Chapter 161 Lumbar Total Disc Arthroplasty
Background
The intervertebral disc is a complex structure that plays a key role in range of motion and load transfer in the lumbar spine. The nucleus pulposus absorbs compressive loads, whereas the anulus fibrosus resists shear forces and contains the nucleus. A disc in the normal lumbar spine bears 80% of compressive loads. It is subjected to 1 to 2.5 times body weight on ambulation and up to 10 times body weight when lifting a heavy load. The lumbar disc also allows for rotation and translation in three orthogonal planes. The characteristics of motion vary according to the level, with more rotation occurring in the upper lumbar spine and more flexion and extension in the lower lumbar spine. The center of rotation in the sagittal plane is usually located dorsal and caudal to the center of the distal end plate, but varies slightly with flexion and extension.1
The gold standard of operative treatment for DDD in patients who fail conservative therapy is arthrodesis of the affected segment and decompression of stenosis, if needed. This may be accomplished through a dorsal approach, ventral approach, or a combination of the two with or without iliac crest autograft. The procedure has been performed since 1911 and carries success rates of between 60% and 90%. This treatment, however, has several significant drawbacks. Pseudarthrosis rates are reported at 14%.2 Evidence of adjacent-segment degeneration is observed in 30% to 40% of fusions.3,4 Iliac crest autograft harvesting results in significant rates of postoperative complications and donor site pain.5
Indications
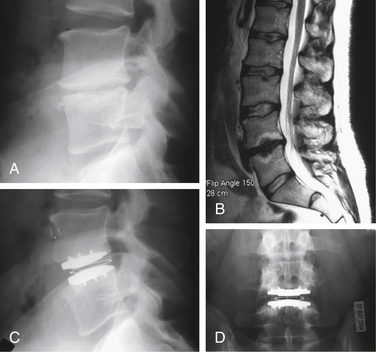
As with many other spine operations, proper patient selection is one of the most important aspects of a successful TDR. The most common indications used in current disc replacement surgeries are listed in Box 161-1.6 Typical indications are illustrated in Figure 161-1. The vast majority of patients who qualify for TDR are younger than 60 years of age. This excludes most patients with degenerative processes in dorsal spinal structures (e.g., facet degeneration, ligamentum flavum hypertrophy, disc herniation) and with inadequate bone stock. Bertagnoli et al. have shown that in a carefully selected group of patients older than 60 years of age, a TDR procedure resulted in equivalent patient satisfaction rates.7 Even in this group of 22 patients, however, there were 2 cases of radiculopathy due to circumferential stenosis and 2 cases of implant subsidence. DDD must be shown to be the main, if not the only, source of the back pain. Positive radiographic findings and a concordant discogram are currently thought to be the best ways to confirm DDD. Because most cases of low back pain from DDD resolve with nonoperative treatment, surgical candidates must have failed those options. They must have significant pain and disability to justify the potential risks and recovery period associated with operative intervention. Because current TDR implants are designed for a ventral surgical approach, patients must be able to tolerate such an approach from the standpoint of prior surgical interventions in that area and medical comorbidities.
BOX 161-1 Common Indications and Contraindications for Lumbar Total Disc Arthroplasty
Absolute Contraindications
DDD, degenerative disc disease.
TDR implants rely on fixation to vertebral end plates and ingrowth. Therefore, the patient’s bone quality must be adequate. Osteoporosis, osteopenia due to a metabolic disorder, or tumor may cause implant failure by dislodgement or subsidence. Because lumbar TDR replaces only ventral elements of the spinal column, a good candidate for disc replacement should not have degeneration of dorsal structures, especially the facet joints. Radiographic studies and facet blocks may be used to diagnose facet arthropathy. Even though ventral insertion of a TDR device may offer some indirect decompression, circumferential stenosis is best treated by direct decompression of neural elements through a dorsal approach with fusion, if needed. Studies have shown that the disc space preparation necessary for insertion of a lumbar TDR increases the rotational instability of the spine. The currently available unconstrained disc replacement implants do not fully restore this stability.8 Therefore, TDR in a spine with preexisting rotational instability (Cobb angle >11 degrees) might be expected to result in higher rates of failure.
Current Designs
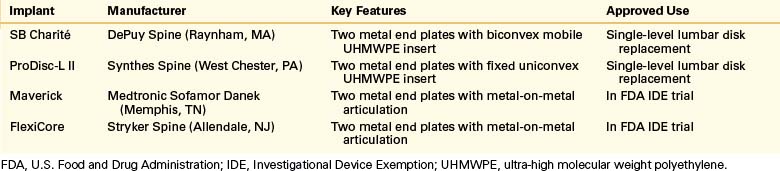
Although only two TDR implants are currently approved by the FDA, there are multiple designs in various stages of testing and development. Charité is the oldest and one of the most extensively studied of the current-generation TDR designs. The first version, SB CHARITÉ I, was first implanted in 1984. The implant underwent two modifications and has been used in its current version, SB CHARITÉ III, since 1994. It consists of two cobalt-chromium end plates with a biconvex sliding central core of ultra-high molecular weight polyethylene (UHMWPE). This results in a floating center of rotation, allowing angular motion and translation. To encourage bony ingrowth, the end plates are covered with plasma-sprayed titanium and electrochemically coated with calcium phosphate. This has been shown in animal studies to result in 48% osseointegration, compared with the 10% to 30% ingrowth seen in successful hip and knee replacement prostheses.9,10 The implant is inserted using a standard ventral retroperitoneal approach and is approved for single-level use.
Another FDA-approved TDR implant, the ProDisc L, was originally developed in 1990 and has undergone one design revision since then. It also contains two cobalt-chromium end plates, but the UHMWPE insert is monoconvex and locked into the distal end plate. This results in a ball-and-socket joint that limits translation and allows rotation. A central keel on the end plates and plasma-sprayed titanium coating allow for bony fixation and ingrowth. Two other TDR implants designed for a ventral approach are currently in FDA Investigational Device Exemptions study stages: FlexiCore (Stryker Spine, Allendale, NJ) and Maverick (Medtronic Sofamor Danek, Memphis, TN). Both products have end plates with metal-on-metal, ball-and-socket articulations. Key features of current TDR designs are listed in Table 161-1.
Because of the known complications that have occurred during device implantation, including dislodgement and the need for revision surgery using the ventral approach, there is extensive work being done on TDR designs implanted by other means.11 Among them is TRIUMPH (Globus Medical, Inc., Audubon, PA), which is inserted through a standard dorsolateral approach. It consists of two metal-on-metal articulating components of cobalt-chromium alloy with a titanium porous coating and multiple keels for bony ingrowth and fixation. The dorsal approach allows the surgeon to address any pathology in the dorsal structures, including facet overgrowth, disc herniation, and soft tissue hypertrophy. The smaller anulotomy and preservation of the anterior longitudinal ligament leaves the segment with greater stability. Such a design might greatly expand the surgical indications for TDR because most patients with DDD also have some pathologic processes in the dorsal structures. Another aspect of TDR research involves the search for alternative bearing surfaces. ORBIT (Globus Medical, Inc.) is an artificial disc that includes articulating polyetheretherketone (PEEK) units with a porous titanium coating for bony ingrowth. This results in nearly complete radiolucency, improved MRI compatibility, and excellent wear characteristics.
Nucleus Replacement
An alternative direction in disc replacement technology involves an artificial nucleus substitute, also known as partial disc replacement. The rationale behind this strategy is to remove the pain generator and restore the disc’s biomechanical properties, while preserving more of the anulus and stability than in classic TDR.12 The original designs by Fernstrom (stainless steel ball), Nachemson (injectable silicone), and Hamby and Glaser (bone cement) in the 1950s and 1960s failed to prevent further disc degeneration.13–15 Better biomaterials have become available recently, prompting a new wave of nucleus replacement technologies. The earliest and most widely used of the current nucleus replacement designs is the Prosthetic Disc Nucleus (PDN, Raymedica Inc., Bloomington, MN). It consists of a hydrogel pellet in a polyurethane envelope. After implantation, the hydrogel absorbs water and expands to the limits of its outer liner, restoring disc height. An earlier design used two implants inserted into the disc space. In response to high rates of dislodgement, the implant was redesigned to a larger single pellet—the PDN-SOLO. New approaches are also being tested to lower dislodgement rates.16 Other constrained, unconstrained, and injectable devices are being tested, but complication rates remain high. In addition, indications for nucleus replacement are more limited than those for TDR, and include at least 50% disc height preservation and an intact anulus.12
Outcomes
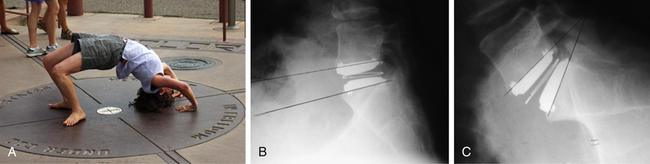
In a prospective, randomized, multicenter, FDA-regulated IDE clinical trial, the Charité disc was compared with the gold standard procedure of anterior interbody fusion using Bagby and Kuslich (BAK) threaded cages (Zimmer Spine, Minneapolis, MN) and iliac crest autograft.6 Three hundred and four patients were enrolled, with approximately 95% follow-up at 12 months and 90% at 24 months. Data analysis demonstrated that the Charité implant was equivalent to the BAK cage regarding Oswestry Disability Index (ODI) scores and visual analogue scale (VAS) scores at 24-month follow-up. The TDR group had significantly shorter hospital stays (3.7 vs. 4.2 days), higher patient satisfaction rates (73.7% vs. 53.1%), and higher overall clinical success rates (57.1% vs. 46.5%). Index-level range of motion was preserved postoperatively, as illustrated in Figure 161-2. The conclusion of the study was that the Charité disc was at least as good as the control procedure in treatment of one-level DDD. Five-year follow-up to this study showed persistence of equivalent improvement in VAS and ODI scores between the two groups.17 It also found that more patients in the TDR group were employed full time (65.6% vs. 46.5%) and fewer patients were on long-term disability (8% vs. 20.9%). Surgery for adjacent-level disease was performed on one Charité patient and two BAK patients (1.1% vs. 4.7%), but these numbers were too small to show statistical significance. There was a significant patient dropout between years 2 and 5, with only 44% follow-up at 5 years. Comparison of the 5-year completers and the patients lost to follow-up showed that at 2 years the two groups were statistically similar in ODI and VAS scores. This demonstrates that the study’s conclusion of the noninferiority of the Charité device to anterior fusion is still valid. Two recently published, nonrandomized, 10-year follow-up studies on the Charité arthroplasty demonstrated 80% to 90% good and excellent clinical outcomes, a 2% rate of adjacent-level degeneration, and close to a 90% return-to-work rate.18,19
Concurrently, a prospective, randomized, multicenter, FDA-regulated IDE clinical trial was conducted to compare the ProDisc L implant with circumferential fusion at one level in the lumbar spine.20 At 24 months, the TDR group had similar improvement in VAS scores (41.6% vs. 36%), but a greater number of patients in the disc replacement group had improvements in ODI scores of more than 15 points (77.2% vs. 64.8%). The experimental group also had a statistically greater number of patients employed or enjoying recreational activities at 24 months after surgery than the control group. Index-level range of motion was preserved in 97% of patients. One limitation of this study was the fact that a ventral-only experimental surgery was compared with a front/back control surgery with an inherently greater operative time and risk of complications.
Leahy et al. used the data from this trial to evaluate whether the presence of prior decompressive surgery had any effect on outcomes of ProDisc L TDR.21 Patients with prior microdiscectomy or traditional laminectomy and discectomy were compared with those without prior lumbar surgeries in the TDR cohort. The outcomes in both groups were similar, indicating that disc replacement is a viable option for patients with prior disc surgeries.
Tropiano et al. reported midterm results from a nonrandomized study on outcomes with the ProDisc I TDR.22 They followed 64 patients with single- or multiple-level disc replacement for an average of 8.7 years. Approximately 75% of patients had good to excellent results in pain and functional improvement and 79% were satisfied or entirely satisfied with the outcome. The authors noted that small but significant negative effects on outcomes were seen in patients younger than 45 years of age and those with prior lumbar surgery. It should be noted that this study used an older ProDisc design that has since been improved.
Siepe et al. analyzed data from an ongoing, nonrandomized study on the ProDisc II to compare outcomes of TDR at different spinal segments.23 Ninety-nine patients had TDR at L4-5, L5-S1, or both levels and were followed for an average of 25.8 months. All patients had significant improvement from baseline in ODI and VAS scores. The best results were achieved with L4-5 TDR, followed by L5-S1 TDR. TDR at both levels resulted in the worst outcomes. The incidence of facet or sacroiliac joint pain confirmed by injection was 9.1% in L4-5 TDR, 28.1% in L5-S1 TDR, and 60% in patients with both levels replaced. Bisegmental disc replacement also led to higher rates of complications and need for revision surgery.
Complications
Complications in TDR surgery can be divided into approach-related, device-related, and patient-related complications.11 Currently available disc replacement devices are designed for the ventral approach. The most serious complications from this approach include injuries to the major vessels and ureters, as well as retrograde ejaculation. In current TDR studies, such complications are seen in up to 10% of patients, which is similar to the rates seen in anterior spinal fusions.6
Device-related complications include implant subsidence, migration or extrusion, malposition, and material wear. Subsidence is by far the most common issue, seen in up to 9% of TDRs in older studies and in 3% of patients in more recent trials.3,5,6 Subsidence has also been observed in up to 56% of failed TDRs.24 Of note, no clear clinical effect of subsidence on outcomes has yet been demonstrated. This complication may be minimized by screening patients with poor bone quality and seating the implants on the more cortical peripheral edges of the vertebral end plates. The risk of device migration or extrusion depends significantly on implant design, placement, and sizing. A TDR implant inserted in the midline that can be fixed to bone with spikes or keels, promotes bony ingrowth to the end plates, and is sized to provide good soft tissue tension would have a very low chance of migrating. In earlier studies, the rates of migration were up to 7%, but with improved designs and surgical experience, more recent studies have shown rates of 0% to 1%.3,17,22 There are no reports of confirmed adverse reactions to particulate wear debris, and significant wear has been mostly associated with implant malpositioning.24,25 Cunningham demonstrated, in an animal model, that only a local histiocytic reaction and no neuropathology or significant systemic or local response was observed in response to the wear particles generated by TDRs.10 Most device-related complications are iatrogenic and can be minimized with proper patient selection, operative technique, and implant sizing.
Patient-related complications include adjacent-level disc degeneration, same-level facet degeneration, and heterotopic ossification. Successful motion preservation is the main mechanical goal of lumbar TDR. This has been well documented in recent studies.17,20 The adjacent-segment degeneration seen after arthrodesis is thought to be related to increased stress at adjacent segments due to transfer of extra motion and loads from the fused segment. There are currently no long-term studies that definitively show that motion preservation using TDR avoids accelerated adjacent-segment degeneration. Intermediate-term data by Huang et al. using the ProDisc implant show that range of motion of at least 5 degrees is needed for improved clinical outcomes and reduction in degeneration of adjacent discs.26
The presence of degenerative disease in the facet joints is a contraindication to lumbar TDR. Early disease in the facets may be missed during initial screening, and patients may continue to experience pain after surgery from persistent motion in these degenerated dorsal structures. In a study assessing 175 patients complaining of persistent pain after ProDisc II implantation, facet joints were confirmed as a source by fluoroscopically guided blocks in 12.6% of patients, mostly at the index level (84%).27 Van Ooij et al. reported facet joint arthrosis in 42% of patients with persistent pain after Charité TDR.24 All 29 patients with failed TDRs in a study by Rosen et al. had evidence of facet distraction or compression on CT.28 Facet distraction was also associated with radiculopathy, likely from capsular stretch.
TDR design may also affect the manner in which the facets are loaded. In the normal lumbar spine, the facets are loaded with ventral shear forces. TDR implants with a floating center of rotation, such as the Charité, maintain such a load on the facets, potentially accelerating degeneration. On the other hand, implants with a fixed center of rotation, such as the ProDisc, absorb anteroposterior shear forces, thus creating greater stress within the implant and at the implant-bone interface, while sparing the facets. The fixed center of rotation creates a greater arc of motion to compensate for the lack of translation. This changes the kinematics of a facet joint, causing greater stresses at the extremes of flexion and extension.1
Heterotopic ossification, limiting the TDR’s range of motion, is of some concern. Although it was observed often with earlier implant designs, studies with modern TDRs show significantly lower rates. In addition, Tortolani et al. have reviewed the data from the Charité IDE study and evaluated the clinical significance of heterotopic ossification. Of 276 patients, 12 (4.3%) had radiographic evidence of new bone formation. All of these patients had ranges of motion and clinical outcomes similar to the rest of the study population.29
The rates of complications with TDR surgeries are relatively low. Nevertheless, strategies for revision have been developed. If additional surgery is necessary, either a ventral or a dorsal approach is used. Repeat ventral surgery carries a significantly higher risk of intraoperative complications. In the Charité IDE study, the incidence of vessel injury during reoperation was significantly greater than that with the index operation (16.7% vs. 3.6%), mostly because of scarring to the vertebral column.30 Other structures at risk due to scarring and distorted anatomy are the ureters and the lumbosacral trunk. These complications may be minimized with use of preoperative angiography and the intraoperative placement of intravascular balloons and ureteral stents.
An alternative revision procedure involves dorsal fusion with dynamic or rigid instrumentation. This strategy avoids the area of previous surgery and solves problems of instability or persistent pain. Cunningham et al. performed an in vitro biomechanical analysis of such a construct. They found that augmentation of a ventrally placed TDR with dorsally placed pedicle screws provided stability similar to that of the combination of ventral cage and dorsal pedicle screws (circumferential fusion construct).31 If TDR components must be removed because of dislodgement or malposition, however, the dorsal approach is inadequate. Alternative approaches, such as transperitoneal, contralateral retroperitoneal, and transpsoas (for levels L4-5 and above), have been described.
A prophylactic strategy involves placing an antiadhesive barrier over the vertebral column after TDR implantation to prevent scarring after a primary anterior approach. Various liquid and solid products are available and have been shown to be effective in adhesion prevention with abdominal and pelvic surgery.32 These products are yet to be evaluated in the setting of lumbar TDR.
Future Direction
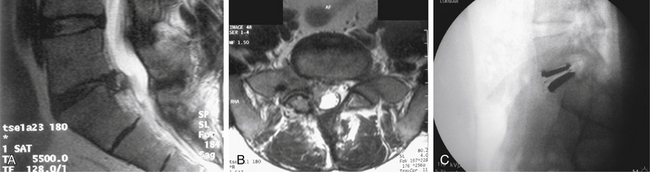
Lumbar TDR is a relatively new field with enormous potential for advancement. Long-term outcome studies are needed to show favorable results and TDR’s ability to decrease the incidence of adjacent-level degeneration. Additional studies are also needed to evaluate the use of lumbar disc replacement in patients not included in current IDE trials. Potential benefits of TDR in a setting of disc herniation are illustrated in Figure 161-3.
Appropriate patient selection and proper surgical technique remain as the key aspects of successful arthroplasty. Future implant designs should improve biomechanics, possibly resulting in modular implants to address varying kinematics at different lumbar spine segments or the need for multiple-level surgery. Alternative approaches for TDR could eliminate risks associated with anterior retroperitoneal primary and revision surgery. Circumferential arthroplasty technology could allow for simultaneous disc and facet joint replacement, which would significantly broaden indications for lumbar joint replacement.
Blumenthal S., McAfee P.C., Guyer R.D., et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disk replacement with the CHARITÉ™ artificial disc versus lumbar fusion. Part I: evaluation of clinical outcomes. Spine (Phila Pa 1976). 2005;30:1565-1575.
Cunningham B.W. Basic scientific considerations in total disc arthroplasty. Spine J. 2004;4(Suppl 6):219S-230S.
Guyer R.D., McAfee P.C., Banco R.J., et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement fusion: five-year follow-up. Spine J. 2009;9:374-386.
Huang R.C., Girardi F.P., Cammisa F.P.Jr., et al. Correlation between range of motion and outcome after lumbar total disc replacement: 8.6-year follow-up. Spine (Phila Pa 1976). 2005;30:1407-1411.
Patel A.A., Brodge D.S., Pimenta L., et al. Revision strategies in lumbar total disc arthroplasty. Spine (Phila Pa 1976). 2008;33:1276-1283.
Siepe C.J., Korge A., Grochulla F., et al. Analysis of post-operative pain patterns following total lumbar disc replacement: results from fluoroscopically guided spine infiltrations. Eur J Spine. 2008;17:44-56.
Zigler J., Delamarter R., Spivak J.M., et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976). 2007;32:1155-1162.
1. Huang R.C., Wright T.M., Panjabi M.M., et al. Biomechanics of nonfusion implants. Orthop Clin North Am. 2005;36:271-280.
2. Turner J.A., Ersek M., Herron L., et al. Patient outcomes after lumbar spinal fusions. JAMA. 1992;268:907-911.
3. Huang R.C., Girardi F.P., Lim M.R., et al. Advantages and disadvantages of nonfusion technology in spine surgery. Orthop Clin North Am. 2005;36:263-269.
4. Gillet P. The fate of the adjacent motion segments after lumbar fusion. J Spinal Disord Tech. 2003;16:338-345.
5. Wipperman B.W., Schratt H.E., Steeg S., et al. Complications of spongiosa harvesting of the iliac crest: a retrospective analysis of 1,191 cases [in German]. Chirurg. 1997;68:1286-1291.
6. Blumenthal S., McAfee P.C., Guyer R.D., et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disk replacement with the CHARITÉ™ artificial disc versus lumbar fusion. Part I: evaluation of clinical outcomes. Spine (Phila Pa 1976). 2005;30:1565-1575.
7. Bertagnoli R., Yue J.J., Nanieva R., et al. Lumbar total disc arthroplasty in patients older than 60 years of age: a prospective study of the ProDisc prosthesis with 2-year minimum follow-up period. J Neurosurg Spine. 2006;4:85-90.
8. McAfee P.C., Cunningham B.W., Hayes V., et al. Biomechanical analysis of rotational motions after disc arthroplasty. Spine (Phila Pa 1976). 2006;31(Suppl):S152-S160.
9. McAfee P.C., Cunningham B.W., Orbegoso C.M., et al. Analysis of porous ingrowth in intervertebral disk prostheses: a nonhuman primate model. Spine (Phila Pa 1976). 2003;28:332-340.
10. Cunningham B.W. Basic scientific considerations in total disc arthroplasty. Spine J. 2004;4(Suppl 6):219S-230S.
11. Bertagnoli R., Zigler J., Karg A., et al. Complications and strategies for revision surgery in total disc replacement. Orthop Clin North Am. 2005;36:389-395.
12. Bertagnoli R., Karg A., Voigt S. Lumbar partial disc replacement. Orthop Clin North Am. 2005;36:341-347.
13. Fernstrom V. Arthroplasty with intercorporal endoprosthesis in herniated disc and painful disc. Acta Chir Scand. 1966;355:154-159.
14. Hamby W.B., Glaser H.T. Replacement of spinal intervertebral discs with locally polymerizing methyl methacrylate. J Neurosurg. 1959;16:311-313.
15. Nachemson A.L. Some mechanical properties of the lumbar intervertebral disc. Bull Hosp Joint Dis. 1962;23:130-132.
16. Shim S.C., Lee S.H., Park C.W., et al. Partial disc replacement with the PDN prosthetic disc nucleus device: early clinical results. J Spinal Disord Tech. 2003;16:398-404.
17. Guyer R.D., McAfee P.C., Banco R.J., et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement fusion: five-year follow-up. Spine J. 2009;9:374-386.
18. Lemaire J.P., Carrier H., SariAli E., et al. Clinical and radiological outcomes with the Charité artificial disc: a 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353-359.
19. David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the CHARITÉ artificial disc in 106 patients. Spine (Phila Pa 1976). 2007;32:661-666.
20. Zigler J., Delamarter R., Spivak J.M., et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976). 2007;32:1155-1162.
21. Leahy M., Zigler J.E., Ohnmeiss D.D., et al. Comparison of results of total disc replacement in postdiscectomy patients versus patients with no previous lumbar surgery. Spine (Phila Pa 1976). 2008;33:1690-1693.
22. Tropiano P., Huang R.C., Girardi F.P., et al. Lumbar total disc replacement: seven to eleven-year follow-up. J Bone Joint Surg [Am]. 2005;87:490-496.
23. Siepe C.J., Korge A., Grochulla F., et al. Analysis of post-operative pain patterns following total lumbar disc replacement: results from fluoroscopically guided spine infiltrations. Eur J Spine. 2008;17:44-56.
24. Van Ooij A., Oner F.C., Verbout A.J. Complications of artificial disc replacement; a report of 27 patients with the SB Charité disc. J Spinal Disord Tech. 2003;16:369-383.
25. van Ooij A., Kurtz S.M., Stessels F., et al. Polyethylene wear debris and long-term clinical failure of the Charité disc prosthesis: a study of 4 patients. Spine (Phila Pa 1976). 2007;32:223-229.
26. Huang R.C., Girardi F.P., Cammisa F.P.Jr., et al. Correlation between range of motion and outcome after lumbar total disc replacement: 8.6-year follow-up. Spine (Phila Pa 1976). 2005;30:1407-1411.
27. Siepe C.J., Korge A., Grochulla F., et al. Analysis of post-operative pain patterns following total lumbar disc replacement: results from fluoroscopically guided spine infiltrations. Eur J Spine. 2008;17:44-56.
28. Rosen C., Kiester D., Lee T.Q. Lumbar disk replacement failures: review of 29 patients and rationale for revision. Orthopedics. 2009;32:562.
29. Tortolani P.J., Cunningham B.W., Eng M., et al. Prevalence of heterotopic ossification following total disc replacement: a prospective, randomized study of two hundred and seventy-six patients. J Bone Joint Surg [Am]. 2007;89:82-88.
30. McAfee P.C., Geisler F.H., Saiedy S.S., et al. Revisability of the CHARITÉ artificial disk replacement. Spine (Phila Pa 1976). 2006;31:1217-1226.
31. Cunningham B.W., Hu N., Beatson H.J., et al. Revision strategies for single- and two-level total disc arthroplasty procedures: a biomechanical perspective. Spine J. 2009;9:773-775.
32. Patel A.A., Brodge D.S., Pimenta L., et al. Revision strategies in lumbar total disc arthroplasty. Spine (Phila Pa 1976). 2008;33:1276-1283.