Chapter 164 Lumbar Spinal Arthroplasty
Clinical Experiences of Motion Preservation
Lumbar spinal arthroplasty was first reported in clinical settings in 1994 by Griffith and colleagues.1 This early experience was acquired with the first lumbar artificial disc, the Charité I, in patients with degenerative disc disease. Since that time, a randomized, controlled trial comparing arthroplasty with the Charité Artificial Disc versus anterior lumbar interbody fusion with the BAK Cage and iliac crest bone was completed. Multiple other lumbar arthroplasty devices have been developed subsequent to the Charité and are undergoing or completing clinical trials.
Unlike other spinal medical devices, lumbar discs are required by the Food and Drug Administration (FDA) to complete randomized, controlled trials prior to market approval in the United States. As a result, lumbar arthroplasty devices have undergone more scrutiny and clinical evaluation than any other spinal medical devices. Specifically, a new device, the ProDisc-L, was granted FDA approval in 2006 and was described in a peer-review publication.2 In addition, the Maverick Total Disc Arthroplasty System (Medtronic Sofamor Danek), Kineflex Lumbar Disc (SpinalMotion), and FlexiCore Intervertebral Disc (SpineCore/Stryker) lumbar discs have both completed their randomized enrollments and are currently in continued access (nonrandomized) mode. All these ongoing and completed randomized clinical trials have generated a large body of evidence on the safety and efficacy of arthroplasty for lumbar spine in clinical applications and, in many cases, in level-1 publications.
Results
General Clinical Outcome Results
The Charité Artificial Disc manuscripts described clinical outcomes as early as 1 year3 and up to 13 years after surgery.4 The short-term and medium-term papers included herein typically disclosed early analyses from single sites involved in the Charité Artificial Disc IDE study comparing arthroplasty with Charité Artificial Disc versus anterior interbody fusion with BAK Cage and autograft.3,5,6 The complete randomized, controlled trial at 2-year follow-up included 205 arthroplasty and 99 fusion patients and was thoroughly described in two manuscripts, one focused on clinical outcomes7 the other on radiographic outcomes.8 Three additional medium- and long-term studies were also found: two papers with 10-year follow-up4,9 and one with an average of 6.6-year follow-up.10
Safety and efficacy of arthroplasty was demonstrated in all short- and medium-term studies. Specifically, at 2 years after surgery, Blumenthal and McAfee reported no device-related complications and a reoperation rate of 5.4% (vs 9.1% in the control arm). Efficacy was also demonstrated using validated disability (Oswestry Disability Index [ODI]) and pain (Visual Analogue Score [VAS]) clinical outcomes tools. At 2 years, the reduction in ODI reached 48.5% (vs. 42.4% in the control group) and the absolute reduction in VAS reached 40.6 points (vs. 34.1 points in the control group).7,8
Two of the three long-term studies confirmed these findings. Lemaire and colleagues reported 10-year follow-up results in 100 patients.4 This study included 54 patients operated on at one level, 45 patients operated on at two levels, and one patient operated on at three levels. Overall, the authors reported excellent or good clinical outcomes in 90% of cases. In a second long-term study, David and colleagues presented 10-year data on 106 patients.9 Only one-level surgeries were performed in this study. Excellent or good clinical outcome was obtained in 82.1% patients. Both papers thus concluded that arthroplasty was a viable option for disc degeneration.
A medium-term paper was published by Ross and colleagues, describing the long-term effect of arthroplasty in 160 patients (226 Charité Artificial Discs). This paper reported a cumulative survival rate at 156 months of 35% and a mean ODI score improvement of 14%. Implant removal was also described in 12 patients.10 These relatively poor findings were further discussed in two Letters to the Editor, which pointed out mathematical inconsistencies and overall clinical flaws in the manuscript and further highlighted the need for proper surgical technique and patient selection for optimal clinical outcome.11,12
The ProDisc-L manuscripts described clinical outcomes as early as 3 months13 and up to 8.7 years after surgery.14 As described earlier for the Charité Artificial Disc, The short-term papers typically disclosed early findings from one or two of the sites that participated in the randomized, controlled trial comparing ProDisc-L against a 360-degree fusion.13,15–21 The complete randomized, controlled trial at 2-year follow-up included 161 arthroplasty and 75 fusion patients.2 The long-term data included 64 patients operated on at one site, of whom 55 were available between 7 and 11 years after surgery for clinical and radiographic follow-up.14 All these studies concluded similarly that disc arthroplasty, at all evaluated time points, was safe and resulted in complication and/or reoperation rates comparable to those generally accepted for spinal surgery (complication rate of 9% at 8.7 years14; there were no major complications, but a reoperation rate of 3.7% at 2 years was reported2). In addition to safety, efficacy of spinal arthroplasty was also shown because all cases presented significant improvements in pain and disability. The final randomized, controlled trial data reported improvements in the arthroplasty group in VAS for pain by an average of 39 points and in disability, as determined by the ODI, by 28 points. It is worth noting, however, that the ODI tool used in this trial was not the validated and widely accepted ODI methodology Version 1.0 as defined by Fairbanks and colleagues.22 In a Letter to the Editor, Fairbanks denounced the use of the Oswestry Disability Index in the ProDisc-L study and thus cast doubt on the validity of the disability improvement outcomes observed herein.23
The two clinical data publications on the Maverick device were both based on the same data set of 64 patients, collected at one site.24,25 The clinical outcomes were described using the ODI Version 1.0 and VAS scores. The efficacy of arthroplasty was once again demonstrated using these tools, as ODI scores decreased by an average of 20.7 points and VAS scores by 4.4 points. As for the FlexiCore Intervertebral Disc, only one paper has been published (2008).26 This manuscript describes the clinical outcomes of 44 patients, of whom only 6 were available for 2-year follow-up. Although the clinical relevance of these data may therefore be questionable, the authors still concluded that the device may be safe and efficacious but that the data were not representative of the entire patient cohort.
Radiographic Analyses: Range of Motion, Heterotopic Ossification, and Sagittal Balance Analyses
Unlike other clinical and radiographic outcomes, accurate measurement of ROM was shown to be challenging and, to some extent, subjective, as patient positioning, imaging staff training, and other factors unrelated to the actual motion potential of the spine were shown to affect final readings.27 Using ProDisc-L cases, Lim and colleagues evaluated different methodologies and associated error margins for the measurement of ROM from radiographic images. Specifically, Lim and colleagues concluded that a ROM of at least 4.6 degrees must be observed to be 95% certain that a given device had any sagittal motion at all. Similarly, changes needed to be at least 9.6 degrees in ROM to confirm at 95% that change in motion really happened.28,29 These technical limitations might explain the inconsistent ROM data, particularly for ProDisc-L cases, found in the published literature. The flexion-extension ROM results from the 2-year randomized, controlled trial were determined at 7.7 degrees and 4.67 degrees and characterized as a normal ROM.2 However, at 8.7 years after surgery, Huang and colleagues reported a ROM less than that reported in asymptomatic normal person, with an average motion of 3.8 degrees.30 In a 2006 prospective study on 41 patients with 2-year follow-up, Leivseth and colleagues also reported that the device fails to restore normal segmental motion, and another retrospective study on 26 patients concluded that sagittal balance and ROM significantly improved after lumbar arthroplasty.31
Less controversy was observed when reviewing ROM data for the Charité Artificial Disc. A complete manuscript was dedicated to the radiographic data of the 2-year randomized, controlled trial of the Charité Artificial Disc.8 In this study, arthroplasty patients had a 13.6% increase in motion from preoperative to the 2-year postoperative time point. The ROM also correlated to device placement, as poor device placement resulted in a statistically significant reduction in motion. At 10-year follow-up, David reported an average 10.1-degree ROM, a value very similar to the 10.3 degrees reported by Lemaire and colleagues in their 10-year follow-up study.4,9
Le Huec and colleagues published the only data available on radiographic findings after arthroplasty with the Maverick Total Disc Arthroplasty System. In their study, Le Huec and colleagues broadened their analysis to include sagittal alignment and pelvic tilt.24,32 Using data related to 35 patients at an average of 14 months after surgery, authors showed maintenance of overall lordosis and unchanged sacral and pelvic tilts following arthroplasty.
More recently, a study by Tournier comparing all three—Charité Artificial Disc, ProDisc-L, and Maverick Total Disc Arthroplasty System—further refined the analyses from Le Huec on pelvic and sagittal tilt. In this study, authors found no difference in ROM among prostheses and observed maintenance of sagittal balance before and after surgery with all devices. However, modifications of the lumbar curvature were observed.33
The issue of heterotopic ossification in clinical cases of lumbar arthroplasty has been presented by McAfee and colleagues and, more recently, by Tortolani and colleagues.34,35 In his 2003 paper, McAfee introduced a novel method to characterize spinal heterotopic ossification. This method was applied by Tortolani and colleagues in reviewing the 276 arthroplasty patients from the Charité Artificial Disc trial (randomized and nonrandomized cases). From this analysis, 4.3% of cases of heterotopic ossification were noted. However, heterotopic ossification was not related to ROM, as the authors concluded that no difference in the ROM at 24 months after surgery was found between the patients who had and those who did not have heterotopic ossification.
Facets and Adjacent-Level Degeneration
Three long-term analyses evaluated adjacent-level degeneration, one with ProDisc-L at 8.7 years, and the other two with the Charité Artificial Disc at 10 years of follow-up. In the ProDisc-L study, 24% of patients developed adjacent level degeneration by the latest follow-up time point. A correlation was also found between a low ROM and the prevalence of adjacent level degeneration: All patients with adjacent-level degeneration had a ROM less than 5 degrees, whereas only 59% of patients without adjacent-level degeneration had a ROM less than 5 degrees.36 Lemaire and colleagues and David reported 2 (2%) and 3 (2.8%) cases of adjacent-level degeneration at the latest time point, respectively.4,9 Lemaire and colleagues and David also disclosed 11 cases (11%) and 5 cases (4.7%) with facet arthrosis at the latest time point, respectively.
The issue of facet degeneration was also recently discussed in a short-term study. From a 13-patient case series with 12 months of follow-up, Trouillier and colleagues alluded to possible maintenance of facet joint integrity following arthroplasty with Charité Artificial Disc based on the favorable results from their series.37 At the other end of the spectrum, Shim and colleagues, at the 3-year time point, observed 36.4% and 32.0% increase in index-level facet degeneration and 19.4% and 28.6% adjacent level disc degeneration with the Charité Artificial Disc and the ProDisc-L, respectively.
Revision and Revision Strategies
The first description of appropriate revision for a Charité Artificial Disc was presented by David.38 In this single-case example, a Charité Artificial Disc was replaced at 9.5 years after surgery with another Charité Artificial Disc. The author concluded that revision of the disc with another disc could be safely and adequately performed and was thus an alternative to a revision fusion procedure. David also noted that because of the inherent difficulty of an anterior approach, only experienced surgeons should undertake this operation.
Further revision and explantation of the disc were also described by McAfee and colleagues and Leary and colleagues.39,40 McAfee and colleagues confirmed David’s experience and concluded that arthroplasty with the Charité Artificial Disc did not preclude any further procedures at the index level during primary insertion, with nearly one third being revisable to a new motion-preserving prosthesis and just over two thirds being successfully converted to anterior lumbar interbody fusion (ALIF) and/or posterior pedicle screw arthrodesis, the original alternative procedure. Leary further implied that technical errors in position and sizing of the implant were largely to blame for further revision surgery. Finally, Punt and colleagues reviewed 75 revision cases from the Dutch experience (estimated by the authors at more than 1000 Dutch patients). In this series, patients received posterior fusion either with removal of the disc or without removal of the disc. No statistically significant difference was observed between these two groups.41 This paper included patients previously described by Van Ooij and colleagues.42
Surgical Technique
The appropriate surgical technique with the Charité Artificial Disc as well as the ProDisc-L was presented in two separate publications. Geisler and colleagues provided a detailed account of the surgical technique for the Charité Artificial Disc and dedicated an entire section of the paper to patient selection and preoperative planning, two critical aspects of successful spinal arthroplasty.43 The authors also strongly recommended the availability of a spinal access surgeon to perform the approach, especially in revision cases. Finally, appropriate midline identification and positioning of the device also represented a critical discussion point in this paper. For the ProDisc-L, Gumbs and colleagues retrospectively reviewed 64 cases of open retroperitoneal exposures and concluded that the approach was safe and, as discussed by Geisler and colleagues, required a multidisciplinary team, such as an orthopedic and an access or general surgeon, to minimize complication rates.44
Complications
Complications from spinal arthroplasty have also been reported for all three devices. Most complications requiring revision surgery were resolved by either fusion or disc replacement surgery. Interestingly, the causes of these complications seemed to be device-specific (i.e., due to the design and/or make of the device).
For the ProDisc-L, the major complication described in the literature referred to the vertical split fracture of the vertebral body following total disc replacement. This occurrence was described by Shim and colleagues in two separate cases that were not revised or treated surgically, but the patients experienced prolonged back pain as a result.45 An additional complication in the form of acquired spondylolysis was described by Schulte and colleagues.46 Authors attributed this complication to inaccurate implant size and positioning.
For the Charité Artificial Disc, the key complications were observed on the earlier devices, which were gamma sterilized in air and thus had a potential for oxidation of the core polyethylene nucleus.38 Complications due to this oxidation process were described by Van Ooij and colleagues (and Punt and colleagues, as this paper reiterates data from the Van Ooij patient population).41,42 This issue was resolved with a process change in 1998 to gamma sterilization in nitrogen. In a review of the randomized, controlled trial patient population, Geisler and colleagues also evaluated the rate of neurologic complications in the arthroplasty group versus the fusion group. The rate of neurologic complications was described as exceedingly low in both groups, with no statistically significant differences between groups.
Little has been published so far on the Maverick Total Disc Arthroplasty System, but one article described an early removal of the Maverick Total Disc Arthroplasty System.47 This removal was performed 1 year after implantation owing to severe persistent back pain. Intraoperatively, gross metallosis around the articulation of the device was observed. The revision was successful and included a 360-degrees fusion. Metallosis was thus cited as a potential complication for devices consisting of a metal-on-metal design.
Zeh and colleagues presented an additional potential complication: Because of this metal-on-metal structure of the Maverick Total Disc Arthroplasty System, cobalt and chromium ions from the device were being released into the bloodstream.48 In this study, cobalt and chromium ions from subjects implanted with the Maverick Total Disc Arthroplasty System were evaluated and compared to ion levels in total hip arthroplasty (THA) patients. Zeh and colleagues found that concentrations of chromium and cobalt measured in the serum were similar in terms of their level to the values measured in THA metal-on-metal combinations or exceed those values reported in the literature. As a result, although Zeh and colleagues did not recommend holding back with the implantation of the device, they did suggest long-term clinical evaluations to determine the clinical impact of high ion levels in serum and also recommended discussions with patients on the potential health effects of the prosthesis.
Special Patient Population Analyses
The clinical outcomes for selected patient populations (e.g., smokers, persons older than 60 years) were discussed in several papers, more specifically for the ProDisc-L device. Bertagnoli and colleagues led these efforts with four publications presenting clinical data on arthroplasty with ProDisc-L in patients with single-level arthroplasty,15 patients with multilevel arthroplasty,16 patients 60 years or older,49 smokers,50 and patients with arthroplasty adjacent to a fused level.51 Although Bertagnoli and colleagues repeatedly stated the importance of proper patient selection in every paper, all the results presented in these studies concluded that spinal arthroplasty with ProDisc-L successfully addressed low-back pain in these specific patient populations. Hannibal and colleagues compared one- versus two-level arthroplasty cases at the 2-year follow-up to try to establish whether one-level cases were experiencing greater clinical improvements as compared to the two-level cases. This hypothesis was not verified because differences in clinical improvements between one- and two-level cases were marginal.52 Yaszay and colleagues approached the problem from a different angle and evaluated patients’ outcomes based on a radiographic observation: preoperative disc height.53 Yaszay and colleagues observed that patients with greater disc collapse experienced a greater benefit from total disc replacement, as compared to patients with less-collapsed intervertebral discs. On average, patients in all of Bertagnoli and colleagues and Yaszay and colleagues series showed significant clinical improvement following arthroplasty.
Using the Charité Artificial Disc IDE randomized, controlled trial patient population, subanalyses by patient type were also published by Guyer and colleagues and Geisler and colleagues. Specifically, patients were stratified by age at surgery (18 to 45 years vs. 46 to 60 years) or whether they had had prior surgery or not.54,55 In both cases, there was no difference in clinical outcome between groups, whether patients were 18 to 45 years old or 46 to 60 years old, or whether patients did or did not have prior surgery. Along the same trend, Geisler and colleagues also evaluated the clinical outcomes of patients from the Charité Artificial Disc randomized, controlled trial who did not improve with arthroplasty and needed revision surgery to a fusion. These patients (7.1% of all arthroplasty cases) did not improve, despite the revision surgery, further highlighting the importance of proper patient selection and, possibly, the fact that patient selection still remains a somewhat approximate science.56
Health Economics Evaluations
The impact of spinal arthroplasty on health care economics was reviewed for both the Charité Artificial Disc and the ProDisc-L. Guyer and colleagues analyzed the costs related to a Charité Artificial Disc arthroplasty compared to an anterior fusion with autograft, anterior fusion with rhBMP-2 (Infuse Bone Graft and LT-Cages), and instrumented posterior lumbar interbody fusion. This analysis included the cost of revision surgery at the rate estimated in the published literature. Guyer and colleagues concluded that all fusion procedures were more costly than the arthroscopy approach by 12.0% (ALIF with autograft) to 36.5% (ALIF with recombinant human bone morphogenetic protein-2 [rhBMP-2] and posterior fusion).57
A similar analysis by Levin and colleagues evaluated the costs of one- and two-level arthroplasty versus 360-degrees fusion. This study did not include possible needs for revisions. Nevertheless, one-level arthroplasty cases were found to be less costly than one-level fusions ($35,592 for arthroplasty vs. $46,280 for 360-degrees fusion), and costs for two-level cases were similar for both groups ($55,524 for arthroplasty vs. $56,823 for fusion).58
Discussion
Surgeon-specific variability in technique and proficiency was also cited in cases of revision. In fact, a study by Regan and colleagues evaluating the occurrence of revision in low-volume versus high-volume centers confirmed that surgeons in low-volume centers can incur greater perioperative complications, although none that affected long-term outcomes.59 Nevertheless, revisions were often found to be associated with technical errors, such as errors in positioning or sizing of the implant.
Total Disc Replacement Surgery
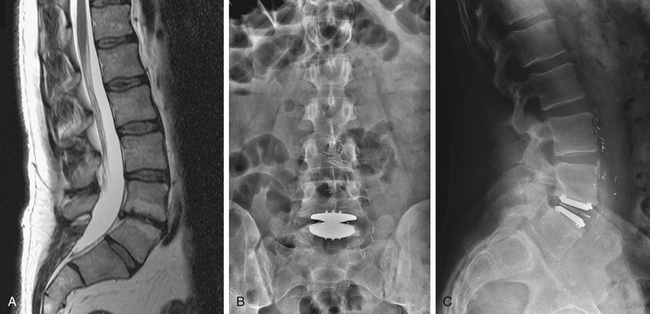
The typical diseased lumbar segment considered for artificial disc technologies at L4–5 or L5–S1 has advanced degenerative disc disease with loss of vertical height and lordosis, dehydration changes, adjacent Modic endplate changes, and little motion on dynamic studies (Fig. 164-1). The natural progression of degenerative disease disc limits the joint mobility, and this biomechanical fact places more forces on the adjacent levels than in the normal situation. The artificial lumbar disc, by restoring normal motion, height, and lordosis, decreases the forces on the adjacent levels. Thus, theoretically, levels adjacent to a dynamically stabilized level might receive beneficial effects compared to the natural history of the unoperated-on degenerative state. Clearly some patients will benefit from the decreased force on the adjacent vertebral level(s) following a dynamic stabilization (arthroplasty) compared to a static stabilization (fusion). Estimates of the rate and groups of patients at maximum benefit need to await long-term clinical follow up studies with lumbar arthroplasty devices similar to the hip and knee arthroplasty registries. Also, although a dynamically stabilized level can be converted to a fusion, a fused level cannot be converted to a dynamic joint. Thus, artificial technology can be thought of as a definitive procedure for the vast majority of patients. It can potentially be converted to a fusion if the pain and functional goals are not met or if degenerative changes occur in the posterior elements and the arthrodesis level is believed to be the pain generator.
Long-Term Follow-Up of Tdr Patients
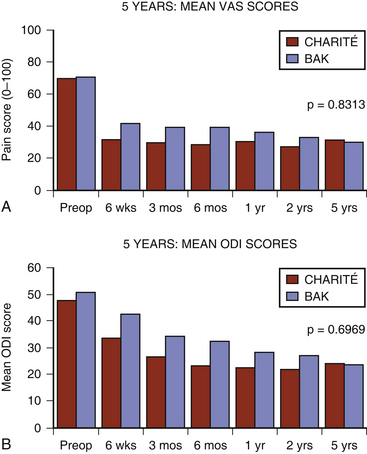
The initial Charité device was planned with a 2-year follow-up period. At the request of the FDA the follow-up period was extended to 5 years and the sites requested to participate in the “new” 2- to 5-year follow-up period. Multiple sites did participate in this extended reporting period and formed the basis of the 5-year Charité results. The Charité 5-year ODI and VAS results (Fig. 164-2) were substantially the same as the 2-years follow-up results.60 Despite the prospective collection of the these results, critics formulated objections to these reported good results.61
Conclusion
Overall, there were only minor differences among devices in terms of overall clinical and radiographic outcome. Significant improvements in clinical outcomes were seen with all evaluated devices, regardless of make or design. An average maintenance of motion after surgery was described, along with relatively low rates of revision. Differences between devices were mostly apparent in complication types: one potential complication for devices with keels was vertebral body split, and devices with metal-on-metal designs could cause metallosis and ion release in the serum. As for metal-on-poly devices, degradation of the polymer core was also mentioned as a potential complication, albeit one that is not relevant for the current metal-on-poly devices. Specific emphasis was found in most publications on proper technique and patient selection, regardless of implant design. Finally, arthroplasty was found to be less costly than fusion.
Bertagnoli R., Yue J.J., Fenk-Mayer A., et al. Treatment of symptomatic adjacent-segment degeneration after lumbar fusion with total disc arthroplasty by using the prodisc prosthesis: a prospective study with 2-year minimum follow up. J Neurosurg Spine. 2006;4(2):91-97.
Bertagnoli R., Yue J.J., Nanieva R., et al. Lumbar total disc arthroplasty in patients older than 60 years of age: a prospective study of the ProDisc prosthesis with 2-year minimum follow-up period. J Neurosurg Spine. 2006;4(2):85-90.
Bertagnoli R., Yue J.J., Shah R.V., et al. The treatment of disabling single-level lumbar discogenic low back pain with total disc arthroplasty utilizing the Prodisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30(19):2230-2236.
Blumenthal S.L., McAfee P.C., Guyer R.D., et al. A prospective, randomized, multicenter food and drug administration investigational device exemptions study of lumbar total disc replacement with the Charité Artificial Disc versus lumbar fusion. Part I: evaluation of clinical outcomes. Spine. 2005;30(14):1565-1575.
Cunningham B.W., McAfee P.C., Geisler F.H., et al. Distribution of in vivo and in vitro range of motion following 1-level arthroplasty with the Charité Artificial Disc compared with fusion. J Neurosurg Spine. 2008;8(1):7-12.
David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the Charité Artificial Disc in 106 patients. Spine. 2007;32(6):661-666.
David T. Revision of a Charité Artificial Disc 9.5 years in vivo to a new Charité Artificial Disc: case report and explant analysis. Eur Spine J. 2005;14(5):507-511.
Geisler F.H. Surgical technique of lumbar Artificial Disc replacement with the Charité Artificial Disc. Neurosurgery. 2005;56:ONS46-ONS57.
Geisler F.H., Blumenthal S.L., Guyer R.D., et al. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: results of a multicenter, prospective, randomized investigational device exemption study of Charité intervertebral disc. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(2):143-154.
Geisler F.H., Guyer R.D., Blumenthal S.L., et al. Effect of previous surgery on clinical outcome following 1-level lumbar arthroplasty. J Neurosurg Spine. 2008;8(2):108-114.
Geisler F.H., Guyer R.D., Blumenthal S.L., et al. Patient selection for lumbar arthroplasty and arthrodesis: the effect of revision surgery in a controlled, multicenter, randomized study. J Neurosurg Spine. 2008;8(1):13-16.
1. Griffith S.L., Shelokov A.P., Buttner-Janz K., et al. A multicenter retrospective study of the clinical results of the LINK SB Charité intervertebral prosthesis. The initial European experience. Spine. 1994;19(16):1842-1849.
2. Zigler J., Delamarter R., Spivak J.M., et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32(11):1155-1162. discussion 1163
3. McAfee P.C., Fedder I.L., Saiedy S., et al. SB Charité disc replacement: report of 60 prospective randomized cases in a US center. J Spinal Disord Tech. 2003;16(4):424-433.
4. Lemaire J.P., Carrier H., Sari Ali E., et al. Clinical and radiological outcomes with the Charité Artificial Disc: a 10-year minimum follow-up. J Spinal Disord. 2005;18(4):353-359.
5. Guyer R.D., McAfee P.C., Hochschuler S.H., et al. Prospective randomized study of the Charité Artificial Disc: data from two investigational centers. Spine J. 2004;4(suppl 6):252S-259S.
6. Geisler F.H., Blumenthal S.L., Guyer R.D., et al. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: results of a multicenter, prospective, randomized investigational device exemption study of Charité intervertebral disc. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(2):143-154.
7. Blumenthal S.L., McAfee P.C., Guyer R.D., et al. A prospective, randomized, multicenter food and drug administration investigational device exemptions study of lumbar total disc replacement with the Charité Artificial Disc versus lumbar fusion. Part I: evaluation of clinical outcomes. Spine. 2005;30(14):1565-1575.
8. McAfee P.C., Cunningham B., Holsapple G.A., et al. A Prospective, randomized, multicenter food and drug administration investigational device exemptions study of lumbar total disc replacement with the Charité Artificial Disc versus lumbar fusion. Part II: evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine. 2005;30(14):1576-1583.
9. David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the Charité Artificial Disc in 106 patients. Spine. 2007;32(6):661-666.
10. Ross R., Mirza A.H., Norris H.E., Khatri M. Survival and clinical outcome of SB Charité III disc replacement for back pain. J Bone Joint Surg Br. 2007;89(6):785-789.
11. Guyer R.D., Blumenthal S.L. Survival and clinical outcome of SB Charité III disc replacement for back pain. J Bone Joint Surg Br. 2007;89(12):1673. author reply 1673-1674
12. Scott-Young M.N. Survival and clinical outcome of SB Charité III disc replacement for back pain. J Bone Joint Surg Br. 2007;89(12):1674. author reply 1674-1675
13. Bertagnoli R., Kumar S. Indications for full prosthetic disc arthroplasty: a correlation of clinical outcome against a variety of indications. Eur Spine J. 2002;11(suppl 2):S131-S136.
14. Tropiano P., Huang R.C., Girardi F.P., et al. Lumbar total disc replacement. Seven to eleven-year follow-up. J Bone Joint Surg Am. 2005;87(3):490-496.
15. Bertagnoli R., Yue J.J., Shah R.V., et al. The treatment of disabling single-level lumbar discogenic low back pain with total disc arthroplasty utilizing the Prodisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30(19):2230-2236.
16. Bertagnoli R., Yue J.J., Shah R.V., et al. The treatment of disabling multilevel lumbar discogenic low back pain with total disc arthroplasty utilizing the ProDisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30(19):2192-2199.
17. Chung S.S., Lee C.S., Kang C.S. Lumbar total disc replacement using ProDisc II: a prospective study with a 2-year minimum follow-up. J Spinal Disord Tech. 2006;19(6):411-415.
18. Delamarter R.B., Fribourg D.M., Kanim L.E., Bae H. ProDisc artificial total lumbar disc replacement: introduction and early results from the United States clinical trial. Spine. 2003;28(20):S167-S175.
19. Tropiano P., Huang R.C., Girardi F.P., Marnay T. Lumbar disc replacement: preliminary results with ProDisc II after a minimum follow-up period of 1 year. J Spinal Disord Tech. 2003;16(4):362-368.
20. Zigler J.E., Burd T.A., Vialle E.N., et al. Lumbar spine arthroplasty: early results using the ProDisc II: a prospective randomized trial of arthroplasty versus fusion. J Spinal Disord Tech. 2003;16(4):352-361.
21. Zigler J.E. Lumbar spine arthroplasty using the ProDisc II. Spine J. 2004;4(suppl 6):260S-267S.
22. Fairbank J.C., Pynsent P.B. The Oswestry Disability Index. Spine. 2000;25(22):2940-2952. discussion 2952
23. Fairbank J.C. Use and abuse of Oswestry Disability Index. Spine. 2007;32(25):2787-2789.
24. Le Huec J.C., Mathews H., Basso Y., et al. Clinical results of Maverick lumbar total disc replacement: two-year prospective follow-up. Orthop Clin North Am. 2005;36(3):315-322.
25. Le Huec J.C., Basso Y., Aunoble S., et al. Influence of facet and posterior muscle degeneration on clinical results of lumbar total disc replacement: two-year follow-up. J Spinal Disord Tech. 2005;18(3):219-223.
26. Sasso R.C., Foulk D.M., Hahn M. Prospective, randomized trial of metal-on-metal artificial lumbar disc replacement: initial results for treatment of discogenic pain. Spine. 2008;33(2):123-131.
27. Cunningham B.W., McAfee P.C., Geisler F.H., et al. Distribution of in vivo and in vitro range of motion following 1-level arthroplasty with the Charité Artificial Disc compared with fusion. J Neurosurg Spine. 2008;8(1):7-12.
28. Lim M.R., Girardi F.P., Zhang K., et al. Measurement of total disc replacement radiographic range of motion: a comparison of two techniques. J Spinal Disord Tech. 2005;18(3):252-256.
29. Lim M.R., Loder R.T., Huang R.C., et al. Measurement error of lumbar total disc replacement range of motion. Spine. 2006;31(10):E291-E297.
30. Huang R.C., Girardi F.P., Cammisa F.P.Jr., et al. Long-term flexion-extension range of motion of the prodisc total disc replacement. J Spinal Disord Tech. 2003;16(5):435-440.
31. Chung S.S., Lee C.S., Kang C.S., Kim S.H. The effect of lumbar total disc replacement on the spinopelvic alignment and range of motion of the lumbar spine. J Spinal Disord Tech. 2006;19(5):307-311.
32. Le Huec J., Basso Y., Mathews H., et al. The effect of single-level, total disc arthroplasty on sagittal balance parameters: a prospective study. Eur Spine J. 2005;14(5):480-486.
33. Tournier C., Aunoble S., Le Huec J.C., et al. Total disc arthroplasty: consequences for sagittal balance and lumbar spine movement. Eur Spine. 2007;16(3):411-421.
34. McAfee P.C., Cunningham B.W., Devine J., et al. Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech. 2003;16(4):384-389.
35. Tortolani P.J., Cunningham B.W., Eng M., et al. Prevalence of heterotopic ossification following total disc replacement. A prospective, randomized study of two hundred and seventy-six patients. J Bone Joint Surg Am. 2007;89(1):82-88.
36. Huang R.C., Tropiano P., Marnay T., et al. Range of motion and adjacent level degeneration after lumbar total disc replacement. Spine J. 2006;6(3):242-247.
37. Trouillier H., Kern P., Refior H.J., Muller-Gerbl M. A prospective morphological study of facet joint integrity following intervertebral disc replacement with the Charité Artificial Disc. Eur Spine J. 2006;15(2):174-182.
38. David T. Revision of a Charité Artificial Disc 9.5 years in vivo to a new Charité Artificial Disc: case report and explant analysis. Eur Spine J. 2005;14(5):507-511.
39. Leary S.P., Regan J.J., Lanman T.H., Wagner W.H. Revision and explantation strategies involving the Charité lumbar artificial disc replacement. Spine. 2007;32(9):1001-1011.
40. McAfee P.C., Geisler F.H., Saiedy S., et al. Revisability of the Charité Artificial Disc replacement—analysis of 688 patients enrolled in the U.S. IDE study of the Charité Artificial Disc. Spine. 2006;31(11):1217-1226.
41. Punt I.M., Visser V.M., van Rhijn L.W., et al. Complications and reoperations of the SB Charité lumbar disc prosthesis: experience in 75 patients. Eur Spine J. 2008;17(1):36-43.
42. van Ooij A., Oner F.C., Verbout A.J. Complications of artificial disc replacement: a report of 27 patients with the SB Charité disc. J Spinal Disord Tech. 2003;16(4):369-383.
43. Geisler F.H. Surgical technique of lumbar artificial disc replacement with the Charité Artificial Disc. Neurosurgery. 2005;56:ONS46-ONS57.
44. Gumbs A.A., Shah R.V., Yue J.J., Sumpio B. The open anterior paramedian retroperitoneal approach for spine procedures. Arch Surg. 2005;140(4):339-343.
45. Shim C.S., Lee S., Maeng D.H., Lee S.H. Vertical split fracture of the vertebral body following total disc replacement using ProDisc: report of two cases. J Spinal Disord Tech. 2005;18(5):465-469.
46. Schulte T.L., Lerner T., Hackenberg L., et al. Acquired spondylolysis after implantation of a lumbar ProDisc II prosthesis: case report and review of the literature. Spine. 2007;32(22):E645-E648.
47. Francois J., Coessens R., Lauweryns P. Early removal of a Maverick disc prosthesis: surgical findings and morphological changes. Acta Orthop Belg. 2007;73(1):122-127.
48. Zeh A., Planert M., Siegert G., et al. Release of cobalt and chromium ions into the serum following implantation of the metal-on-metal Maverick-type artificial lumbar disc (Medtronic Sofamor Danek). Spine. 2007;32(3):348-352.
49. Bertagnoli R., Yue J.J., Nanieva R., et al. Lumbar total disc arthroplasty in patients older than 60 years of age: a prospective study of the ProDisc prosthesis with 2-year minimum follow-up period. J Neurosurg Spine. 2006;4(2):85-90.
50. Bertagnoli R., Yue J.J., Kershaw T., et al. Lumbar total disc arthroplasty utilizing the ProDisc prosthesis in smokers versus nonsmokers: a prospective study with 2-year minimum follow-up. Spine. 2006;31(9):992-997.
51. Bertagnoli R., Yue J.J., Fenk-Mayer A., et al. Treatment of symptomatic adjacent-segment degeneration after lumbar fusion with total disc arthroplasty by using the prodisc prosthesis: a prospective study with 2-year minimum follow up. J Neurosurg Spine. Feb 2006;4(2):91-97.
52. Hannibal M., Thomas D.J., Low J., et al. ProDisc-L total disc replacement: a comparison of 1-level versus 2-level arthroplasty patients with a minimum 2-year follow-up. Spine. 2007;32(21):2322-2326.
53. Yaszay B., Bendo J.A., Goldstein J.A., et al. Effect of intervertebral disc height on postoperative motion and outcomes after ProDisc-L lumbar disc replacement. Spine. 2008;33(5):508-512. discussion 513
54. Guyer R.D., Geisler F.H., Blumenthal S.L., et al. Effect of age on clinical and radiographic outcomes and adverse events following 1-level lumbar arthroplasty after a minimum 2-year follow-up. J Neurosurg Spine. 2008;8(2):101-107.
55. Geisler F.H., Guyer R.D., Blumenthal S.L., et al. Effect of previous surgery on clinical outcome following 1-level lumbar arthroplasty. J Neurosurg Spine. 2008;8(2):108-114.
56. Geisler F.H., Guyer R.D., Blumenthal S.L., et al. Patient selection for lumbar arthroplasty and arthrodesis: the effect of revision surgery in a controlled, multicenter, randomized study. J Neurosurg Spine. 2008;8(1):13-16.
57. Guyer R.D., Tromanhauser S.G., Regan J.J. An economic model of one-level lumbar arthroplasty versus fusion. Spine J. 2007;7(5):558-562.
58. Levin D.A., Bendo J.A., Quirno M., et al. Comparative charge analysis of one- and two-level lumbar total disc arthroplasty versus circumferential lumbar fusion. Spine. 2007;32(25):2905-2909.
59. Regan J.J., McAfee P.C., Blumenthal S.L., et al. Evaluation of surgical volume and the early experience with lumbar total disc replacement as part of the ide study of the Charité Artificial Disc. Spine. 2006;31(19):2270-2276.
60. Guyer R.D., McAfee P.C., Banco R.J., et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the Charité Artificial Disc versus lumbar fusion: five-year follow-up. Spine J. 2009;9(5):374-386.
61. van den Eerenbeemt K.D., Ostelo R.W., van Royen B.J., et al. Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur Spine J Aug. 2010;19(8):1262-1280.