Lung Defense Mechanisms
Before the discussion of infectious disorders of the respiratory system in Chapters 23 through 26, it is appropriate to first consider how the lung protects itself against the infectious agents to which it is exposed. Although this chapter focuses on protective mechanisms against infection, defenses against noninfectious substances, especially inhaled particulate material, also are addressed. The major categories of defense mechanisms to be discussed include (1) physical or anatomic factors relating to deposition and clearance of inhaled material, (2) antimicrobial peptides, (3) phagocytic and inflammatory cells that interact with the inhaled material, and (4) adaptive immune responses, which depend on prior exposure to and recognition of the foreign material. The chapter concentrates on the aspects of the host defense system specific to the lung and then proceeds with a discussion of several ways the system breaks down, resulting in an inability to handle microorganisms and an increased risk for certain types of respiratory tract infection. The chapter concludes by briefly considering how we can activate or augment specific immune responses through immunization, thus enhancing defenses against selected respiratory pathogens.
Physical or Anatomic Factors
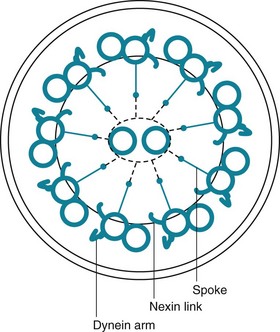
The term mucociliary transport or mucociliary clearance refers to a process of waves of beating cilia moving a blanket of mucus (and any material trapped within the mucus) progressively upward along the tracheobronchial tree. From the trachea down to the respiratory bronchioles, the most superficial layer of epithelial cells lining the airway has cilia projecting into the airway lumen. These cilia have a structure identical to that of cilia found elsewhere in the body, consisting of longitudinal microtubules with a characteristic architecture. Specifically, a cross-sectional view of cilia shows two central microtubules surrounded by nine pairs of microtubules arranged around the periphery (Fig. 22-1). Small projecting side arms from each doublet, called dynein arms, are crucial to the contractile function of the microtubules and hence to the beating of the cilia.
Antimicrobial Peptides
The sol layer contains a number of substances that are important in innate immunity. The innate immune system can be thought of as a fast-acting system that is ready to quickly protect the lungs without prior sensitization and ideally avoid activation of the adaptive immune system (discussed in the Adaptive Immune Responses section). In addition to mucociliary clearance, the innate immune system is composed of small molecules, proteins, and cells that are able to respond to inhaled particles in a way that does not require any previous exposure to the particle. These molecules are generally highly conserved in evolution and are present in many invertebrate species as well as in humans. They are able to immediately interact with microorganisms through recognition of conserved structures on the microbes, and they can act directly to kill the invader and stimulate a further host immune response. They provide a fast, energy-efficient, effective frontline defense, with broad overlap in actions. There are many components of innate immunity in the lung, and a full description is beyond the scope of this chapter. The interested reader is referred to the in-depth reviews listed in the references. For the reader to get a sense of the system, this chapter focuses on a few of the best described of these molecules: lysozyme, lactoferrin, defensins, collectins (surfactant protein A [SP-A] and surfactant protein D [SP-D]), and immunoglobulin (Ig)A.
Respiratory IgA can be considered part of the innate immune system because it is also constitutively produced by the respiratory epithelium and does not require prior exposure. IgA is further discussed in the section on humoral immune mechanisms.
Phagocytic and Inflammatory Cells
Dendritic Cells
Dendritic cells are present throughout the body in various forms. They are bone marrow–derived cells that, in the lung, are located in the airway epithelium as well as in alveolar walls and peribronchial connective tissue. These cells have long and irregular cytoplasmic extensions that form a contiguous network. The primary function of dendritic cells is to sample the airway microenvironment, ingest and process antigens, and then migrate to regional lymph nodes. In the lymph nodes, dendritic cells present antigen to T cells, a critical step for the later immunologic defense provided by lymphocytes. Langerhans cells, a type of dendritic cell with a particular ultrastructural appearance, are the cells with abnormal proliferation that appear to be responsible for Langerhans cell histiocytosis of the lung (also called eosinophilic granuloma; see Chapter 11).
Natural Killer Cells
Natural killer (NK) cells are part of the rapid initial response and are capable of killing microorganisms without prior sensitization. These cells lack surface markers characteristic of either T or B lymphocytes (discussed in the section on cellular immune mechanisms). NK cells are important in rapid protection against viral infections. They act by recognizing and killing virus-infected cells that have been transformed and no longer express certain markers of cellular health on the cell surface. NK cells also are important in surveillance for neoplasms, and they use the same methods to detect and kill malignantly transformed cells.
Adaptive Immune Responses
Humoral Immune Mechanisms
The overall role of the humoral immune system in respiratory defenses includes protecting the lung against a variety of bacterial and, to some extent, viral infections. The clinical implications of this role and the consequences of impairment in the humoral immune system are discussed in the section on defects in the adaptive immune system.
Cellular Immune Mechanisms
One important role for the cellular immune system is to protect against bacteria that have a pattern of intracellular growth, especially M. tuberculosis (see discussion of tuberculosis in Chapter 24). In addition, the cellular immune system has a critical role in the handling of many viruses, fungi, and protozoa.
Failure of Respiratory Defense Mechanisms
Impairment of Physical Clearance
Other physical or anatomic factors that influence deposition and clearance of particles include genetic abnormalities and environmental factors affecting the mucociliary transport system. Especially interesting information has been provided by a genetic abnormality termed the dyskinetic cilia syndrome, also sometimes called the immotile cilia syndrome. In this disorder, a defect in ciliary structure and function leads to absent or impaired ciliary motility and hence to ineffective mucociliary clearance. More than 20 types of defects are recognized, but the most common is absence of dynein arms on the microtubules. Clinically, the impairment in mucociliary clearance is associated with chronic sinusitis, chronic bronchitis, and bronchiectasis. In males, the sperm tail, which has a structure similar to that of cilia, is abnormal, resulting in poor sperm motility and infertility. The disorder called Kartagener syndrome, which consists of the triad of chronic sinusitis, bronchiectasis, and situs inversus, is a variant of the dyskinetic cilia syndrome (see Chapter 7). Normal ciliary motion in a specific direction is believed to be responsible for the proper rotation of the heart and positioning of intraabdominal organs during embryogenesis. When ciliary function is significantly disturbed, positioning of the heart and intraabdominal organs becomes random, thus accounting for the situs inversus found in approximately 50% of patients with dyskinetic cilia syndrome.
Management of patients with respiratory failure often involves insertion of a tube into the trachea (an endotracheal tube) and support of gas exchange with a mechanical ventilator (see Chapter 29). Endotracheal tubes pose a significant risk for bacterial infection of the lower respiratory tract, often called ventilator-associated pneumonia, in part by preventing glottic closure, a critical component of the sequence of events leading to an effective cough. In addition, the endotracheal tube provides a direct conduit into the trachea for any bacteria that have colonized or contaminated the ventilator tubing or the endotracheal tube itself.
Impairment of Phagocytic and Inflammatory Cells
Cigarette smoking depresses the ability of alveolar macrophages to take up and kill bacteria. Hypoxia, starvation, alcoholism, and cold exposure similarly appear to be conditions in which impaired bacterial killing is at least partly due to depressed macrophage function. Treatment with corticosteroids, given for myriad diseases, seems to depress migration and function of macrophages, and this may compound additional adverse effects of steroids on lymphocytes and the immune system. Some data suggest that macrophage migration is impaired in AIDS, possibly complicating the other host defense defects recognized in the disease (see Chapter 26).
Bals, R. Epithelial antimicrobial peptides in host defects against infection. Respir Res. 2000;1:141–150.
Barbato, A, Frischer, T, Kuehni, CE, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–1276.
Boyton, RJ, Openshaw, PJ. Pulmonary defences to acute respiratory infection. Br Med Bull. 2002;61:1–12.
Curtis, JL. Cell-mediated adaptive immune defense of the lungs. Proc Am Thorac Soc. 2005;2:412–416.
Fahy, JV, Dickey, BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247.
Hiemstra, PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp Lung Res. 2007;33:537–542.
Holt, PG. Pulmonary dendritic cells in local immunity to inert and pathogenic antigens in the respiratory tract. Proc Am Thorac Soc. 2005;2:116–120.
Kim, S, Shao, MXG, Nadel, JA. Mucous production, secretion and clearance. In: Mason RJ, Broaddus VC, Murray JF, et al, eds. Textbook of pulmonary medicine. ed 4. Philadelphia: Elsevier Saunders; 2005:330–354.
Kingma, PS, Whitsett, JA. In defense of the lung: surfactant protein A and surfactant protein D. Curr Opin Pharmacol. 2006;6:277–283.
Lambrecht, BN. Alveolar macrophage in the driver’s seat. Immunity. 2006;24:366–368.
Martin, TR, Frevert, CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2:403–411.
Mason, CM, Nelson, S. Pulmonary host defenses. Implications for therapy. Clin Chest Med. 1999;20:475–488.
Mizgerd, JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716–727.
Mizgerd, JP. Respiratory infection and the impact of pulmonary immunity on lung health and disease. Am J Respir Crit Care Med. 2012;186:824–829.
Moore, BB, Moore, TA, Toews, GB. Role of T- and B-lymphocytes in pulmonary host defences. Eur Respir J. 2001;18:846–856.
Opitz, B, van Laak, V, Eitel, J, et al. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. 2010;181:1294–1309.
Pilette, C, Ouadrhiri, Y, Godding, V, et al. Lung mucosal immunity: immunoglobulin-A revisited. Eur Respir J. 2001;18:571–588.
Riches, DW, Fenton, MJ. Monocytes, macrophages and dendritic cells of the lung. In: Mason RJ, Broaddus VC, et al, eds. Textbook of pulmonary medicine. ed 4. Philadelphia: Elsevier Saunders; 2005:355–376.
Tsai, KS, Grayson, MH. Pulmonary defense mechanisms against pneumonia and sepsis. Curr Opin Pulm Med. 2008;14:260–265.
Underwood, MA, Bevins, CL. Defensin-barbed innate immunity: clinical associations in the pediatric population. Pediatrics. 2010;125:1237–1247.
Vareille, M, Kieninger, E, Edwards, MR, et al. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24:210–229.
Vermaelen, K, Pauwels, R. Pulmonary dendritic cells. Am J Respir Crit Care Med. 2005;172:530–551.
Augmentation of Respiratory Defense Mechanisms
Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1128–1132.
Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59:1102–1106.
Huss, A, Scott, P, Stuck, AE, et al. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48–58.
Lambert, LC, Fauci, AS. Influenza vaccines for the future. N Engl J Med. 2010;363:2036–2044.
Tosh, PK, Jacobson, RM, Poland, GA. Influenza vaccines: from surveillance through production to protection. Mayo Clin Proc. 2010;3:257–273.