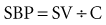6 Low Systemic Arterial Blood Pressure
 Initial Evaluation
Initial Evaluation
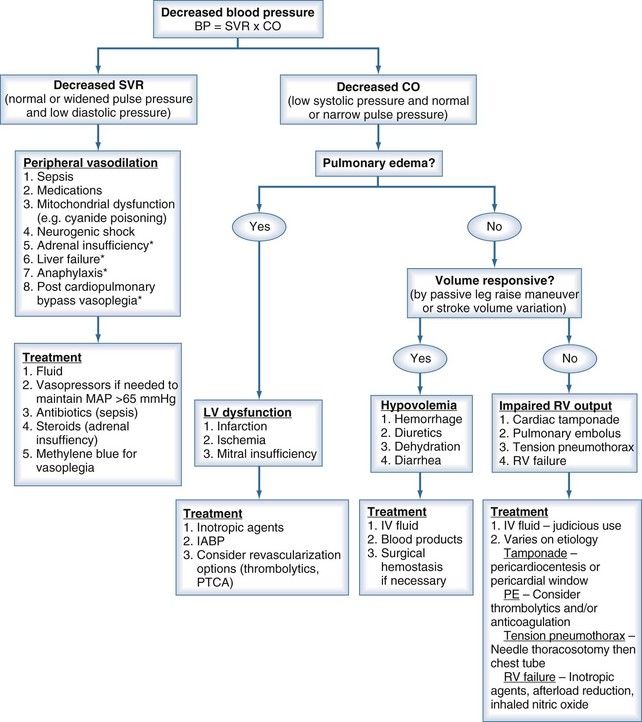
A clinician’s initial evaluation should be a global assessment (Figure 6-1). When walking into a patient’s room, you should think, “What do I see?” and quickly determine whether the patient is in distress or has problems related to the airway or breathing. Look for obvious signs of external hemorrhage, look for evidence of hypoperfusion, and assess the adequacy of intravenous (IV) access. Do not rely solely on blood pressure (BP) readings, as there is no “normal” BP for all patients, and a BP value in the “normal” range does not always equate with adequate tissue perfusion. A patient with a history of poorly controlled chronic hypertension may have signs of hypoperfusion even when the BP is within the normal range (for nonhypertensive patients). Conversely, a patient with cirrhosis may have adequate perfusion despite having a lower-than-normal BP. A quick bedside assessment of tissue perfusion should include evaluation of mental status, urine output, and skin findings (e.g., temperature, diaphoresis, mottling, and capillary refill). If any of these parameters are abnormal, a more urgent approach to treatment must be taken.
 What Is the Cause?
What Is the Cause?
To help focus the differential diagnosis of a hypotensive patient, it is important to review basic cardiovascular physiology. The first concept to remember is that pressure = flow × resistance, where flow is cardiac output, and resistance is SVR. Because cardiac output is determined by stroke volume (SV) × heart rate, the presence of hypotension means that at least one of these parameters (e.g., SV, SVR, or heart rate) is abnormal.1 Disturbances in heart rate should be obvious by feeling the peripheral pulse, looking at the cardiac monitor, or evaluating a 12-lead electrocardiogram (ECG). The focus of this chapter is evaluating and treating conditions associated with decreased SV or SVR. By properly measuring pulse pressure and diastolic pressure, the clinician can determine whether the primary cause is a change in SVR or SV.
During systole, the SV is ejected into the proximal arterial conduits. Because more blood is being ejected than the peripheral circulation can accommodate in the arterioles, the arterial walls distend, increasing SBP in a way that is directly proportional to the SV and indirectly proportional to the capacitance (C) of the arterial wall. This relationship is represented by the formula1:
That is, for a fixed SV, if capacitance is higher, the SBP is lower.
During diastole, the portion of the SV that was “stored” by the distention of the arterial walls during systole fills the peripheral arterioles, leading to a progressive decrease in BP until the next systolic phase. This is the diastolic pressure, a parameter that is directly related to the SVR and capacitance (i.e., low diastolic pressure = low SVR and/or capacitance).1 When using these basic cardiovascular principles to understand the cause of hypotension, it is important to remember the following: (1) capacitance does not change from heartbeat to heartbeat, and (2) SV depends on preload, afterload, and contractility.
Low SVR is characteristic of a number of pathologic conditions, including sepsis, adrenal insufficiency, vasodilating medications, neurogenic shock, post–cardiopulmonary bypass (CPB) vasoplegia, and severe liver dysfunction. Decreased SVR should be suspected in the presence of a widened pulse pressure and low diastolic pressure.2,3
Reduced SV can be due to decreased preload, decreased contractility, or increased afterload. The most common cause of inadequate preload is hypovolemia. Other causes of inadequate preload include increased intrathoracic pressure due to dynamic hyperinflation in mechanically ventilated patients4,5 or tension pneumothorax, pulmonary embolism,6 mitral valve stenosis,7 cardiac tamponade,8 and right ventricular failure.9 Decreased contractility can be caused by myocardial ischemia or infarction, cardiomyopathy, myocarditis, negative inotropic drugs, myocardial stunning after CPB, and direct myocyte toxins, such as chemotherapeutic agents and inflammatory mediators (e.g., tumor necrosis factor [TNF] and interleukin 1-beta [IL-1β).10 A reduction in SV can be identified by decreased systolic BP and normal or narrow pulse pressure.
 Treatment
Treatment
There are several tools that aid in the workup of the hypotensive patient. One option is the use of ultrasound to evaluate inferior vena cava diameter variation during the inspiratory and expiratory phases of the respiratory cycle. Patients with a large variation (>50%) will most likely respond to additional volume.11 Ultrasound, when used in a focused cardiac examination, can also identify the global quality of contractility, ventricular size and volume, obvious wall motion abnormalities, significant valvular abnormalities, and the presence of a pericardial effusion.12
An IV fluid bolus should be a first-line option in treating hypotension, but not every patient will have the desired response to fluid administration. The clinician can evaluate “volume responsiveness” by noninvasive or minimally invasive measures. In the nonintubated, supine patient, elevating the patient’s legs in a 45-degree angle above the plane of the bed will cause a rapid temporary increase in venous return to the heart. If the patient’s condition is dependent on additional volume, one will see an increase in SBP that also correlates to an increase in stroke volume. This maneuver increases pulse pressure in “responders.” An increase in pulse pressure of more than 9% noted before and after the passive leg lifts will identify patients who are likely to respond to additional IV fluid administration.13,14 A more invasive option is to measure pulse pressure or stroke volume variation in the intubated and mechanically ventilated patient. In these patients, a decrease in stroke volume of 13% or more during the inspiratory cycle correlates with preload responsiveness of stroke volume (i.e., stroke volume and therefore cardiac output are likely to increase if intravascular volume is increased by infusing IV colloid or crystalloid solutions). This variation represents a decrease in venous return in conjunction with the increased intrathoracic pressure during the inspiratory phase of the ventilator. This measurement is only accurate when the heart rhythm is regular, so it is an unreliable index of preload responsiveness in patients with many kinds of arrhythmias, in the presence of an intraaortic balloon pump, or when there is loss of integrity in the arterial waveform. It is also only accurate in mechanically ventilated patients who are not experiencing large variations in intrathoracic pressures.15,16
In those patients where a low SVR is suspected as the primary cause of hypotension, the treatment is different. Large amounts of additional IV fluid will not adequately increase the BP to maintain tissue perfusion alone. Vasoconstrictor agents (e.g., norepinephrine, dopamine, phenylephrine, vasopressin) will be required in these patients. In certain specific cases, other pharmacologic adjuncts may be helpful. Low-dose hydrocortisone in vasoconstrictor-resistant septic shock17 and methylene blue in post CPB vasoplegia are two examples.18
Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock. Part I. Clinical manifestation of cardiovascular dysfunction. J Cardiothorac Vasc Anesth. 2001;15:364-376.
Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345:588-595.
An excellent basic science review of the physiology of vasodilatory shock.
Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358:1037-1052.
Pinsky MR. Heart-lung interaction. Curr Opin Crit Care. 2007;13:528-531.
Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003;349:684-690.
A thorough review of cardiac tamponade that covers cause, diagnosis, and treatment.
Monett X, Teboul JL. Volume responsiveness. Curr Opin Crit Care. 2007;13:549-553.
1 Wood L. The pathophysiology of the circulation in critical illness. In: Hall J, Schmidt G, Wood L, editors. Principles of Critical Care. New York: McGraw-Hill; 1998:259-276.
2 Astiz ME, Rackow EC, Weil MH. Pathophysiology and treatment of circulatory shock. Crit Care Clin. 1993;9(2):183-203.
3 Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345(8):588-595.
4 Pinsky MR, Desmet JM, Vincent JL. Effect of positive end-expiratory pressure on right ventricular function in humans. Am Rev Respir Dis. 1992;146(3):681-687.
5 Pinsky MR. Heart lung interaction. Curr Opin Crit Care. 2007;13(5):528-531.
6 Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358(10):1037-1052.
7 Carabello BA. Modern management of mitral stenosis. Circulation. 2005;112(3):432-437.
8 Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003;349(7):684-690.
9 Woods J, Monteiro P, Rhodes A. Right ventricular dysfunction. Curr Opin Crit Care. 2007;13(5):532-540.
10 Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock. Part I. Clinical manifestation of cardiovascular dysfunction. J Cardiothorac Vasc Anesth. 2001;15(3):364-376.
11 Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834-1837.
12 Beaulieu Y. Bedside echocardiography in the assessment of the critically ill. Crit Care Med. 2007;35(Suppl. 5):S235-S249.
13 Preau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38(3):819-825.
14 Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34(5):1402-1407.
15 Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162(1):134-138.
16 Monett X, Teboul JL. Volume responsiveness. Curr Opin Crit Care. 2007;13(5):549-553.
17 Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296-327.
18 Shanmugam G. Vasoplegic syndrome – the role of methylene blue. Eur J Cardiothorac Surg. 2005;28(5):705-710.