Chapter 13 Local Anesthetics
| Abbreviations | |
|---|---|
| CNS | Central nervous system |
| Epi | Epinephrine |
| IV | Intravenous |
| VGSC | Voltage-gated sodium channels |
Therapeutic Overview
Local anesthetics reversibly block the generation and conduction of action potentials in all excitable cells. These drugs have many uses in medicine ranging from dental to obstetric procedures, because they prevent painful stimuli from being perceived in a specific part of the body into which they have been locally administered. Local anesthetics can also affect an entire region of the body when injected (e.g., when injected into the spinal cord). The first known local anesthetic, cocaine, is a natural product, but its abuse liability (see Chapter 37) precludes most of its clinical uses except in ophthalmology. These anesthetics may be applied locally by subcutaneous infiltration for the removal of a superficial skin lesion or may be applied to the spinal cord as a regional anesthetic for a hip replacement procedure or for the management of postoperative pain. Thus the selection of a local anesthetic depends on the pharmacokinetic profile required for each specific use and on the side effect profile of the compound, factors summarized in the Therapeutic Overview Box.
| Therapeutic Overview |
|---|
| Factors in drug selection |
| Speed of onset |
| Duration of effect |
| Side effects |
| Seizures |
| Cardiovascular depression |
Mechanisms of Action
and all neurons in the central nervous system (CNS). Thus the actions of these compounds can be restricted by administering them locally. Certain practical pharmacokinetic properties make these agents particularly useful in temporarily blocking the sensory transmission of pain impulses. The greatest advantage of local anesthetics is their reversibility—that is, once the drug is eliminated by metabolism, diffusion from its injection site, or excreted, the nerve resumes normal function. There are generally no long-term consequences from the use of local anesthetics. Thus these drugs are highly effective in providing regional or localized reversible pain relief.
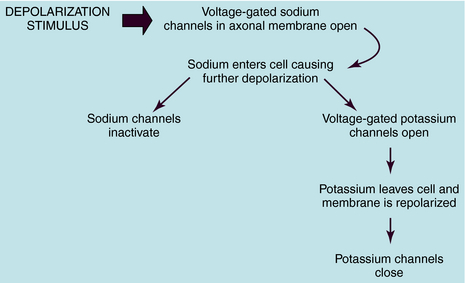
The resultant K+ efflux through these and non–voltage-gated K+ channels returns the membrane potential to its normal resting value. This mechanism is depicted in Figure 13-1.
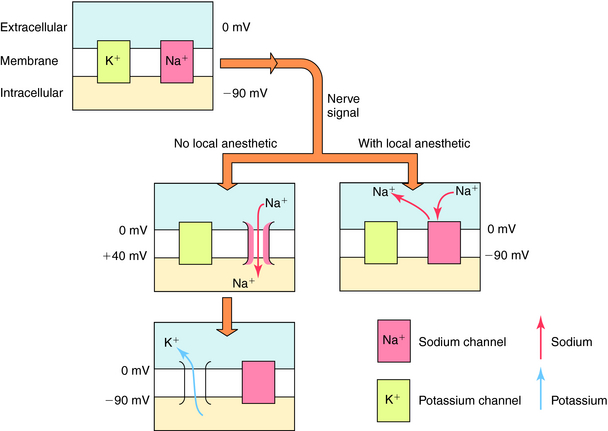
The cellular physiological mechanism by which local anesthetics block the conduction of nerve impulses is well understood. These drugs bind selectively to VGSCs at the intracellular surface of the channel pore near the pore’s vestibule and thereby block the pathway for Na+ and prevent the channels from opening (Fig. 13-2). By blocking Na+ influx, these drugs prevent the depolarization necessary for action potential propagation and, at sufficient concentrations, block impulse conduction. Because local anesthetics dissociate from the VGSC very rapidly, once drug administration is stopped, the drug diffuses away from the nerve and/or is absorbed by the local circulation, and impulse activity is restored.
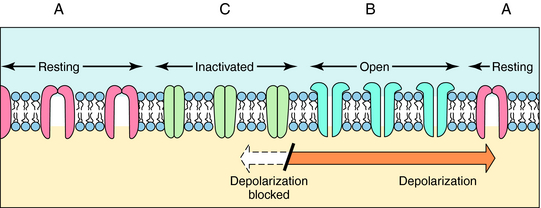
The ability of local anesthetics to block VGSC is highly dependent on the activity (or state) of the channel. Na+ channels exist in three major states (Fig.13-3). In the resting closed state, the channels do not allow Na+ influx and are highly sensitive to depolarization-induced opening. In the open state, the channels allow Na+ influx, whereas in the inactivated closed state, they do not allow Na+ influx and are not opened by depolarization. Local anesthetics have different potencies for binding to these different states. They are much more likely to bind when the channels are active, open, or inactivated and are less likely to bind to the resting state. This modulation of affinity is called activity (or state) dependence and has great practical importance. Because local anesthetics preferentially block nerves in which Na+ channels are open or inactivated (activity-dependent), they are more potent in rapidly firing nerves than in nerves in which action potentials occur less frequently. Because sensory neurons fire at greater frequencies in response to more intense noxious stimuli, the effectiveness of impulse blockade is greater under these conditions.
Pharmacokinetics
The structures of the local anesthetics have a direct bearing on their therapeutic actions (Fig. 13-4). All of these agents contain a hydrophobic group linked by either an ester or an amide bond to a relatively hydrophilic group (usually a tertiary or quaternary amine). This hydrophilic group makes the drug H2O-soluble, enabling the agent to diffuse to the nerve. The hydrophobic group makes the agent lipid soluble and enables the drug to penetrate the lipid membranes (sheath, perineural tissue, and nerve membrane) to reach its binding site on the inner surface of the VGSC. The more hydrophobic local anesthetics bind more tightly to the channel.
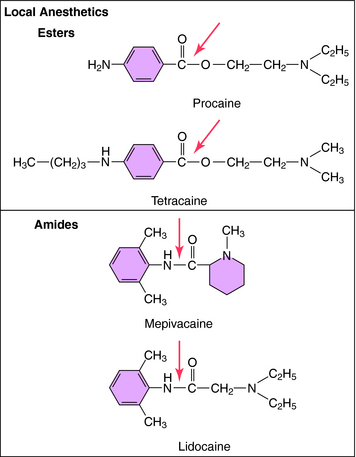
FIGURE 13–4 Structures of the two types of local anesthetics depicting sites of hydrolysis (shown with arrows).
Local anesthetics must cross the neuronal cell membrane to reach the VGSC; therefore sufficient amounts of the drugs must be in the unionized form to gain entry to the cell. However, the charged form of the compound is thought to be most active. Thus an important chemical aspect of local anesthetics is that they are weak bases, with pKa values of 8 to 9. At physiological pH (~7.4), most (80% to 90%) of the drug is in the ionized (charged) form (see Chapter 2). Once inside the cell, the predominant ionized form of the agent binds to the Na+ channel proteins.
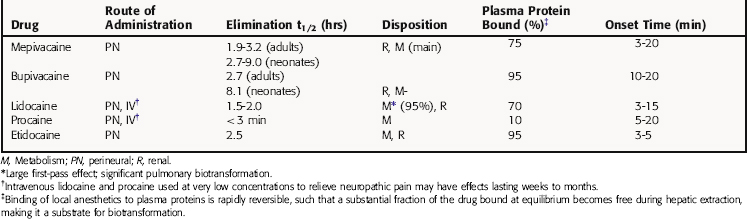
The ester local anesthetics, including procaine and tetracaine (see Fig. 13-4), are rapidly hydrolyzed to inactive products by plasma cholinesterase (butyrylcholinesterase) and liver esterases. Thus these compounds have a very short t1/2 in the body (Table 13-1). Certain body sites lack esterase activity, including the spinal fluid, which causes the duration of local anesthetic action with “spinal” (intrathecal) anesthesia to be considerably extended. The amide local anesthetics, by contrast, are metabolized by hepatic cytochrome P450s through N-dealkylation, followed by hydrolysis. The relative rates of biotransformation of these local anesthetics vary depending on their specific chemical structure (see Table 13-1).
Relationship of Mechanisms of Action to Clinical Response
Anatomical, physiological, and chemical factors all play important roles in determining the susceptibility of nerve fibers to blockade by local anesthetics during clinical procedures. Because the Na+ channels present in different types of neurons have similar affinities for local anesthetics, these drugs block impulse conduction in all nerve cells, including sensory, motor, autonomic, and CNS neurons. Na+ channels also play an important role in other electrically excitable cells. Thus local anesthetics can also have prominent effects on skeletal and cardiac muscle. Their effects on cardiac Na+ channels form the basis for their therapeutic use in the treatment of certain cardiac dysrhythmias (see Chapter 22). Their effects on smooth and cardiac muscle also contribute to their toxicity. However, local anesthetics are not equally potent and effective in all cells where Na+ channels are present, and they exhibit some selectivity for different neuronal and muscle cell types. This selectivity is determined by the rate of drug penetration, firing rate, and axonal size and location within the nerve bundle and importantly, by the “margin of safety” for nerve impulse transmission. This last factor, a measure of how well transmission can overcome deficits in excitability, differs among different fiber types and also at different zones in a neuron. It is likely to be highest in the trunk region of an axon, lower at distal and central branch regions, and lower also at the impulse generating sites such as the distal terminals of sensory fibers.
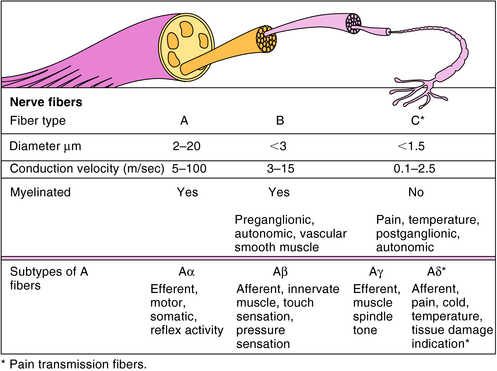
Neurons differ substantially in terms of their diameter, degree of myelination, and frequency of firing, and all of these factors influence the ability of local anesthetics to produce blockade. The three major types of nerve fibers are classified as A, B, or C (Fig. 13-5). A fibers are myelinated and have the fastest conduction velocity, B fibers are myelinated with a slower conduction velocity, and C fibers are unmyelinated and have the slowest conduction velocity. Most pain impulses in humans are carried by the Aδ and C fibers. The Aδ fibers, which are distributed primarily in skin and mucous membranes, are the smallest subtype of A fibers (2 to 5 μm diameter) and are associated with a sharp, pricking pain termed “fast pain.” C fibers, which are more widely distributed, are smaller than Aδ fibers (< 1.5 μm diameter) and are associated with a duller, long-lasting, burning pain termed “slow pain.”
The anatomical location of different nerve types also influences the ability of local anesthetics to produce nerve block. For example, peripheral nerves are never affected uniformly by a local anesthetic, because a concentration gradient of the drug from the mantle to the core of the nerve is established during the onset of the block; a steady-state distribution of drug in the nerve is rarely achieved. Thus a dynamic block results from both the rapid influx of the drug into the nerve and a slower efflux from the nerve after injection of a concentrated bolus. Axons in the mantle are generally exposed to higher drug concentrations than are axons in the core, where drug diffusion is more restricted. Although these principles help explain the differential sensitivity of nerve fibers to local anesthetics, it is difficult to predict what will happen in every given situation. Sensory modalities are lost in the following general order: cold, warmth, pain, touch, and deep pressure. As indicated above, motor functions appear to be more resistant to blockade by local anesthetics, but this may result from the relatively complex motor tasks that are tested in the clinical setting, wherein the patient can recruit several different groups of muscles to accomplish a similar movement. Indeed, when simple muscle movements—for example, extensor postural thrust—are isolated in experimental animals during peripheral nerve block, their deficiencies are greater and longer in duration than the loss of pain sensitivity.
Local anesthetics are generally less effective in inflamed tissues than in normal tissues, because inflammation usually results in a local metabolic acidosis, which decreases the pH in surrounding tissues. At an acidic pH, the portion of drug in the nonionized form is reduced markedly, resulting in reduced penetration of cell membranes and reducing the amount of drug reaching its site of action (see Chapter 2).
Local anesthetics are used widely to provide temporary pain relief in localized regions of the body. By varying the drug, its concentration and dose, and the method of administration, a wide range of effects can be obtained. A localized, intensely numb area of skin can be achieved for a short time by infiltrating the area with a short-acting drug. On the other hand, a sensory block can be achieved by administering a dilute solution of a long-acting local anesthetic through an indwelling catheter to the epidural space, providing complete anesthesia while preserving most motor function, including uterine motility. Anesthetics are used to simultaneously provide analgesia and muscle relaxation through spinal or epidural administration, and this route is also used to administer dilute solutions of local anesthetics mixed with opioids for postoperative pain relief (see Chapter 36). These combinations provide effective analgesia and have the advantage of using a lower total dose of the opioid drug than normally would be required alone. Local anesthetics are used as both diagnostic and therapeutic tools in the management of more complicated acute and chronic pain states.
Pharmacovigilance: Side Effects, Clinical Problems, and Toxicity
Local anesthetics have potentially deleterious effects on cardiac pacemaker activity, electrical excitability,
CNS seizures and convulsions at high concentrations of agent
Blockade of cardiac Na+ channels (also used therapeutically for antiarrhythmic effects; see Chapter 22)
conduction times, and contractile force. Dysrhythmias are possible when high blood concentrations of anesthetic agents are attained. For example, cocaine is a potent local anesthetic and is a prominent drug of abuse, which has extensive adverse effects on the heart and the CNS (see Chapter 37). Bupivacaine is the most cardiotoxic of the local anesthetics, whereas lidocaine is used therapeutically in certain arrhythmias (see Chapter 22).
Amir R, Argoff CE, Bennett GJ, et al. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain. 2006;7(5 Suppl 3):S1-S29.
Corman SL, Skledar SJ. Use of lipid emulsion to reverse local anesthetic-induced toxicity. Ann Pharmacother. 2007;41:1873-1877.
Heavner JE. Local anesthetics. Curr Opin Anaesthesiol. 2007;20:336-342.