleural line, and given an adequate depth setting, several of these parallel lines can be seen at regular intervals. When visualized in the presence of lung sliding, an A-line–dominant pattern is said to occur and is suggestive of normal lung parenchyma. In addition, an A-line–dominant pattern is noted to correlate with low pulmonary capillary wedge pressure and hence represents a low likelihood for pulmonary edema.1
Evaluation for pneumothorax
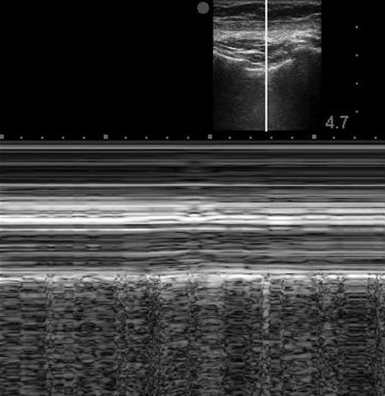
Either low- or high-frequency transducers can be used, although with older machines, use of a low-frequency transducer in a thin patient may produce insufficient near-field detail. In our practice a high-frequency (vascular) transducer is routinely used for evaluation of pneumothorax and is preferred in thin subjects. In a supine patient, examination of the anterior chest wall at six to eight positions in each hemithorax and identification of lung sliding at each point would essentially rule out pneumothorax. In contrast, the absence of lung sliding (representing parietal pleura without visceral pleura to slide against) or the presence of a nonspecific air artifact without the normal aeration pattern is suggestive of pneumothorax. Confirmation of lung sliding can be verified with M-mode. The area under investigation, between the ribs shadows, is centered on the screen. M-mode is initiated after aligning the vertical line through the area in question while holding the probe as still as possible to avoid motion artifact. The “barcode sign” (or “stratosphere sign”) (Figure 19-4) is the appearance of parallel horizontal lines extending through the entire field of view and indicates lack of normal motion of an inflated lung.
If lung sliding is absent and the barcode sign is noted on M-mode, any condition separating the two viscera from their physiologic juxtaposition or impeding their normal movement should be suspected. Causes include massive pneumonia, effusion, atelectasis, apnea, severe acute obstructive lung disease, and previous pleurodesis or pleurectomy. Even though lack of lung sliding is not specific for pneumothorax, the presence of lung sliding effectively rules out pneumothorax at the intercostal locations under the applied ultrasound probe.2
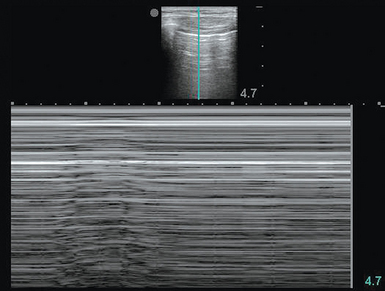
A lung point is a less commonly found but highly specific finding that rules in pneumothorax.2,3 The operator would see findings compatible with pneumothorax (absent lung sliding) juxtaposed to aerated lung (lung sliding, A- or B-lines) and moving with each respiratory cycle. This finding not only makes the diagnosis but also, since a lung point defines the margin of the pneumothorax, helps quantify the size of the pneumothorax within the chest cavity. It can also be helpful for procedural guidance in evacuating a pneumothorax. A lung point can be viewed in M-mode (Figure 19-5), with the screen fluctuating over time between the seashore and barcode (or stratosphere) patterns with the transducer stationary. The position on the hemithorax at which the lung point is noted is further informative. Extensive pneumothorax with retraction of the majority of lung would produce a lung point very posteriorly or not produce one at all because of lack of lung reaching the chest wall in any location. A small pneumothorax would generate a lung point at an anterior and caudal location. In general, the more lateral and posterior a lung point is noted, the larger and more clinically relevant the pneumothorax.2
Evaluation for pulmonary edema (Interstitial Syndrome)
Lung ultrasound can contribute to the diagnosis of pulmonary edema when supported by a suggestive clinical scenario. A normal, A-line–dominant pattern, as described earlier, has been associated with low pulmonary artery occlusion pressure and is likely to represent a “dry” lung. Several authors have described a correlation between extravascular lung water and a specific air artifact pattern known as the B-line pattern.1,4
A B-line, or comet tail (Figure 19-6), is a horizontal hyperechoic beam emanating from the inferior margin of the pleural line and extending through to the deep edge of the screen. The B-line fans out, obliterates visualization of A-lines, and moves with the motion of the pleural line. Vertical lines that do not meet these criteria are artifacts of no clinical value. When diffusely identified, a B-line pattern correlates with thickened interlobular septa or ground-glass areas on computed tomography (CT). Though not reproduced in the literature, two distinct B-line patterns have been described, each correlating with a specific pulmonary parenchymal process. The first pattern consists of multiple B-lines discernible on screen, separated by 3 mm at a smooth pleural line. Sometimes referred to as B3-lines, this pattern is thought to correlate with subpleural ground-glass lesions, as would be the case with pulmonary edema, whether cardiogenic or related to acute respiratory distress syndrome (ARDS). In the case of ARDS, a mixed pattern of B-lines anteriorly and consolidation at gravity-dependent regions of the lung is expected. The second pattern consists of fewer visible B-lines, separated by approximately 7 mm at a ragged pleural line. These B7-lines are thought to correspond to thickened subpleural interlobular septa, as would be the case with interstitial lung disease.5
A single B-line in isolation or a B-line pattern confined to the last intercostal space is unlikely to be indicative of any pathology.5 The lung bases, which have a greater mass of lung parenchyma, will often display a B-line pattern, and therefore an upper lung field examination is more informative in directed evaluation for pulmonary edema.
Evaluation for consolidation (alveolar syndrome)
Atelectasis and infiltrate
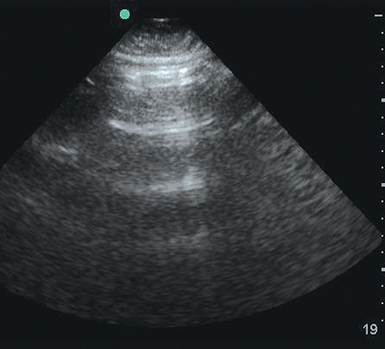
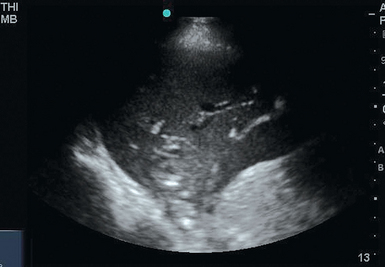
Consolidated lung offers numerous neighboring fluid-filled alveoli for the ultrasound beam to reflect off, thereby giving rise to a tissue-like appearance on the ultrasound screen. The term hepatization is applied when the lung’s density and pattern resemble that of the liver. Distinguishing infiltrate from atelectasis can be difficult. The finding of sonographic air bronchograms suggests an infiltrative process with alveolar consolidation. Sonographic air bronchograms (Figure 19-7) are tubular bright artifactual structures within a tissue, such as occurs with consolidation, that correspond to air bronchograms on radiographs. On M-mode these structures can be further characterized as dynamic or static. Static bronchograms produce straight lines, whereas dynamic bronchograms produce a sinusoidal pattern. Although static air bronchograms can occasionally be seen in atelectatic lung, dynamic air bronchograms effectively differentiate pulmonary consolidation from atelectasis.6,7
Goal-directed applications
Approach to ventilator-dependent patients
In a patient in whom weaning from ventilator support is difficult, ultrasound can be used to evaluate for diaphragmatic dysfunction.8 The low-frequency transducer is applied in conventional fashion to the lateral chest wall of a supine, intubated patient. The diaphragm is identified and the patient is directed to perform a sniff maneuver. Diaphragmatic motion is visualized in real time, similar to the fluoroscopic test, and its strength and function can be evaluated. In a mildly sedated or delirious patient who cannot produce a sniff on command, the ventilator can be transiently occluded at end-expiration and diaphragmatic motion at inspiratory effort observed. In a passively ventilated patient, paradoxic movement of the diaphragm can be detected. If the diaphragm is not well visualized, movement of the superior edge of the liver or spleen can be used as a surrogate for motion of the diaphragm. Diaphragmatic thickness is informative of function and can be assessed by ultrasound.8 Conventionally, the patient is positioned upright and the diaphragm is identified at the zone of apposition, where it is easily visualized surrounded by tissue. Measurements are taken in either M-mode or two-dimensional scanning at a range of lung volumes extending from residual volume to total lung capacity. In a critically ill patient, semirecumbent positioning can be used.9 If mechanically ventilated, measurements can be made at the points of end-inspiration and end-expiration by using brief hold maneuvers on the ventilator, if needed, to capture a still image.
Other applications
Additional applications of lung ultrasound are recognized. After lung recruitment measures, ultrasound can be used to assess the degree of re-aeration of the lung regions in question.10 Adequate positioning of the endotracheal tube above the carina after intubation is suggested by identification of bilateral pleural sliding or motion of B-lines in concert with manual ventilation or ventilator-delivered breaths. Pneumothorax can be ruled out after procedures such as central line placement or thoracentesis immediately and with greater accuracy than with the standard chest radiograph.
Limitations
Sonographic assessment of critical care patients has multiple challenges, as mentioned in Chapter 1. Unlike ambulatory patients, the critically ill are commonly confined to the supine position and are often sedated and unable to follow commands, thus requiring operators to adapt their technique. The anterior and lateral chest wall is usually readily available for placement of the transducer, and these regions are often adequately informative. With support from staff, patients could be transiently repositioned for rapid assessment of the posterior chest wall as needed.
Pearls and highlights
• A high-frequency probe often produces clearer images of pleural lines, lung sliding, and A- and B-lines at the anterior chest wall in thin patients.
• A lung point rules in pneumothorax, whereas the absence of lung sliding could have several causes.
• The presence of lung sliding rules out pneumothorax, but only at the intercostal space under examination.
• Given the high sensitivity of lung ultrasound for detection of pneumothorax as compared with supine chest radiography, scanning for lung sliding before and after placement of a central venous catheter is advised.
• Dynamic air bronchograms, when identified on M-mode, rule out atelectasis and suggest consolidation.
References
1. Lichtenstein, DA, Mezière, GA, Lagoueyte, J-F, et al. A-lines and B-lines. Lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009; 136(4):1014–1020.
2. Lichtenstein, DA, Pneumothorax. In: Whole body ultrasonography in the critically ill. Springer-Verlag, Berlin, 2010.
3. Lichtenstein, D, The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434–1440.
4. Noble, VE, Murray, AF, Capp, R, et al, Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: time course for resolution. Chest. 2009;135(6):1433–1439.
5. Lichtenstein, DA, Lung and interstitial syndrome. In: Whole body ultrasonography in the critically ill. Springer-Verlag, Berlin, 2010.
6. Lichtenstein, D, Mezière, G, Seitz, J. The dynamic air bronchogram. a lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest. 2009; 135(6):1421–1425.
7. Lichtenstein, DA, Lung: alveolar syndrome. InWhole body ultrasonography in the critically ill. Springer-Verlag, Berlin, 2010.
8. Cohn, D, Benditt, JO, Eveloff, S, et al. Diaphragm thickening during inspiration. J Appl Physiol. 1997; 83(1):291–296.
9. Baldwin, CE, Paratz, JD, Bersten, AD, Diaphragm and peripheral muscle thickness on ultrasound: intra-rater reliability and variability of a methodology using non-standard recumbent positions. Respirology. 2011;16(7):1136–1143.
10. Bouhemad, B, Brisson, H, Le-Guen M, et al, Bedside ultrasound assessment of positive end-expiratory pressure–induced lung recruitment. Am J Respir Crit Care Med. 2011;183(3):341–347.