CHAPTER 285 Lumbar Spine Stenosis
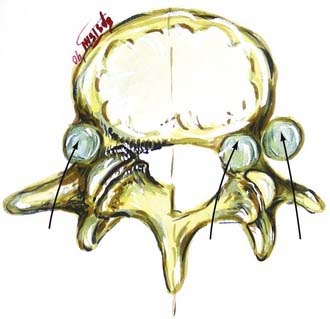
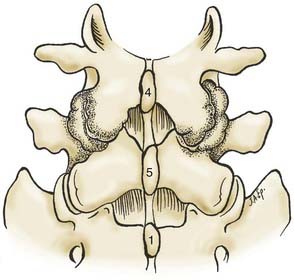
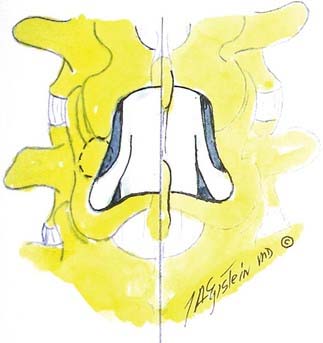
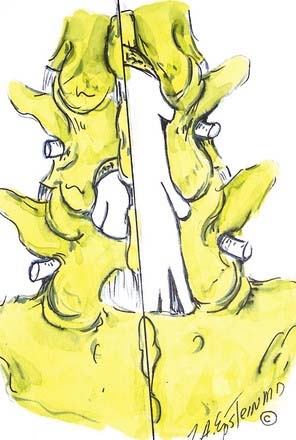
Verbiest1–4 was one of the first to define central lumbar spinal stenosis, noting that laminectomy alone relieved radiculopathic symptoms for patients with narrowed spinal canals. He defined congenital stenosis, characterized by shortened pedicles and a shallow sagittal diameter, in two ways: absolute stenosis (≤10 mm) or relative stenosis (>10 to 12 mm).3–5 In a trefoil-shaped spinal canal, lateral recess or subarticular stenosis also contributes to lateral thecal sac and nerve root entrapment beneath the superior articular facets.6 Acquired stenosis is defined by the development of progressive degenerative changes superimposed on an originally normal or narrowed spinal canal. Single and multilevel stenosis is variously attributed to thickened laminae, arthrotic facets, exaggerated lordotic curvatures, laminar shingling, infolding of the hypertrophied yellow ligament, and ossification of the posterior longitudinal ligament, all of which contribute to central, lateral, or foraminal stenosis.7 Stenosis typically occurs at the L4-5 level, followed in descending order of frequency at L3-4, L2-3, L5-S1, and L1-2.8 Other factors more rarely contribute to spinal canal stenosis. Endocrinopathies, including Paget’s disease, acromegaly, and fluorosis, contribute to increased narrowing of the spinal canal, as does achondroplasia (characterized by trapezoidal vertebrae; short, thick pedicles and vertebral end plates; hypertrophied lamina; and increased periosteal bone formation).9
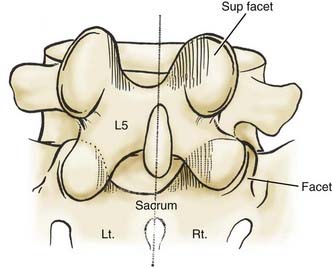
Anatomy
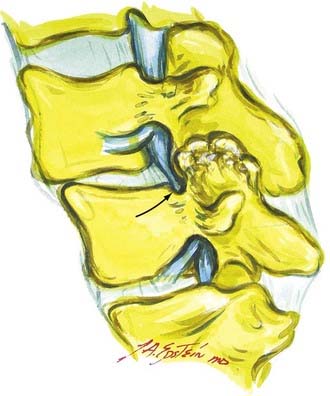
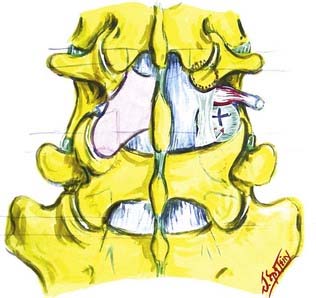
Hypertrophy or ossification of the ligamentum flavum contributes to dorsal compression of the thecal sac, whereas ventrally and centrally situated disks, spurs, or osteophytes, and, rarely, hypertrophy of the posterior longitudinal ligament may compromise the available canal space. Although congenital benign Tarlov cysts (small or large) may occasionally balloon into neural foramina, they are not considered pathologic and rarely require surgery.10,11
Symptoms of stenosis or neurogenic claudication are attributed to direct mechanical compression or indirect vascular insufficiency involving the lumbar nerve roots or cauda equina.12 Standing and walking increase lordosis and transiently exaggerate infolding of the ligamentum flavum, while sitting and recumbence reverse the lordosis, open the canal, improve blood flow, and often temporarily relieve complaints.
Symptoms and Signs
Patients with congenital lumbar stenosis may become symptomatic early, in the third to fifth decades of life, whereas patients with acquired stenosis typically develop symptoms later, in the sixth to seventh decades. Unilateral or bilateral radiculopathy may be associated with neurogenic claudication, characterized by leg pain, numbness, tingling, and weakness, that is exacerbated by walking specific distances (measured in blocks). When 100 consecutive patients with disk disease, lateral recess stenosis, and central stenosis were evaluated, all three groups of patients demonstrated comparable complaints of pain at rest, pain at night, and pain on coughing.13 Many patients without significant neurological deficits can be managed conservatively with rehabilitation or multidisciplinary pain centers.14
Neurological findings include mechanical, motor, reflex, and sensory signs that reflect the level or levels of involvement. In descending order, these are L5 root syndromes (L4-5 disease), L4 root syndromes (L3-4), L3 root syndromes (L2-3), and S1 root pathology (L5-S1).15 Bladder dysfunction is rare in young patients but is frequently encountered in the geriatric population with lumbar spinal stenosis. Patients averaging 71 years of age and undergoing two- to four-level laminectomies for severe lumbar stenosis exhibited a significant degree of both preoperative and postoperative bladder compromise.16 Six months following laminectomies, although only 45% of patients showed cystoscopic or urodynamic evidence of improved urinary function (i.e., decreased postvoiding residual volumes), 60% of patients reported a subjective recovery of urinary function.
Diagnostic Studies
Radiography
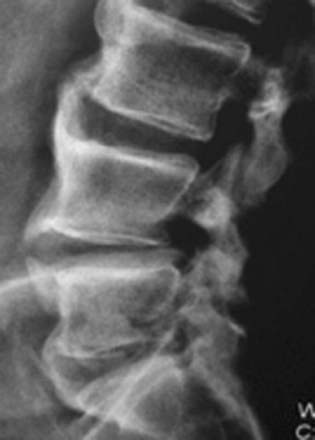
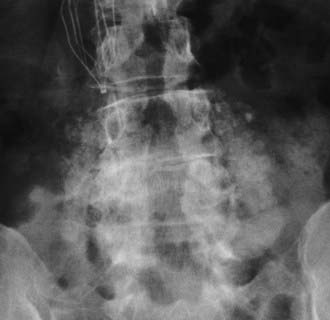
Plain anteroposterior radiographs demonstrate the number of lumbar vertebrae and help determine whether there is a lumbosacral bony anomaly (frequency, 7%).17 Lateral radiographs reveal the curvature of the lumbar spine and the presence or absence of static or dynamic olisthesis or instability, defined by greater than 4 mm of translation and greater than 10 to 12 degrees of angulation at the level of olisthy.18
Magnetic Resonance Imaging
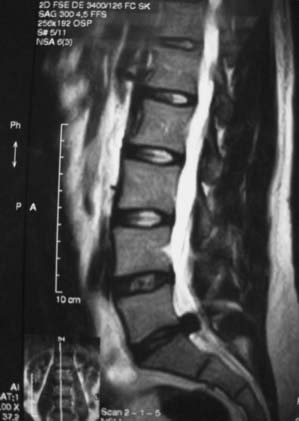
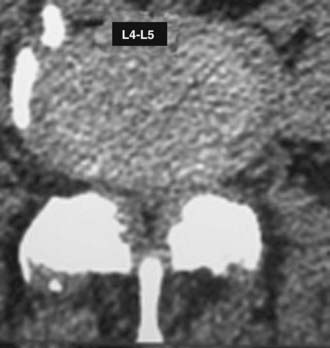
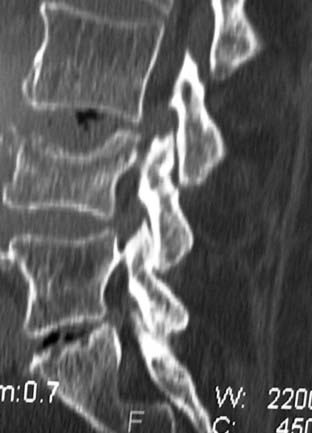
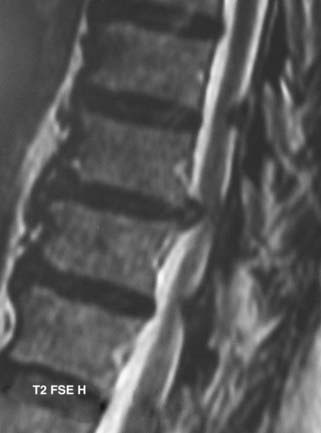
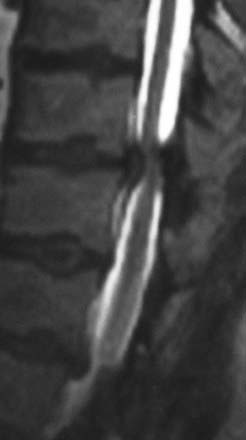
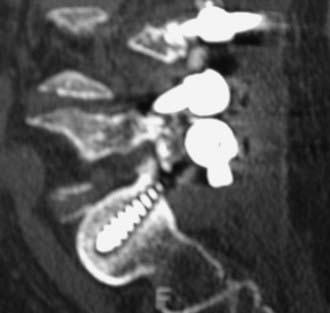
MRI offers the best noninvasive assessment of soft tissue structures but not bone-related pathology (![]() Figs. 285-E1 to 285-E4). MRI studies best demonstrate the soft tissue compressive changes of the thecal sac or nerve roots attributed to disk disease, ligamentous compression (hypertrophy of the yellow ligament or posterior longitudinal ligament), olisthesis, and other intrinsic neural pathology.19 MRI also offer simultaneous longitudinal, sagittal, and transaxial views of soft tissue pathology that may be located centrally, laterally, foraminally, or far laterally. It does not, however, directly demonstrate calcific or ossific changes often associated with disk or limbus fractures, spondylosis, and ossification of the posterior longitudinal or yellow ligament. Many otherwise asymptomatic lesions are also identified on MRI in the older population. In one series of patients older than 60 years, herniated disks (36%) and spinal stenosis (21%) were symptomatic in only one third of patients.19
Figs. 285-E1 to 285-E4). MRI studies best demonstrate the soft tissue compressive changes of the thecal sac or nerve roots attributed to disk disease, ligamentous compression (hypertrophy of the yellow ligament or posterior longitudinal ligament), olisthesis, and other intrinsic neural pathology.19 MRI also offer simultaneous longitudinal, sagittal, and transaxial views of soft tissue pathology that may be located centrally, laterally, foraminally, or far laterally. It does not, however, directly demonstrate calcific or ossific changes often associated with disk or limbus fractures, spondylosis, and ossification of the posterior longitudinal or yellow ligament. Many otherwise asymptomatic lesions are also identified on MRI in the older population. In one series of patients older than 60 years, herniated disks (36%) and spinal stenosis (21%) were symptomatic in only one third of patients.19
Lumbar instability associated with lumbar stenosis can also be documented on MRI by the visualization of increased fluid within the facet joints.20 The volume of facet fluid can be calculated on routine axial MRI by adding the width of the fluid in both facet joints and dividing that by the sum of the width of both facets. Increased lumbar facet fluid (indicating instability) positively correlated with sagittal instability documented on dynamic x-ray studies obtained at the L4-5 level (focus of degenerative changes). Of 50 patients entered in the study, 28 (55%) had facet fluid; 23 (82%) were unstable, and 5 (18%) were not. The investigators concluded that a close linear relationship exists between the facet fluid and instability as seen on MRI and dynamic radiographs, respectively. Enhanced MRI offers “myelographic” views that correlate with operative findings 82.6% of the time.21
Contrast-enhanced MRI also accurately differentiates scar from disk (96%), visualizes the cervicothoracic or thoracolulmbar junction, and helps differentiate among tumor, demyelinating syndromes, adhesive arachnoiditis, and infection.22 Magnetic resonance myelography offers more specific data in the preoperative assessment of foraminal stenosis. In one study it documented foraminal stenosis that was surgically confirmed in 35 of 990 patients.23
Computed Tomography
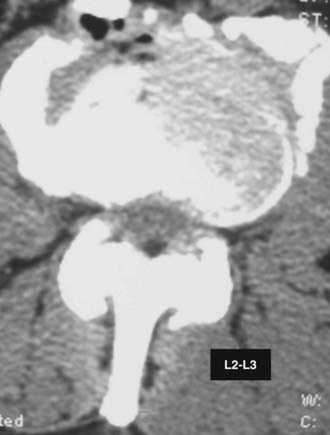
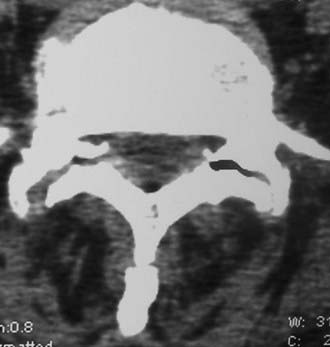
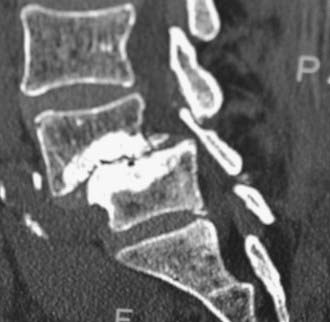
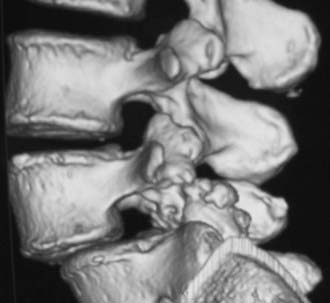
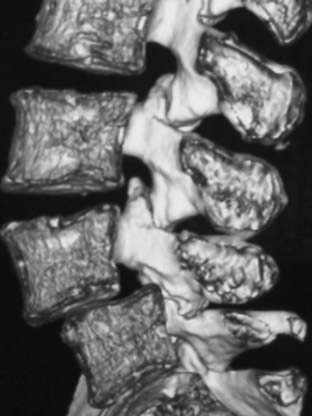
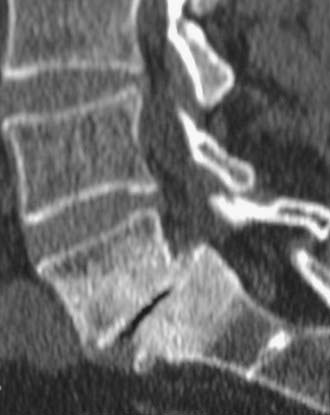
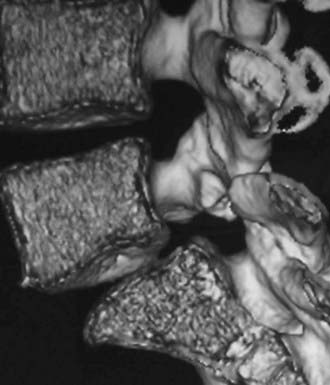
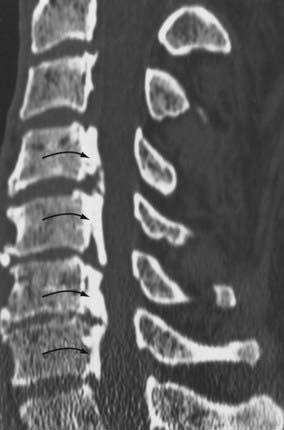
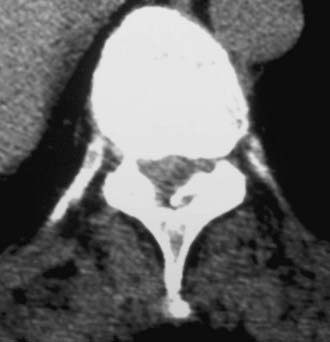
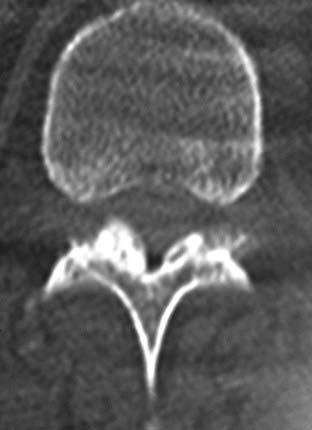
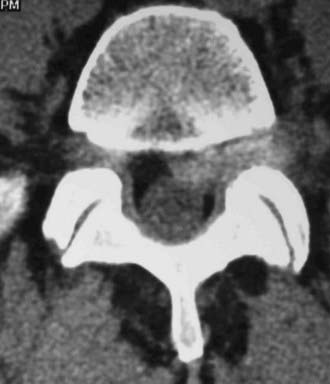
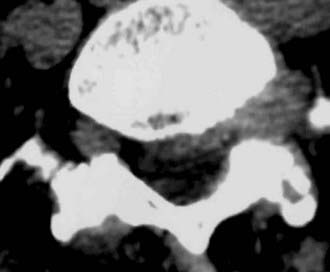
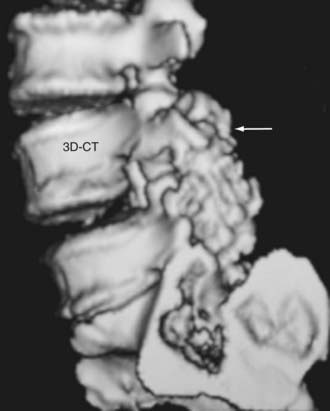
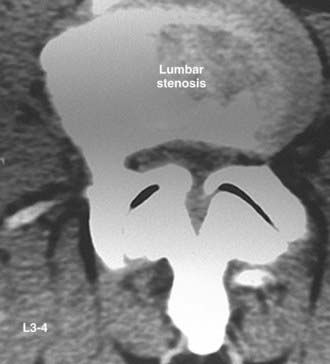
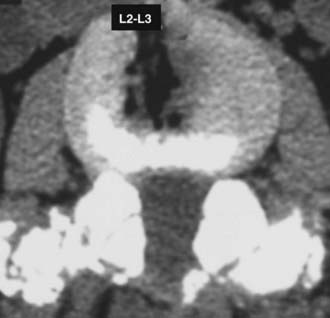
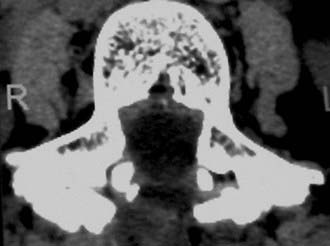
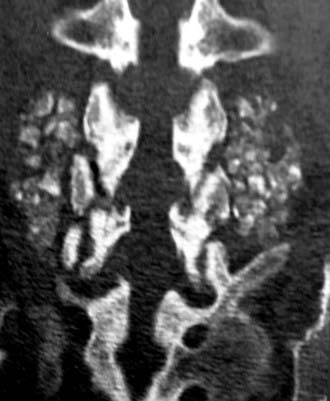
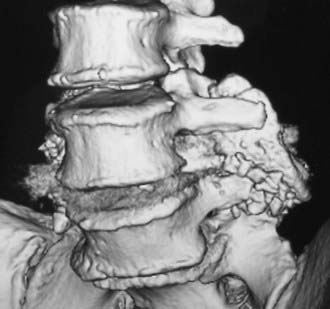
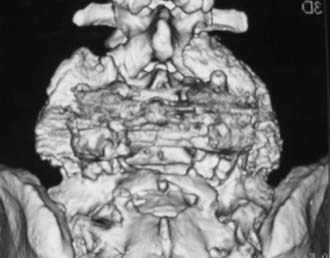
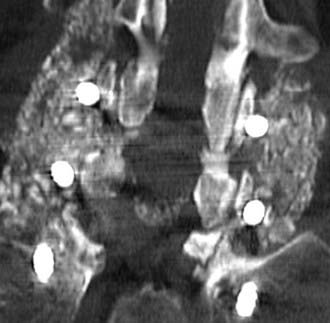
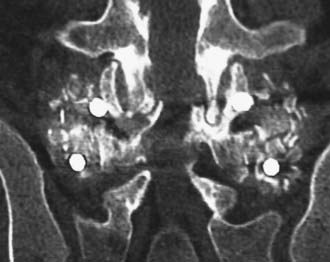
Routine and two- and three-dimensional reconstructed CT images in multiple planes (axial, coronal, sagittal) confirm the diagnosis of lumbar stenosis (![]() Figs. 285-E5 to 285-E14). They also contribute to the recognition of accompanying disk disease, limbus fractures, olisthesis or instability, ossification of the posterior longitudinal ligament (OPLL), ossification of the yellow ligament (OYL), and progression of fusion in a posterolateral fusion mass. In a series of 48 patients who were symptomatic following lumbar fusion, CT identified 157 abnormalities; 12 of 27 major lesions included fusion mass fractures, hairline pseudarthroses, and residual spinal stenosis (all confirmed during second operations).24 Myelographic CT studies are now rarely performed because the majority of pathologic findings can be confirmed noninvasively with varying combinations of MRI and CT evaluations.
Figs. 285-E5 to 285-E14). They also contribute to the recognition of accompanying disk disease, limbus fractures, olisthesis or instability, ossification of the posterior longitudinal ligament (OPLL), ossification of the yellow ligament (OYL), and progression of fusion in a posterolateral fusion mass. In a series of 48 patients who were symptomatic following lumbar fusion, CT identified 157 abnormalities; 12 of 27 major lesions included fusion mass fractures, hairline pseudarthroses, and residual spinal stenosis (all confirmed during second operations).24 Myelographic CT studies are now rarely performed because the majority of pathologic findings can be confirmed noninvasively with varying combinations of MRI and CT evaluations.
Pathology Mimicking Lumbar Stenosis
Some patients with lumbar stenosis may have more cephalad tumors contributing to seemingly “lumbar” complaints. These typically include ependymomas, neurofibromas, meningiomas, and metastatic lesions (![]() Fig. 285-E15). In one study, a patient with an unresolved right footdrop following lumbar surgery was found to have a left-sided parasagittal convexity meninigoma; resection resulted in complete resolution of the deficit.25 Other degenerative, metabolic, endocrine, and vascular disorders may mimic the signs and symptoms of lumbar stenosis. These include thoracic disk herniation, Scheuermann’s disease, Paget’s disease, and arthritis of the hips. In one series, amyloidosis and its characteristic crystals contributed to hypertrophy of the yellow ligament in 12 of 97 patients undergoing lumbar surgery for stenosis.26 Diabetes, contributing to diabetic peripheral neuropathy, femoral amyotrophy, or angiopathy, can also be misdiagnosed as lumbar stenosis. Of note, among diabetics with lumbar stenosis, only 39% exhibited good or excellent outcomes, compared with 95% good or excellent results for those without diabetes.27 Peripheral vascular diseases resulting in vascular rather than neurogenic claudication, characterized by pain associated with ambulation but relieved with rest alone, may be present alone or exist simultaneously with lumbar stenosis.
Fig. 285-E15). In one study, a patient with an unresolved right footdrop following lumbar surgery was found to have a left-sided parasagittal convexity meninigoma; resection resulted in complete resolution of the deficit.25 Other degenerative, metabolic, endocrine, and vascular disorders may mimic the signs and symptoms of lumbar stenosis. These include thoracic disk herniation, Scheuermann’s disease, Paget’s disease, and arthritis of the hips. In one series, amyloidosis and its characteristic crystals contributed to hypertrophy of the yellow ligament in 12 of 97 patients undergoing lumbar surgery for stenosis.26 Diabetes, contributing to diabetic peripheral neuropathy, femoral amyotrophy, or angiopathy, can also be misdiagnosed as lumbar stenosis. Of note, among diabetics with lumbar stenosis, only 39% exhibited good or excellent outcomes, compared with 95% good or excellent results for those without diabetes.27 Peripheral vascular diseases resulting in vascular rather than neurogenic claudication, characterized by pain associated with ambulation but relieved with rest alone, may be present alone or exist simultaneously with lumbar stenosis.
Associated Conditions
Tandem Stenosis
For patients with tandem lesions, decompression or excision of the cephalad pathology may result in partial and occasionally marked improvement in lumbar complaints.25,28–32 In one series, following an L4-5 instrumented fusion, one patient was found to have additional C4-7 and T11-12 stenosis; he underwent laminectomies to decompress all levels and finally improved.25 In some series of tandem cervical and lumbar stenosis, the order of surgery is dictated by the severity of the stenosis.31 For patients with absolute stenosis (canal ≤ 10 mm) and myelopathy, often with superimposed pathology such as OPLL, cervical surgery should typically precede lumbar intervention (![]() Fig. 285-E16). For patients with relative stenosis and a canal depth of 11 to 13 mm correlated with radiculopathy rather than myelopathic symptoms, lumbar surgery often takes precedence over cervical intervention. For the former patients, preliminary cervical decompression often results in improvement of seemingly “lumbar” complaints in more than one third. In another series, 8 of 230 patients with lumbar stenosis were also found to have cervical stenosis.32 Three patients underwent cervical surgery first, and five had initial lumbar procedures; all patients exhibited excellent or good results, and none deteriorated. Although tandem cervical-lumbar stenosis occurs in only 10% of patients, it should be anticipated in patients older than 65 years, and these individuals should undergo more stringent screening for tandem cervical disease.
Fig. 285-E16). For patients with relative stenosis and a canal depth of 11 to 13 mm correlated with radiculopathy rather than myelopathic symptoms, lumbar surgery often takes precedence over cervical intervention. For the former patients, preliminary cervical decompression often results in improvement of seemingly “lumbar” complaints in more than one third. In another series, 8 of 230 patients with lumbar stenosis were also found to have cervical stenosis.32 Three patients underwent cervical surgery first, and five had initial lumbar procedures; all patients exhibited excellent or good results, and none deteriorated. Although tandem cervical-lumbar stenosis occurs in only 10% of patients, it should be anticipated in patients older than 65 years, and these individuals should undergo more stringent screening for tandem cervical disease.
Ossification of the Yellow Ligament
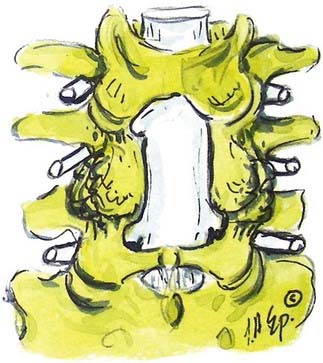
OYL may significantly contribute to lumbar stenosis. OYL presents as an initial ingrowth of fibrocartilage attributed to the proliferation of type II collagen (![]() Figs. 285-E17 to 285-E20). Hypertrophy or OYL begins laterally at the enthesis and extends medially.33 In 110 predominantly geriatric individuals undergoing multilevel laminectomies (average, five levels) with noninstrumented fusion, the 10 who developed intraoperative dural tears exhibited severe OYL; this extended to or through the dura in 3.34 For the remaining 100 patients, 57 showed moderate OYL, and 22 showed marked OYL.
Figs. 285-E17 to 285-E20). Hypertrophy or OYL begins laterally at the enthesis and extends medially.33 In 110 predominantly geriatric individuals undergoing multilevel laminectomies (average, five levels) with noninstrumented fusion, the 10 who developed intraoperative dural tears exhibited severe OYL; this extended to or through the dura in 3.34 For the remaining 100 patients, 57 showed moderate OYL, and 22 showed marked OYL.
Ossification of the Posterior Longitudinal Ligament
The frequency of OPLL in the proximal lumbar spinal canal is 10%, with another 10% being found in the proximal thoracic spine (T1-4) spine; the majority (80%) of OPLL is found in the cervical spinal canal.35 OPLL and OYL may both contribute to thoracic or lumbar stenosis.29,30,35,36 Of 1100 patients having surgery for spinal stenosis from 1986 to 1997, 26 (2.3%) had OYL or OPLL (11 OPLL, 12 OYL, 3 OYL and OPLL).35 Analysis of a fluid collection in the lumbar spine using β2-transferrin may help document the presence of a fistula.37
Ankylosing Hyperostosis
Ankylosing hyperostosis may also contribute to lumbar stenosis (![]() Fig. 285-E21). The characteristic CT findings include anterior or posterolateral marginal, somatic osseous proliferation and proliferative changes involving the posterior facet joints, articular capsules, yellow ligament, or supraspinal ligaments.38
Fig. 285-E21). The characteristic CT findings include anterior or posterolateral marginal, somatic osseous proliferation and proliferative changes involving the posterior facet joints, articular capsules, yellow ligament, or supraspinal ligaments.38
Disk Herniation with and without Olisthesis
Disk herniations occur in up to 45% of patients undergoing surgery for lumbar stenosis with or without olisthesis (in a series of 857 individuals).39,40 Disk herniations in conjunction with stenosis alone were reported in 15% to 45% of cases.39,41–43 In 60 patients undergoing multilevel laminectomies (average, 5.4 levels) for lumbar stenosis using lamina autograft and β-tricalcium phosphate (β-TCP) for one- to two-level noninstrumented posterolateral lumbar fusion, disk herniations were observed in 20 patients (33.3%).44 Of 95 patients undergoing one-level instrumented posterolateral lumbar fusion using lamina autograft supplemented with demineralized bone matrix, 13 patients (13.6%) without preoperative olisthesis underwent unilateral or bilateral facetectomy for far lateral disks or stenosis.45 Among 100 patients undergoing multilevel laminectomy (3.6 levels) with one-level (78 patients) and two-level (22 patients) instrumented fusion using lamina autograft and β-TCP, 57 herniated disks were found in 50 patients: 21 were routine disks, 7 were foraminal disks, 24 were far lateral disks, and 5 were recurrent disk herniations.
Lower frequencies of disk herniation (4.3% to 20%) have been reported for patients undergoing lumbar decompression in the presence of olisthesis.39,42 In one study evaluating 290 patients with olisthesis, 20% had disk herniations; 47 were routine, and 12 were foraminal or far lateral.42
Limbus Vertebral Fracture
Patients with lumbar stenosis may also exhibit one of four types of limbus vertebral fractures, which are better visualized on CT than MRI.46–48 Type I consists of a shelf of cortical bone traversing the canal, type II involves predominantly a large central fragment, type III is characterized by a lateral or far lateral calcified fragment, and type IV is defined by a massive fragment extending across the entire width of the spinal canal from one interspace to the next. These fragments are typically extremely large, include both cortical and cancellous elements, and warrant more extensive resection to afford access for adequate decompression. For foraminal and far lateral lesions, unilateral facetectomy is typically warranted. Furthermore, resection requires piecemeal removal; this is most safely carried out by first creating a defect or depression at the level of the disk space and then morcellating the limbus fracture into fragments using a down-biting curet, tamp, and mallet technique, which allows delivery into the defect and safe removal. In some cases, intraoperative monitoring (somatosensory evoked potentials [SEPs] or electromyography [EMG]) may be useful to minimize undue retraction or manipulation and subsequent neurological injury.
Far Lateral Disk Pathology
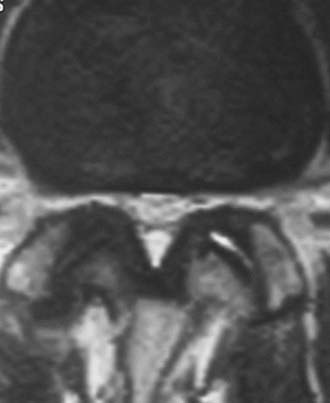
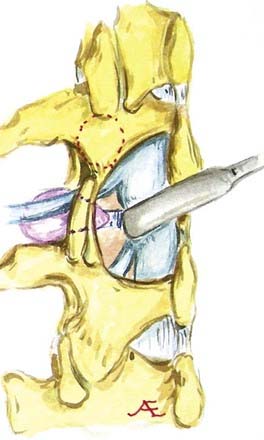
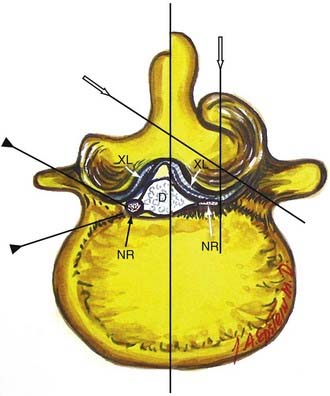
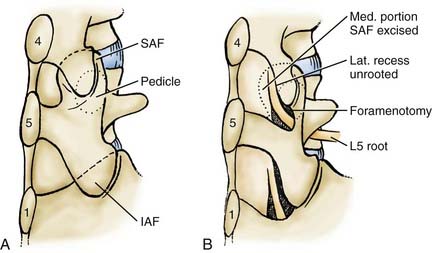
In the lumbar spinal canal, lumbar nerve roots may become trapped by disk herniation or stenosis extending into the far lateral compartment, which is bordered superiorly by the pedicle, anteriorly by the disk, medially by the vertebral body and superior articular facet, and laterally by fat (![]() Figs. 285-E22 to 285-E28).49,50 Far lateral disks, which typically originate at the inferior interspace and migrate superolaterally, constitute 7% to 12% of all disk herniations.51 Other factors contributing to far lateral pathology include spondylostenosis, arthrosis, limbus vertebral fractures, olisthy with or without lysis, and scoliotic deformity.49,52
Figs. 285-E22 to 285-E28).49,50 Far lateral disks, which typically originate at the inferior interspace and migrate superolaterally, constitute 7% to 12% of all disk herniations.51 Other factors contributing to far lateral pathology include spondylostenosis, arthrosis, limbus vertebral fractures, olisthy with or without lysis, and scoliotic deformity.49,52
Three surgical techniques are used to approach far lateral disk herniations that accompany lumbar stenosis.49,51 Only rarely can a far lateral disk be removed through a medial facetectomy at the L5-S1 level; most cases require either the intertransverse approach or a full facetectomy.49,51 Notably, the L5-S1 level is the widest and least stenotic, and very laterally located facet joints may allow adequate access to the foraminal and proximal far lateral portion of a far lateral disk. The intertransverse procedure combines a medial facetectomy-foraminotomy and far lateral exposure (Wiltse approach), thus preserving the pars interarticularis; risks, however, include delayed fracture of the pars interarticularis, retention of disk material, or damage to the nerve root secondary to incomplete exposure. Finally, the full facetectomy, which is the safest approach, fully visualizes the root along its entire course, along with OYL, synovial cysts, spondylostenosis, and olisthesis; however, it does increase the risk of instability.51 Although in the past many patients undergoing full facetectomies for far lateral disks were not fused, these patients typically undergo simultaneous noninstrumented or instrumented fusion today.
Over a 10-year period (1984 to 1994), 170 patients underwent surgery for far lateral lumbar disks accompanied by lateral recess stenosis (134 patients) or central stenosis (36 patients), far lateral stenosis (30 patients), or degenerative spondylolisthesis (23 patients).51 Most far lateral disks were found at L4-5 (68 patients), L3-4 (63 patients), and L5-S1 (33 patients).49,51 Of note, only 4 of 170 patients (2.4%) underwent secondary pedicle-screw arthrodesis.51 All 4 patients had become unstable following L4-5 laminectomy–unilateral facetectomy for decompression of stenosis accompanied by grade I olisthy. Of interest, outcomes of far lateral disk surgery (Odom’s criteria) were comparable for the three approaches to facet excision: good to excellent in 68% with medial facetectomy, 79% with the intertransverse approach, and 70% with full facetectomy.
Of the original 170 patients, 76 were evaluated by both the surgeon and the Short Form 36 (SF-36) questionnaire (preoperatively and 3, 6, and 12 months postoperatively) for an average of 2.8 years.52 For the 56 patients who were examined within 4.5 years of surgery, significant positive correlations were found between the surgeon’s assessment and six of the SF-36 Health Scales; General Health and Social Function were excluded.
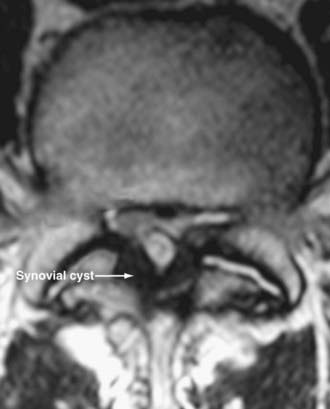
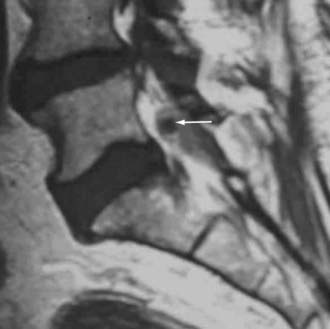
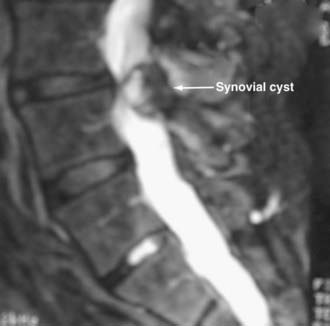
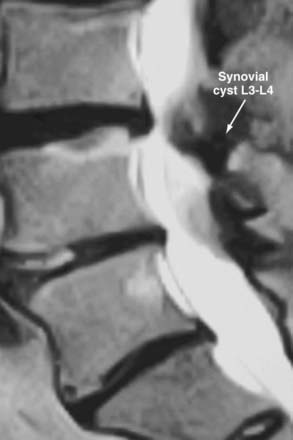
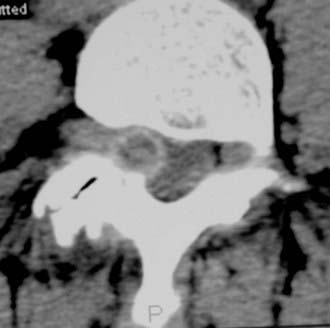
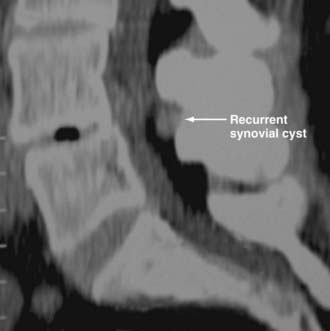
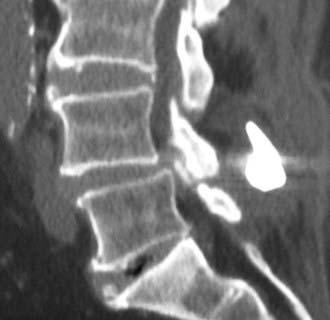
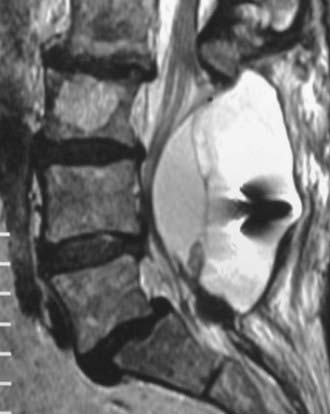
Synovial Cysts
Synovial cysts may contribute to the pathology of lumbar spinal stenosis (![]() Figs. 285-E29 to 285-E36).53,54 In one series, outcomes were assessed in 45 patients with stenosis and synovial cysts versus 35 with stenosis and degenerative spondylolisthesis using the SF-36 questionnaire.53 The procedures in these patients required laminectomy at an average of 3.8 and 3.5 levels, respectively. Five of the 45 with synovial cysts developed instability postoperatively, and 11 of 35 with preoperative olisthesis developed further progression. After a minimum of 2 postoperative years, good or excellent results were observed in only 58% and 63% of patients, respectively (Physical Function Scale +44 and +38). Because synovial cysts indicate intrinsic disruption of the facet joint and, therefore, instability, it is likely that more primary fusions should be considered in these patients to improve outcomes.53,54
Figs. 285-E29 to 285-E36).53,54 In one series, outcomes were assessed in 45 patients with stenosis and synovial cysts versus 35 with stenosis and degenerative spondylolisthesis using the SF-36 questionnaire.53 The procedures in these patients required laminectomy at an average of 3.8 and 3.5 levels, respectively. Five of the 45 with synovial cysts developed instability postoperatively, and 11 of 35 with preoperative olisthesis developed further progression. After a minimum of 2 postoperative years, good or excellent results were observed in only 58% and 63% of patients, respectively (Physical Function Scale +44 and +38). Because synovial cysts indicate intrinsic disruption of the facet joint and, therefore, instability, it is likely that more primary fusions should be considered in these patients to improve outcomes.53,54
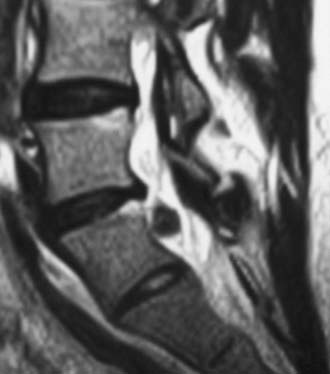
FIGURE 285-E33 On this parasagittal T2-weighted image, a right-sided synovial cyst arising from the L4-5 facet joint is visualized.
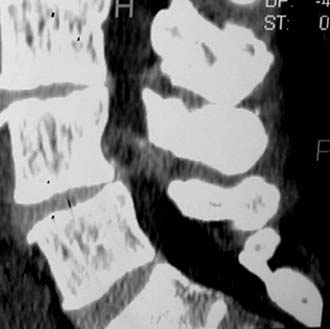
FIGURE 285-E35 On this parasagittal two-dimensional computed tomography scan (soft tissue window), the right-sided synovial cyst seen on the axial study (see Fig. 285-E34), arising from the L4-5 facet joint and extending into the foramen, is observed.
In a subsequent series of 110 mostly geriatric patients undergoing, on average, five-level laminectomy with primary noninstrumented fusion, a high frequency of synovial cysts was encountered in those who developed dural tears.34 Of note, 5 of 10 patients with dural tears had synovial cysts (with severe OYL in all 10), whereas only eight synovial cysts were encountered among the remaining 100 without dural tears.
Degenerative Spondylolisthesis
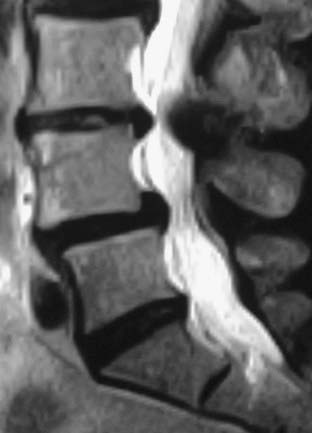
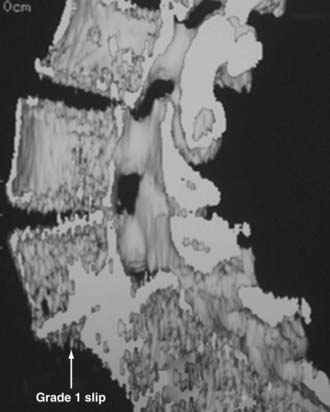
In the lumbar spine, degenerative olisthesis or spondylolisthesis (with an intact neural arch) occurs when facet joints are congenitally oriented in a sagittal rather than a coronal position (![]() Figs. 285-E37 to 285-E43).39,43 Progressive arthrotic changes of the facet joints contribute to a grade I or 25% anterolisthesis or olisthesis, frequently resulting in progressive cauda equina and nerve root compression. Resultant hypertrophied facet joints contribute to dorsolateral intrusion on the thecal sac and superiorly exiting nerve roots as they exit the spinal canal foraminally and far laterally. Simultaneously, the inferiorly exiting nerve root is compressed in the lateral recess by hypertrophied yellow ligament, disk “bulges,” or arthrotic spurs.
Figs. 285-E37 to 285-E43).39,43 Progressive arthrotic changes of the facet joints contribute to a grade I or 25% anterolisthesis or olisthesis, frequently resulting in progressive cauda equina and nerve root compression. Resultant hypertrophied facet joints contribute to dorsolateral intrusion on the thecal sac and superiorly exiting nerve roots as they exit the spinal canal foraminally and far laterally. Simultaneously, the inferiorly exiting nerve root is compressed in the lateral recess by hypertrophied yellow ligament, disk “bulges,” or arthrotic spurs.
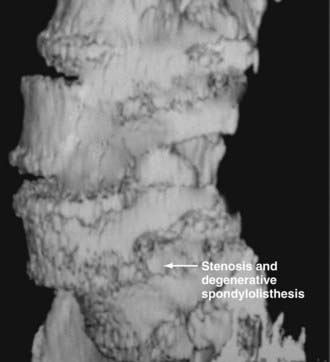
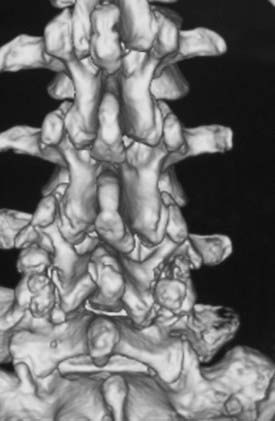
FIGURE 285-E37 Lateral three-dimensional computed tomography image of grade I degenerative spondylolisthesis at the L4-5 level.
Patients with degenerative spondylolisthesis are typically women (2 : 1 female-to-male ratio) 50 to 60 years of age whose symptoms have evolved over decades but have been exacerbated over months to years. Neurological deficits appear late in the clinical course and may be correlated with the onset of neurogenic claudication or radiculopathy associated with proximal weakness or a partial footdrop. The L4-5 level is most commonly involved, followed in descending order by L3-4, L2-3, and L5-S1.43 Olisthesis is rare at L5-S1 because this level is typically located below the intercrestal line and is therefore supported by longer transverse processes and the iliotransverse ligaments. Of 290 patients with degenerative spondylolisthesis, 86% had olisthy at one level, and the remaining 14% had two-level olisthesis.42
Nonsurgical Versus Surgical Management
A subset of patients with spinal stenosis of differing severities might be managed conservatively without surgery. For example, both young and old patients with severe comorbidities that may preclude risky surgical intervention and with severe MRI- and CT-documented stenosis or olisthesis may benefit from treatment at multimodality pain centers.14 In one series, the Oswestry Disability Index (ODI) and surgeons’ clinical assessments were used to compare outcomes for 54 matched pairs with lumbar stenosis treated conservatively (nonoperatively) versus with laminectomy.55 No statistically significant differences in outcomes between the two groups were revealed.
Other studies have documented the superiority of surgery for lumbar stenosis, including decompression or decompression with fusion, compared with nonsurgical alternatives. In a randomized controlled trial involving 94 patients with stenosis, the results of no surgery (44 patients) versus surgical decompression (50 patients) were compared; the latter included undercutting laminectomies for stenosis, with 10 undergoing additional fusions.56 Outcomes, based on the ODI and self-reported measures, revealed that patients in both groups improved over the 2-year postoperative interval, but surgical patients achieved greater relief of leg and back pain and demonstrated less overall disability. In a nonrandomized cohort study of patients with lumbar stenosis, better outcomes were observed following decompression (54 patients) and decompression with fusion for degenerative spondylolisthesis (42 patients) compared with nonoperative intervention (29 patients).57 Based on Roland Morris questionnaires to assess outcomes, patients undergoing either of the surgical procedures exhibited higher scores than those treated conservatively (6.9 and 6.1 versus 1.2). Similarly, better outcomes were observed for the decompression and decompression and fusion groups compared with those who chose nonsurgical alternatives (63.3% and 61.5% versus 25%).
In another study, patients with stenosis and degenerative spondylolisthesis from 13 centers, randomly enrolled in two treatment groups, demonstrated substantial gains in pain relief and function following decompressive laminectomy with or without fusion (304 patients) compared with no surgery (303 patients).58 Patents were evaluated with the SF-36 and a modified ODI 1.5, 3, 6, 12, and 24 months postoperatively. Surgical patients demonstrated significant advantages at 3 months, which increased at 1 year and were only minimally diminished at 2 years postoperatively. On the SF-36, Bodily Pain and Physical Function scores showed a net gain of 18.1 and 18.3 points, respectively, whereas the net ODI was –16.7.
Pre- and Postoperative Considerations
Comorbid Factors
In an observational study of 3482 patients undergoing surgery for lumbar stenosis, medical (headache, depression, central nervous system disorders) and psychosocial comorbid factors (active compensation cases, self-reported poor health, smoking) negatively impacted 3-month and 1-year SF-36 and ODI postoperative outcomes.59 In another study involving 122 lumbar decompressions performed for stenosis in geriatric patients averaging 78.8 years of age, a grade I or II rating based on the American Society of Anesthesiologists’ scale indicated limited comorbid factors and a greater likelihood that surgery would prove safe and effective.60 The advisability for such lumbar procedures in patients deemed grade III proved less certain.
Preoperative Psychiatric Clearance
Depression significantly affects the outcome of patients undergoing lumbar spine surgery. In one study of 99 patients undergoing surgery for lumbar stenosis, questionnaires were completed preoperatively and 3 months postoperatively.61 The Beck Depression Inventory, ODI, Stucki Questionnaire, and Visual Analogue Scale were used. Before surgery, 20% were considered depressed. This factor positively correlated with increased postoperative disability based on the multiple questionnaires; those with continuous depression showed less improvement in symptom severity, pain intensity, walking capacity, and overall disability score. When preoperative depression improved or resolved, the postoperative Beck Depression Inventory outcomes were comparable to those in patients without a history of depression. In another series, 66% of 95 patients with lumbar stenosis were satisfied with their postoperative results; they were typically younger and exhibited less severe preoperative symptoms and disabilities. Depression in particular had a uniquely negative impact on their outcomes.62 In another series, the perceived quality of life (expectations, level of optimism) was evaluated in 57 patients before and 3 months after lumbar spine surgery; higher preoperative expectations and optimism correlated with a better quality of life postoperatively.63
Prophylactic Antibiotics
Although the Centers for Disease Control and Prevention recommends prophylactic antibiotics for lumbar surgery to limit spine infections, protocols vary from single-dose to multiple-dose regimens.64–68 In one study involving patients undergoing comparable but varied lumbar procedures, including stenosis with and without olisthesis and with and without instrumentation, the efficacy of multiple- (5 to 7 days) versus single-dose prophylactic antibiotic regimens employing a first-generation cephalosporin were compared.64 Of interest, although the infection rates for the two groups proved comparable (0.8% of 1133 multidose versus 0.4% of 464 single-dose patients), five of the six infections in the multidose patients proved to be caused by resistant bacteria, compared with none of the three organisms seen in the single-dose patients. In another retrospective series of patients undergoing lumbar diskectomy alone, comparable infection rates were encountered in the multidose (5 of 434 patients [1.15%] who received one preoperative and at least three postoperative doses of antibiotics) and single-dose (3 of 201 [1.49%] patients who received a single dose of preoperative antibiotics) populations.65 Based on laboratory studies in an ovine model, the ideal prophylactic antibiotic for spine surgery is a second-generation cephalosporin, typically cefazolin, administered at a dose of 2 g within 15 to 30 minutes of the skin incision.67 Nevertheless, the reported incidence of infection may vary from 3% for noninstrumented to 12% for instrumented procedures.68 Additionally, the frequency of infection is higher for posterior spine procedures than for anterior operations.
In addition to 2 g of a second-generation cephalosporin 15 minutes before the incision, 80 mg of gentamicin is given by intravenous Soluset over 30 minutes before surgery, providing increased protection against methicillin-resistant Staphylococcus aureus (MRSA). Other adjuncts include irrigation with bacitracin and polymyxin B sulfate every 15 minutes during the actual operative procedure. In one study, constant irrigation with saline and 50,000 units each of bacitracin and polymyxin B sulfate was used for clean procedures performed at two community hospitals; the infection rate was impressively reduced to 0%.69 The frequency of intraoperative bacterial growth (cultures from the wound and other sources) with this irrigation was reduced from 64% to 4% when both antibiotics were used.
Silver-Impregnated Dressings
Postoperatively, a silver-impregnated dressing should be applied to the wound over the staples. Silver has been used as an effective topical wound therapy for centuries.70 Silver easily binds to negatively charged proteins. Nanocrystals slowly release the silver ion for up to 7 days (sustained release). Silver inhibits proinflammatory cytokines and upregulates zinc metabolism, thereby increasing epithelialization and potentiating wound healing. These dressings are uniquely effective against resistant organisms (MRSA, Staphylococcus epidermidis, Pseudomonas aeruginosa, vancomycin-resistant enterococcus, and others).
One of several products on the market is Silverlon (Argentum Medical, Willowbrook, IL), a polymeric fabric coated with metallic silver that coats a large surface area and is flexible. Directions for application include the following: (1) wash the wound daily with a sterile gauze soaked in sterile water; (2) wash the Silverlon dressing daily with sterile water and squeeze gently, but leave the dressing wet; (3) apply the Silverlon dressing with the silver side down; (4) reuse each dressing, washing it daily as described, up to 7 days, unless the drainage is purulent; and (4) apply a dry gauze dressing over the Silverlon dressing. Alcohol, iodine compounds, saline, and peroxide should be avoided when using silver dressings. Two large series of patients undergoing multilevel lumbar laminectomies for stenosis with instrumented (one- or two-level) lumbar fusions were sequentially compared: 3 of 128 patients (2.3%) managed without Silverlon dressings developed postoperative infections managed with 6 weeks of intravenous antibiotics, whereas none of 106 patients treated with Silverlon dressings became infected.71
Management of Coexisting Cervical Disease
Patients with significant cervical spondylostenosis and cord compression documented on MRI or CT studies may require prophylactic awake intubation, awake positioning (three-pin head holder), and electrophysiologic monitoring before lumbar surgery.72 After the patient has been mildly sedated, the three-pin head holder is placed using local anesthesia at each pin site following skin preparation in the supraorbital and retroauricular regions with alcohol or iodine-based products. Once the patient is prone, the head holder is affixed to the Mayfield headrest, taking care to maintain the patient’s neck in a neutral position. All pressure points are appropriately padded. Before induction of anesthesia, the patient’s neurological examination is checked and correlated with SEPs that have been monitored throughout the entire process.73–77
Intraoperative Monitoring
Somatosensory evoked potentials (SEPs), electromyography (EMG), and motor evoked potentials (MEPs) monitoring contribute to the safety and efficacy of lumbar canal decompression with or without fusion. Preoperative SEPs have been used diagnostically to determine the presence and severity of lumbar root involvement or cauda equina compromise. In a prospective study of 54 patients with lumbar stenosis, 87% exhibited SEP changes consistent with multilevel stenosis, with 78% demonstrating abnormal anterior tibial responses.76 In one study of 61 patients with mostly spinal cord tumors (61%) or adult tethered cord syndromes (25%), new postoperative deficits were observed in 3 patients (4.9%) and were permanent in 1.77 One patient experienced a significant decrease in SEP amplitude, whereas 84% showed spontaneous EMG changes in lower extremity muscles or in external anal or urethral sphincters sometime during surgery. The investigators concluded that all modalities were useful during complex spine procedures involving the conus and cauda equina and that surgical decisions and microsurgery were impacted by the presence of intraoperative monitoring, which helped reduce the risk of permanent intraoperative complications.
Intraoperative monitoring has been emphasized during cervical spine surgery. In 1993 Epstein and coworkers78 noted that the introduction of SEP monitoring to scoliosis surgery reduced neural injuries from a range of 4% to 6.9% to 0% to 0.7%. When comparable cervical spine procedures were performed in 218 unmonitored and 100 prospectively monitored patients, the risk of quadriplegia was 3.7% for unmonitored patients, including one death (0.5%); no quadriplegic injuries or deaths occurred in monitored individuals. In this and a subsequent study, immediate notification of intraoperative SEP changes was thought to contribute to the reduction in neural injuries observed; in addition, early notification of changes contributed to resuscitative maneuvers, including reversal of hypotension, change in positioning, release of distraction, cessation of manipulation, and alteration of graft placement.78,79
During surgical procedures for degenerative lumbar spine disease, the majority of clinical postoperative root injuries positively correlates with intraoperative SEP changes.80 Furthermore, when both SEPs (posterior tibial or peroneal) and EMG scans (lower extremity muscles) were recorded in 44 patients during lumbosacral decompression with instrumentation, in two cases abnormal SEPs and spontaneous EMG activity correlated with new postoperative deficits.81 The authors advocated that both SEPs and EMG be used to provide immediate feedback during surgery regarding the sensory and motor function of the nerve tissue, cord, and cauda equina.
Prophylaxis for Deep Venous Thrombosis and Pulmonary Embolism
There is an increased risk of deep venous thrombosis (DVT) and pulmonary embolism (PE) in patients undergoing lumbar surgery, particularly if there is a history of malignancy, DVT, or PE during previous surgery, hypercoagulable syndromes, significant cardiac disease, obesity, or long surgical procedures. For lumbar surgery performed without any type of prophylaxis, the risk of DVT approaches 43%.82–84 Despite the efficacy of low-dose heparin or low-molecular-weight heparin derivatives in the prevention of DVT and PE, the associated risk of major hemorrhage (0.7%) must be carefully considered. Notably, 10 of 13 patients developed significant neurological deficits in a series, 4 of which were permanent.85 In another study of 720 spinal operations performed using minidose heparin 5000 units subcutaneously every 12 hours, two major epidural hematomas warranted surgical extirpation.86
For patients undergoing lumbar decompressive procedures with or without fusion, DVT and PE prophylaxis may consist of alternating compression stockings alone. Studies of compression stockings for prophylaxis have revealed a 2% incidence of DVT.87 Prophylaxis with compression stockings in 139 patients undergoing multilevel laminectomies (average, 3.8 levels) and instrumented fusion (average, 1.4 levels) resulted in 4 cases (2.8%) of DVT (2 to 6 days postoperatively) and 1 case (0.7%) of PE.84 Hematologic screening for hypercoagulation syndromes revealed that only the patient who developed PE tested positive for factor V Leiden hypercoagulation mutation. Of interest, the frequency of hypercoagulation syndromes in the overall population approaches 10%. It is therefore worthwhile to carefully screen patients with histories of DVT or PE, either themselves or their families, to determine whether they are appropriate candidates for prophylactic placement of removable inferior vena cava filters.
Bloodless Surgery or Normovolemic Hemodilution
The technique of normovolemic hemodilution in lumbar surgery limits allogeneic transfusion requirements by allowing blood salvage maneuvers to begin at the outset of the surgical procedure.88–90 Normovolemic hemodilution involves the controlled removal of a volume of whole blood (1 to 3 units), based on the patient’s preoperative hematocrit; each milliliter of blood is then replaced with 3 to 4 mL of colloid or crystalloid. Typically, normovolemic hemodilution decreases the hematocrit to 28% intraoperatively, which is generally high enough to avoid hemodynamic compromise.88–90
In a series of 68 patients undergoing multilevel laminectomies (three to six levels) with instrumented fusion (one to two levels), 52 patients with average preoperative hematocrit of 41.3% required no homologous blood transfusions.89 Alternatively, the 16 patients who required an average of 3.1 transfused units exhibited a lower average preoperative hematocrit of 38.5%. Several factors that contributed to greater transfusion requirements in the latter population included lower average preoperative hematocrit, older average age (59 versus 50 years), a greater percentage of females (12 of 16 patients), and slightly more extensive operations.89
Minimally Invasive Surgery
Older Patients
There is much discussion regarding the safety and efficacy of minimally invasive surgical approaches for decompressing lumbar spinal stenosis in the geriatric population. In one study, 57 patients with a mean age of 75 years were assessed before and after minimally invasive procedures using the Visual Analogue Pain Scale, ODI, and SF-36.91 Two shortcomings of the study included the short-term follow-up (average, 10 months; median, 7 months) and the loss of 50 of the original 107 patients from the study. For the remaining 57 patients, there were no major complications or deaths, and postoperative scores improved for patients using the listed outcome questionnaires. From this, the authors concluded that minimally invasive procedures were safe and effective for managing spinal stenosis in the elderly population.91
However, in geriatric patients, OYL, synovial cysts, spondyloarthrosis, stenosis, and olisthesis increase the risk of cerebrospinal fluid (CSF) fistulas, neural damage, and residual disease when using minimally invasive procedures. Even with open operations performed under an operating microscope with full maneuverability, these procedures (laminectomy, decompression) may prove extremely difficult.92
Younger Patients
Younger patients undergoing minimally invasive surgery for stenosis may be at greater risk for developing stress fractures (secondary to ligamentous laxity) or facet joint degeneration in the absence of degenerative disease or remodeling, which in older patients might avert these complications. In a simulated study comparing multiple minimally invasive approaches to the distal lumbar spinal canal, a marked increase in pars stress fractures occurred following limited decompression (extension, rotation).93
X-Stop Procedures
X-Stop devices (Kyphon, Sunnyvale, CA; Medtronic, Memphis, TN) are being used to treat one- to two-level lumbar spinal stenosis (![]() Figs. 285-E44 and 285-E45). By reversing the lumbar lordotic curvature and placing the patient in a greater degree of flexion, the device theoretically “opens” the spinal canal, thereby relieving the symptoms of stenosis.
Figs. 285-E44 and 285-E45). By reversing the lumbar lordotic curvature and placing the patient in a greater degree of flexion, the device theoretically “opens” the spinal canal, thereby relieving the symptoms of stenosis.
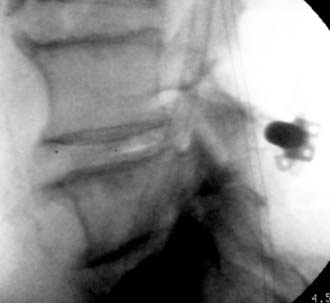
FIGURE 285-E44 Lateral fluoroscopic image documents extrusion of an X-Stop device placed at the L4-5 level.
The literature regarding the safety and efficacy of the X-Stop includes several preliminary studies. In an initial randomized, multicenter trial involving 191 patients with lumbar stenosis, implantation of the X-Stop device resulted in a 73.1% satisfaction rate, compared with only a 35.9% satisfaction rate among those managed nonsurgically (control patients).94 In a 2-year prospective, randomized, multicenter study, the SF-36 was used to compare outcomes for patients who received either the X-Stop device or no surgery (control patients).95 Two years postoperatively, X-Stop patients improved on five of eight health scales; the exceptions were General Health, Role Emotional, and Mental Health. In another randomized controlled series of 70 patients with stenosis or degenerative spondylolisthesis, the clinical success rate was 63.4% with the X-Stop device, compared with 12.9% for control patients.2,96
Nevertheless, the safety, efficacy, and value of these devices when used to treat lumbar stenosis with or without degenerative spondylolisthesis remains unclear. One must also consider that X-Stop–related complications and failures are likely underreported in the literature, because few journals accept and even fewer surgeons report negative outcomes.92,97,98 One series examined 1-year outcomes for 24 patients with lumbar stenosis undergoing X-Stop placement; 29% of patients experienced sufficiently severe residual pain or neurogenic claudication to warrant caudal epidural injections.97 A second series of 65 patients with lumbar stenosis who received the X-Stop device experienced only a 31.1% incidence of good outcomes.98 In a third series, 7 of 12 patients (58%) with lumbar stenosis or degenerative spondylolisthesis who received the X-Stop implant and were followed for 2 years required additional open surgery (decompression, posterolateral fusion).99 The authors of the latter series recommended that the X-Stop device be avoided in patients with severe stenosis attributed to degenerative spondylolisthesis.
Medical and neurological complications encountered with the X-Stop device are rarely reported but appear to be more prevalent in the geriatric population with more comorbidities.6 In one study, two patients in their 80s undergoing X-Stop placement for stenosis or spondylolisthesis (at outside institutions) developed an infection and hematoma, respectively; both had multiple significant comorbid factors predisposing them to these complications, which required prolonged hospitalizations.92 I recently treated an 84-year-old man who had undergone X-Stop placement for MRI-documented L4-5 stenosis and degenerative spondylolisthesis at another institution; postoperatively, he developed an immediate complete bilateral footdrop. Despite the acute deficit, nothing was done until 3 months later, when the X-Stop dislodged; it was removed, but no further surgery was performed. Finally, 9 months later, I performed an L1-S1 laminectomy and in situ L4-5 fusion; the left footdrop resolved, but the right-sided deficit remains partial.
Endoscopic Decompression
Endoscopic modalities have been applied to decompression of lumbar spinal stenosis. In one series of 10 patients prospectively undergoing unilateral endoscopic facetectomy to treat severe lumbar spinal stenosis, dynamic radiographs documented no postoperative instability when compared with control patients.100 The investigators concluded that endoscopic removal of a unilateral facet joint did not significantly contribute to instability, and other surrounding structures that contributed to stability were preserved.
Expansive Laminaplasty
Some surgeons advocate expansive laminaplasty to decompress lumbar stenosis. An expansive lumbar laminaplasty opens the spinal canal by means of rotatory lifting of the involved laminae; bone grafts from the spinous processes or iliac bone are then placed on the surface of the operated levels.101 Lumbar laminaplasty maintains stability while sufficiently enlarging the spinal canal, providing 119% canal expansion for up to 3 years postoperatively, with a 73% incidence of good or excellent outcomes.102 In another retrospective study of 71 patients undergoing lumbar laminaplasty followed an average of 5.4 years, only 8 patients (11%) developed adjacent-segment disease that became symptomatic.103 Disease-free survival rates were 95.7% at 5 years, 63.1% at 10 years, and 42.1% at 13 years. Those with spondylolisthesis were more prone to develop adjacent-level disease and exhibited a greater degree of preoperative range of motion at the adjacent levels.
Open Decompression Procedures
Laminotomy
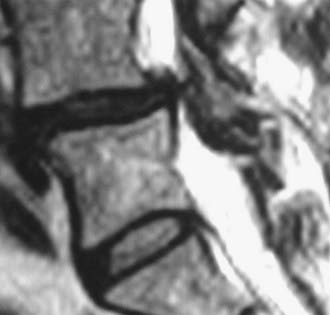
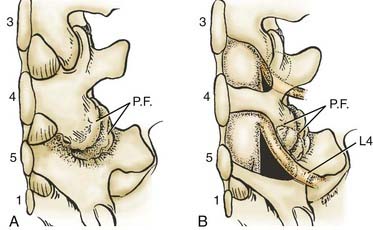
Unilateral laminotomy is another alterative for decompressing focal one-sided spinal stenosis (![]() Figs. 285-E46 to 285-E53). When 102 patients averaging 63 years of age were followed for an average of 5.6 years, 92.2% remained improved following unilateral laminotomy, and 85.3% showed excellent to fair results.104 Second operations, which were rarely performed (11.7%), addressed recurrent stenosis (seven patients) and instability (two patients).
Figs. 285-E46 to 285-E53). When 102 patients averaging 63 years of age were followed for an average of 5.6 years, 92.2% remained improved following unilateral laminotomy, and 85.3% showed excellent to fair results.104 Second operations, which were rarely performed (11.7%), addressed recurrent stenosis (seven patients) and instability (two patients).
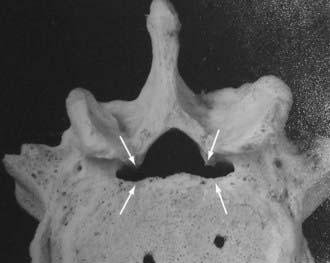
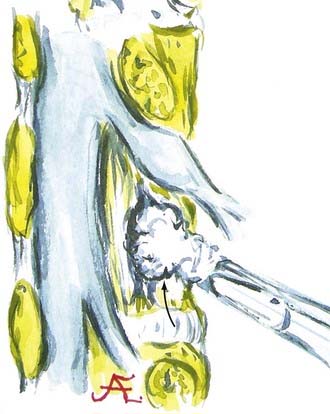
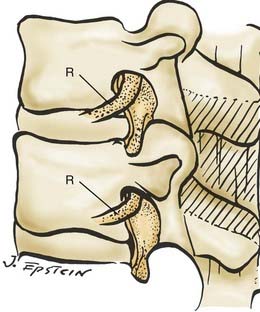
FIGURE 285-E47 In this anatomic specimen, the S1 superior articular facets (arrows) compress the lateral recesses.
Bilateral Laminotomy (Fenestration Procedure)
For a subset of patients with moderate rather than severe central stenosis, fenestration approaches, defined as bilateral laminotomies, may be considered. Stenosis in these patients may be variously attributed to congenital or acquired spondylostenosis, OYL, disk herniation, limbus fracture, scoliosis, or degenerative olisthesis. Bilateral laminotomies involve the preservation of the spinous processes, interspinous ligament, and lateral two thirds of the facet joints; all three elements contribute to continued stability. In one series, the bilateral fenestration approach provided symptomatic relief for up to 5.5 postoperative years; although new bone deposition contributed to stability, there was no significant evidence of recurrent stenosis.105 In another study, the bilateral fenestration procedure adequately decompressed nerve roots and preserved stability, yielding a 71% incidence of good or excellent results on surgeon-based outcome measures and 76% good or excellent results on patient-based outcome questionnaires.106
Coronal Hemilaminectmoy
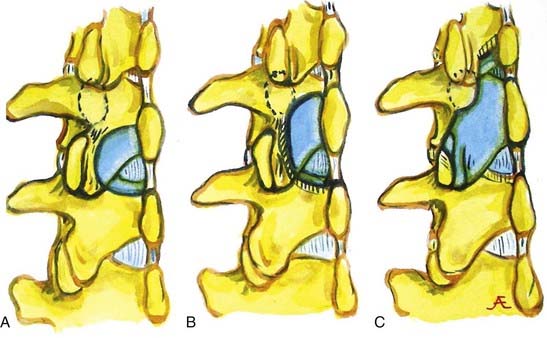
An alternative to the fenestration procedure, particularly for one-level stenosis, is to perform a coronal hemilaminectomy (![]() Figs. 285-E54 and 285-E55). This involves the removal of two thirds of the cephalad spinous process and lamina and one third of the caudad spinous process and lamina. This provides excellent decompression of central and lateral recess stenosis while facilitating lateral and foraminal excision of OYL under an operating microscope.39–41 This surgical alterative is particularly useful in older patients with more severe stenotic changes. It maximizes the safety and efficacy of the decompression while minimizing the risk of instability by undercutting the facet joints and avoiding more extensive facet disruption.
Figs. 285-E54 and 285-E55). This involves the removal of two thirds of the cephalad spinous process and lamina and one third of the caudad spinous process and lamina. This provides excellent decompression of central and lateral recess stenosis while facilitating lateral and foraminal excision of OYL under an operating microscope.39–41 This surgical alterative is particularly useful in older patients with more severe stenotic changes. It maximizes the safety and efficacy of the decompression while minimizing the risk of instability by undercutting the facet joints and avoiding more extensive facet disruption.
Fenestration Approaches for Grade I Degenerative Spondylolisthesis
For patients with degenerative spondylolisthesis (without an active slip > 4 mm or > 10 to 12 degrees of angulation) and more moderate stenosis, fenestration procedures may provide sufficient decompression.42,43 Alternatively, for geriatric patients with severe stenosis and marked OYL, and particularly those with synovial cyst herniations into the spinal canal, this procedure may increase the risk of CSF fistula formation and neural injury, particularly to the cephalad, far laterally exiting nerve root. In a 1983 study Epstein and associates43 treated 60 patients with stenosis and degenerative spondylolisthesis using 6 fenestration procedures (15% of patients), 40 multilevel laminectomies, and 14 coronal hemilaminectomies. In a later series of 290 patients with stenosis and degenerative spondylolisthesis, a comparable 14.1% of patients (41) was treated with a fenestration technique (3.2 levels of stenosis, 1.7 levels of olisthy).42
Nevertheless, the majority of patients with lumbar stenosis and degenerative spondylolisthesis will be offered decompressive laminectomy with or without fusion for sciatica accompanied by neurogenic claudication.58,107,108 In a nonrandomized “as treated comparison” assessing lumbar decompression with or without fusion versus nonsurgical management of 304 patients with stenosis and degenerative spondylolisthesis from 13 centers in 11 states, “substantially greater improvement” in pain and function (SF-36, ODI) was documented for the surgical patients.58
Lumbar Laminectomy without Fusion
Lumbar laminectomy remains a safe and effective alternative for the management of severe lumbar spinal stenosis, particularly in the geriatric population (![]() Fig. 285-E56).39–41 The number of surgical levels undergoing decompression should be based on a full assessment of radiographic, MRI, and CT evaluations to avoid the most common pitfall leading to failed lumbar stenosis surgery: inadequate decompression. Additional associated stenotic pathology documented in 32 patients older than 65 years (average age, 71) included disk degeneration or herniation, facet arthropathy, OYL, synovial cyst extrusion, spondylosis, and spondylolisthesis.109 These patients demonstrated uniquely stenotic spinal canals measuring less than 11.5 mm (71.9% of patients), requiring laminectomies in 68.6% of patients (87.5% total, 12.5% partial) and laminectomies with diskectomies in the remainder.
Fig. 285-E56).39–41 The number of surgical levels undergoing decompression should be based on a full assessment of radiographic, MRI, and CT evaluations to avoid the most common pitfall leading to failed lumbar stenosis surgery: inadequate decompression. Additional associated stenotic pathology documented in 32 patients older than 65 years (average age, 71) included disk degeneration or herniation, facet arthropathy, OYL, synovial cyst extrusion, spondylosis, and spondylolisthesis.109 These patients demonstrated uniquely stenotic spinal canals measuring less than 11.5 mm (71.9% of patients), requiring laminectomies in 68.6% of patients (87.5% total, 12.5% partial) and laminectomies with diskectomies in the remainder.
Success rates associated with laminectomy for stenosis with or without olisthesis range from 80% to 91%. For 50 patients with stenosis and degenerative spondylolisthesis, laminectomy and facetectomy contributed to a 91% frequency of good to excellent results at 3 years and an 87% success rate at 6 years.110 Other studies have quoted nearly comparable 80% to 84% success rates following decompression alone for stenosis, without fusion.111,112
Older studies showed that spinal stenosis with or without degenerative spondylolisthesis could be successfully managed with decompression alone without fusion.65,76,108,113–117 In Turner and colleagues’118 meta-analysis of stenosis surgery, including those with olisthy, 64% of cases yielded excellent results with or without fusion. Their study included the evaluation of 74 papers involving multiple surgeons and outcome measures (e.g., Odom’s criteria and Prolo and coworkers’119 scheme). Of 290 patients with stenosis or olisthesis undergoing laminectomy alone, 2.4% developed postoperative instability following full unilateral facetectomies to resect far lateral disks at L4-5 levels having already demonstrated preoperative olisthesis (![]() Figs. 285-E57 and 285-E58).108 Furthermore, 82% of patients demonstrated good or excellent results over an average 10-year postoperative interval. In another series of 258 laminectomies performed to address stenosis with or without olisthesis, Silvers and associates117 observed a 75% incidence of good or excellent results over an average of 4.7 years following surgery. Additionally, Deyo and colleagues120,121 documented comparable clinical outcomes, morbidity, mortality, and resource utilization for patients undergoing laminectomy with or without fusion. However, for geriatric patients, fusion increased morbidity by 20%, blood transfusion requirements were 5.4 times greater, there was a higher incidence of nursing home referrals, and there was a twofold greater mortality rate.
Figs. 285-E57 and 285-E58).108 Furthermore, 82% of patients demonstrated good or excellent results over an average 10-year postoperative interval. In another series of 258 laminectomies performed to address stenosis with or without olisthesis, Silvers and associates117 observed a 75% incidence of good or excellent results over an average of 4.7 years following surgery. Additionally, Deyo and colleagues120,121 documented comparable clinical outcomes, morbidity, mortality, and resource utilization for patients undergoing laminectomy with or without fusion. However, for geriatric patients, fusion increased morbidity by 20%, blood transfusion requirements were 5.4 times greater, there was a higher incidence of nursing home referrals, and there was a twofold greater mortality rate.
Outcome
There are primarily two types of outcome measures used to evaluate the results of lumbar surgery: surgeon-based and patient-based questionnaires. A surgeon-based outcome study that included 140 patients demonstrated significant relief of leg pain (82%) and back pain (71%) over an average of 42 postoperative months.122 Notably, poorer outcomes were observed in females, those involved in litigation or other compensation cases, those with prior failed surgery, and those with new postoperative sensory deficits.
The patient-based ODI administered an average of 4.3 years after lumbar laminectomy for stenosis (438 patients) revealed that only 62% of women and 57% of men experienced good or excellent outcomes.123 Poor prognostic factors included diabetes, prior total hip replacement, and preoperative spinal fracture or osteoporosis. Another study revealed less optimal long-term results; questionnaires administered to 119 patients an average of 4.6 years after laminectomy showed marked improvement in 37%, mild to moderate improvement in 29%, and no change in 17%; 5% were somewhat worse, and 12% were much worse.124
The SF-36 was used to evaluate 2-year postoperative patient-based outcomes for those undergoing decompressive laminectomies for the excision of synovial cysts and lumbar stenosis (45 patients; 3.8-level laminectomies) or for synovial cysts, stenosis, and degenerative spondylolisthesis (35 patients; 3.5-level laminectomies).53 Five of the 45 patients with cysts and stenosis developed a new grade I olisthesis postoperatively. However, of the 35 with grade I olisthesis preoperatively accompanied by stenosis and cysts, 11 developed new or increased grade II olisthesis postoperatively. Nevertheless, nearly comparable rates of excellent results (58% and 63%) and SF-36 improvement on Physical Function (+44 and +38) were documented for both groups. Because only moderate improvement was documented for patients with synovial cysts and stenosis with or without olisthesis, perhaps primary fusion addressing instability should be considered to improve the operative results for both groups.53 Lumbar instability measured on dynamic lateral radiographs is defined as greater than 4 mm of translation (or 8%) or greater than 10 to 12 degrees of angular displacement.125
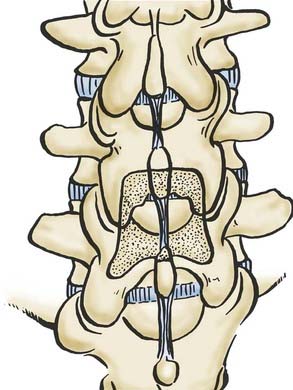
Noninstrumented Fusion
Many studies have advocated performing noninstrumented posterolateral fusion following lumbar decompressive laminectomy to improve both short- and long-term outcomes (![]() Figs. 285-E59 to 285-E64).126,127 In the series of Katz and coworkers,127 patients undergoing noninstrumented fusions demonstrated better symptomatic relief 6 and 24 months postoperatively compared with those having laminectomy alone or instrumented fusion. In McCulloch’s128 study, which evaluated 21 patients undergoing microsurgical decompression with single-level noninstrumented fusion for stenosis and degenerative spondylolisthesis, the overall satisfaction rate was 86%. The authors of another study observed 90% good or excellent results following intertransverse process fusion without instrumentation, 80% good or excellent results following laminectomy alone, but only 33% good or excellent results after laminectomy and complete facetectomy.129
Figs. 285-E59 to 285-E64).126,127 In the series of Katz and coworkers,127 patients undergoing noninstrumented fusions demonstrated better symptomatic relief 6 and 24 months postoperatively compared with those having laminectomy alone or instrumented fusion. In McCulloch’s128 study, which evaluated 21 patients undergoing microsurgical decompression with single-level noninstrumented fusion for stenosis and degenerative spondylolisthesis, the overall satisfaction rate was 86%. The authors of another study observed 90% good or excellent results following intertransverse process fusion without instrumentation, 80% good or excellent results following laminectomy alone, but only 33% good or excellent results after laminectomy and complete facetectomy.129
Adjunctive Demineralized Bone Matrix
High fusion rates and excellent or good outcomes were observed for 75 patients (49 women, 26 men; average age, 69 years) undergoing multilevel laminectomies (average, 4.9 levels) with an average two-level noninstrumented posterolateral fusion using a 50-50 mix of lamina autograft and demineralized bone matrix (Osteofil/ICM; Medtronic, Memphis, TN) (see ![]() Figs. 285-E61 to 285-E64).130 Two-dimensional CT studies documented fusion in 90.6% of patients (no lucency in the fusion mass, presence of bony trabeculation); dynamic films revealed no motion (fusion) in 97.3% of patients an average of 4.8 months postoperatively. Only one patient who failed to fuse on both studies required a secondary arthrodesis. Furthermore, 1- and 2-year outcomes were nearly comparable; patients exhibited maximal recovery on six of eight health scales (excluding General Health and Mental Health).
Figs. 285-E61 to 285-E64).130 Two-dimensional CT studies documented fusion in 90.6% of patients (no lucency in the fusion mass, presence of bony trabeculation); dynamic films revealed no motion (fusion) in 97.3% of patients an average of 4.8 months postoperatively. Only one patient who failed to fuse on both studies required a secondary arthrodesis. Furthermore, 1- and 2-year outcomes were nearly comparable; patients exhibited maximal recovery on six of eight health scales (excluding General Health and Mental Health).
Adjunctive β-Tricalcium Phosphate
The use of β-TCP as an adjunct to lamina autograft in performing noninstrumented posterolateral lumbar fusion has been established (see ![]() Figs. 285-E61 to 285-E64).44,131,132 Most recently, adequate fusion rates and outcomes were achieved in 60 patients (average age, 70 years) undergoing multilevel lumbar laminectomies (average, 5.4 levels) with one- or two-level noninstrumented fusion using lamina autograft in a 50-50 mix with β-TCP (Vitoss/β-TCP, Malvern, PA).44 Dynamic radiographs and two-dimensional CT studies were obtained by two independent neuroradiologists to document fusion 3, 6, and up to 12 months postoperatively; SF-36 outcomes were assessed over the same intervals. Pathologies included stenosis (60 patients) accompanied by OYL (46), herniated disks (20), and synovial cysts (8). Instability was associated with degenerative spondylolisthesis (48 patients), spondylolisthesis with lysis (2 patients), and degenerative scoliosis (10 patients). Fusion was documented on both studies in 85% of patients, and pseudarthrosis on one or both studies was seen in the remaining 15%. Two years postoperatively, Odom’s criteria revealed 51 good or excellent results; SF-36 outcomes showed maximal improvement on six of eight health scales.
Figs. 285-E61 to 285-E64).44,131,132 Most recently, adequate fusion rates and outcomes were achieved in 60 patients (average age, 70 years) undergoing multilevel lumbar laminectomies (average, 5.4 levels) with one- or two-level noninstrumented fusion using lamina autograft in a 50-50 mix with β-TCP (Vitoss/β-TCP, Malvern, PA).44 Dynamic radiographs and two-dimensional CT studies were obtained by two independent neuroradiologists to document fusion 3, 6, and up to 12 months postoperatively; SF-36 outcomes were assessed over the same intervals. Pathologies included stenosis (60 patients) accompanied by OYL (46), herniated disks (20), and synovial cysts (8). Instability was associated with degenerative spondylolisthesis (48 patients), spondylolisthesis with lysis (2 patients), and degenerative scoliosis (10 patients). Fusion was documented on both studies in 85% of patients, and pseudarthrosis on one or both studies was seen in the remaining 15%. Two years postoperatively, Odom’s criteria revealed 51 good or excellent results; SF-36 outcomes showed maximal improvement on six of eight health scales.
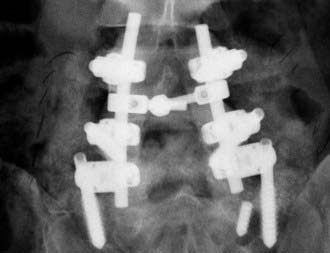
Instrumented Lumbar Fusion
In some series, performing instrumented fusion following decompressive laminectomy improves outcomes (![]() Figs. 285-E65 to 285-E68). In one study of 50 patients with stenosis and olisthesis treated with laminectomy alone (25 patients) versus laminectomy with fusion (25 patients), better results were observed in the latter an average of 3 years postoperatively.133 Other studies recommend that even unilateral decompression for stenosis with degenerative spondylolisthesis include contralateral noninstrumented autologous bone fusion.134 In another study of 28 patients with spinal stenosis and clearly unstable olisthesis, average four-level laminectomies and one- or two-level instrumented fusion (grade I, 25 patients; grade II, 3 patients) yielded an 85% incidence of good or excellent outcomes.125 Nevertheless, careful patient selection is critical because fusion places patients at greater risk for perioperative morbidity and morality.120,121
Figs. 285-E65 to 285-E68). In one study of 50 patients with stenosis and olisthesis treated with laminectomy alone (25 patients) versus laminectomy with fusion (25 patients), better results were observed in the latter an average of 3 years postoperatively.133 Other studies recommend that even unilateral decompression for stenosis with degenerative spondylolisthesis include contralateral noninstrumented autologous bone fusion.134 In another study of 28 patients with spinal stenosis and clearly unstable olisthesis, average four-level laminectomies and one- or two-level instrumented fusion (grade I, 25 patients; grade II, 3 patients) yielded an 85% incidence of good or excellent outcomes.125 Nevertheless, careful patient selection is critical because fusion places patients at greater risk for perioperative morbidity and morality.120,121
Adjunctive Demineralized Bone Matrix
Adequate fusion rates and outcomes were observed following one- or two-level posterolateral fusion using lamina autograft supplemented with demineralized bone matrix (Osteofil/ICM; Medtronic, Memphis, TN) in a 50-50 mix.45 SF-36 outcomes and the frequency of fusion were both evaluated following multilevel laminectomies and one-level (95 patients) or two-level (45 patients) instrumented pedicle-rod-screw (3D System; Medtronic, Memphis, TN) fusions. At 3, 6, and up to 12 months postoperatively, two radiologists independently documented fusion using dynamic radiographs and two-dimensional CT studies. SF-36 outcomes were also assessed for these intervals. Patients were followed an average of 3 years (minimum, 1.5 years). One year postoperatively, patients in both fusion groups comparably improved on six of eight SF-36 health scales. For one-level fusion, CT studies documented arthrodesis an average of 5.2 months postoperatively in 92.6% of patients; dynamic radiographs showed a 98% fusion rate. Two patients with pseudarthrosis on both studies required secondary fusions an average of 8 months postoperatively. Two-level fusions were demonstrated on CT studies an average of 6.1 months postoperatively in 91.2% of patients; dynamic radiographs showed a 96% fusion rate. The two patients with pseudarthrosis in both studies required secondary fusions an average of 10 months postoperatively.
Adjunctive β-Tricalcium Phosphate
β-TCP is increasingly used to perform posterolateral lumbar fusions.135,136 Fusion rates and outcomes were initially evaluated following 40 multilevel laminectomies (average, 3.7 levels) and one-level (27 patients) or two-level (13 patients) posterolateral instrumented fusion (pedicle-screw 3D System; Medtronic, Memphis, TN) using lamina autograft supplemented with β-TCP (Vitoss/β-TCP; Orthovita, Malvern, PA).136 Dynamic radiographs and two-dimensional CT studies were obtained 3, 6, and up to 12 months postoperatively until fusion or pseudarthrosis was achieved. SF-36 outcome questionnaires were also administered 3, 6, and 12 months postoperatively. For single-level fusion, 6-month radiologic data revealed fusion in 26 of 27 patients on both studies; for two-level fusion, 11 of 13 fused. Odom’s criteria showed continued improvement for all patients. SF-36 data showed worsening on two health scales (Role Physical, Role Emotional) and minimal to marked improvement on the remaining six health scales. At 12 months, improvement was noted on all eight health scales. Of interest, only one patient with pseudarthrosis following a two-level fusion had secondary surgery. A subsequent analysis of 100 patients undergoing three-dimensional instrumented posterolateral fusion with lamina autograft and β-TCP (50-50 mix) revealed that 5% exhibited pseudarthrosis (all were prior smokers who continued to smoke) an average of 6.5 months postoperatively. Of interest, another 7% demonstrated delayed fusion (six of seven were prior smokers), and 88% showed completed fusions within the first postoperative year.
Instrumented Fusion for Lumbar Stenosis with Spondylolisthesis or Spondylolysis
The management of lumbar stenosis and isthmic spondylolisthesis warrants treatment with laminectomy, instrumented fusion, or both. In one series, good or excellent outcomes (96%) were achieved in patients undergoing instrumented fusion for grades I and II olisthesis and lysis.137 In another study involving laminectomy and instrumented fusion (25 patients), good or excellent results (76%) correlated with a 96% frequency of solid fusion.138
Secondary Lumbar Surgery
Although initial success rates for decompressive laminectomy for lumbar stenosis with or without fusion vary from 80% to 85%, the success rate declines to 50% for secondary procedures.39,41,120–122,139–141 In one study, ODI results revealed good or excellent outcomes in 67% of patients undergoing first procedures but in only 46% following secondary procedures.139 Furthermore, when 41 similar patients (average age, 51 years; followed up to 4.6 years) answered questionnaires, significantly better outcomes were demonstrated for those undergoing one rather than two operations, especially when the interval between operations was relatively short.140
Recurrent Stenosis or Instability
Secondary decompression with or without fusion addresses recurrent stenosis or instability in 9.3% to 28% of patients.126,127,139,140,142 In one series of 100 patients averaging 67 years of age and followed for less than 5 years, 16 demonstrated recurrent stenosis at or above previously decompressed levels.142 In 108 patients in another series followed for an average of 6.8 years, 9.3% required secondary surgery for recurrent stenosis.143 In another study, 6% of men but 28% of women required additional surgery for recurrent stenosis with or without olisthesis.144 In my series of 290 patients undergoing laminectomy for stenosis with degenerative spondylolisthesis, 5 patients required secondary fusions due to increased olisthesis or instability.42 Notably, the longer the postoperative interval, the higher the reoperation rate; in Katz and coworkers’126 series, 6% warranted repeat surgery (for instability or recurrent stenosis) at 1 year and 15% at 3 years. Additionally, significant cormorbidities including osteoarthritis, cardiac disease, pulmonary disease, rheumatoid arthritis, and insufficient prior decompression correlated with poorer long-term clinical outcomes.
Other Pathology Contributing to Secondary Surgery
Multiple factors may contribute to the need for second operative procedures. One series involving intertransverse process fusions (184 patients) yielded a 2.7% incidence of new L5-S1 disk herniations mandating secondary disk excision and L4-S1 fusion.145 In another series, patients who required secondary surgery to address recurrent disk herniations (19 patients) or focal recurrent stenosis (19 patients) did well, whereas those with recurrent central stenosis (20 patients) or perineural stenosis (35 patients) did poorly.13 Caputy and Luessenhop’s142 5-year evaluation of patients undergoing decompressive laminectomies alone revealed an initial 27% failure rate; projected over a lifetime, this could approach 50%. Half the failures were associated with recurrent stenosis observed at previously operated sites, and the other half were associated with new neurological deficits attributed to adjacent stenosis. Unsatisfactory outcomes were evaluated in 103 patients after lumbar surgery with instrumented fusion; these included 26 cases of recurrent stenosis, 13 adjacent-segment instability, 15 pseudarthrosis, 11 screw misplacement, 4 epidural fibrosis, 5 arachnoiditis, 7 disk herniations, and 22 miscellaneous.95 The use of lumbar interbody fusion cages to address new or recurrent disk pathology was also associated with substantial complications.146 The best improvement occurred after second procedures to address recurrent or new stenosis and disk herniation; few improved in the other categories. An additional factor was a 14.4% risk for developing adjacent-segment degenerative disease at asymptomatic levels over an average 44.5-month interval.147
Postoperative instability and fusion-related complications also contributed to the need for secondary surgery. Two studies cited a 13% to 43% frequency of instability and poor clinical outcomes following laminectomy with or without preoperative olisthesis.148,149 Other series observed olisthesis progression 13% to 18% of the time.150 High reoperation and complication rates approaching 63% were recorded.151 Inaccurate screw placement was observed 21% of the time.152 Additionally, an 11% incidence of new postoperative motor deficits, a 3.5% frequency of new postoperative sensory deficits, and a 4.18% to 5.7% incidence of screw breakage were noted.153,154
Failures of Instrumentation
Failed spinal fusion results in poorer postoperative outcomes. In one series, the rate of instability following laminectomy for stenosis was 54%, with 62% of patients requiring assistance for ambulation.155 Another consideration is cost; one study revealed that the typical laminectomy costs $12,615, a laminectomy and noninstrumented fusion costs $18,495, and a laminectomy with instrumentation costs $25,914.156
Complications: Cerebrospinal Fluid Fistulas
The risk of CSF fistulas following surgery for lumbar stenosis ranges from 5% to 10%.34 Ten patients in a series of 110 undergoing multilevel laminectomies with noninstrumented fusion exhibited dural tears intraoperatively attributed to severe OYL (10 patients) in conjunction with synovial cysts (5 of 10 patients). Patients with dural tears were older, averaging 74 years of age, and included more women (90% versus 75%), who underwent slightly more extensive laminectomies (5.5 versus 5.0 levels).
Damage to the cauda equina and nerve roots and creation of a CSF fistula can best be avoided by routinely using the operating microscope and dissecting from “normal to abnormal.” This often requires maintaining close contact with the lateral bony margins and use of Penfield elevators or small curets. Small Kerrison punches should be introduced only when margins and an underlying soft tissue plane are evident. When CSF fistulas occur, direct or indirect repair can be accompanied by several adjuncts (![]() Fig. 285-E69). If a CSF leak occurs, one must first assess (under the microscope) whether direct suturing of the leakage site is feasible. If dural margins are accessible, or if further decompression will make a sufficient dural margin available, primary repair should be performed. Typically, a 7-0 Gore-Tex suture (Ethicon; Johnson and Johnson, Somerville, NJ) is optimal because the needle is smaller than the suture. Once the dural edges are held together with Penfield forceps and averted, the suture is applied either separately or individually to either side and tied in six knots. If there is room, additional titanium staples (medium, 1.4 mm) should be applied. When closing longer CSF fistulas, fewer sutures and more staples can be used as long as the dural margin is clear and edges can be readily apposed. Before proceeding to the next step, the Valsalva maneuver should be used to ensure that the closure is watertight. If not, additional staples or sutures may be needed. If there is no additional space for either of these or if there is friable dura, a free muscle patch graft can be placed over the suture line. Of note, during the placement of the 7-0 sutures, several ends of these sutures are typically kept on clamps so that at the end of the closure they can be used to go through the muscle patch graft and facilitate closure. Additionally, the use of DuraGen (Integra Life Sciences, Plainsboro, NJ) may warrant suturing over the closure line using these additional sutures. This should then be followed by application of a fibrin sealant. Hemovac drains are usually employed even when CSF leaks are present because they facilitate watertight closure where DuraGen and fibrin sealant have been used.
Fig. 285-E69). If a CSF leak occurs, one must first assess (under the microscope) whether direct suturing of the leakage site is feasible. If dural margins are accessible, or if further decompression will make a sufficient dural margin available, primary repair should be performed. Typically, a 7-0 Gore-Tex suture (Ethicon; Johnson and Johnson, Somerville, NJ) is optimal because the needle is smaller than the suture. Once the dural edges are held together with Penfield forceps and averted, the suture is applied either separately or individually to either side and tied in six knots. If there is room, additional titanium staples (medium, 1.4 mm) should be applied. When closing longer CSF fistulas, fewer sutures and more staples can be used as long as the dural margin is clear and edges can be readily apposed. Before proceeding to the next step, the Valsalva maneuver should be used to ensure that the closure is watertight. If not, additional staples or sutures may be needed. If there is no additional space for either of these or if there is friable dura, a free muscle patch graft can be placed over the suture line. Of note, during the placement of the 7-0 sutures, several ends of these sutures are typically kept on clamps so that at the end of the closure they can be used to go through the muscle patch graft and facilitate closure. Additionally, the use of DuraGen (Integra Life Sciences, Plainsboro, NJ) may warrant suturing over the closure line using these additional sutures. This should then be followed by application of a fibrin sealant. Hemovac drains are usually employed even when CSF leaks are present because they facilitate watertight closure where DuraGen and fibrin sealant have been used.
Postoperative Rehabilitation
Following decompressive lumbar surgery with or without fusion, rehabilitation efforts appear to be of questionable benefit. Nevertheless, few have quantitated this. Mannion and colleagues157 evaluated 159 patients following decompression for spinal stenosis who were randomized into two groups: 2 months postoperatively, one group was told to keep active (54 patients); the others underwent physiotherapy (PT) with spine stabilization exercises (56 patients) or PT and mixed techniques (49 patients).157 The PT programs were two 30-minute sessions per week for up to 3 months, with home exercises. Pain levels for back and leg (separately) and Roland Morris disability questionnaires were evaluated 2, 5, 12, and 24 months postoperatively. At 2 years, 151 patients remained in the study (97%). After surgery, significant reductions in leg and back pain were noted; pain did not change up to 24 months thereafter. Most critically, 12 weeks of postoperative PT did not impact the postoperative course in terms of pain or disability. Allowing patients to keep active was as effective as formal PT programs, without the inherent cost.
Deyo RA, Ciol MA, Cherkin DC, et al. Lumbar spinal fusion: A cohort study of complications and resource use in the Medicare population. Spine. 1993;18:1463-1470.
Epstein JA, Epstein BS, Lavine L. Nerve root compression associated with narrowing of the lumbar spinal canal. J Neurol Neurosurg Psychiatry. 1962;25:165-170.
Epstein JA, Epstein BS, Rosenthal AD, et al. Sciatica caused by nerve root entrapment in the lateral recess: the superior facet syndrome. J Neurosurg. 1972;36:584-589.
Epstein NE. Fusion rates and SF-36 outcomes after multilevel laminectomy and non-instrumented lumbar fusion in a predominantly geriatric population. J Spinal Disord Tech. 2008;21:159-164.
Epstein NE. The frequency and etiology of intraoperative dural tears in 110 predominantly geriatric patients undergoing multilevel laminectomy with non-instrumented fusion. J Spinal Disord Tech. 2007;20:380-386.
Epstein NE. SF-36 Outcomes and fusion rates following multilevel laminectomies and 1-2 level instrumented posterolateral fusions utilizing lamina autograft and demineralized bone matrix. J Spinal Disord Tech. 2007;20:139-145.
Epstein NE. Efficacy of pneumatic compression stocking prophylaxis in the prevention of deep venous thrombosis and pulmonary embolism following 139 lumbar laminectomies with instrumented fusions. J Spinal Disord Tech. 2006;19:28-31.
Epstein NE. A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19:424-429.
Epstein NE. Lumbar laminectomy for the resection of synovial cysts and coexisting lumbar spinal stenosis or degenerative spondylolisthesis: an outcome study. Spine. 2004;29:1049-1055.
Epstein NE. Decompression in the surgical management of degenerative spondylolisthesis: Advantages of a conservative approach in 290 patients. J Spinal Disord. 1998;11:116-122.
Epstein NE. Evaluation of varied surgical approaches used in the management of 170 far lateral lumbar disc herniations: Indications and results. J Neurosurg. 1995;83:648-656.
Epstein NE, Danto J, Nardi D. Evaluation of intraoperative somatosensory evoked potential (SSEP) monitoring during 100 cervical operations. Spine. 1993;18:737-747.
Epstein NE, Epstein JA, Carras RC, et al. Coexisting cervical and lumbar spinal stenosis: diagnosis and management. Neurosurgery. 1984;15:489-496.
Epstein NE, Hood DC. A comparison of surgeon’s assessment to patient’s self analysis (Short Form 36) after far lateral lumbar disc surgery: an outcome study. Spine. 1997;22:2422-2428.
Epstein NE, Peller A, Korsh J, et al. Impact of intraoperative normovolemic hemodilution on transfusion requirements for 68 patients undergoing lumbar laminectomies with instrumented posterolateral fusion. Spine. 2006;31:2227-2230.
Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802-808.
Prolo DJ, Oklund SA, Butcher M. Toward uniformity in evaluating the results of lumbar spine operations. A paradigm applied to posterior lumbar interbody fusions. Spine. 1986;11:601-606.
Shenkin HA, Nash CJ. Spondylolisthesis after multiple bilateral laminectomies and facetectomies for lumbar spondylosis: follow-up review. J Neurosurg. 1979;50:45-47.
Silvers HR, Lewis PJ, Asch HL. Decompressive lumbar laminectomy for spinal stenosis. J Neurosurg. 1993;78:695-701.
Turner JA, Ersek M, Herron L, et al. Surgery for lumbar spinal stenosis: attempted meta-analysis of the literature. Spine. 1992;17:1-8.
Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36:230-237.
Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(2):2257-2270.
White AA, Panjabi MM. Biomechanical considerations in the surgical measurement of the spine. In: White AA, Panjabi MM, editors. Clinical Biomechanics of the Spine. 2nd ed. Philadelphia: Lippincott; 1990:511-631.
Wilste LL, Kirkaldy-Willis WH, McIvor GW. The treatment of spinal stenosis. Clin Orthop. 1976;115:83-91.
Zucherman JF, Hsu KY, Hartien CA, et al. A multicenter, prospective, randomized trial evaluating the X-Stop interspinous process decompression system for the treatment of neurogenic claudication: two-year follow-up results. Spine. 2005;30:1351-1358.
1 Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36:230-237.
2 Verbiest H. Neurogenic intermittent claudication in cases with absolute and relative stenosis of the lumbar vertebral canal (ASLC, RSLC) in cases with narrow lumbar intervertebral foramina, and in cases with both entities. Clin Neurosurg. 1972;20:204-210.
3 Verbiest H. Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral canal. A review of 27 years experience. J Bone Joint Surg Br. 1977;59:181-188.
4 Verbiest H. Introduction: Spinal stenosis. In: Hopp E, editor. Spine: State of The Art Reviews. Philadelphia: Hanley and Belfus; 1987:361-368.
5 Verbiest H. Stenosis of the lumbar vertebral canal and sciatica. Neurosurg Rev. 1980;3:75-89.
6 Epstein JA, Epstein BS, Rosenthal AD, et al. Sciatica caused by nerve root entrapment in the lateral recess: the superior facet syndrome. J Neurosurg. 1972;36:584-589.
7 Epstein JA, Epstein BS, Lavine LS, et al. Lumbar nerve root compression at the intervertebral foramina caused by arthritis of the posterior facets. J Neurosurg. 1973;39:362-369.
8 Getty CJM. Lumbar spinal stenosis: The clinical spectrum and the results of operation. J Bone Joint Surg Br. 1980;62:481-485.
9 Epstein JA, Malis LI. Compression of spinal cord and cauda equina in achondroplastic dwarfs. Neurology. 1955;5:875-881.
10 Hogan QH. Lumbar epidural anatomy. A new look by cryomicrotome section. Anesthesiology. 1991;75:767-775.
11 Tarlov IM. Spinal perineural and meningeal cysts. J Neurol Neurosurg Psychiatry. 1970;33:833-843.
12 Wanatabe R, Park WW. Vascular and neural pathology of lumbosacral spinal stenosis. J Neurosurg. 1986;64:64-70.
13 Jonsson B, Stromqvist B. Symptoms and signs in degeneration of the lumbar spine. A prospective, consecutive study of 300 operated patients. J Bone Joint Surg Br. 1993;75:381-385.
14 Rosomoff H, Rosomoff RS. Nonsurgical aggressive treatment of lumbar spinal stenosis. In: Hopp E, editor. Spine: State of the Art Reviews. Philadelphia: Hanley and Belfus; 1987:383-400.
15 Epstein JA, Epstein BS, Lavine L. Nerve root compression associated with narrowing of the lumbar spinal canal. J Neurol Neurosurg Psychiatry. 1962;25:165-170.
16 Deen HGJr, Zimmerman BS, Swanson SK, et al. Assessment of bladder function after lumbar decompressive laminectomy for spinal stenosis: a prospective study. J Neurosurg. 1994;80:971-974.
17 Elster AD. Bertolotti’s syndrome revisited. Transitional vertebrae of the lumbar spine. Spine. 1989;14:1373-1377.
18 Dvorak J, Panjabi MM, Novotny JE, et al. Clinical validation of functional flexion-extension roentgenograms of the lumbar spine. Spine. 1991;16:943-950.
19 Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
20 Rihn JA, Lee JY, Khan M, et al. Does lumbar facet fluid detected on magnetic resonance imaging correlate with radiographic instability in patients with degenerative lumbar disease? Spine. 2007;32:1555-1560.
21 Ross JS, Modic MI. Current assessment of spinal degenerative disease with magnetic resonance imaging. Clin Orthop. 1992;279:68-81.
22 Djukic S, Lang P, Morris J, et al. The postoperative spine. Magnetic resonance imaging. Orthop Clin North Am. 1990;21:603-624.
23 Aota Y, Niwa T, Yoshikawa K, et al. Magnetic resonance imaging and magnetic resonance myelography in the presurgical diagnosis of lumbar foraminal stenosis. Spine. 2007;32:896-903.
24 Laasonen EM, Soini J. Low-back pain after lumbar fusion. Surgical and computed tomographic analysis. Spine. 1989;14:210-213.
25 Wethout FD, Pare LS, Linskey ME. Central causes of foot drop: rare and underappreciated differential diagnoses. J Spinal Cord Med. 2007;30:62-66.
26 D’Agostino AN, Mason MS, Quinn SF. Lumbar spinal stenosis and spondylosis associated with amyloid deposition in the ligamentum flavum. Clin Neuropathol. 1992;11:146-150.
27 Simpson JM, Silveri CP, Balderston RA, et al. The results of operations on the lumbar spine in patients who have diabetes mellitus. J Bone Joint Surg Am. 1993;75:1823-1829.
28 Epstein NE, Epstein JA, Carras RC, et al. Coexisting cervical and lumbar spinal stenosis: diagnosis and management. Neurosurgery. 1984;15:489-496.
29 Epstein N, Schwall G. Thoracic spinal stenosis: diagnostic treatment challenges. J Spinal Disord. 1994;7:259-269.
30 Epstein N. Clinical Opinion: Thoracic ossification of the posterior longitudinal ligament, ossification of the yellow ligament from T9-T12 with superimposed acute T10/T11 disc herniation: Controversies in surgical management. J Spinal Disord. 1996;9:446-450.
31 LaBan MM, Green ML. Concurrent (tandem) cervical and lumbar spinal stenosis: a 10-year review of 54 hospitalized patients. Am J Phys Med Rehabil. 2004;83:187-190.
32 Aydogan M, Ozturk C, Mirzanli C, et al. Treatment approach in tandem (concurrent) cervical and lumbar spinal stenosis. Acta Orthop Belg. 2007;73:234-237.
33 Baba H, Maezawa Y, Furusawa N, et al. The role of calcium deposition in the ligamentum flavum causing a cauda equina syndrome and lumbar radiculopathy. Paraplegia. 1995;33:219-223.
34 Epstein NE. The frequency and etiology of intraoperative dural tears in 110 predominantly geriatric patients undergoing multilevel laminectomy with non-instrumented fusion. J Spinal Disord Tech. 2007;20:380-386.
35 Epstein NE. Ossification of the yellow (OYL) and spondylosis and/or ossification of the posterior longitudinal (OPLL) ligament of the thoracic and lumbar spine. J Spinal Disord. 1999;12:250-256.
36 Okada K, Oka S, Tohge K, et al. Thoracic myelopathy caused by ossification of the ligamentum flavum. Clinicopathologic study and surgical treatment. Spine. 1991;16:280-287.
37 Haft GF, Mendoza SA, Weinstein SL, et al. Use of Beta-2 Transferrin to diagnose CSF leakage following spinal surgery; a case report. Iowa Orthop J. 2004;24:115-118.
38 Leroux JL, Legeron P, Moulinier L, et al. Stenosis of the lumbar spinal canal in vertebral ankylosing hyperostosis. Spine. 1993;18:2368-2369.
39 Epstein NE, Epstein JA. Surgery for spinal stenosis, 2nd ed. Wiesel SW, Weinstein JN, Herkowitz H, et al, editors. The Lumbar Spine, vol 2. Philadelphia: Saunders. 1995:737-756.
40 Epstein NE, Epstein JA. Lumbar spinal stenosis, 4th ed. Julian R, editor. Neurological Surgery, vol 3. Philadelphia: Saunders. 1996:2390-2415.
41 Epstein NE, Epstein JA. Lumbar decompression for spinal stenosis: surgical indications and techniques with and without fusion, 2nd ed. Frymoyer JW, editor. The Adult Spine, Principles and Practice, vol 2. New York: Lippincott-Raven. 1997:2055-2088.
42 Epstein NE. Decompression in the surgical management of degenerative spondylolisthesis: Advantages of a conservative approach in 290 patients. J Spinal Disord. 1998;11:116-122.
43 Epstein NE, Epstein JA, Carras R, et al. Degenerative spondylolisthesis with an intact neural arch: a review of 60 cases with an analysis of clinical findings and the development of surgical management. Neurosurgery. 1983;13:555-561.
44 Epstein NE. An analysis of non-instrumented posterolateral lumbar fusions in predominantly elderly patients utilizing lamina autograft and beta tricalcium phosphate. Spine J. 2008;8:882-887.
45 Epstein NE. SF-36 Outcomes and fusion rates following multilevel laminectomies and 1-2 level instrumented posterolateral fusions utilizing lamina autograft and demineralized bone Matrix. J Spinal Disord Tech. 2007;20:139-145.
46 Epstein NE, Epstein JA, Mauri T. Treatment of fractures of the vertebra limbus and spinal stenosis in five adolescents and five adults. Neurosurgery. 1989;24:595-604.
47 Epstein NE, Epstein JA. Limbus lumbar vertebral fractures in 27 adolescents and adults. Spine. 1991;16:962-966.
48 Epstein NE. Lumbar surgery for 56 limbus fractures emphasizing non-calcified type III lesions. Spine. 1992;17:1489-1496.
49 Epstein NE, Epstein JA, Carras R, et al. Far lateral disc herniations and associated structural abnormalities: Evaluation of 60 patients and the comparative value of CT, MRI, and myelo-CT in the diagnosis and management. Spine. 1990;15:534-539.
50 Epstein NE, Epstein JA. The surgical management of lumbar foraminal and far lateral herniated discs complicated by vertebral limbus fractures. Neuro-Orthopedics. 1995;17/18:173-178.
51 Epstein NE. Evaluation of varied surgical approaches used in the management of 170 far lateral lumbar disc herniations: Indications and results. J Neurosurg. 1995;83:648-656.
52 Epstein NE, Hood DC. A comparison of surgeon’s assessment to patient’s self analysis (Short Form 36) after far lateral lumbar disc surgery: An outcome study. Spine. 1997;22:2422-2428.
53 Epstein NE. Lumbar laminectomy for the resection of synovial cysts and coexisting lumbar spinal stenosis or degenerative spondylolisthesis: an outcome study. Spine. 2004;29:1049-1055.
54 Epstein NE. Lumbar synovial cysts: a review of diagnosis, surgical management, and outcome assessment. J Spinal Disord Tech. 2004;17:321-325.
55 Herno A, Airaksinen O, Saari T, et al. Lumbar spinal stenosis: a matched-pair study of operated and non-operated patients. Br J Neurosurg. 1996;10:461-465.
56 Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32:1-8.
57 Athiviraham A, Yen D. Is spinal stenosis better treated surgically or nonsurgically? Clin Orthop Relat Res. 2007;458:90-93.
58 Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257-2270.
59 Slover J, Abdu WA, Hanscom B, et al. The impact of comorbidities on the change in Short-Form 36 and Oswestry scores following lumbar spine surgery. Spine. 2006;31:1974-1980.
60 Freedman B, Arinzon Z, Zohar E, et al. Observations on the safety and efficacy of surgical decompression for lumbar spinal stenosis in geriatric patients. Eur Spine J. 2002;11:571-574.
61 Sinikallio S, Aalto T, Airaksinen O, et al. Depression is associated with poorer outcome of lumbar spinal stenosis surgery. Eur Spine J. 2007;16:905-912.
62 Sinikallio S, Aalto T, Airaksinen O, et al. Lumbar spinal stenosis patients are satisfied with short-term results of surgery—younger age, symptom severity, disability, and depression decrease satisfaction. Disabil Rehabil. 2007;29:537-544.
63 Saban KL, Penckofer SM. Patient expectations of quality of life following lumbar spinal surgery. J Neurosci Nurs. 2007;39:180-189.
64 Kanayama M, Hashimoto T, Shigenobu K, et al. Effective prevention of surgical site infection using a Centers for Disease Control and Prevention guideline-based antimicrobial prophylaxis in lumbar surgery. J Neurosurg Spine. 2007;6:327-329.
65 Dobzyniak MA, Fischgrund JS, Hankins S, et al. Single versus multiple dose antibiotic prophylaxis in lumbar disc surgery. Spine. 2003;18:E453-E455.
66 Barker FG. Efficacy of prophylactic antibiotic therapy in spinal surgery—a meta-analysis. Neurosurgery. 2002;51:391-401.
67 Walters R, Moore R, Fraser R. Penetration of cephazolin in human lumbar intervertebral disc. Spine. 2006;31:567-570.
68 Brown EM, Pople IK, De Louvois J, et al. Spine update: prevention of postoperative infection in patients undergoing spinal surgery. Spine. 2004;29:938-945.
69 Savitz SI, Savitz MH, Goldstein HB, et al. Topical irrigation with polymyxin and bacitracin for spinal surgery. Surg Neurol. 1998;50:208-212.
70 Mooney EK, Lippitt C, Friedman J. Plastic Surgery Educational Foundation DATA Committee. Silver Dressings. Plast Reconstr Surg. 2006;117:666-669.
71 Epstein NE. Technical note: do silver impregnated dressings limit infections following lumbar laminectomy with instrumented fusion? Surg Neurol. 2007;68:483-485.
72 Epstein NE. Circumferential surgery for the management of ossification of the posterior longitudinal ligament. J Spinal Disord. 1998;11:200-207.
73 Epstein NE. The value of anterior cervical plating in preventing vertebral fracture and graft extrusion following multilevel anterior cervical corpectomy with posterior wiring/fusion: indications, results, and complications. J Spinal Disord. 2000;13:9-15.
74 Epstein NE. Circumferential cervical surgery for ossification of the posterior longitudinal ligament: a multianalytic outcome study. Spine. 2004;29:1340-1345.
75 Epstein NE. Complication avoidance in 116 dynamic-plated single level anterior corpectomy and fusion. J Spinal Disord Tech. 2007;20:347-351.
76 Egli D, Hausmann O, Schmid M. Lumbar spinal stenosis: assessment of cauda equina involvement by electrophysiological recordings. J Neurol. 2007;254:741-750.
77 Krassioukov AV, Sarjeant R, Arkia H, et al. Multimodality intraoperative monitoring during complex lumbosacral procedures: indications, techniques, and long-term follow-up review of 61 consecutive cases. J Neurosurg Spine. 2004;1:243-253.
78 Epstein NE, Danto J, Nardi D. Evaluation of intraoperative somatosensory evoked potential (SSEP) monitoring during 100 cervical operations. Spine. 1993;18:737-747.
79 Epstein NE. Somatosensory evoked potential monitoring (SSEPs) in 173 cervical operations. Neuro-Orthopedics. 1996;20:2-21.
80 Resnick DK, Choudhri TF, Failey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine. 2005;2:725-732.
81 Balzer JR, Rose RD, Welch WC, et al. Simultaneous somatosensory evoked potential and electromyographic recordings during lumbosacral decompression and instrumentation. Neurosurgery. 1998;42:1318-1324.
82 Epstein NE. A review article on the risks and benefits of differing prophylaxis regimens for the treatment of deep venous thrombosis and pulmonary embolism in neurosurgery. Surg Neurol. 2005;64:295-301.
83 Epstein NE. Intermittent pneumatic compression stocking prophylaxis against deep venous thrombosis in anterior cervical surgery: a prospective efficacy study in 200 Patients and Literature Review. Spine. 2005;30:2538-2543. 15
84 Epstein NE. Efficacy of pneumatic compression stocking prophylaxis in the prevention of deep venous thrombosis and pulmonary embolism following 139 lumbar laminectomies with instrumented fusions. J Spinal Disord Tech. 2006;19:28-31.
85 Gerlach R, Raabe A, Beck J, et al. Postoperative nadroparin administration for prophylaxis of thrombosis events is not associated with an increased risk of hemorrhage after spinal surgery. Eur Spine J. 2004;13:9-13.
86 Wen DY, Hall WA. Complications of subcutaneous low-dose heparin therapy in neurosurgical patients. Surg Neurol. 1998;50:521-525.
87 Feree BA, Wright AM. Deep venous thrombosis following posterior lumbar spinal surgery. Spine. 1993;18:1079-1082.
88 Epstein NE, Peller A, Korsh, et al. Normovolemic hemodilution: an effective method for limiting postoperative transfusion following complex lumbar spinal decompression with instrumented fusion. Spinal Surg. 2004;18:179-188.
89 Epstein NE, Peller A, Korsh J, et al. Impact of intraoperative normovolemic hemodilution on transfusion requirements for 68 patients undergoing lumbar laminectomies with instrumented posterolateral fusion. Spine. 2006;31:2227-2230.
90 Epstein NE. Bloodless spinal surgery: a review of the normovolemic hemodilution technique. Surg Neurol. 2008;70:614-618.
91 Rosen DS, O’Toole JE, Eicholz KM, et al. Minimally invasive lumbar spinal decompression in the elderly: outcomes of 50 patients aged 75 years and older. Neurosurgery. 2007;60:503-509.
92 Epstein NE. How often is minimally invasive minimally effective: what are the complication rates for minimally invasive surgery? Surg Neurol. 2008;70:386-388.
93 Ivanov A, Faizan A, Sairyo K, et al. Minimally invasive decompression for lumbar spinal canal stenosis in younger age patients could lead to higher stresses in the remaining neural arch-a finite element investigation. Minim Invasive Neurosurg. 2007;50:18-22.
94 Zucherman JF, Hsu KY, Hartien CA, et al. A multicenter, prospective, randomized trial evaluating the X-Stop interspinous process decompression system for the treatment of neurogenic claudication: two-year follow-up results. Spine. 2005;30:1351-1358.
95 Hsu KY, Zucherman JF, Hartien CA, et al. Quality of life of lumbar stenosis-treated patients in whom the X-Stop interspinous device was implanted. J Neurosurg Spine. 2006;5:500-507.
96 Anderson PA, Tribus CB, Kitchel SH, et al. Treatment of neurogenic claudication by interspinous decompression: application of the X-Stop device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2006;4:463-471.
97 Siddiqui M, Smith FW, Wardlaw D. One-year results of X-Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine. 2007;32:1345-1348.
98 Brussee P, Hauth J, Donk RD, et al. Self-rated evaluation of outcome of the implantation of interspinous process distraction (X-Stop) for neurogenic claudication. Eur Spine J. 2008;17:200-203.
99 Verhoof O, Bron JL, Wapstra FH, et al. High failure rate of the interspinous distraction device (X-Stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesis. Eur Spine J. 2008;17:188-192.
100 Haufe SM, Mork AR. Effects of unilateral endoscopic facetectomy on spinal instability. J Spinal Disord Tech. 2007;20:146-148.
101 Kawaguchi Y, Ishihara H, Kanamori M, et al. Adjacent segment disease following expansive lumbar laminoplasty. Spine J. 2007;7:273-279.
102 Tsuji H, Itho T, Sekido H, et al. Expansive laminoplasty for lumbar spinal stenosis. Int Orthop. 1990;14:308-314.
103 Matsui H, Tsuji H, Sekido H, et al. Results of expansive laminoplasty for lumbar spinal stenosis in active manual workers. Spine. 1992;17(suppl 3):S37-S40.
104 Oertel MF, Ryang YM, Korinth MC, et al. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral lanimotomy for bilateral decompression. Neurosurgery. 2006;59:1269-1279.
105 Nakai O, Okawa A, Yamaura I. Long-term roentgenographic and functional changes in patients who were treated with wide fenestration for central lumbar stenosis. J Bone Joint Surg Am. 1991;73:1184-1191.
106 Caspar W, Papavero L, Sayler MK, et al. Precise and limited decompression for lumbar spinal stenosis. Acta Neurochir (Wien). 1994;131:130-136.
107 Martin CR, Gruszczynski AT, Braunsfurth HA, et al. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine. 2007;32:1791-1798.
108 Maheshwaran S, Davies AM, Evans N, et al. Sciatic in degenerative spondylolisthesis of the lumbar spine. Ann Rheum Dis. 1995;54:539-543.
109 Kaptan H, Kasimcan O, Cakiroglu K, et al. Lumbar spinal stenosis in elderly patients. Ann N Y Acad Sci. 2007;1100:173-178.
110 Alexander E, Kelly DL, Davis CH, et al. Intact arch spondylolisthesis: A review of 50 cases and description of surgical treatment. J Neurosurg. 1985;63:840-844.
111 Hall S, Bartelson JD, Onofrio BM, et al. Spinal stenosis: clinical features, diagnostic procedures, and results of surgical treatment in 68 patients. Ann Intern Med. 1985;103:271-275.
112 Mauersberger W, Nietgen T. Surgical treatment of lumbar stenosis: long-term results. Neurosurg Rev. 1989;12:291-295.
113 Shenkin HA, Nash CJ. Spondylolisthesis after multiple bilateral laminectomies and facetectomies for lumbar spondylosis: follow-up review. J Neurosurg. 1979;50:45-47.
114 Epstein NE. Surgical management of lumbar stenosis: Decompression and indications for fusion. Neurosurg Focus. 1997;3:1-14.
115 Johnsson KE, Willner S, Jonsson K. Postoperative instability after decompression for lumbar spinal stenosis. Spine. 1986;11:107-110.
116 Johnsson KE, Redlund-Johnell I, Uden A, et al. Preoperative and postoperative instability in lumbar spinal stenosis. Spine. 1989;14:591-593.
117 Silvers HR, Lewis PJ, Asch HL. Decompressive lumbar laminectomy for spinal stenosis. J Neurosurg. 1993;78:695-701.
118 Turner JA, Ersek M, Herron L, et al. Surgery for lumbar spinal stenosis: Attempted meta-analysis of the literature. Spine. 1992;17:1-8.
119 Prolo DJ, Oklund SA, Butcher M. Toward uniformity in evaluating the results of lumbar spine operations. A paradigm applied to posterior lumbar interbody fusions. Spine. 1986;11:601-606.
120 Deyo RA, Cherkin DC, Loeser JD, et al. Morbidity and mortality in association with operations on the lumbar spine: the influence of age, diagnosis, and procedure. J Bone Joint Surg Am. 1992;74:536-543.
121 Deyo RA, Ciol MA, Cherkin DC, et al. Lumbar spinal fusion: a cohort study of complications and resource use in the Medicare population. Spine. 1993;18:1463-1470.
122 Herron LD, Mangelsdorf C. Lumbar spinal stenosis: results of surgical treatment. J Spinal Disord. 1991;4:26-33.
123 Airaksinen O, Herno A, Turunen V, et al. Surgical outcome in 438 patients treated surgically for lumbar spinal stenosis. Spine. 1997;22:2278-2282.
124 Tuite GF, Stern JD, Doran SE, et al. Outcome after laminectomy for spinal stenosis. Part I: clinical correlations. J Neurosurg. 1995;82:912-918.
125 Epstein NE. Primary fusion for the management of “unstable” degenerative spondylolisthesis. Neuro-Orthopedics. 1998;23:45-52.
126 Katz JN, Lipson SJ, Larson MG, et al. The outcome of decompressive laminectomy for degenerative lumbar stenosis. J Bone Joint Surg Am. 1991;73:809-816.
127 Katz JN, Lipson SJ, Lew RA, et al. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis. Patient selection, costs, and surgical outcomes. Spine. 1997;22:1123-1131.
128 McCulloch JA. Microdecompression and uninstrumented single-level fusion for spinal canal stenosis with degenerative spondylolisthesis. Spine. 1998;23:2243-2252.
129 Lombardi JS, Wilste LL, Reynolds J, et al. Treatment of degenerative spondylolisthesis. Spine. 1985;10:821-827.
130 Epstein NE. Fusion rates and SF-36 outcomes after multilevel laminectomy and non-instrumented lumbar fusion in a predominantly geriatric population. J Spinal Disord Tech. 2008;21:159-164.
131 Epstein NE. Non-instrumented posterolateral lumbar fusions utilizing combined lamina autograft and beta tricalcium phosphate in a predominantly geriatric population: an outcome assessment. Spinal Surg. 2006;20:219-231.
132 Epstein NE. Efficacy of different bone volume expanders for augmenting lumbar fusions. Surg Neurol. 2008;69:16-19.
133 Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802-808.
134 Pierro CG, Helm GA, Shaffrey CI, et al. Treatment of lumbar stenosis by extensive unilateral decompression and contralateral autologous bone fusion: operative technique and results. J Neurosurg. 1996;84:166-173.
135 Epstein NE. An artificial bone substitute (beta tricalcium phosphate) utilized as a bone void filler or supplement in lumbar fusions. Winthrop-University Hosp Med J. 2006;28:469-472.
136 Epstein NE. A preliminary study of the efficacy of beta tricalcium phosphate as a bone expander for instrumented posterolateral lumbar fusions. J Spinal Disord Tech. 2006;19:424-429.
137 Markwalder TM, Saager C, Reulen HJ. Isthmic spondylolisthesis—an analysis of the clinical and radiological presentation in relation to intraoperative findings and surgical results in 72 consecutive cases. Acta Neurochir. 1991;110:154-159.
138 Boos N, Marchesi D, Aebi M. Treatment of spondylolysis and spondylolisthesis with Cotrel-Dubousset instrumentation: a preliminary report. J Spinal Disord. 1991;4:472-479.
139 Herno A, Airaksinen O, Saari T, et al. Surgical results of lumbar spinal stenosis. A comparison of patients with or without previous back surgery. Spine. 1995;20:964-969.
140 Herno A, Airaksinen O, Saari T, et al. The effect of prior back surgery on surgical outcome in patients operated on for lumbar spinal stenosis. A matched-pair study. Acta Neurochir (Wien). 1996;138:357-363.
141 Wilste LL. Salvage of failed lumbar spinal stenosis surgery. In: Hopp E, editor. Spine: State of the Art Reviews. Philadelphia: Hanley and Belfus; 1987:421-450.
142 Caputy AJ, Luessenhop AJ. Long-term evaluation of decompressive surgery for degenerative lumbar stenosis. J Neurosurg. 1992;77:669-676.
143 Herno A, Airaksinen O, Saari T. Long-term results of surgical treatment of lumbar spinal stenosis. Spine. 1993;18:1471-1474.
144 McCullen GM, Bernini PM, Bernstein SH, et al. Clinical and roentgenographic results of decompression for lumbar spinal stenosis. J Spinal Disord. 1994;7:380-387.
145 Brodsky AE. Post-laminectomy and post-fusion stenosis of the lumbar sine. Clin Orthop Rel Res. 1976;115:130-139.
146 Zigler JE, Boden S, Anderson PA, et al. Specialty update: what’s new in spine surgery. J Bone Joint Surg Am. 2002;84:1282-1288.
147 Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg Spine. 1999;90:163-169.
148 White AA, Panjabi MM. Biomechanical considerations in the surgical measurement of the spine. In: White AA, Panjabi MM, editors. Clinical Biomechanics of the Spine. 2nd ed. Philadelphia: JB Lippincott; 1990:511-631.
149 Wilste LL, Kirkaldy-Willis WH, McIvor GW. The treatment of spinal stenosis. Clin Orthop. 1976;115:83-91.
150 Lee CK. Lumbar spinal instability (olisthesis) after extensive posterior spinal decompression. Spine. 1983;8:429-433.
151 Whitecloud TS3d, Butler JC, Cohen JL, et al. Complications with the variable spinal plating system. Spine. 1989;14:472-476.
152 Weinstein JN, Spratt KF, Spendler D, et al. Spinal pedicle fixation: reliability and validity of roentgenogram-based assessment and surgical factors on successful screw placement. Spine. 1988;13:1012-1018.
153 McAfee PC, Weiland DJ, Carlow JJ. Survivorship analysis of pedicle spinal instrumentation. Spine. 1991;16(suppl 8):S422-S427.
154 Matsuzaki H, Tokuhashi Y, Matsumoto F, et al. Problems and solutions of pedicle screw plate fixation of the lumbar spine. Spine. 1990;15:1159-1165.
155 Mulline BB, Rea GL, Irsik R, et al. The effect of postlaminectomy spinal instability on the outcome of lumbar spinal stenosis patients. J Spinal Disord. 1996;9:107-116.
156 Nork SE, Hu SS, Workman KL, et al. Patient outcomes after decompression and instrumented posterior spinal fusion for degenerative spondylolisthesis. Spine. 1999;24:561-569.
157 Mannion AF, Denzier R, Dvorak J, et al. A randomized controlled trial of post-operative rehabilitation after surgical decompression of the lumbar spine. Eur Spine J. 2007;16:1101-1117.