6 Low Grade Astrocytomas
Introduction
In the 1970s, 80% of nonglioblastoma gliomas were considered to be diffuse astrocytomas. The percentages of oligodendrogliomas and oligoastrocytomas have risen progressively subsequently; these tumors now account for 65% of grade II primary glioma diagnoses in the SEER database from 1994 to 2001.1 This increase reflects not a biological change, but, instead, an evolution in pathological interpretation prompted by the identification of a favorable-risk chemosensitive oligodendroglioma; this has created an “incentive” to find oligodendroglial elements and to determine whether there are deletions in 1p and 19q.
Diffuse Astrocytoma, WHO Grade II
PATHOLOGICAL AND MOLECULAR ASPECTS
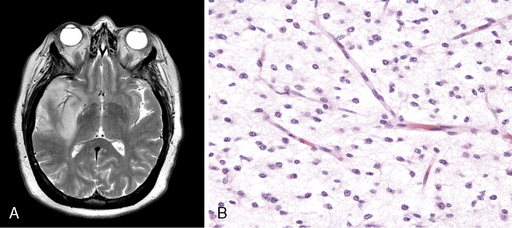
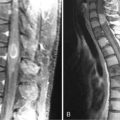
Diffuse astrocytoma is the most common astrocytoma. Because its cells produce neuroglial fibrils, this tumor is firm and rubbery. Its epicenter is typically in white matter. Cytologically, the more common fibrillary subtype is composed of atypical fibrillary astrocytes with hyperchromatic, elongated nuclei that appear “naked” within a fibrillary background or display discernable eosinophilic cytoplasmic processes. Fine and coarse neuroglial fibrils occupy the matrix and lie in random orientation among the cells from which they are derived (Figure 6-1). Mitotic activity is, by definition, absent in small biopsies and rare even in large resection specimens. Vasculature is inconspicuous, and necrosis is characteristically absent. Two pathological variants of the diffuse astrocytoma are the protoplasmic and the gemistocytic. The former is composed of cells resembling protoplasmic astrocytes. Protoplasmic tumors generally involve the cerebral cortex. Gemistocytic astrocytomas, by contrast, have cells with prominent eosinophilic cytoplasm that imparts a distended or globoid appearance and strong glial fibrillary acidic protein (GFAP) immunopositivity.
Unlike oligodendrogliomas, which frequently demonstrate losses of 1p and 19q and rarely have p53 mutations, many WHO grade II diffuse astrocytomas demonstrate loss of chromosome 17p, and roughly half harbor p53 mutations.2,3 The prognostic value of a p53 mutation is debated.2,4,5
Many WHO grade II diffuse astrocytomas harbor an alteration of a component of either the RB1 or the TP53 pathway, but few have alterations in both pathways. One study of 46 patients found a defect in the TP53 pathway in 70% and in the Rb pathway in 13%.6 The authors concluded that disruption of either the TP53 or the p14ARF pathway is frequent in low-grade astrocytomas and correlates with an unfavorable clinical course.
Even more common in WHO grade II diffuse astrocytomas is abnormal p53 protein immunoreactivity, which can occur even when mutations are not present.2 PDGFr-alpha overexpression is also common7 in these tumors.
A recent study comparing WHO grade II diffuse astrocytomas with primary and secondary GBMs using cDNA array analysis noted that WHO grade II diffuse astrocytomas had highly specific and homogenous expression profiles compared with the less specific and heterogenous profiles of primary GBMs; those of secondary GBM were intermediate.8 The distinguishing factor for primary GBMs was the upregulation of genes involved in angiogenesis. Such genes were not upregulated in WHO grade II diffuse astrocytomas, suggesting that angiogenesis is intimately linked with aggressive behavior.
CLINICAL ASPECTS
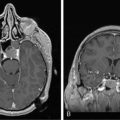
WHO grade II diffuse astrocytomas are generally slowly growing, locally infiltrative tumors in young or middle-aged adults.9,10 They rarely metastasize within11 or outside12 the CNS. They usually present as nonenhancing lesions on MRI (Figure 6-1). They are best viewed as “diffuse”; in other words, they are infiltrative, ill-defined tumors that can spread for many centimeters throughout the cortex and along white matter tracts, perivascular spaces, and periventricular ependyma in the form of secondary structures of Scherer, namely in perivascular and perineuronal locations. Regional heterogeneities in morphology, proliferative activity, ploidy and genetic aberrations are common.13 Although some patients survive for decades,14,15 these tumors have a propensity to evolve gradually into more aggressive anaplastic gliomas or GBMs,16,17 especially in older patients.16
Patients with supratentorial WHO grade II diffuse astrocytomas have a much higher incidence of seizures as a presenting symptom than do those with higher grade gliomas. Epilepsy is by far the most common and important symptom in WHO grade II diffuse astrocytomas. Seizures occur as a presenting symptom in approximately 50% of cases, and have a prevalence greater than 80%.18 Furthermore, patients with WHO grade II diffuse astrocytomas often suffer from neuropsychological and psychological problems that are aggravated by epilepsy and its treatment.19
The seizures originate not from the mass lesion but from adjacent brain tissue.20,21 Nevertheless, both radiation therapy22 and lesionectomy21,23,24 may significantly reduce or even eliminate medically refractory seizures.
Anticonvulsants, even multiple agents in combination, fail to control seizures in up to 50% of patients.19,25 They may also impair cognitive functioning.19 Since differences in efficacy are small, clinicians should choose an anticonvulsant with few side effects. Due to their effects on cytochrome P450 metabolism, the potential for interaction between anticonvulsants and other drugs (e.g., chemotherapeutics) can make it difficult to maintain adequate drug levels. Newer nonenzyme-inducing antiepileptic drugs such as levetiracetam and lamotrigine have equal efficacy, fewer side effects, and less frequent drug interactions than the more conventional agents, and are thus preferable to phenytoin and phenobarbital.
In a recent review of patients with intractable epilepsy and supratentorial tumors, including many with WHO grade II diffuse astrocytomas, improved seizure outcome was associated with a short duration of epilepsy before surgery, a single EEG focus, and the absence of hippocampal sclerosis or cortical dysplasia.26 The authors concluded that tumor resection was indicated for seizure control and prevention of malignant progression.
PROGNOSTIC FEATURES
Before CT scanning became readily available, reported median survival times for patients with WHO grade II diffuse astrocytomas were 5 years or less.27–30 More recent studies, however, show much longer median survival times, usually between 7 and 9 years, and 5- and 10-year survival rates range from 25% to 97% and 10% to 63% respectively.14,17,31–37 Two explanations have been offered for this longer reported survival: either patients are being diagnosed earlier or they are being more effectively treated. One recent study38 noted that use of newer imaging methods added no more than 6 months to the duration of survival following imaging diagnosis. Because radiation therapy has not been shown to improve survival, the authors speculated that longer survival resulted from more effective surgery, although they did note that macroscopically complete resections did not significantly improve survival duration as compared to less complete resections.
The outcome for patients with low-grade glioma (LGG) is intimately linked to anaplastic transformation. Pathologic progression of a diffuse astrocytoma to GBM produces an abrupt change in clinical behavior. The incidence of malignant transformation has been reported in various clinical studies to range from 13% to 86%.28,39–43 Because many of these series included previously treated patients, the natural history of this transformation is difficult to determine. A recent study reviewed 40 patients with progressive tumors who had undergone surgery but no further therapy for at least 3 months after the diagnosis of diffuse astrocytoma was made.44 Half had grade III or IV tumors when a second biopsy or operation was performed. Patients with progression had longer median times to reoperation (47 months vs. 22.5 months). The authors noted that complete resection delayed the time to reoperation but did not affect whether progression occurred.
The prognosis of adult patients with diffuse astrocytomas is largely determined by the inherent biology of the tumor27,32,37,43,45 and patient age.14,32,37,39,46,47 Adverse prognostic factors include poor performance status, tumor contrast enhancement, cognitive dysfunction, focal neurological deficits, and steroid dependency.14,27,32,37,39,43,46,48 In one study of several hundred patients accrued to two EORTC studies, multivariate analysis identified age greater than 40 years, astrocytoma histology subtype, largest diameter of tumor greater than 6 cm, tumor crossing the midline, and neurologic deficit present prior to surgery as the most important poor prognostic factors.49
TREATMENT ISSUES
Surgery provides tissue for histopathological analysis and can be helpful in relieving mass effect. However, because of the infiltrative nature of this tumor, gross total resection is often not possible. Nevertheless, most neurosurgeons favor attempting maximal resection at the time of surgery.49–52 However, it must be emphasized that these observations are difficult to interpret because of a bias in comparing these patients to those for whom it was not possible to do a maximal resection; therefore, the data should not be considered definitive and less extensive surgeries or even observation can still be justified.
Radiation therapy is also frequently advocated for diffuse astrocytomas, but the data supporting this approach are weak. An important study from the EORTC examined the effect of either immediate or delayed RT (54 Gy in 6 weeks) in a randomized study that included over 300 patients.53,54 Although immediate RT prolonged progression-free survival and seemed to result in better seizure control, no difference in survival was noted. The absence of a survival benefit suggested that although RT seemed to slow progression, it did not avert transformation into more aggressive GBMs. In addition, escalating the RT dose does not improve survival for these patients.46,50
Thus, there are no firm recommendations concerning RT in diffuse astrocytoma. Generally, clinicians use prognostic factors to choose patients who might benefit from immediate RT. These include persistence of significant disease-associated neurological dysfunction (including seizures), recurrent or progressive disease, age over 40, tumor size greater than 6 cm or crossing midline, and MIB proliferation index greater than 3%.49,50,55
There is no evidence that administration of chemotherapy prolongs survival in patients with diffuse astrocytoma.56 However, a recent report from the RTOG suggested some improvement in progression-free survival when RT was combined with procarbazine, CCNU, and vincristine (PCV), although 5-year survival was not significantly altered.57
Pilocytic Astrocytoma
PATHOLOGICAL ASPECTS
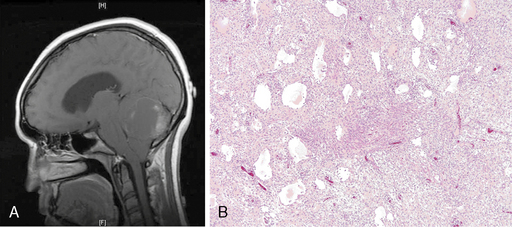
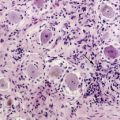
Pilocytic astrocytomas (PA) differ microscopically from fibrillary astrocytomas in that there is a tendency for biphasic morphology, alternating between compact perivascular and microcystic regions. The hallmark, although quite nonspecific, is the piloid or hairlike neoplastic astrocyte, appearing as a slender unipolar cell. Tumor cells remote from blood vessels have a rarefied and sparsely cellular appearance and tend to undergo microcystic degeneration. Rosenthal fibers and eosinophilic granular bodies are commonly seen and are useful pathological hallmarks in differentiating these tumors from other astrocytomas (Figure 6-2).
CLINICAL ASPECTS
PAs are well-circumscribed tumors that occur mostly in children. They occur most frequently in the cerebellum (Figure 6-2), but also arise in supratentorial regions, chiefly the optic-hypothalamic region, as well as dorsally exophytic brainstem lesions.
PAs are frequently cystic and well-demarcated radiographically and almost always enhance on MRI58 due to the paradoxical prominence of microvasculature, an important histological feature distinguishing the PA from a WHO grade II glioma.
PROGNOSTIC ASPECTS
PAs are distinguished from WHO grade II through IV gliomas by their excellent prognosis; over 95% of patients survive 10 years or more.59 Furthermore, they are much less likely to transform into GBM, although there are several reports of such an occurrence many years after diagnosis. Multicentric spread has also been reported, especially with hypothalamic tumors.60
TREATMENT ISSUES
Surgery is the primary treatment. Since prognosis is excellent, even after partial resection, irradiation minimally impacts survival or the risk of tumor progression.59 Additionally, since RT may be associated with eventual malignant transformation of PAs,61 RT should be reserved for symptomatic patients with extensive residual disease.
Chemotherapy has been used to treat young patients with PAs at inaccessible sites such as the optic pathways,62 but evidence supporting a routine role for this modality in PAs is lacking.
Other Astrocytoma Subtypes
PLEOMORPHIC XANTHOASTROCYTOMA (PXA) WHO GRADE II
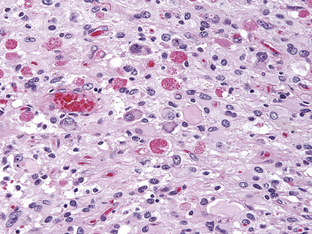
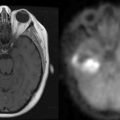
Once termed giant cell glioblastoma or monstrocellular sarcoma, PXAs were officially recognized as a distinct entity in the 1993 WHO classification. They account for less than 1% of astrocytic neoplasms. Although their histological features include marked cellular pleomorphism, nuclear atypia and the presence of bizarre, multinucleate giant cells (Figure 6-3), they behave in a comparatively benign fashion. Long-term survival is common following complete resection, which is generally possible due to their superficial location, relative circumscription, and cyst/mural architecture.63–65 Nonetheless, although the 10-year survival rate for patients is greater than 70%, PXAs are distinguished from the truly benign astrocytomas, such as PAs, by their tendency to recur and to transform into more anaplastic forms (as often as 20% of the time). They are thus assigned a WHO grade of II. Pathological correlates of this more malignant behavior include less pleomorphism and a higher mitotic rate (more than 5 mitoses/HPF).65
SUBEPENDYMAL GIANT CELL ASTROCYTOMAS (SEGA) WHO GRADE I
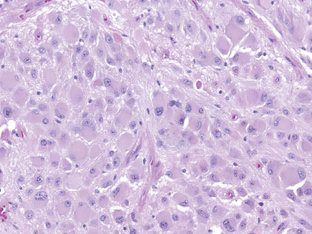
SEGAs occur in approximately 5% to 10% of patients with tuberous sclerosis, an autosomal dominant, multisystem, neurocutaneous syndrome characterized by a triad of seizures, adenoma sebaceum, and mental retardation. They are presumed to arise from the distinctive cortical tubers that are characteristics of these tumors. Although the characteristic giant cells are of mixed glioneuronal lineage,66 SEGAs are still grouped with the astrocytomas. They are histologically benign (Figure 6-4), although their location in the lateral ventricles at the level of the foramen of Munro can often result in hydrocephalus. Surgery provides for a better outcome when performed before, rather than after, obstructive hydrocephalus occurs.67,68
Although most studies have addressed the SEGAs in the context of tuberous sclerosis, at least half of SEGAs occur sporadically.69 It is not clear whether spontaneously occurring SEGAs are as benign, as several cases have been reported wherein spontaneous SEGAs behaved aggressively.
NEW ASTROCYTOMA VARIANTS
The fourth edition of the WHO classification of CNS tumors identifies two new subtypes of astrocytoma.70 The angiocentric glioma is a WHO grade I neoplasm that occurs in children and young adults. It presents as a hemispheric mass in a patient with epilepsy. It does not enhance on neuroimaging. Pathologically, tumors are composed of bipolar cells intimately associated with blood vessels, often oriented perpendicular to the vascular lumen. They are probably curable by surgery.71,72
The pilomyxoid astrocytoma is a variant of the PA that often arises in the hypothalamus.73 Pilomyxoid astrocytomas are well circumscribed on MRI and typically enhance. Histologically, this tumor is dominated by a hypercellular monomorphous population of piloid cells without Rosenthal fibers and eosinophilic granular bodies (EGBs).
Despite their being a PA variant, these tumors are often aggressive; in the new classification schema, they are therefore classified as WHO grade II neoplasms.74–76
ASTROCYTOMAS IN NF1 PATIENTS
Neurofibromatosis type 1 is the most common inherited neurocutaneous syndrome, whose manifestations usually include CNS and peripheral neural tumors. The most common site of CNS tumor origin is the optic pathways, and most optic tumors are PAs. However, NFI patients may also develop diffuse astrocytomas in later life.77,78
PAs in NF1 have a distinctive predilection to involve the optic nerve, optic chiasm, and hypothalamus. In young patients with NF1,15% to 20% have an optic PA.79 Tumors arising in these areas, even those with high mitotic activity, are often indolent, suggesting that this location affects clinical behavior.
Patients with NF1 also develop tumors, in other locations, with indeterminate features. They are low grade but lack typical PA features and can be diffusely infiltrative; they tend to behave similarly to the PA.77 Diffusely infiltrating astrocytomas are rare, but their frequency rises with age.78 They can resemble a GBM. While RT is discouraged for PAs, it appears necessary for these more aggressive infiltrative astrocytomas, especially those with malignant histology.
1. E.B. Claus, P.M. Black. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973–2001. Cancer. 2006;106:1358-1363.
2. K. Watanabe, K. Sato, W. Biernat, et al. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin Cancer Res. 1997;3:523-530.
3. J. Reifenberger, G.U. Ring, U. Gies, et al. Analysis of p53 mutation and epidermal growth factor receptor amplification in recurrent gliomas with malignant progression. J Neuropathol Exp Neurol. 1996;55:822-831.
4. A. Peraud, F.W. Kreth, O.D. Wiestler, et al. Prognostic impact of TP53 mutations and p53 protein overexpression in surpratentorial WHO grade II astrocytomas and oligoastrocytoma. Clin Cancer Res. 2002;8:1117-1124.
5. N. Ishii, M. Tada, M-F. Hamou, et al. Cells with TP53 mutations in low grade astrocytic tumors evolve clonally to malignancy and are an unfavorable prognostic factor. Oncogene. 1999;18:5870-5878.
6. T. Watanabe, Y. Katayama, A. Yoshino, et al. Deregulation of the TP53/p14(ARF) tumor suppressor pathway in low-grade diffuse astrocytomas and its influence on clinical course. Clin Cancer Res. 2003;9:4884-4890.
7. M. Hermanson, K. Funa, J. Koopmann, et al. Association of loss of heterozygosity on chromosome 17p with high platelet-derived growth factor a expression in human malignant gliomas. Cancer Res. 1996;56:164-171.
8. S. Godard, G. Getz, M. Delorenzi, et al. Classification of human astrocytic gliomas on the basis of gene expression: A correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63:6613-6625.
9. R.E. McLendon, D.S. Enterline, R.D. Tien, et al. Tumors of central neuroepithelial origin. In: D.D. Bigner, R.E. McLendon, J.M. Bruner, editors. Russel and Rubinstein’s Pathology of Tumors of the Nervous System. 6th ed. New York: Arnold; 1998:307-572.
10. P. Kleihues, R.L. Davis, H. Ohgaki, et al. Diffuse astrocytoma. In: P. Kleihues, W.K. Cavenee, editors. Pathology and Genetics of Tumours of the Nervous System. Lyon: IARC Press; 2000:22-26.
11. O.D. Laerum, S.J. Mork. Mechanisms of altered growth control: invasion and metastasis. D.D. Bigner, R.E. McLendon, J.M. Bruner, editors. Russell and Rubinstein’s Pathology of Tumors of the Nervous System, 6th ed, vol. 1. New York: Arnold, 1998;117-140.
12. H.J. Hoffman, P.K. Duffner. Extraneural metastases of central nervous system tumors. Cancer. 1985;56:1778-1782.
13. A. Perry. Pathology of low-grade gliomas: An update of emerging concepts. Neuro-Oncology. 2003;5:168-178.
14. G. Bauman, K. Lote, D. Larson, et al. Pretreatment factors predict overall survival for patients with low-grade glioma: A recursive partitioning analysis. Int J Rad Oncol Biol Phys. 1999;45:923-929.
15. F.G. Davis, S. Freels, J. Grutsch, et al. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histologic type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973-1991. J Neurosurg. 1998;88:1-10.
16. S. Shafqat, E.T. Hedley-Whyte, J.W. Henson. Age-dependent rate of anaplastic transformation in low-grade astrocytoma. Neurology. 1999;52:867-869.
17. F.T. Vertosick, R.G. Selker, V.C. Arena. Survival of patients with well-differentiated astrocytomas diagnosed in the era of computed tomography. Neurosurgery. 1991;28:496-501.
18. K. Lote, A.E. Stenwig, K. Skullerud, H. Hirschberg. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer. 1998;34:98-102.
19. M. Klein, N.HJ. Engelberts, H.M. van der Ploeg, et al. Epilepsy in low-grade gliomas: The impact on cognitive function and quality of life. Ann Neurol. 2003;54:514-520.
20. M.M. Haglund, M.S. Berger, D.D. Kunkel, et al. Changes in gamma-aminobutyric acid and somatostatin in epileptic cortex associated with low-grade gliomas. J Neurosurg. 1992;77:209-216.
21. M.S. Berger, S. Ghatan, M.M. Haglund, et al. Low-grade gliomas associated with intractable epilepsy: seizure outcome utilizing electrocorticography during tumor resection. J Neurosurg. 1993;79:62-69.
22. L.R. Rogers, H.H. Morris, K. Lupica. Effect of cranial irradiation on seizure frequency in adults with low-grade astrocytoma and medically intractable epilepsy. Neurology. 1993;43:1599-1601.
23. J. Zentner, A. Hufnagel, H.K. Wolf, et al. Surgical treatment of neoplasms associated with medically intractable epilepsy. Neurosurgery. 1997;41:378-386.
24. M.J. Hennessy, R.D. Elwes, M. Honavar, et al. Predictors of outcome and pathological considerations in the surgical treatment of intractable epilepsy associated with temporal lobe lesions. J Neurol Neurosurg Psychiatr. 2001;70:450-458.
25. F. Semah, M.C. Picot, C. Adam, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence. Neurology. 1998;51:1256-1262.
26. C. Luyken, H. Blumcke, R. Fimmers, et al. The spectrum of long-term epilepsy-associated tumors: Long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003;44:822-830.
27. R. Soffietti, A. Chio, M.T. Giordana, et al. Prognostic factors in well-differentiated cerebral astrocytomas in the adult. Neurosurgery. 1989;24:686-692.
28. E.R. Laws, W.F. Taylor, M.B. Clifton, H. Okazaki. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984;61:665-673.
29. J.T. Fazekas. Treatment of grades I and II brain astrocytomas. The role of radiotherapy. Intl J Rad Oncol Biol Phys. 1977;2:661-666.
30. C. Silverman, J.E. Marks. Prognostic significance of contrast enhancement in low-grade astrocytomas of the adult cerebrum. Radiology. 1981;139:211-213.
31. C. Leighton, B. Fisher, G. Bauman, et al. Supratentorial low-grade glioma in adults: An analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15:1294-1301.
32. K. Lote, T. Egeland, B. Hager, et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: A retrospective study in 379 patients. J Clin Oncol. 1997;15:3129-3140.
33. B.M. McCormack, D.C. Miller, G.N. Budzilovich, et al. Treatment and survival of low-grade astrocytoma in adults—1977–1988. Neurosurgery. 1992;31:636-642.
34. C.A. Medbery, K.L. Straus, S.M. Steinberg, et al. Low-grade astrocytomas: Treatment results and prognostic variables. Intl J Rad Oncol Biol Phys. 1988;15:837-841.
35. M. Scerrati, R. Roselli, M. Iacoangeli, et al. Prognostic factors in low grade (WHO grade II) gliomas of the cerebral hemispheres: the role of surgery. J Neurol Neurosurg Psychiatr. 1996;61:291-296.
36. E.G. Shaw, B.W. Scheithauer, D.T. Gilbertson, et al. Postoperative radiotherapy of supratentorial low-grade gliomas. Intl J Rad Oncol Biol Phys. 1989;16:663-668.
37. M.L.C. van Veelen, C.JJ. Avezaat, J.M. Kros, et al. Supratentorial low grade astrocytoma: prognostic factors, dedifferentiation, and the issue of early versus late surgery. J Neurol Neurosurg Psychiatr. 1998;64:581-587.
38. T.B. Johannesen, F. Langmark, K. Lote. Progress in long-term survival in adult patients with supratentorial low-grade tumors: a population-based study of 993 patients in whom tumors were diagnosed between 1970 and 1993. J Neurosurg. 2003;99:854-862.
39. J.M. Piepmeier. Observations on the current treatment of low-grade astrocytic tumors of the cerebral hemispheres. J Neurosurg. 1987;67:177-181.
40. D. Afra, W. Muller, G. Benoist, et al. Supratentorial recurrences of gliomas. Results of reoperations on astrocytomas and oligodendrogliomas. Acta Neurochir. 1978;43:217-227.
41. W. Muller, D. Afra, R. Schroder. Supratentorial recurrences of gliomas. Morphological studies in relation to time intervals with astrocytomas. Acta Neurochir. 1977;37:75-91.
42. W. Muller, D. Afra, R. Schroder. Supratentorial recurrences of gliomas. Morphological studies in relation to time intervals with oligodendrogliomas. Acta Neurochir. 1977;39:15-25.
43. J.H. Philippon, S.H. Clemenceau, F.H. Fauchon, J.F. Foncin. Supratentorial low-grade astrocytomas in adults. Neurosurgery. 1993;32:554-559.
44. M. Schmidt, M.S. Berger, K. Lamborn, et al. Repeated operations for infiltrative low-grade gliomas without intervening therapy. J Neurosurg. 2003;98:1165-1169.
45. E.G. Shaw, B.W. Scheithauer, J. O’Fallon. Supratentorial gliomas: a comparative study by grade and histologic type. J Neuro-Oncol. 1997;31:273-278.
46. A.B. Karim, B. Maat, R. Hatlevoll, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) study 22844. Intl J Rad Oncol Biol Phys. 1996;36:549-556.
47. C.J. Vecht. The influence of age on treatment decisions in low grade glioma. J Neurol Neurosurg Psychiatr. 1993;56:1259-1264.
48. K. Lote, T. Egeland, B. Hager, et al. Prognostic significance of CT contrast enhancement within histological subgroups of intracranial glioma. J Neuro-Oncol. 1998;40:161.
49. F. Pignatti, M. van den Bent, D. Curran, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076-2084.
50. E. Shaw, R. Arusell, B. Scheithauer, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: Initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267-2276.
51. G.E. Keles, K.R. Lamborn, M.S. Berger. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95:735-745.
52. J.S. Smith, E.F. Chang, K.R. Lamborn, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338-1345.
53. A.B. Karim, D. Afra, P. Cornu, et al. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council Study BR04: An interim analysis. Intl J Rad Oncol Biol Phys. 2002;52:316-324.
54. M. van den Bent, D. Afra, O. De Witte, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985-990.
55. M.A. Papagikos, E.G. Shaw, V.W. Stieber. Lessons learned from randomised clinical trials in adult low-grade glioma. Lancet Oncol. 2005;6:240-244.
56. H.J. Eyre, J.J. Crowley, J.J. Townsend, et al. A randomized trial of radiotherapy versus radiotherapy plus CCNU for incompletely resected low-grade gliomas: a Southwest Oncology Group study. J Neurosurg. 1993;78:909-914.
57. E.G. Shaw, M. Wang, S. Coons, et al. Final report of Radiation Therapy Oncology Group (RTOG) protocol 9802: Radiation therapy (RT) versus RT + procarbazine, CCNU, and vincristine (PCV) chemotherapy for adult low-grade glioma (LGG) (abstract). J Clin Oncol. 2008;26:90s.
58. Y-Y Lee, P. Van Tassel, J.M. Bruner, et al. Juvenile pilocytic astrocytomas: CT and MR characteristics. AJR Am J Roentgenol. 1989;152:1263-1270.
59. C. Burkhard, P-L Di Patre, D. Schuler, et al. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg. 2003;98:1170-1174.
60. A.N. Mamelak, M.D. Prados, W.G. Obana, et al. Treatment options and prognosis for multicentric juvenile pilocytic astrocytoma. J Neurosurg. 1994;81:24-30.
61. P.B. Dirks, V. Jay, L.E. Becker, et al. Development of anaplastic changes in low-grade astrocytomas of childhood. Neurosurgery. 1994;34:68-78.
62. M.T. Brown, H.S. Friedman, J. Oakes, et al. Chemotherapy for pilocytic astrocytomas. Cancer. 1993;71:3165-3172.
63. J.J. Kepes. Pleomorphic xanthoastrocytoma: the birth of a diagnosis and a concept. Brain Pathol. 1993;3:269-274.
64. P.A. Pahapill, D.A. Ramsay, RF. Del Maestro. Pleomorphic xanthoastrocytoma: Case report and analysis of the literature concerning the efficacy of resection and the significance of necrosis. Neurosurgery. 1996;38:822-829.
65. C. Giannini, B. Scheithauer, P. Burger, et al. Pleomorphic xanthoastrocytoma: What do we really know about it? Cancer. 1998;85:2033-2045.
66. S. Goh, W. Butler, E.A. Thiele. Subependymal giant cell tumors in tuberous sclerosis complex. Neurology. 2004;63:1457-1461.
67. M.J. Clarke, A.B. Foy, N. Wetjen, C. Raffel. Imagine characteristics and growth of subependymal giant cell astrocytomas. Neurosurg Focus. 2006;20:E5.
68. M.G. Nagib, S.J. Haines, D.L. Erickson, et al. Tuberous sclerosis: a review for the neurosurgeon. Neurosurgery. 1984;14:93-98.
69. M. Sharma, A. Ralte, R. Arora, et al. Subependymal giant cell astrocytoma: a clinicopathological study of 23 cases with special emphasis on proliferative markers and expression of p53 and retinoblastoma gene proteins. Pathology. 2004;36:139-144.
70. D.N. Louis, H. Ohgaki, O.D. Weistler, W.K. Cavenee. WHO Classification of Tumours of the Central Nervous System. Lyon: IARC Press, 2007.
71. A. Lellouch-Tubiana, N. Boddaert, M. Bourgeois, et al. Angiocentric neuroepithelial tumor (ANET): a new epilepsy-related clinicopatological entity with distinctive MRI. Brain Pathol. 15, 2005.
72. M. Wang, T. Tihan, A.M. Rojiani, et al. Monomorphous angiocentric glioma: a distinctive epileptogenic neoplasm with features of infiltrating astrocytoma and ependymoma. J Neuropathol Exp Neurol. 64, 2005.
73. T. Tihan, P.G. Fisher, J.L. Kepner, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999;58:1061-1068.
74. R.J. Komotar, J. Mocco, B.S. Carson, et al. Pilomyxoid astrocytoma: a review. MedGenMed. 2004;6:42.
75. C. Fernandez, D. Figarella-Branger, N. Girard, et al. Clinico-pathological features of pilomyxoid astrocytoma of the optic pathway. Neurosurgery. 2003;53:544-553.
76. K. Chikai, A. Ohnishi, T. Kato, et al. Clinico-pathological features of pilomyxoid astrocytoma of the optic pathway. Acta Neuropathol. 2004;108:109-114.
77. F.J. Rodriguez, A. Perry, D.H. Gutmann, et al. Gliomas in neurofibromatosis type 1: A clinicopathologic study of 100 patients. J Neuropathol Exp Neurol. 2008;67:240-249.
78. D.H. Gutmann, S.A. Rasmussen, P. Wolkenstein, et al. Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1). Neurology. 2002;59:759-761.
79. R. Listernick, R.E. Ferner, G.T. Liu, D.H. Gutmann. Optic pathway gliomas in neurofibromatosis-1: Controversies and recommendations. Ann Neurol. 2007;61:189-198.