Chapter 13 Lung Transplantation
The first human lung transplant was performed in 1963 by Dr. James Hardy at the University of Mississippi.1 The recipient was a prisoner with lung cancer who survived for 18 days before succumbing to renal failure. Between 1963 and 1974, 36 patients underwent lung transplantation but only two recipients lived longer than a month. It was not until the introduction of cyclosporine in the early 1980s that lung transplantation became a realistic treatment option. In recent years the outcome following lung transplantation has remained relatively stable, with survival rates at 3 months of 90%, at 1 year of 80%, at 3 years of 60%, and at 5 years of 45%.2
The number of patients on the waiting list for lung transplantation in the United States is about 3500,3 but only about 1000 patients undergo the procedure each year, resulting in a median waiting time of just over 3 years.2 Furthermore, because of the limited number of transplants performed, only a small proportion of patients who could benefit from lung transplantation are actually listed for surgery. Although donor shortage is a problem for all solid-organ transplant programs, it is a particular problem for lung transplantation, because only 10% to 15% of multiple organ donors have lungs that are suitable for transplantation. In the United States, approximately 15% of patients die each year while awaiting lung transplantation.2
SINGLE, DOUBLE, OR HEART-LUNG TRANSPLANT
For most conditions in which lung transplantation is indicated, either a single or a bilateral sequential lung transplant may be performed. Outcomes of bilateral sequential lung transplantation are slightly better, but single-lung transplantation represents the most economic use of available organs. For conditions associated with pulmonary sepsis (cystic fibrosis, bronchiectasis), bilateral sequential lung transplant is required. For pulmonary hypertension, either single or bilateral sequential lung transplant may be performed, although bilateral sequential lung transplant is the preferred option worldwide.4,5 Even in the presence of significant right ventricular dysfunction, patients with pulmonary hypertension do not usually require heart-lung transplantation.6 The number of patients with primary pulmonary hypertension who undergo lung transplantation is becoming smaller with the development of more effective medical therapy.
Selection Criteria and Preoperative Management
Recipient Criteria
Candidates for lung transplantation must have end-stage pulmonary disease and limited life expectancy for which there is no other suitable treatment.7 Recommended age limits are 55 years for heart-lung transplantation, 60 years for bilateral lung transplantation, and 65 years for single lung transplantation. Disease-specific criteria for lung transplantation are summarized in Table 13-1. Contraindications to lung transplantation (Table 13-2) are related primarily to the presence of other end-organ disease.
Table 13-1 Disease-Specific Criteria for Lung Transplantation
| COPD |
| FEV1 <25% predicted (without reversibility) |
| PaCO2 >7.3 kPa (55 mmHg) |
| Elevated pulmonary artery pressures ± cor pulmonale |
| Cystic Fibrosis and Bronchiectasis |
| FEV1 <30% predicted |
| Rapidly progressive deterioration in respiratory status (even if FEV1 >30%), including recurrent admissions, massive hemoptysis, and increasing cachexia |
| PaCO2 >6.7 kPa (50 mmHg) and PaO2 <7.3 kPa (55 mmHg) |
| Idiopathic Pulmonary Fibrosis |
| Symptomatic desaturation with rest or exercise |
| Progressive disease |
| Abnormal pulmonary function tests, particularly FVC <70% and DLco <50%-60% of predicted |
| Systemic Disease with Pulmonary Fibrosis |
| As for idiopathic pulmonary fibrosis, but with stable/quiescent systemic disease |
| Pulmonary Hypertension (without congenital heart disease) |
| Symptomatic or progressive disease despite optimal medical treatment and NYHA functional class III or IV |
| CI <2 l/min/m2, RAP>15 mmHg, mean PAP >55 mmHg |
| Eisenmenger Syndrome |
| Severe progressive symptoms and NYHA III or IV functional class despite optimal treatment |
FEV1, forced expiratory volume in one second; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; FVC, forced vital capacity; DLCO, diffusing capacity of carbon monoxide; CI, cardiac index; RAP, right atrial pressure; PAP, pulmonary artery pressure; NYHA, New York Heart Association.
Table 13-2 Contraindications to Lung Transplantation
Preoperative Recipient Optimization
Nutritional status has an important bearing on the outcomes of lung transplantation. Patients with pretransplant body mass indexes less than 17 kg/m2 or more than 25 kg/m2 are at increased risk for early postoperative death.8 Patients with low body mass indexes should receive nutritional supplements. Enteral feeding via a percutaneous gastrostomy tube is commonly used for patients with cystic fibrosis and should be continued during the postoperative period. Compliance with a pulmonary rehabilitation program is a prerequisite for lung transplantation in patients with chronic obstructive pulmonary disease (COPD) and has been shown to improve outcomes of surgery.9 Corticosteroids should be discontinued or weaned to doses less than 20 mg/day of prednisone at the time of listing for transplantation because of their adverse effects on bone and muscle mass and the increased risk for colonization or infection by an opportunistic organism.10 Lung transplant recipients with cystic fibrosis and bronchiectasis may require regular courses of intravenous antibiotics to control pulmonary infection.
Donor Criteria
The optimal donor criteria11–14 for lung transplantation are summarized in Table 13-3. However, because of the limited number of suitable organs, there is substantial pressure to use marginal donors. Although good outcomes have been obtained with graft ischemic times longer than 6 hours, data indicate a reduction in survival rates when the ischemic time exceeds 5.5 hours.15 Donor lungs with a positive gram stain on tracheal aspirate or bronchial washings may be considered for transplantation depending on other factors, such as the chest radiograph appearances and the gas exchange. Marginal oxygenation in the donor can be managed with various ventilatory strategies to improve gas exchange (see Chapter 29). If there is evidence of unilateral chest sepsis in a potential donor, a single-lung transplant using the noninfected donor lung may be considered. The decision to use a marginal organ is difficult and depends not only on donor factors but also on the sickness of the potential recipient and the likelihood of another organ becoming available.
| Ischemic time < 6 hours |
| Age < 55 years |
| ABO blood group compatible |
| Clear chest radiograph |
| PaO2 > 40 kPa (300 mmHg) with 100% oxygen and 5 cm |
| H2O PEEP |
| Smoking history <20 pack years |
| Absence of chest trauma |
| Absence of purulent secretions at bronchoscopy |
| Absence of organisms on sputum gram stain |
| No prior cardiopulmonary surgery |
PEEP, positive end expiratory pressure; PaO2, arterial partial pressure of oxygen.
From Orens JB, Boehler A, de Perrot M, et al: A review of lung transplant donor acceptability criteria. J Heart Lung Transplant 22:1183-1200, 2003.
Recipient-Donor Matching
The matching of donors and recipients of thoracic organs (heart and lungs) is based on compatibility, geographic location, and medical urgency, as outlined in Chapter 14. For lung transplantation, a number of criteria are used to ensure size matching, including height, thoracic circumference, and chest radiograph dimensions. A recent study has shown that predicted total lung capacity based on height and gender provides more accurate size matching than height alone.16
Living Lobar Lung Donors
Living lobar lung transplantation was introduced in 1993 for individuals too unwell to await cadaveric organs. In this procedure, right and left lower lobes from two healthy donors are implanted in the recipient in the place of the whole right and left lungs. A recent review of the outcomes of this technique in a single center showed survival rates at 1 year of 70%, at 3 years of 54%, and at 5 years of 45%.17 These rates are comparable to those obtained in conventional lung transplantation.
Intraoperative Management
Surgical access for bilateral sequential lung transplant is usually made by performing a transsternal bilateral thoracotomy (a “clamshell” incision) with the patient in a supine position. The procedure involves performing two single-lung transplants, one after the other. The side with the worse lung function is typically resected first.
Immunosuppression
Standard immunosuppression18 in lung transplantation consists of triple therapy, including a calcineurine inhibitor (cyclosporine, tacrolimus), an antiproliferative agent (azathioprine, mycophenolate mofetil), and a corticosteroid (methylprednisolone, prednisone). In a recent survey of North American practice, the most commonly used regimen consisted of tacrolimus, mycophenolate mofetil, and prednisone.5 The first doses of immunosuppression are given prior to surgery and continued postoperatively. If there is evidence of postoperative acute renal dysfunction, the calcineurine inhibitor may be withheld for a few days or another class of drug may be substituted. In some centers, induction therapy that is commonly either an anti-lymphocyte antibody or interleukin 2 (IL-2) blocker is administered during the early postoperative period.
Calcineurine Inhibitors
Cyclosporine can be given as a continuous infusion or in divided doses (1 to 3 mg/kg/day). The oral dose is calculated at 3 to 9 mg/kg/day in two divided doses or converted from the intravenous dose based on a bioavailability of about 30%. Patients with cystic fibrosis have poor absorption of cyclosporine (bioavailability may be as low as 20%) and should take the drug with pancreatic enzyme supplementation. Cyclosporin may be given three (rather than two) times a day in this group to combat reduced absorption. Traditionally, trough cyclosporine serum levels are obtained immediately prior to the next dose (C0 levels). Recently, dose optimization based on serum levels obtained 2 hours postdose (C2) has been shown to reduce the incidence of nephrotoxicity without increasing the incidence of acute rejection.19,20 The target serum level varies according to the assay used and whether C0 or C2 levels are obtained. Tacrolimus can be given intravenously (0.01 to 0.05 mg/kg/day) or orally 0.1 to 0.3 mg/kg/day in two divided doses.21 Trough levels obtained immediately prior to the next dose are used to attain dose optimization.
The main side effect of the calcineurine inhibitors is renal dysfunction.22,23 Renal impairment is dose related and is reversible on drug withdrawal. If renal impairment develops, it may be appropriate to withhold the drug temporarily, reduce the dose, or use an alternative such as a target-of-rapamycin (TOR) inhibitor (see later material). Other side effects include acute delirium, hypertension, dyslipidemia, impaired glucose tolerance, gout, gingival hypertrophy, and hirsutism. Tacrolimus commonly causes a fine tremor but results in less hirsutism and gingival hypertrophy than does cyclosporine.
Calcineurine inhibitors (and sirolimus) are metabolized by the cytochrome P450 (CYP) 3A enzyme system, which is subject to inhibition (decreased metabolism) and induction (increased metabolism) by certain drugs (see Table 4-3). CYP3A inhibition is exploited therapeutically by using diltiazem (an inhibitor of CYP3A) as an antihypertensive agent, thereby reducing the required dose of the calcineurine inhibitor.
Antilymphocytic Antibodies
A number of antibodies directed against human T lymphocytes are available for immunosuppression, including polyclonal antithymyoctye globulin (ATG) and monoclonal anti-CD3 antibody (OKT3) and the recently developed monoclonal antibodies directed against interleukin-2 receptors (e.g., dacluzimab and basiliximab). These agents are used for induction therapy and for the treatment of refractory acute rejection. Induction therapy is used in 40% to 50% of heart and lung transplant programs.4,24
Routine Postoperative Care
After lung transplantation, up to 90% of patients have some degree of reperfusion injury, which manifests as an increased alveolar-arterial oxygen gradient and perihilar edema on the chest radiograph.25 In most patients this reperfusion injury is mild and does not delay progress. However, to avoid exacerbating reperfusion injury, all patients should be ventilated with a lung-protective strategy (see Chapter 29). Many patients with chronic lung disease have compensated respiratory acidosis, and attempts to ventilate to a normal carbon dioxide tension will result in marked alkalosis (and possibly high airway pressures).
Some have recommended the routine use of nitric oxide to prevent reperfusion injury, but in a randomized trial, inhaled nitric oxide at a dose of 20 parts per million was not associated with a reduction in reperfusion injury when compared to controls.26 (Nitric oxide may have a role in the treatment of reperfusion injury; see later discussion). In patients who have undergone singlelung transplantation for emphysematous disease, low ventilation rates should be used to avoid dynamic hyperinflation in the native lung (see later discussion). Bronchospasm can occur both in the graft and in the remaining native lung and can be treated by inhaled bronchodilators.
Routine measures to prevent nosocomial infection are extremely important; these are described in Chapter 14. Institution of enteral nutrition within 2 to 3 days of surgery is important, because patients are commonly malnourished and catabolic. Graduated compression stockings to help prevent deep venous thrombosis should be used in all patients, but routine use of anticoagulants is probably not justified except when there is prolonged immobility. Bronchoscopy is usually performed within the first 2 to 4 weeks following surgery. At this time the bronchial anastomoses are inspected, washings are obtained for microbiologic analysis, and transbronchial biopsies are taken to look for acute rejection.
Analgesia
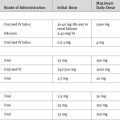
Effective analgesia is absolutely essential to facilitate early extubation, coughing and deep breathing, and compliance with physiotherapy. This goal is best achieved by a thoracic epidural blockade. It is a common practice to insert an epidural catheter when the patient is in the operating room prior to surgery. However, if this has not been done—for instance, because of an anticipated need for CPB with full anticoagulation—it is usually appropriate to insert the catheter when the patient is in the ICU, prior to waking the patient. The presence of normal blood clotting should be established prior to the procedure. Epidural dosing regimes are shown in Table 12-5. To minimize epidural-induced hypotension in patients who are being kept “dry,” epidural infusions containing high concentrations of local anesthetic solutions should be avoided. The epidural is usually discontinued once the chest drains have been removed, but it should not remain in place longer than 5 to 7 days because of the risk of infection.
Antimicrobials
Antibiotic prophylaxis following lung transplant surgery is similar to that required following any cardiothoracic procedure (see Chapter 35). There are two situations in which additional antibiotic coverage is required. First, when infection is identified in the donor lung, and second, in a recipient with suppurative lung disease. Results of sputum samples obtained preoperatively are used to guide antibiotic therapy.
The lungs of patients with cystic fibrosis and bronchiectasis are typically colonized with multiresistant organisms, particularly Pseudomonas aeruginosa and Burkholderia species. Although colonization by panresistant P. aeruginosa does not appear to increase the risk for mortality in lung transplant recipients,27 colonization by certain Burkholderia species, particularly B. cenocepacia (formerly genomovar III), is associated with increased mortality rates,28 and in some centers it constitutes a contraindication to lung transplantation.
Early Complications
Reperfusion Injury
Severe reperfusion injury (primary graft failure, ischemia-reperfusion injury) is the most common cause of early death following lung transplantation.24,29 The condition occurs in about 15% of all lung transplant recipients and has a mortality rate in excess of 40%.23,29 The cause of reperfusion injury is poorly understood and in many circumstances appears to be idiosyncratic. In one study, length of ischemic time, recipient age, underlying pulmonary disease, and the use of CPB were not identified as risk factors.29 Reperfusion injury is not a process mediated by the immune system, but it is associated with an increased risk for developing early acute rejection.30
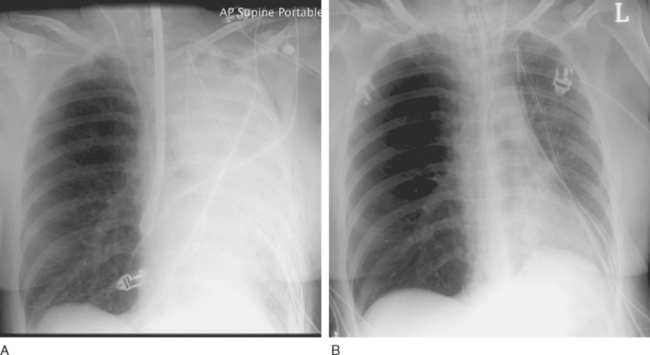
Reperfusion injury presents as severe noncardiogenic pulmonary edema with widespread alveolar shadowing on the chest radiograph (Fig. 13-1), reduced lung compliance, impaired gas exchange, and frothy edema fluid in the endotracheal tube. There may be copious serous drainage from the chest tubes. The condition may be apparent immediately after graft reperfusion or, more commonly, it may develop slowly during the first few hours in the ICU.
Other causes of pulmonary edema must also be considered (Table 13-4). In particular, pulmonary vein obstruction due to an anastomotic problem or thrombus formation can also cause gross pulmonary edema. If pulmonary venous obstruction is suspected, TEE examination with careful inspection of the pulmonary veins should be performed. The finding of increased velocity within one or more pulmonary veins and loss of the usual phasic flow pattern on Doppler flow mapping are suggestive of the diagnosis.
Table 13-4 Differential Diagnosis of Pulmonary Edema in the First 48 Hours Following Lung Transplantation
| Reperfusion injury |
| Pulmonary vein obstruction |
| Excess fluid administration |
| Cardiogenic pulmonary edema (e.g., due to myocardial ischemia) |
| Aspiration (rare) |
| Hyperacute rejection |
In contrast to other solid-organ transplantation, hyperacute rejection is not well described following lung transplantation. However, it is likely that some cases of overwhelming pulmonary edema that occur very soon after surgery are due to hyperacute rejection and not to reperfusion injury.31 There is no specific treatment for hyperacute rejection other than retransplantation, and death is likely.
The treatment of reperfusion injury is supportive. A lung-protective ventilatory strategy should be employed, with high levels of positive end-expiratory pressure (PEEP). Inhaled nitric oxide is beneficial, at least during the acute period.30 Other pulmonary vasodilators such as nitroglycerin may reduce pulmonary vascular resistance (and improve right ventricular function) but at the cost of inhibiting hypoxic pulmonary vasoconstriction and exacerbating hypoxemia.
Diuretics and fluid restriction should be employed to achieve the lowest pulmonary artery wedge pressure that is consistent with acceptable hemodynamics. Vasopressors may be required to support the blood pressure. Hemoglobin should be maintained above 90 g/l. Renal dysfunction secondary to modest hypovolemia may be preferable to exacerbating the reperfusion injury by means of fluid resuscitation.
For patients with life-threatening reperfusion injury, extracorporeal membrane oxygenation (ECMO) should be considered (see Chapter 22). A number of centers have reported good outcomes by using this technique.32–34 Ideally, ECMO should be initiated early, before cardiovascular collapse occurs. This has the advantage of allowing ECMO to be commenced in a controlled fashion, and it may also permit the use of venovenous ECMO, which is associated with a lower incidence of complications than is found with arteriovenous ECMO.
Dynamic Hyperinflation of the Native Lung
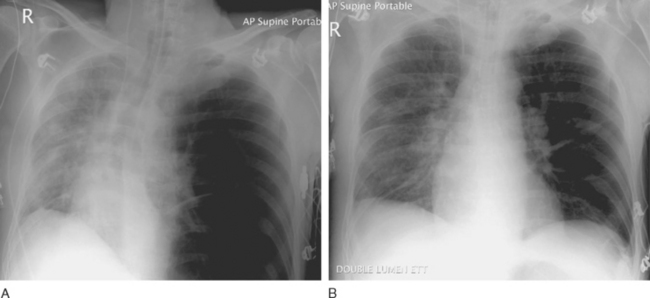
Dynamic hyperinflation of the native lung is a problem that occurs in patients with emphysematous disease undergoing single-lung transplantation, particularly when there is significant reperfusion injury in the graft. The problem arises because of differences in the compliance between the native lung (high compliance) and the transplanted lung (low compliance). With positive pressure ventilation through a single-lumen endotracheal tube, there is preferential ventilation of the native emphysematous lung. This can result in gross overdistension of the native lung, underventilation of the graft, and mediastinal shift (Fig. 13-2A). Moderate degrees of dynamic hyperinflation are surprisingly well tolerated. However, severe dynamic hyperinflation is associated with hypoxemia, hypercarbia, and hemodynamic collapse. The differential diagnosis includes tension pneumothorax in the native lung and mucus plugging in the graft (Table 13-5). Inappropriate insertion of a chest drain in this situation can cause a major air leak, potentially compounding the ventilatory problems.
Table 13-5 Differential Diagnosis of Hyperexpansion of the Native Lung Following Single-Lung Transplant
| Air trapping within the native lung |
| Tension pneumothorax on the side of the native lung |
| Mucus plugging within the transplanted lung |
| Phrenic nerve injury on the operative side |
If dynamic hyperinflation is apparent on the chest radiograph but the patient is stable, simple interventions (e.g., β agonists) are usually sufficient. These include avoiding ventilatory strategies that promote air trapping within the native lung (such as rapid rates, high PEEP, and short expiratory times; see Chapter 29) and converting the patient to a spontaneous breathing mode. However, severe dynamic hyperinflation that is associated with impaired gas exchange and hypotension requires urgent institution of independent lung ventilation (see Fig. 13-2B).
Independent Lung Ventilation.
Independent lung ventilation is required in up to 10% of single-lung transplant recipients.35 The technique is performed via a double-lumen endotracheal tube placement and is described in Chapter 40. Accurate and timely placement of double-lumen endotracheal tubes can be difficult and should be undertaken by someone experienced in their use. The appropriate ventilatory strategies for the two lungs are quite different because the transplanted lung typically has restrictive physiology, whereas the native lung has obstructive physiology. The implications of this are explained in Chapter 29. Suggested initial ventilator settings are outlined in Table 13-6.
Table 13-6 Initial Ventilator Settings at the Start of Independent Lung Ventilation for Dynamic Hyperinflation in Single-Lung Transplant Recipients
| Native (emphysematous) Lung |
| Ventilatory rate of 4-8 breaths/min |
| Inspiratory time of 1.5 sec |
| Expiratory time of 6-13.5 sec |
| PEEP ≤ 5 cm H2O |
| Tidal volume of 4-8 ml/kg (depending on plateau pressure) |
| Graft |
| Ventilatory rate of 14-18 breaths/min |
| Inspiratory time of 1.2 sec |
| Expiratory time of 2-3 sec |
| PEEP 10-15 cm H2O |
| Tidal volume of 3-5 ml/kg (depending on plateau pressure) |
PEEP, positive end expiratory pressure.
Occasionally, prolonged independent lung ventilation is required. In one report, good outcomes were reported in two patients after 25 and 35 days of independent lung ventilation.36 One treatment option for intractable native lung hyperinflation is lung volume reduction surgery in the native lung. Concomitant single-lung transplantation and lung volume reduction surgery has been suggested as a way of avoiding postoperative dynamic hyperinflation.35
Hemodynamic Instability
Raised Intrathoracic Pressure.
Obstructed venous return resulting from high ventilatory pressures and high PEEP, dynamic hyperinflation, or ventilator dysynchrony can precipitate hemodynamic collapse. The problem is exacerbated if the graft is relatively large for the recipient. The diagnosis of obstructed venous return may be suspected if there is high central venous pressure, marked pulsus paradoxus on the arterial waveform, and high airway pressures. If this condition is suspected, the patient should be briefly disconnected from the ventilator; if this does not resolve the problem, the patient should be paralyzed and a chest radiograph, and possibly a TEE examination, should be obtained.
Surgical Complications
Specific surgical complications include pulmonary vein obstruction (see earlier material), airway dehiscence or obstruction, and phrenic nerve injury. Together, surgical complications account for 10% of early deaths.24
Hyperammonemia Syndrome
Hyperammonemia syndrome37 is a rare complication of transplantation; there is a reported incidence of its occurrence after lung transplantation of 4.1%.38 The condition is characterized by an elevated serum ammonia level (4 to 150 times normal), relatively normal liver function tests, and encephalopathy. Patients initially develop confusion and agitation, which may progress to seizures, coma, cerebral edema, and death. The cause is unknown but the condition is more likely in patients who have had “catabolic” stressors, such as gastrointestinal bleeding, reperfusion injury, and sepsis. Renal failure and high protein intake (as enteral or parenteral nutrition) contribute to the syndrome.
The differential diagnosis includes other causes of delirium, such as the side effects of immunosuppressive drugs (corticosteroids and calcineurine inhibitors); impaired gas exchange (hypoxemia and hypercarbia); sepsis; electrolyte disorder; and withdrawal syndromes (see Chapter 37).
Later Complications
Acute Rejection
The incidence of acute rejection following lung transplantation is higher than that in other solid-organ transplants.39,40 The first episode typically occurs after the first week, the peak incidence occurs 2 to 4 weeks following surgery.
Acute rejection is often asymptomatic and is detected in transbronchial biopsies performed during routine surveillance bronchoscopy. Symptoms, if present, are usually nonspecific, such as deterioration in respiratory function, low-grade fever, and malaise. The main differential diagnosis is infection. If either diagnosis is suspected, bronchoscopy with transbronchial biopsy should be performed. Treatment of acute rejection is a short course of high-dose corticosteroids and, possibly, augmentation of baseline immunosuppression. The frequency and severity of episodes of acute rejection are risk factors for the development of chronic rejection.40
Infection
The combination of poor nutritional status, major surgery, prolonged hospital stay, preoperative colonization, and the use of potent immunosuppressive drugs all predispose to the development of postoperative infection. The risk for infection is further increased by augmented immunosuppression used to treat acute and chronic rejection, and bronchial anastomotic problems. Infection accounts for nearly 40% of all deaths in the first year and for about 20% of deaths overall.4
Bacterial Infections.
Bacterial infections that occur in the first month following surgery are usually caused by gram-negative organisms (particularly Pseudomonas aeruginosa) and Staphylococcus aureus. In patients with cystic fibrosis, organisms that had previously colonized their upper airways are potential sources of infection. Bacterial infections that occur after discharge from the hospital are usually caused by community-acquired pathogens such as Hemophilus influenzae, Streptococcus pneumoniae, or Legionella species.23
Viral Infections.
CMV is the most common viral pathogen in lung transplant recipients. Clinical infection can occur due to reactivation of latent disease in an antibody-positive recipient or, more commonly, to new infection in an antibodynegative recipient who has received an organ from a CMV antibody-positive donor. The peak incidence of clinical infection occurs 1 to 12 months after surgery,4 typically after cessation of antiviral prophylaxis. CMV infection can cause tracheobronchitis and pneumonitis. Patchy infiltrates may be visible on a chest radiograph but this is a nonspecific finding. The diagnosis is confirmed by identifying viral DNA from blood, from bronchial washings using gene amplification with the polymerase chain reaction, or from typical changes (CMV inclusion bodies) on transbronchial biopsy. Treatment involves 2 to 3 weeks of intravenous ganciclovir followed by oral valganciclovir.
Community-acquired respiratory viruses (respiratory syncytial virus, parainfluenza virus, influenza virus, and adenovirus) are significant causes of morbidity in lung transplant recipients. These viruses are usually diagnosed in samples taken during bronchoscopy that has been performed for clinical indications such as dyspnea or pulmonary infiltrates in chest radiographs. Treatment is usually supportive, although specific antiviral agents can be used if the diagnosis is made early. Infection by community-acquired respiratory viruses has been identified as a risk factor for chronic rejection.41
Fungal Infections.
Aspergillus species are the most common and lethal fungal pathogens in transplant recipients.23 These organisms can cause ulcerative tracheobronchitis (particularly in the presence of bronchial anastomotic problems) and pneumonia. Tracheobronchitis presents with fever, cough, wheeze, and hemoptysis. On bronchoscopy, there may be evidence of mucosal ulcerations, pseudomembranes, or frank necrosis. Invasive pneumonia results in severe respiratory failure and is associated with high mortality rates. Infection by Aspergillus species typically occurs within the first 6 months following surgery. Coinfection by CMV also occur.
Chronic Rejection
The bronchiolitis obliterans syndrome, thought to be a manifestation of chronic rejection, is the most common cause of death beyond 1 year following lung transplantation. Nearly 50% of survivors will have developed bronchiolitis obliterans by 5 years.42 The syndrome is diagnosed clinically based on lung function testing—when the FEV1 falls irreversibly by more than 20% from the best posttransplant value.
Malignancy
The most common malignancies that occur early following lung transplantation are lymphoproliferative diseases. These malignancies are associated with excess immunosuppression and infection by the Epstein-Barr virus. The highest incidence occurs in recipients who lack the antibody for the Epstein-Barr virus and who receive an organ from an antibody-positive donor. Treatment includes reduction in immunosuppression, antiviral therapy (acyclovir), and rituximab.
Skin cancers are the most common malignancies that occur in long-term lung transplant survivors. Posttransplant lung cancer in the residual native lung occurs almost exclusively in patients with underlying COPD or pulmonary fibrosis who have a history of smoking.22 By 7 years after transplant almost 20% of lung transplant recipients who are still alive have some type of cancer.4
Chronic Renal Failure and Other Complications
Chronic renal dysfunction secondary to treatment with calcineurine inhibitors occurs in more than one third of lung transplant recipients by 5 years, with 3.4% requiring dialysis.4 The risk for renal dysfunction is reduced by aggressive control of hypertension and diabetes (both side effects of corticosteroids and calcineurine inhibitors). Reducing the dose of the calcineurine inhibitor or substituting a TOR inhibitor has been shown to facilitate renal recovery.22,43
1 Trulock EP. Lung transplantation. Am J Respir Crit Care Med. 1997;155:789-818.
2 Scientific Registry of Transplant Recipients: www.ustransplant.org.
3 The Organ Procurement and Transplantation Network: www.optn.org
4 Trulock EP, Edwards LB, Taylor DO, et al. The registry of the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report —2004. J Heart Lung Transplant. 2004;23:804-815.
5 Levine SM. A survey of clinical practice of lung transplantation in North America. Chest. 2004;125:1224-1238.
6 Kasimir MT, Seebacher G, Jaksch P, et al. Reverse cardiac remodelling in patients with primary pulmonary hypertension after isolated lung transplantation. Eur J Cardiothorac Surg. 2004;26:776-781.
7 Maurer JR, Frost AE, Estenne M, et al. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. Heart Lung. 1998;27:223-229.
8 Madill J, Gutierrez C, Grossman J, et al. Nutritional assessment of the lung transplant patient: body mass index as a predictor of 90-day mortality following transplantation. J Heart Lung Transplant. 2001;20:288-296.
9 Mahler DA. Pulmonary rehabilitation. Chest. 1998;113:263S-268S.
10 Anonymous. International guidelines for the selection of lung transplant candidates. The American Society for Transplant Physicians (ASTP)/American Thoracic Society (ATS)/European Respiratory Society (ERS)/International Society for Heart and Lung Transplantation (ISHLT). Am J Respir Crit Care Med. 1998;158:335-339.
11 Orens JB, Boehler A, de Perrot M, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22:1183-1200.
12 Pierre AF, Sekine Y, Hutcheon MA, et al. Marginal donor lungs: a reassessment. J Thorac Cardiovasc Surg. 2002;123:421-427.
13 Weill D. Donor criteria in lung transplantation: an issue revisited. Chest. 2002;121:2029-2031.
14 Gabbay E, Williams TJ, Griffiths AP, et al. Maximizing the utilization of donor organs offered for lung transplantation. Am J Respir Crit Care Med. 1999;160:265-271.
15 Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171:786-791.
16 Ouwens JP, van der Mark TW, van der Bij W, et al. Size matching in lung transplantation using predicted total lung capacity. Eur Respir J. 2002;20:1419-1422.
17 Starnes VA, Bowdish ME, Woo MS, et al. A decade of living lobar lung transplantation: recipient outcomes. J Thorac Cardiovasc Surg. 2004;127:114-122.
18 Knoop C, Haverich A, Fischer S. Immunosuppressive therapy after human lung transplantation. Eur Respir J. 2004;23:159-171.
19 Morton JM, Aboyoun CL, Malouf MA, et al. Enhanced clinical utility of de novo cyclosporine C2 monitoring after lung transplantation. J Heart Lung Transplant. 2004;23:1035-1039.
20 Glanville AR, Morton JM, Aboyoun CL, et al. Cyclosporine C2 monitoring improves renal dysfunction after lung transplantation. J Heart Lung Transplant. 2004;23:1170-1174.
21 Zuckermann A, Reichenspurner H, Birsan T, et al. Cyclosporine A versus tacrolimus in combination with mycophenolate mofetil and steroids as primary immunosuppression after lung transplantation: one-year results of a two-center prospective randomized trial. J Thorac Cardiovasc Surg. 2003;125:891-900.
22 Kotloff RM, Ahya VN. Medical complications of lung transplantation. Eur Respir J. 2004;23:334-342.
23 Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2004;170:22-48.
24 Taylor DO, Edwards LB, Boucek MM, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-first official adult heart transplant report —2004. J Heart Lung Transplant. 2004;23:796-803.
25 Fisher AJ, Wardle J, Dark JH, et al. Non-immune acute graft injury after lung transplantation and the risk of subsequent bronchiolitis obliterans syndrome (BOS). J Heart Lung Transplant. 2002;21:1206-1212.
26 Meade MO, Granton JT, Matte-Martyn A, et al. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am J Respir Crit Care Med. 2003;167:1483-1489.
27 Aris RM, Gilligan PH, Neuringer IP, et al. The effects of panresistant bacteria in cystic fibrosis patients on lung transplant outcome. Am J Respir Crit Care Med. 1997;155:1699-1704.
28 Aris RM, Routh JC, LiPuma JJ, et al. Lung transplantation for cystic fibrosis patients with Burkholderia cepacia complex: survival linked to genomovar type. Am J Respir Crit Care Med. 2001;164:2102-2106.
29 Christie JD, Bavaria JE, Palevsky HI, et al. Primary graft failure following lung transplantation. Chest. 1998;114:51-60.
30 de Perrot M, Liu M, Waddell TK, et al. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490-511.
31 Choi JK, Kearns J, Palevsky HI, et al. Hyperacute rejection of a pulmonary allograft: immediate clinical and pathologic findings. Am J Respir Crit Care Med. 1999;160:1015-1018.
32 Oto T, Rosenfeldt F, Rowland M, et al. Extracorporeal membrane oxygenation after lung transplantation: evolving technique improves outcomes. Ann Thorac Surg. 2004;78:1230-1235.
33 Meyers BF, Sundt TM3rd, Henry S, et al. Selective use of extracorporeal membrane oxygenation is warranted after lung transplantation. J Thorac Cardiovasc Surg. 2000;120:20-28.
34 Dahlberg PS, Prekker ME, Herrington CS, et al. Medium-term results of extracorporeal membrane oxygenation for severe acute lung injury after lung transplantation. J Heart Lung Transplant. 2004;23:979-984.
35 Mitchell JB, Shaw AD, Donald S, et al. Differential lung ventilation after single-lung transplantation for emphysema. J Cardiothorac Vasc Anesth. 2002;16:459-462.
36 Gavazzeni V, Iapichino G, Mascheroni D, et al. Prolonged independent lung respiratory treatment after single lung transplantation in pulmonary emphysema. Chest. 1993;103:96-100.
37 Chakinala MM, Kollef MH, Trulock EP. Critical care aspects of lung transplant patients. J Intens Care Med. 2002;17:8-33.
38 Lichtenstein GR, Yang YX, Nunes FA, et al. Fatal hyperammonemia after orthotopic lung transplantation. Ann Intern Med. 2000;132:283-287.
39 Schulman LL, Weinberg AD, McGregor CC, et al. Influence of donor and recipient HLA locus mismatching on development of obliterative bronchiolitis after lung transplantation. Am J Respir Crit Care Med. 2001;163:437-442.
40 Schulman LL, Weinberg AD, McGregor C, et al. Mismatches at the HLA-DR and HLA-B loci are risk factors for acute rejection after lung transplantation. Am J Respir Crit Care Med. 1998;157:1833-1837.
41 Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170:181-187.
42 Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440-444.
43 Snell GI, Levvey BJ, Chin W, et al. Rescue therapy: a role for sirolimus in lung and heart transplant recipients. Transplant Proceed. 2001;33:1084-1085.