CHAPTER 83 Loose Bodies
INTRODUCTION
First described in the knee by Ambroise Paré32 in 1558, loose bodies occur in the elbow with a frequency second only to that in the knee.33 As in other joints, it is sometimes difficult to distinguish with certainty between ossification centers and acquired lesions (Box 83-1).5
ACCESSORY OSSIFICATION
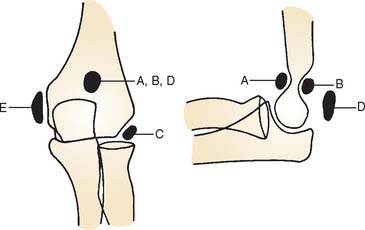
Confusion with acquired processes is caused by accessory ossicles that occur at the elbow as normal variations. Three sites for accessory ossicles about the elbow have been described: distal to the medial epicondyle; proximal to the tip of the olecranon (the patella cubiti); and in the olecranon fossa (the os supratrochleare dorsale8) (Fig. 83-1).
MEDIAL EPICONDYLE ACCESSORY OSSICLE
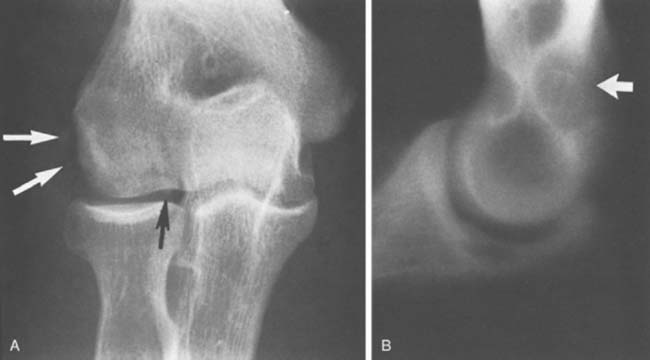
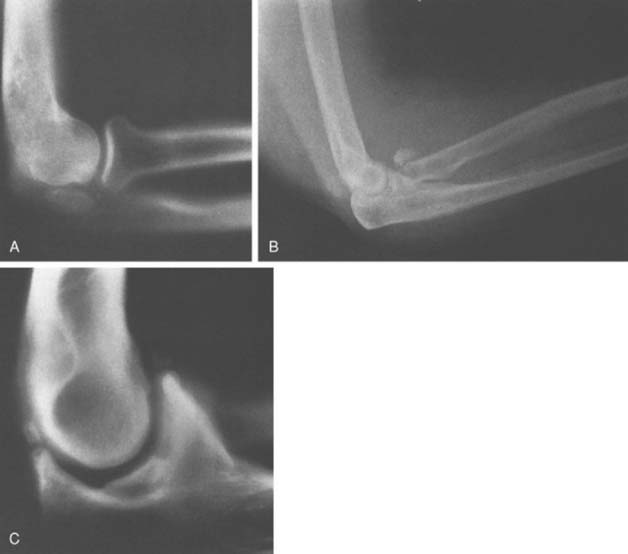
An accessory ossicle to the medial epicondyle19 is sometimes seen as a smooth, rounded ossification just distal to this structure. Because calcification can follow injury to the medial collateral ligament, whether the radiographic lesion reflects a traumatic or a congenital insult is often confusing,40 possibly more than at any other site. However, a discrete, rounded, smooth ossicle in patients who have no history of injury to this region suggests an accessory structure (Fig. 83-2). An irregular or misshapen medial epicondyle suggests a traumatic origin. Nevertheless, the formation of a fully developed medial epicondyle does not necessarily prove the diagnosis, because in a child, the medial epicondyle can remodel and appear normal in later years.
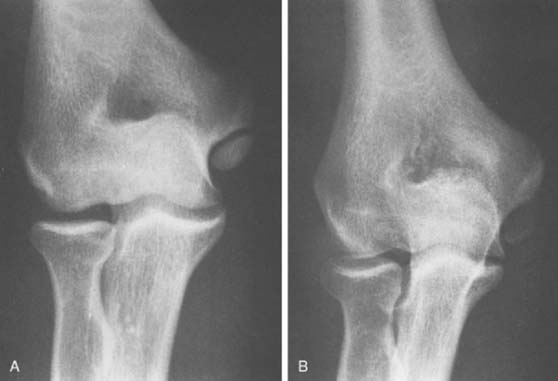
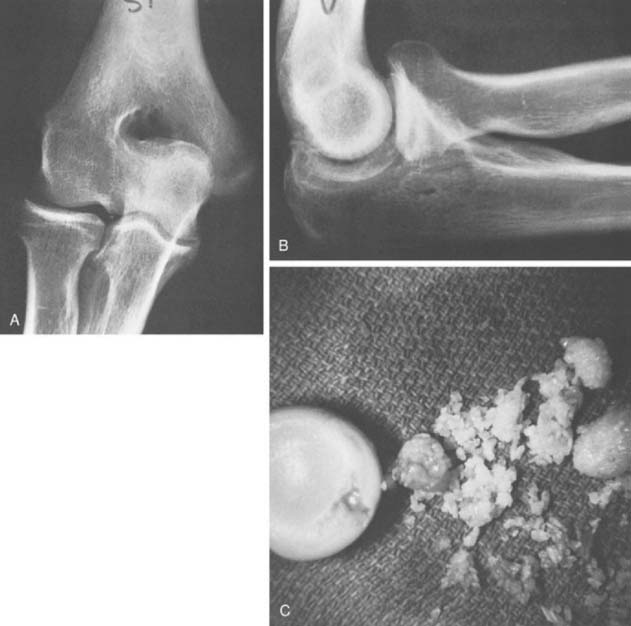
FIGURE 83-2 A, The occurrence of a smooth ossicle at the inferior aspect of the medial epicondyle without a history of injury represents an accessory ossicle. B, An earlier medial epicondyle fracture may have the same appearance, but the epicondyle may remodel in young patients (see Chapter 16). Note the loose bodies in the olecranon fossa.
PATELLA CUBITI
The so-called patella cubiti is rare but has been thoroughly described in earlier literature,16,20,37 and a thorough review has been presented by Habbe.16 This ossicle occurs in the triceps tendon near its insertion and is considered a true sesamoid bone.21 The proximal position is so characteristic in appearance that there should be little doubt about its origin (Fig. 83-3A). This structure should be distinguished from an avulsed olecranon apophysis, which appears farther distal (see Fig. 83-3B), and from a calcified olecranon bursa. Because of its superficial location, this ossicle may be subject to direct trauma and even fracture,4 but the injury generally responds to symptomatic treatment. Confusion with a fractured olecranon spur may exist.
OS SUPRATROCHLEARE DORSALE
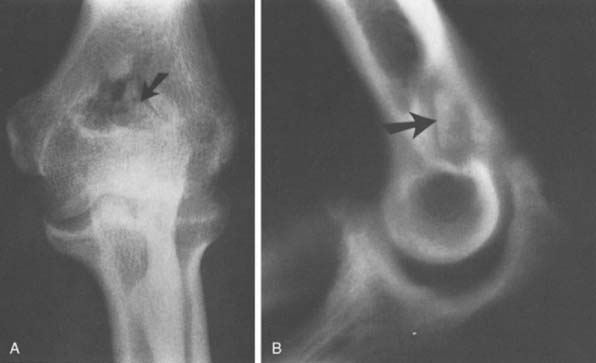
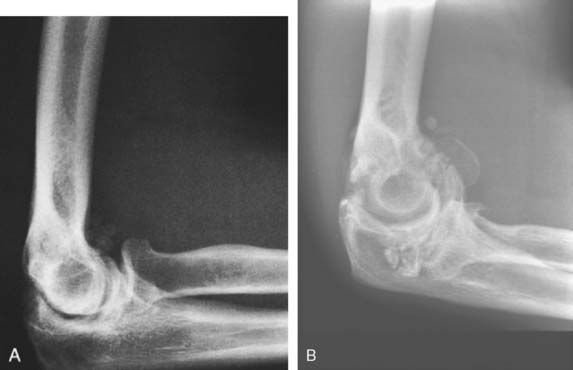
The radiographic density observed in the olecranon fossa has been a source of controversy. Characteristically, the ossicle has a smooth and round or oval shape that is often best seen in the lateral projection but is also demonstrated in the anteroposterior view (Fig. 83-4). In early descriptions of this entity it was considered to be a form of osteochondritis dissecans of the trochlea.29 The precise origin of this osseous structure is still the subject of some discussion, and trauma is often blamed.2 The supposed mechanism of injury is that with hyperextension, impaction of the tip of the olecranon into the olecranon fossa may cause a spur to develop at the tip of the olecranon. Conceivably it can be dislodged, form a nidus and grow into the characteristic os supratrochleare. Certainly, this mechanism has been implicated in the formation of loose bodies in the olecranon fossa (Fig. 83-5).39 The problem has been discussed in detail and clarified by Obermann and Loose, who concluded that the os supratrochleare dorsale is most likely a congenital accessory bone.30 Rather than being caused by trauma, it is subject to injury that produces secondary chondrometaplasia and resulting symptoms. When this occurs, the ossicle may look damaged and have an irregular margin.
Thus, the distinction between an ossicle caused by trauma and an existing one subjected to trauma remains obscure (see Fig. 83-5). Regardless of the source, the treatment is obvious. Mere radiographic evidence of the osseous density does not imply that it needs to be removed. If it is painful owing to injury caused by hyperextension or a direct blow, symptomatic treatment should resolve the pain. If catching, locking, or persistent pain is present, the ossicle is easily excised through a limited posterolateral incision or, preferably, arthroscopically (see Chapter 38).31
ACQUIRED LOOSE BODIES
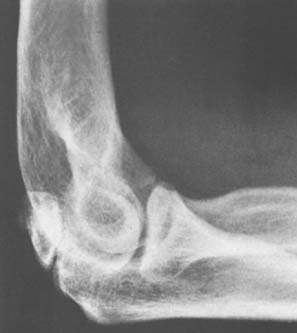
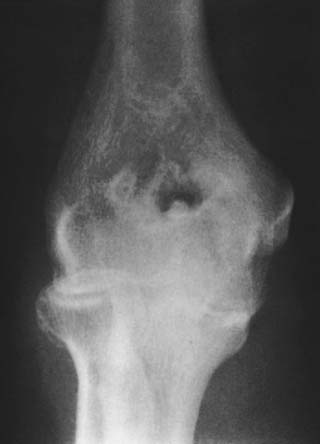
Loose or pedunculated cartilaginous or osseocartilaginous bodies are believed to originate from a small nidus.33 The sequence of morphologic alterations that ensue is common to all free bodies, regardless of their origin.25 Surface proliferation of chondroblasts and osteoblasts nourished by the synovial fluid creates a laminar or layered effect that is seen in about 87% of such bodies that are predominantly cartilaginous and in 80% of those that are predominantly osseous (Fig. 83-6).25The growth process continues as long as the free or pedunculated body is exposed to the synovial fluid.
ETIOLOGY
Our understanding of intra-articular loose bodies has been much enhanced by the work of Milgram.24–26 By clinical findings and presentation, acquired free or pedunculated bodies can be divided into three groups: (1) osteochondral fractures, (2) degenerative disease of the articular surface, and (3) a proliferative disorder of the synovium, synovial chondromatosis. Milgram25 defines three types of cartilage associated with loose bodies based on their supposed site of origin: (1) articularcartilage cells, (2) osteophytic cells from a proliferating osteophyte in a degenerative joint, and (3) lobular cartilage from the synovial lining cell. Pathologically, these loose bodies can originate from a joint fracture, from degenerative osteophytes, or de novo as a proliferative disease of the synovium.
OSTEOCHONDRAL FRACTURE
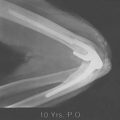
Fracture of the joint surface may be acute or the result of a chronic process such as osteochondritis dissecans. A shear fracture of the capitellum may involve little osseous substance, as in type II lesions (see Chapter 19). If in the acute stage, this fracture is missed, an intra-articular loose body may develop (Fig. 83-7). Elbow dislocation often leads to fractures of the coronoid, capitellum, or radial head and subsequent development of loose or attached intra-articular osseous bodies. Postreduction ossification of collateral ligaments is common, even without fracture. Avulsion of the medial epicondyle at the time of dislocation can also cause entrapment of the loose body in the joint with reduction. This complication is usually obvious (Fig. 83-8) and requires open treatment and fixation.
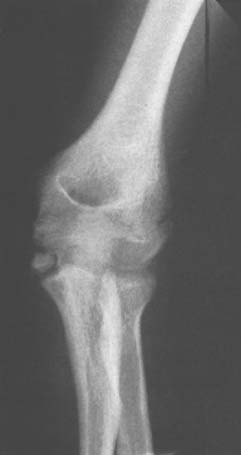
FIGURE 83-8 Entrapment of the medial epicondyle in the ulnohumeral joint after fracture-dislocation of the elbow.
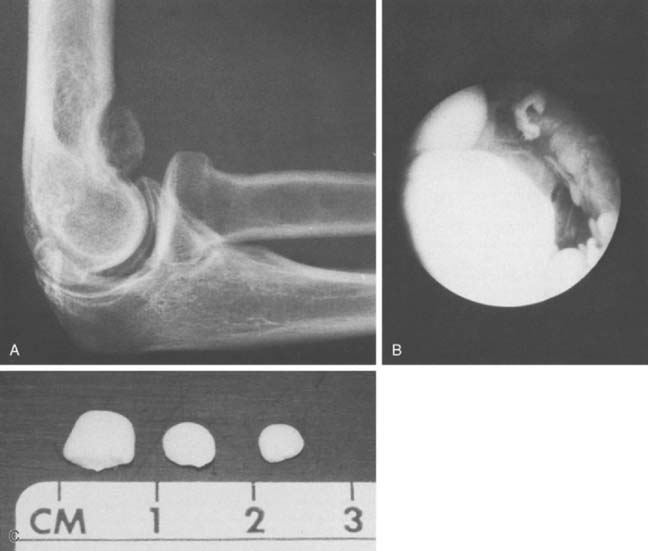
The development of osteochondritis dissecans of the capitellum is discussed thoroughly in Chapters 19 and 49. It can progress to fragment detachment, with formation of a loose body in the joint (Fig. 83-9). Clinical manifestations include the occurrence of subtle pain, loss of extension, grating, snapping, and frank locking of the joint that is due to separation of a loose osteocartilaginous fragment of osteochondritis dissecans or a lesion of the capitellum or other portions of the articular surface or margin (Fig. 83-10). In fact, because trauma has become the accepted cause for loose bodies in the elbow joint, some researchers attribute all extraneous ossific densities, even accessory ossicles, to trauma.1,27,29,40 Although the clinical features and precise causes vary, a bony nidus is the common pathologic finding in each of these traumatic lesions.24
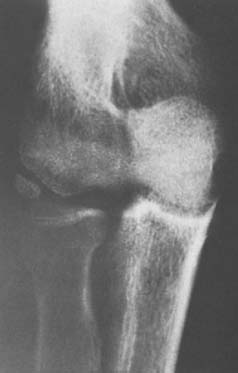
FIGURE 83-9 Osteochondritis dissecans of the capitellum has caused an osteocartilaginous loose body in the joint.
LOOSE BODIES OF DEGENERATIVE ORIGIN
Degenerative joint disease can induce loose body formation by creating a nidus from a fragmented joint surface, from a degenerative osteophyte, or from the synovium of a joint involved with degenerative cartilaginous changes.25 Primary degenerative arthritis is discussed in detail in Chapter 76. Bullock and Goodfellow7 have studied primary articular changes of the radiohumeral joint; this process could certainly give rise to the formation of a nidus and the subsequent development of a loose body. Bell3 reviewed 52 instances of loose bodies in the elbow and concluded that most were related to primary or secondary osteoarthritis. He observed that most occurred in the anterior aspect of the joint (Fig. 83-11), but we cannot confirm this from our experience. With degenerative disease, small osteophytes may be observed at the tips of both the olecranon and the coronoid process, either of which could eventually give rise to the development of an intra-articular loose body (see Fig. 83-7C). In fact, the presence of loose bodies is recognized to be an integral pathologic feature of primary osteoarthritis (Fig. 83-12).28
TREATMENT
The use of the arthroscope has enhanced the diagnosis, localization, and removal of loose bodies (Fig. 83-13)(see Chapter 38). Arthroscopic removal is the treatment of choice for symptomatic loose bodies.31,35 I prefer replacement of a large fragment of the capitellum in osteochondritis dissecans by a suture technique, and the topic is discussed in Chapter 39.
SYNOVIAL CHONDROMATOSIS
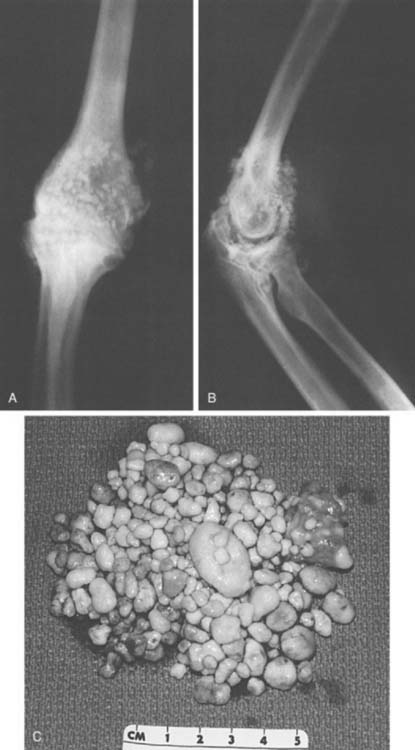
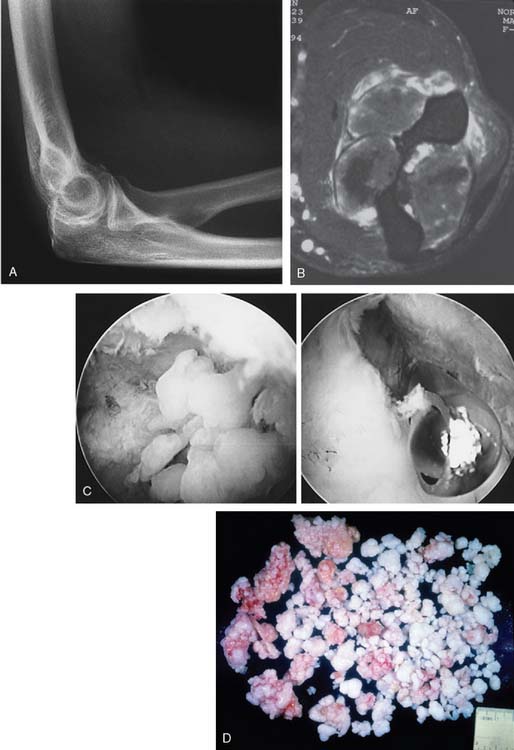
Since the initial description of the entity in 1558,32 much has been written about synovial chondromatosis or osteochondromatosis, and we have recently reviewed our experience with this condition at the elbow.18 In this review, an 80% expectation of successful management with arthroscopic or open débridement was documented. The topic is discussed further in Chapter 76.9 Henderson and Jones reviewed the literature and reported the initial Mayo Clinic experience of 25 cases in 1923.17 They concluded that this entity was separate from traumatic or degenerative loose body formation and that the nidus originated from the synovial tissue (Fig. 83-14). More specifically, the condition is believed to be a proliferative disorder of the subsynovial soft tissue.14 Thus, some risk of malignant transformation is recognized, possibly as high as 5%.9 Milgram has identified three phases of the process.26 In the active initial phase, no free or loose bodies are present. In the transitional phase, osteochondral nodules form in the synovial membrane and nonossified free bodies are found in the joint (Fig. 83-15). In the final phase, free, sometimes very extensive ossified, osteochondral bodies apparently herald the quiescent phase of the disease (Fig. 83-16).
In the second phase, the cartilaginous component becomes symptomatic before it ossifies, producing symptoms of elbow pain and loss of motion even in the presence of a relatively normal radiograph (see Fig. 83-16). Enchondral bone formation replaces the cartilaginous component, and this gives rise to the obvious radiographic demonstration of multiple loose bodies (see Fig. 83-16). Thus, the condition may present with multiple (as many as 100) radiolucent cartilaginous (see Fig. 83-15) or radiopaque ossified (see Fig. 83-16) loose bodies. When the bodies are ossified, a possible cartilaginous neoplasm may be considered, but as discussed in Chapter 82, with the exception of the experience reported by Davis and coworkers,10 the occurrence of cartilaginous sarcomas about the elbow is extremely rare. Yet the distinction can be difficult and may be resolved only by histologic examination of the tissue.11 More recently, computed tomography has been demonstrated to be effective in confirming this diagnosis in the nonossified phase.34 The disease is usually considered a self-limiting process that runs a rather predictable course, but this is not always the case and the process may be very aggressive.26 More recent reports have emphasized the development of nerve compression from capsular distension.36,38 The large volume occupied by the chondromatous tissue has been shown to cause anterior distension and compression of the radial nerve at the arcade of Frohse, which results in partial paralysis of the posterior interosseous nerve.13 A similar mechanism might be attributed to the ulnar neuropathy reported by Roth and others12,23 and even the cutaneous branch of this nerve has been involved.38
SIGNS AND SYMPTOMS
Loose bodies are more common in men than in women, regardless of the etiology, traumatic, degenerative, or proliferative.17 Among Bell’s 52 cases and in our experience, most patients present with loss of motion, usually extension.3 Symptoms may be of catching but rarely are disabling. Pain may or may not be a finding and usually occurs with a sensation of locking or grating. The discomfort is usually generalized. Radiography is helpful for large lesions but can be deceptive if it fails to demonstrate loose bodies that have not yet calcified, if small ossicles are present in the ulnohumeral joint (see Fig. 83-5B), if the location is obscured by surrounding structures, or if the traumatic lesion contains little osseous tissue. These difficulties in diagnosis have been solved with magnetic resonance imaging.6,22
TREATMENT
Regardless of the stage of presentation, arthroscopy, and synovectomy, if required, is now clearly the treatment of choice for removal of the loose bodies (Fig. 83-17). If the process extends beyond the capsule, an open procedure is of course required.
1 Atsatt S. Loose bodies of the elbow joint: An unusual location and form. J. Bone Joint Surg. 1933;15:1008.
2 Bassett L.W., Mirra J.M., Forrester D.M., Gold R.H., Bernstein M.L., Rollins J.S. Post-traumatic osteochondral “loose body” of the olecranon fossa. Radiology. 1981;141:635.
3 Bell M.S. Loose bodies in the elbow. Br. J. Surg. 1975;62:921.
4 Birsner J.W., DeSmet D.H. Patella cubiti with fracture. Ann. West. Med. Surg. 1950;4:744.
5 Broberg M.A., Morrey B.F. Results of treatment of fracture dislocations of the elbow. Clin. Orthop. Relat. Res. 1987;216:109.
6 Brunton L.M., Anderson M.W., Pannunzio M.E., Khanna A.J., Chhabra A.B. Magnetic resonance imaging of the elbow: Update on current techniques and indications. J. Hand Surg. 2006;31A:1001.
7 Bullock P.G., Goodfellow J.W. Pattern of aging of the articular cartilage of the elbow joint. J. Bone Joint Surg. 1967;49B:175.
8 Burman M.S. Unusual locking of the elbow joint by the sesamum cubiti and a free joint body. Am. J. Radiol. 1941;45:731.
9 Christensen J.H., Poulsen J.O. Synovial chondromatosis. Acta Orthop. Scand. 1975;46:919.
10 Davis R.I., Hamilton A., Biggart J.D. Primary synovial chondromatosis: A clinicopathologic review and assessment of malignant potential. Hum. Pathol. 1998;79:683.
11 Dufour J.P., Hamels J., Maldague B., Noel H., Pestiaux B. Unusual aspects of synovial chondromatosis of the elbow. Clin. Rheumatol. 1984;3:247.
12 Fahmy N.R.M., Noble J. Ulnar nerve palsy as a complication of synovial osteochondromatosis of the elbow. Hand. 1981;13:308.
13 Field J.H. Posterior interosseous nerve palsy secondary to synovial chondromatosis of the elbow joint. J. Hand Surg. 1981;6:336.
14 Fisher A.G.T. A study of loose bodies composed of cartilage or of cartilage and bone occurring in joints. Br. J. Surg. 1931;8:493.
15 Gudmundsen T.E., Ostensen H. Accessory ossicles in the elbow. Acta Orthop. Scand. 1987;58:130.
16 Habbe J.E. Patella cubiti, a report of four cases. A. J. R. 1942;48:513.
17 Henderson M.S., Jones H.T. Loose bodies in joints and bursae due to synovial osteochondromatosis. J. Bone Joint Surg. 1923;5:400.
18 Kamineni S., O’Driscoll S.W., Morrey B.F. Synovial osteochondromatosis of the elbow. J. Bone Joint Surg. 2002;84B(7):961-966.
19 Keates T.E. An Atlas of Normal Roentgen Variants That May Simulate Disease. Chicago: Year Book Medical Publishers, 1979.
20 Kienbock R., Desenfans G. Uber Anomalien am Ellbogengelenk Patella cubiti. Beitr. Klin. Chir. 1937;165:524.
21 Kohler A., Zimmer E.A. Borderlands of the Normal and Early Pathologic in Skeletal Anatomy, 3rd ed. New York: Grune & Stratton, 1968.
22 Martinoli C., Bianchi S., Zamorani M.P., Zunzunegui J.L., Derchi L.E. Ultrasound of the elbow. Europ. J. Ultrasound. 2001;14:21.
23 Lister J.R., Day A.L., Ballinger W. Ulnar palsy caused by synovial chondromatosis. Surg. Neurol. 1981;15:428.
24 Milgram J.W. The classification of loose bodies in human joints. Clin. Orthop. Relat. Res. 1977;124:282.
25 Milgram J.W. The development of loose bodies in human joints. Clin. Orthop. Relat. Res. 1977;124:292.
26 Milgram J.W. Synovial osteochondromatosis. J. Bone Joint Surg. 1977;59B:492.
27 Morgan P.W. Osteochondritis dissecans of the supratrochlear septum. Radiology. 1953;60:241.
28 Morrey B.F. Primary osteoarthritis of the elbow: Ulno-humeral arthroplasty. J. Bone Joint Surg. 1992;74B:409.
29 Morton H.S., Crysler W.E. Osteochondritis dissecans of the supratrochlear septum. J. Bone Joint Surg. 1945;27:12.
30 Obermann W.R., Loose H.W.C. The os supratrochleare dorsale: A normal variant that may cause symptoms. A.J.R. 1963;141:123.
31 O’Driscoll S.W., Morrey B.F. Arthroscopy of the elbow: A critical analysis. J. Bone Joint Surg. 1992;74A:84.
32 Paré A. As quoted by Henderson, M.S., and Jones, H. T. J. Bone Joint Surg. 1923;5:400.
33 Phemister D.B. The causes and changes in loose bodies arising from the articular surface of the joint. J. Bone Joint Surg. 1924;6:278.
34 Rao J.P., Spingola C., Mastromonaco E., Villacin A. Synovial osteochondromatosis: Computerized axial tomography, frozen section and arthrography in diagnosis and management. Orthop. Rev. 1986;15:94.
35 Rupp S., Tempelhof S. Arthroscopic surgery of the elbow. Therapeutic benefits and hazards. Clin. Orthop. Relat. Res. 1995;313:140-145.
36 Ruth R.M., Groves R.J. Synovial osteochondromatosis of the elbow presenting with ulnar nerve neuropathy. Am. J. Orthop. 1996;25:843.
37 Sachs J., Degenskein G. Patella cubiti. Arch. Surg. 1948;57:675.
38 Slater R.N.S., Koka S.R., Ross K.R. Cheiralgia paraesthetica secondary to synovial osteochondromatosis of the elbow. J. Orthop. Rheumatol. 1993;6:179-181.
39 Tullos H.S., King J.W. Lesions of the pitching arm in adolescents. J. A. M. A. 1972;220:264.
40 Zietlin A. The traumatic origin of accessory bones at the elbow. J. Bone Joint Surg. 1935;17:933.