Lung Cancer
After reading this chapter you will be able to:
 Describe the epidemiology of lung cancer in the United States, particularly current trends.
Describe the epidemiology of lung cancer in the United States, particularly current trends.
 Identify risk factors for lung cancer.
Identify risk factors for lung cancer.
 State the classification of lung cancer types and the cellular features of the four common types of lung cancer.
State the classification of lung cancer types and the cellular features of the four common types of lung cancer.
 Describe current understanding of the pathophysiology of lung cancer.
Describe current understanding of the pathophysiology of lung cancer.
 Identify the clinical features of the common types of lung cancer.
Identify the clinical features of the common types of lung cancer.
 Describe the diagnostic approach to lung cancer.
Describe the diagnostic approach to lung cancer.
 State the staging system for lung cancer.
State the staging system for lung cancer.
 Describe the treatment and outcomes for the common types of lung cancer by stage.
Describe the treatment and outcomes for the common types of lung cancer by stage.
 State the role of the respiratory therapist in managing patients with lung cancer.
State the role of the respiratory therapist in managing patients with lung cancer.
Lung cancer is a major public health problem. In the United States, approximately 28% of cancer deaths are due to lung cancer.1 Most of these deaths could be avoided if people did not smoke tobacco-related products. Worldwide tobacco consumption has not been declining, however, suggesting lung cancer will remain an epidemic for years to come. Advances in early detection and treatment have been slow, leaving the overall prognosis very poor when lung cancer has been detected. Just over one in eight lung cancer patients is still living 5 years after diagnosis. This chapter provides an overview of lung cancer for the respiratory therapist (RT).
Epidemiology
New Cases
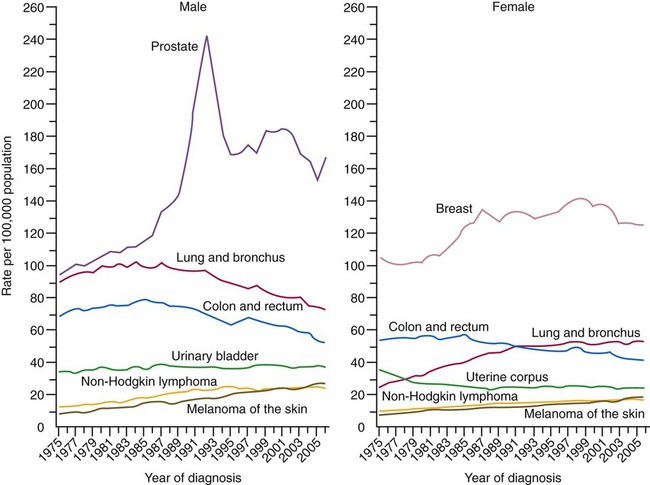
In 2010, an estimated 222,520 new cases of lung cancer were diagnosed in the United States.1 Lung cancer is the second most frequently diagnosed cancer in men and women (prostate and breast cancers are most frequent in men and women) (Figure 28-1). The incidence of lung cancer peaked in men in 1984 (86.5 per 100,000 men) and has since been declining (69.1 per 100,000 in 1997). In women, the incidence increased during the 1990s, with a leveling off toward the end of the decade (43.1 per 100,000 women). These trends parallel the smoking patterns of men and women.1 The World Health Organization estimates that there are 2 million cases of lung cancer worldwide each year.
Deaths
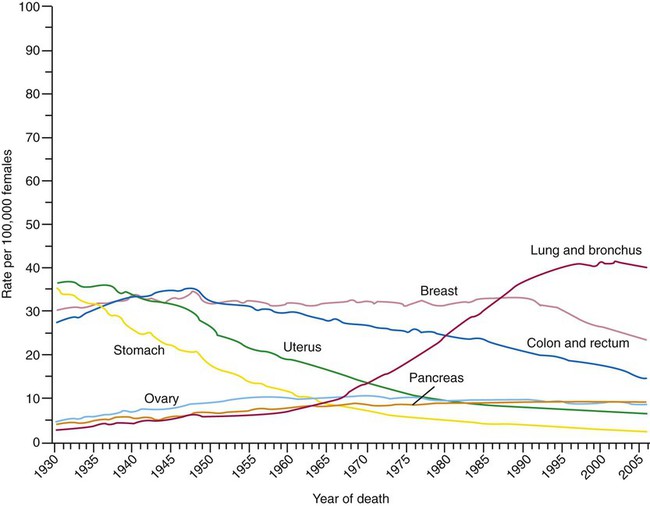
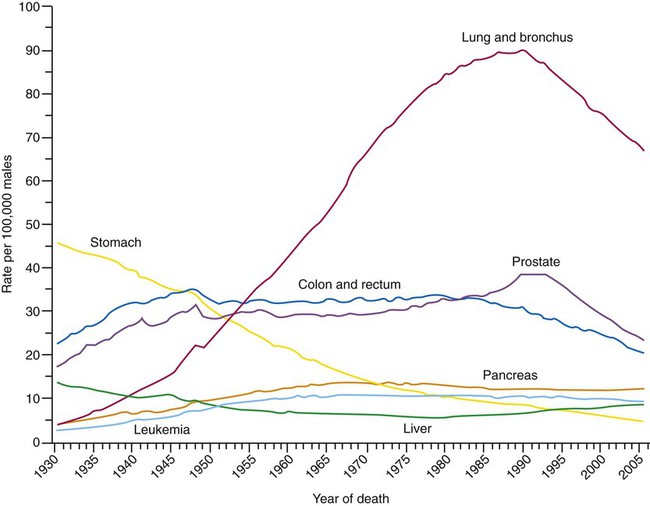
Lung cancer is the leading cause of cancer-related mortality in men and women; it surpassed colon cancer in the early 1950s in men and breast cancer in the late 1980s in women. Mortality rates in men declined significantly in the 1990s, whereas a slow increase occurred in women. These rates parallel the smoking patterns of men and women (Figures 28-2 and 28-3). In 2010 in the United States, an estimated 157,300 deaths were due to lung cancer. In men, lung cancer is the leading cause of cancer-related mortality from age 40 on. In women, lung cancer surpasses breast cancer in the age group of 60 and older.1
Risk Factors
Tobacco-Related Products
There is evidence that nicotine, a chemical in tobacco, is highly addictive.2 Approximately one-fifth of all adults in the United States smoke cigarettes. Progress had been made in the fight against cigarette use; in the decades from 1970-1990, the percentage of women who smoked declined from 33% to 25%, and the rate of smoking among men decreased from 43% to 28%. The annual decline that had occurred since the early 1970s began to slow through the 1990s despite mounting evidence associating smoking with disease and death.3 In addition, a decrease in smoking has not been observed among adults 18 to 24 years old. Cigarette smoking among young people remains a major public health concern. Of young adults (18 to 24 years old), 33% have been reported to be current users of tobacco,4 and 3000 teenagers begin smoking each day.5 Approximately 13% of middle school children and 28% of high school students use tobacco products. In the context that a person who has not started smoking as a teenager is unlikely ever to become a smoker, the tobacco industry has focused on young people and developing countries as the primary sources of new customers.6,7
Other forms of exposure to tobacco-related products also pose risk of promoting lung cancer. Cigar smoking, which has increased considerably over the past several years, is known to be an independent risk factor for developing lung cancer.8
Exposure to sidestream smoke, or passive smoking, may also lead to an increased risk of lung cancer. The risk is generally much lower than active smoking but varies with the intensity of exposure.7 The risk of developing lung cancer has been reported to be 30% higher in individuals exposed to sidestream smoke. It has been estimated that 3000 to 5000 deaths in the United States and 21,400 deaths worldwide from lung cancer occur each year because of second-hand smoke exposure.9,10
Occupational Agents and Other Risks
Many other risk factors have been identified (Box 28-1). Occupational agents are known to act as lung cancer carcinogens. Arsenic, asbestos, and chromium confer the highest risk. Of lung cancers, 2% to 9% have been estimated to be related to occupational exposures. This risk is increased when there is concomitant exposure to tobacco products. Indoor radon exposure is also a risk factor for developing lung cancer.11 Radon is a product generated by the breakdown of uranium. Particulates in the atmosphere (i.e., pollution) can increase the risk of lung diseases including lung cancer.
An inherited genetic predisposition has epidemiologic support as a risk factor, but the mechanisms are not proven. Family members of people who develop lung cancer have an increased risk.12,13 Women seem to have a higher baseline risk of developing lung cancer and a greater susceptibility to the effects of smoking. Differences in the metabolism of tobacco-related carcinogens and their metabolites, an effect of hormone differences, or both are thought to account for the increased susceptibility.14 Dietary factors can also modify risks. Higher consumption of fruits and vegetables is associated with a reduced lung cancer risk, and increased dietary fat intake may lead to a higher risk.15,16 Supplementation with vitamin A, vitamin E, or beta-carotene has not positively influenced risk.15 The presence of chronic obstructive pulmonary disease (COPD) is an independent risk factor.17 This risk increases as the forced expiratory volume in 1 second (FEV1) decreases.18,19
Lung Cancer Classification
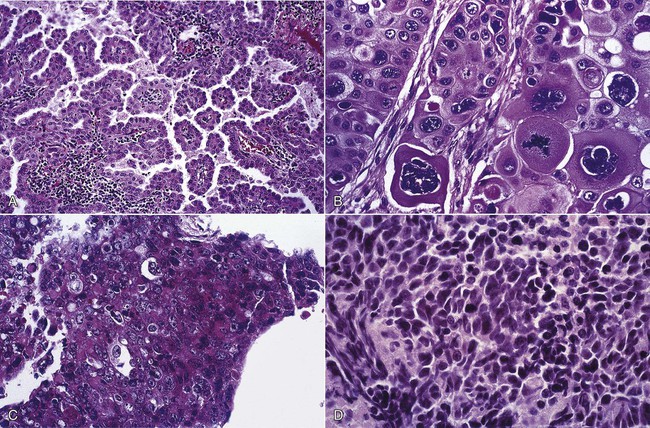
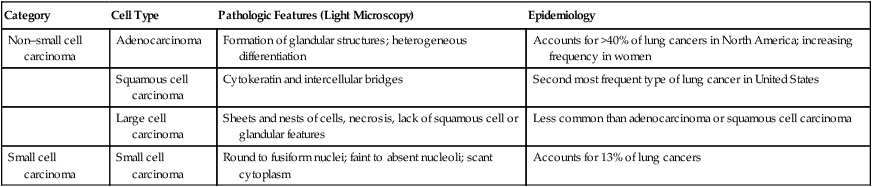
Lung cancers are divided into two major groups—small cell carcinoma and non–small cell carcinoma—based on pathologic features that are visible under light microscopy. The evaluation and management of a patient are guided by the category and stage (see later) of lung cancer. The non–small cell cancer category consists of adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and variants (Figure 28-4). Table 28-1 presents the pathologic and epidemiologic features of the four most common types of lung cancer: adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and small cell carcinoma.
TABLE 28-1
Classification of Most Common Types of Lung Cancer
| Category | Cell Type | Pathologic Features (Light Microscopy) | Epidemiology |
| Non–small cell carcinoma | Adenocarcinoma | Formation of glandular structures; heterogeneous differentiation | Accounts for >40% of lung cancers in North America; increasing frequency in women |
| Squamous cell carcinoma | Cytokeratin and intercellular bridges | Second most frequent type of lung cancer in United States | |
| Large cell carcinoma | Sheets and nests of cells, necrosis, lack of squamous cell or glandular features | Less common than adenocarcinoma or squamous cell carcinoma | |
| Small cell carcinoma | Small cell carcinoma | Round to fusiform nuclei; faint to absent nucleoli; scant cytoplasm | Accounts for 13% of lung cancers |
Pathophysiology
The pathophysiology of lung cancer development is complex and incompletely understood. Damage to genetic material in lung cells is the result of exposure to chemical carcinogens such as the carcinogens contained in tobacco smoke.20 People who develop lung cancer may have a genetic predisposition to the effects of these carcinogens. The genes influenced in the pathogenesis of lung cancer produce proteins involved in cell growth and differentiation, cell cycle processes, apoptosis (programmed cell death), angiogenesis (production of new blood vessels), tumor progression, and immune regulation. If enough of these pathways have been affected, the uncontrolled growth of cells that defines cancer occurs. If the mechanisms that lead to genetic damage can be identified and the means by which the pathways involved are controlled, novel means of risk stratification, prevention, early detection, and therapy should be able to be developed.
Clinical Features
The clinical features of lung cancer result from the effects of local growth of the tumor, regional growth or spread through the lymphatic system, hematogenous (blood-borne) distant metastatic spread, and remote paraneoplastic effects from tumor products or immune cross reaction with tumor antigens (Box 28-2). Some manifestations occur more commonly with a particular cell type. Despite modern imaging advances, only approximately 15% of patients with a diagnosis of lung cancer do not have symptoms at the time of presentation. Some of the initial symptoms may be related to accompanying illnesses because these patients are at risk for other medical problems in addition to lung cancer (e.g., COPD, heart disease).
When symptoms develop that are the result of the presence of cancer but are not related to the growth or spread of the cancer, these symptoms constitute a paraneoplastic syndrome. Paraneoplastic syndromes can result from the effects of proteins produced by the tumor that circulate through the body to have their effects on distant organs or result from the immune response of the body to a tumor antigen that is similar to antigens in other parts of the body, causing immune injury to the distant organ. Paraneoplastic syndromes may occur before the primary tumor appears and be the first sign of disease or an indication of tumor recurrence. Examples include production of excess glucocorticoids (ectopic Cushing syndrome), parathyroid hormone (hypercalcemia of malignancy), and antidiuretic hormone (syndrome of inappropriate antidiuretic hormone [SIADH]). Paraneoplastic neurologic syndromes can affect all parts of the neurologic system resulting in emotional lability (limbic encephalitis), loss of balance (cerebellar degeneration), and muscle weakness with a characteristic recruitment of strength on electrical stimulation (Lambert-Eaton syndrome). Other paraneoplastic syndromes include skeletal and connective tissue syndromes (clubbing, hypertrophic pulmonary osteoarthropathy), coagulation and hematologic disorders, cutaneous and renal manifestations, and systemic symptoms (anorexia, cachexia, and weight loss).21
Diagnosis
Radiographic features are also used to determine the probability of cancer. The larger the lung abnormality, the more likely it is to be cancer. When the abnormality has reached the size of a mass (3 cm), it needs to be considered a cancer until proven otherwise. The rate of growth of the lesion is also helpful. If a nodule grows rapidly (doubles in size in <1 month) or grows very slowly or not at all over a couple of years, it is unlikely to be due to cancer. If the nodule appears to be heavily calcified on imaging, it has likely been present for quite some time and is unlikely to represent cancer. If the abnormality has an irregular border, is lobulated, or is spiculated, it is more likely to be a cancer than if the border is smooth and rounded. Finally, if the lesion is cavitary, the thickness of the wall of the cavity can suggest cancer. A wall thickness of 14 mm or greater is likely to represent a cancer.22
After the clinical and standard imaging features are reviewed, a probability of malignancy can be determined. If the probability is very high, the potential cancer does not seem to have spread, and the individual is fit, proceeding directly to surgery would be reasonable. If the previously mentioned features suggest a very low probability of malignancy, the clinician and patient might choose to follow along with serial chest imaging over time to assess for further growth. When the probability falls between these extremes, adjunctive imaging and invasive procedures can be used to help alter the probability. The most commonly used additional imaging technique is positron emission tomography (PET) with fluorodeoxyglucose (FDG-PET). Because malignant cells are metabolically very active, they take up the glucose analogue more avidly than nonmalignant cells. The attached radioactive tracer becomes trapped in the cells, allowing it to be imaged. When this test is used to help predict the presence of lung cancer, it has a sensitivity of 97% and a specificity of 78%.23 PET imaging can produce false-positive results in other metabolically active conditions such as infections. It can be falsely negative if the lesion is too small (<10 mm) or if the tumor is slow growing and not very metabolically active (e.g., some adenocarcinomas, carcinoid tumor). Single photon emission computed tomography (SPECT) and lung nodule enhancement with contrast-enhanced CT are other imaging techniques that have been studied but are not being used clinically.22
Addition of needle aspiration to the conventional sampling techniques (washing, brushing, and forceps biopsy) improves the yield. The diagnostic yield from lesions in the periphery of the lung, beyond where the camera is able to see, is lower. Conventional sampling techniques and peripheral transbronchial needle aspiration complement each other. Factors that influence the diagnostic yield of flexible bronchoscopy for peripheral lesions include the size of the lesion, its location, and the presence of a “bronchus sign” on CT (an airway leading directly into the lesion). Smaller, more peripheral lesions, without a visible bronchus within or leading directly to them, are unlikely to be diagnosed by flexible bronchoscopy.22 More recent technologic advances, such as multiplanar imaging, electromagnetic navigation of the bronchoscopy instruments, and peripheral endobronchial ultrasound, have been able to improve the yield of flexible bronchoscopy for these small peripheral lesions.24 Endobronchial ultrasound can also be used to guide biopsies of hilar and mediastinal lymph nodes.25–26
Transthoracic needle biopsy, using fluoroscopic or CT guidance, can also be used to obtain tissue. With this procedure, an aspirating needle is passed through the skin into the lung lesion under the guidance of chest imaging. The positive predictive value of this procedure is high, the negative predictive value is modest, and the rate of establishing a specific benign diagnosis is low. Smaller nodules in central locations have lower diagnostic rates. A higher rate of pneumothorax occurs with transthoracic needle biopsy.24 The choice of which procedure to use is guided by the size and location of the lesion and by the local expertise with each technique.
Staging
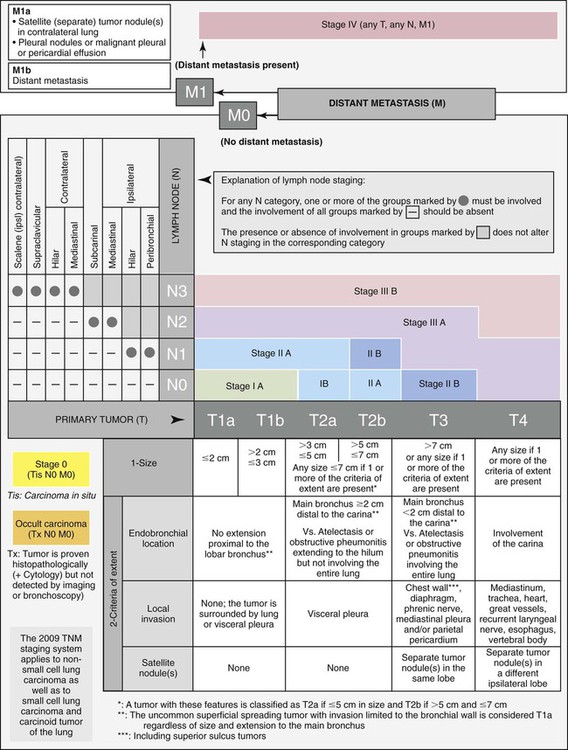
The T component of the staging system is divided into T1 through T4 lesions, as follows:
• A T1 tumor is a small tumor confined to the lung. It must be less than 3 cm in diameter and be surrounded by lung or visceral pleura and cannot extend into a main bronchus. T1a tumors are less than 2 cm in diameter, and T1b tumors are 2 to 3 cm.
• A T2 tumor is larger than a T1 lesion, but its local growth remains minimally invasive. It is greater than 3 cm in diameter (3 to 5 cm for T2a, 5 to 7 cm for T2b), may invade the visceral pleura, or may extend into the main bronchus, but it remains greater than 2 cm from the main carina. It may cause segmental or lobar atelectasis.
• A T3 tumor is locally advanced or invasive up to but not including the major intrathoracic structures. It can be any size, and it may involve the chest wall, diaphragm, mediastinal pleura, parietal pericardium, or main bronchus within 2 cm of the main carina (but not involving the main carina). It may cause atelectasis of an entire lung. There may be separate tumor nodules in the same lobe as the primary tumor. A tumor larger than 7 cm in diameter is considered T3 even if unaccompanied by local invasion.
• A T4 tumor is a tumor of any size that has invaded one of the major intrathoracic structures, such as the mediastinum, heart, great vessels, trachea, esophagus, vertebral body, or main carina. The tumor is also classified T4 if there are tumor nodules in a different ipsilateral lobe of the lung.
• N0 spread does not involve any lymph nodes.
• N1 spread indicates the presence of cancer in nodes within the ipsilateral lung (the same side as the tumor).
• N2 spread signifies cancer in nodes in the mediastinum ipsilateral to the primary tumor.
• N3 spread signifies cancer in nodes distant to the nodes included in N2 such as contralateral (“opposite side”) mediastinal or hilar nodes, ipsilateral (“same side”) or contralateral scalene, or supraclavicular nodes.27–29
The most recent revision27 to this staging system occurred in 2009 (Table 28-2 and Figure 28-5). The stages are labeled from stage IA to stage IV based on the combination of T, N, and M features.
TABLE 28-2
| Stage | |
| IA | T1a,bN0M0 |
| IB | T2aN0M0 |
| IIA | T1a,bN1M0, T2aN1M0, T2bN0M0 |
| IIB | T2bN1M0, T3N0M0 |
| IIIA | T3N1M0, T(1-3)N2M0, T4N0-1 |
| IIIB | T4N2M0, T(1-4)N3M0 |
| IV | T(any)N(any)M1a,b |
For patients with small cell lung cancer, the TNM staging system was previously thought to be less useful. Instead, small cell lung cancer has been staged as limited or extensive disease. Limited stage disease is present when the tumor is confined to a hemithorax (including ipsilateral mediastinal and supraclavicular lymph nodes) and can be contained within a radiotherapy port. Extensive stage disease is present when the tumor extends beyond these boundaries. The recent lung cancer staging revision recognized a benefit to applying the TNM staging system used for non–small cell cancer to small cell cancer as well.27,29
The proper use of testing to stage a patient with lung cancer is addressed in a more recent set of guidelines.30 The history and physical examination are important in guiding testing. The extent of spread is best evaluated using CT of the chest extending to the upper abdomen to include the liver and adrenal glands; this should be ordered in all patients. Magnetic resonance imaging (MRI) has not proved to be more accurate except in the setting of a Pancoast tumor. The sensitivity and specificity of CT for the evaluation of regional lymph node involvement are modest, commonly noted to be 60% and rarely greater than 75%. Imaging with FDG-PET scanning seems to have better test characteristics for staging mediastinal nodes, with sensitivity and specificity greater than 90%.31 Integrated PET/CT scanning seems to have better test characteristics than PET and CT used alone or together.32
Because noninvasive tests can have false-positive results, tissue confirmation is necessary. Bronchoscopy with transbronchial needle aspiration is useful to stage the mediastinum. The addition of endobronchial and endoscopic ultrasound has increased the yield of nonsurgical mediastinal staging.26,33 If such staging is negative, mediastinoscopy, mediastinotomy, or thoracoscopy can confirm the nodal status. Despite the advances in imaging technology and sampling techniques, definitive staging with surgical resection and mediastinal dissection remains the “gold standard” in a patient with resectable disease. The assigned clinical stage (determined by the previously listed testing, including mediastinoscopy) can be lower than the pathologic staging (assigned after surgery).
Per guidelines, a head CT or MRI scan should be performed if symptoms or signs of metastatic disease are present or when evaluating what appears to be stage IIIA through IV disease. Although there is no proven survival benefit from CT versus MRI, many clinicians prefer to use MRI of the brain because it has greater sensitivity to detect metastatic disease.30,34 Bone scanning was previously performed if symptoms or signs suggest bone involvement, if the patient has an elevated calcium or alkaline phosphatase level, or if the patient is in stage IIIA/B disease. This scan has been largely replaced by PET. PET has been used to stage all but brain metastases. The rate of detecting distant metastases using PET appears to be higher than using non-PET approaches.35
Further evaluation of performance status may be necessary in patients for whom surgical resection is indicated. To determine if a patient would tolerate lung resection, reports of activity tolerance and pulmonary function testing are used. Although no one pulmonary function study or absolute cutoff has proved to be ideal, FEV1 and diffusing capacity for carbon monoxide (DLCO) are the most frequently used measures. Traditional preoperative cutoff values are being replaced by percent predicted postoperative values. Percent predicted postoperative values of FEV1 and DLCO can be calculated by multiplying the percent predicted preoperative value by the fraction of the total number of lung segments that will remain postoperatively. Alternatively, quantitative perfusion imaging can be used to guide the calculation. If the percent predicted postoperative FEV1 and DLCO are greater than 40%, the patient should be able to tolerate surgery. As would be expected, a pneumonectomy requires better preoperative lung function than a lobectomy. When doubt remains or when measured values and predictions seem discordant with an individual’s reported activity tolerance, a cardiopulmonary exercise test should be performed. If the maximum oxygen consumption is greater than 15 ml/kg/min, a lobectomy should be reasonably well tolerated. If the maximum oxygen consumption is less than 10 ml/kg/min, conventional surgery should not be performed. Patients with a maximum oxygen consumption value between these two limits should be considered on a case-by-case basis.36
Screening For Lung Cancer
Given the poor prognosis for advanced stage lung cancer and the high proportion of patients who present in an advanced stage, there has been great interest in screening for lung cancer. The earliest efforts at radiographic screening involved the analysis of mass chest radiograph screenings from the population of an individual city. Subsequently in the 1970s, there were large efforts to use chest radiograph, sputum, or a combination of the two as screening tools. Despite considerable ongoing debate about the design and analysis of these randomized studies, they have been interpreted as not showing that screening with plain chest radiograph or sputum examination has a beneficial effect on mortality from lung cancer.37
Given the disappointing overall results from studies of chest radiograph as a screening technique, efforts have centered on the use of low-dose CT imaging as a screening tool. Important highlights of several available CT cohort studies include the ability to find many early stage lung cancers and the long survival times of patients whose lung cancer is diagnosed at an early stage. Limitations of CT and the trial designs that have been identified in these studies are an inability to comment on lung cancer–specific mortality, numerous benign nodules being identified (5% to 50% of participants on the initial scan), intense testing protocols required to follow the identified nodules to ensure they are not cancer, invasive procedures performed on some benign nodules, and questionable cost-effectiveness.38–45
These limitations have prompted the performance of a few large-scale randomized screening trials of CT imaging as the screening tool. One trial, the National Lung Screening Trial, reported a 20% reduction in lung cancer–specific mortality in subjects with very high risk. This finding may change the standard recommendations for lung cancer screening. Remaining issues include identifying individuals at high risk for developing lung cancer, the appropriate management of the numerous small lung nodules identified on CT, the potential harms of radiation from the CT scan, and the cost-effectiveness of the screening program.46
To address these issues, researchers are attempting to develop other tests, including safe and noninvasive tests of the blood and breath. Autoantibodies directed at tumor-associated antigens have been identified in the blood of some patients with primary lung cancer. A positive blood test such as this may be an early indicator of lung cancer before a tumor appears in the lungs and could be a potential tool in selecting patients for screening programs.47 Studies are under way to determine how these blood tests may best be used clinically. In addition, the analysis of exhaled human breath has become an area of interest in lung cancer. Compounds within patients’ exhaled breath form unique chemical signatures. These signatures have shown measurable, specific patterns in lung cancer compared with controls. As research continues in breath analysis, we may see its use in the screening, diagnosis, and treatment monitoring of lung cancer.48
Treatment And Outcomes
Non–Small Cell Lung Cancer
Three types of treatment are used to treat non–small cell lung cancer: surgical resection, radiotherapy, and chemotherapy (Box 28-3). The first two treatments provide local control of the cancer, and the last is used to treat systemic disease. Which therapy or combination of therapies is recommended depends on the stage of the cancer, the patient’s ability to tolerate treatment, and the type of cancer (or its histology). Molecular changes within the tumor are beginning to influence treatment choices as well.
Early Stage Non–Small Cell Carcinoma
Surgical resection offers the best chance of cure for early stage non–small cell lung cancer (stages I and II) (Table 28-3). Survival after resection in pathologic stage IA approaches 70% at 5 years; in pathologic stage IB, 5-year survival is closer to 55%. The surgery of choice is a lobectomy, in which the entire lobe of the lung containing the cancer is removed. If the tumor is very central, a pneumonectomy may be required. Lesser resections, such as segmentectomy, or wedge resection, can be performed in patients with modest lung function to spare as much lung tissue as possible. In most patients, limited resection leads to a slightly lower survival rate and a higher rate of local recurrence of cancer.49,50 In elderly patients and in patients with the poorest lung function, much of the decrease in survival is due to mortality not related to lung cancer.51 In the smallest cancers and in patients who are older than 70 years, a limited resection may be as effective as a lobectomy.51,52 Vascular invasion and tumor differentiation may be prognostic factors. There does not seem to be a difference in survival between patients who have adenocarcinoma and patients who have squamous cell carcinoma. Recurrence usually involves distant metastases.
TABLE 28-3
Non–Small Cell Lung Cancer: 5-Year Survival by Stage
| Stage | Clinical | Pathologic |
| IA | 50% | 73% |
| IB | 43% | 58% |
| IIA | 36% | 46% |
| IIB | 25% | 36% |
| IIIA | 19% | 24% |
| IIIB | 7% | 9% |
| IV | 2% | 13% |
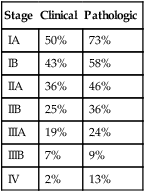
Adapted from Goldstraw P, Crowley J, Chansky K, et al; International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions: The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2:706–714, 2007.
Survival after resection in pathologic stage IIA is 50% to 55% at 5 years and in pathologic stage IIB is approximately 40% (see Table 28-3). Limited resections are not typically an option in stage II cancers. Patients with adenocarcinoma may have poorer survival than patients with squamous cell carcinoma. Most recurrences involve distant metastases.
Radiotherapy has been used with curative intent in early stage non–small cell lung cancer in patients who cannot tolerate surgery or in patients who elect not to undergo surgery. The 5-year survival rate in stage I and II disease approaches 15% with standard radiotherapy alone. There is a high rate of local recurrence, and most deaths are due to lung cancer. Stereotactic body radiotherapy is a novel radiation therapy technique in which multiple convergent beams of radiation are precisely targeted on the tumor. This targeting allows very high doses of radiation to be delivered to the tumor while sparing the normal lung tissue. Rates of local control and survival are impressive in selected groups reported in many case series, approaching the rates of lung resection.53 Lung resection and stereotactic body radiotherapy have not been compared head to head. Lung resection remains the standard of care in patients able to tolerate it. Adjuvant (“applied after initial treatment”) radiotherapy in patients who have undergone surgical resection may improve local control but does not improve survival (with the possible exception of patients who have undergone incomplete resection).
Adjuvant platinum-based chemotherapy leads to a significant survival benefit in selected patients with completely resected stage II lung cancers.54 The potential benefit of adjuvant chemotherapy in patients with stage IB disease is debated.
Locally and Regionally Advanced Non–Small Cell Carcinoma
The approach to N2 (stage IIIA) disease varies among institutions. Unselected patients have a low rate of complete resection with primary surgery, and patients with incomplete resection do poorly. Patients without radiographic evidence of N2 disease but who are found at surgery to have N2 disease do better than patients with preoperative evidence of N2 disease. Adjuvant chemotherapy should be offered to this group. Generally, the more advanced the node involvement (number, extension, or location), the poorer the prognosis; protocols using multimodal therapy are being investigated. Induction with chemotherapy with or without radiotherapy leads to objective responses in most patients, some of whom go to a more favorable stage and become candidates for surgical resection. Patients who have bulky nodes or who require a pneumonectomy are less likely to benefit from resection after induction therapy. At the present time, concurrent chemoradiotherapy should be considered the standard of care, with resection included in specialized centers, often in the setting of a study. Survival rates are 5% to 13% at 5 years. It is suggested that newer agents may be as effective with less toxicity. With advances in each of the modes of therapy, treatment will evolve over time.55–58
T4 disease without advanced nodal status (stage IIIB) may be considered for surgical treatment in only a few settings. T4 disease involving the main carina may be considered for resection at centers with expertise. The role of induction therapy in this setting has not yet been defined. Disease at the N3 level (stage IIIB) is generally considered nonsurgical. Advances in induction therapy may alter this notion in time, and trials of multimodality therapy are ongoing.57,58
Metastatic Non–Small Cell Carcinoma
In stage IV lung cancer, platinum-based chemotherapy regimens have been shown to improve survival and enhance quality of life. They are also cost-effective. This treatment is most appropriate for individuals with a good performance status. Resection of an isolated brain metastasis in patients with a good performance status can improve survival. Standard chemotherapy typically involves two agents administered in cycles, each approximately 3 weeks apart, for a total of four to six cycles. The addition of a third agent or additional cycles has traditionally added risk without benefit. More recently, agents with improved tolerance have been shown to benefit patients who have shown a good response to treatment when administered as maintenance treatment, until progression is noted.59
Standard chemotherapy targets all growing cells, not just cancer cells (hence the common side effects seen). Targeted therapies have been developed where the mechanism of action is more specific to the cancer cell. In lung cancer, inhibitors of epidermal growth factor receptors (EGFRs), known to be highly expressed on lung cancer cells, and vascular endothelial growth factor (VEGF) have been studied. EGFR inhibitors have been most successful in patients with EGFR activating mutations in their cancer tissue. The patients most likely to have EGFR mutations include female never-smokers with adenocarcinoma, in particular, patients of Asian origin. This subgroup has been found to have an improved survival overall, which is improved further by the use of an EGFR inhibitor. The VEGF receptor inhibitor has been shown to improve survival when added to standard chemotherapy in patients with nonsquamous cell histology.59,60 These treatments can be continued until progression is noted. Other promising agents in late phase development include inhibitors of insulin-like growth factor receptor and the EML4-anaplastic lymphoma kinase (ALK) gene rearrangement.59–62 Further successes with targeted therapy are expected in the future.
Small Cell Lung Cancer
Treatment of small cell lung cancer is based on its staging (see Box 28-3). In limited stage disease, combination chemotherapy with concurrent hyperfractionated radiotherapy is recommended. The drug etoposide and a platinum agent are standard, but trials with newer agents are ongoing. Prophylactic cranial radiation is generally recommended for patients who have a complete response to chemoradiotherapy. Surgery is limited to cases in which the diagnosis is in doubt or in cases that have not responded to chemoradiotherapy but remain resectable. In patients with extensive stage disease, combination chemotherapy improves the quality of life and median survival. A poor performance status and an elevated lactate dehydrogenase level portend a poor prognosis. Radiotherapy to the chest may be used in patients who have a complete response to chemotherapy in disease outside the chest.59,63
Future Scenario
The prospect of major advances in the prevention, detection, and treatment of lung cancer is strong. An attainable vision for 2031 could be as follows: Primary prevention campaigns have successfully minimized the number of individuals who are smoking, legislation has passed broadly to prevent exposure to tobacco smoke in public places, progress has been made in occupational exposure avoidance, and successful measures have been enacted to clean the air. Individuals are now identified who have changes in lung cells that suggest lung cancer could develop and are being treated with medication to prevent it from developing. Individuals at risk for developing lung cancer are part of a screening program that detects early stage lung cancer with a test that is inexpensive and acceptable to all. Technology has improved diagnostic abilities by making imaging more specific and biopsies more accurate. Noninvasive diagnostics have expanded with advances in blood and breath testing.47,48,64,65 In addition to tumor appearance, researchers are identifying characteristics of tumor biology that allow more selection in choosing treatments.66,67 The best form of local control for a given tumor (resection, radiation) is known, and means have been developed to minimize the effect of these interventions on the quality of life. Novel agents have been developed that can reach and kill tumor cells, while avoiding injury to healthy tissue. As evidence of successes to date, lung cancer is no longer the leading cause of cancer-related mortality in the United States.