Local anesthetic agents: Pharmacology
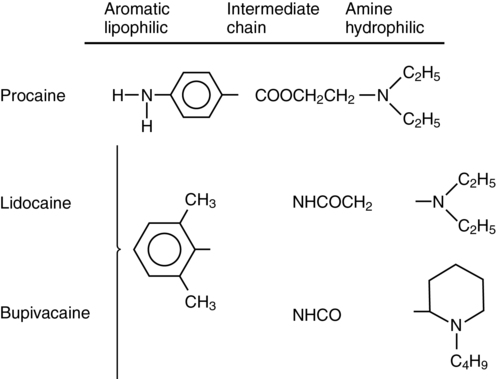
Local anesthetic agents consist of three major chemical moieties (Figure 116-1): a lipophilic aromatic ring, a hydrophilic tertiary amine, and an ester or amide linkage. Changes in the amine or ring chemical structure result in marked alterations in lipid/aqueous solubility, potency, and protein binding. Local anesthetics are classified into two major groups based on the linkage between the lipophilic and hydrophilic components: amino esters and amino amides. Though they exert their effect by the same mechanism, they are metabolized differently (esters in the blood by pseudocholinesterase; amides by normal hepatic pathways) and have different allergic potential (ester greater than amide). The most commonly used local anesthetic agents and their physiochemical properties are described in Table 116-1.
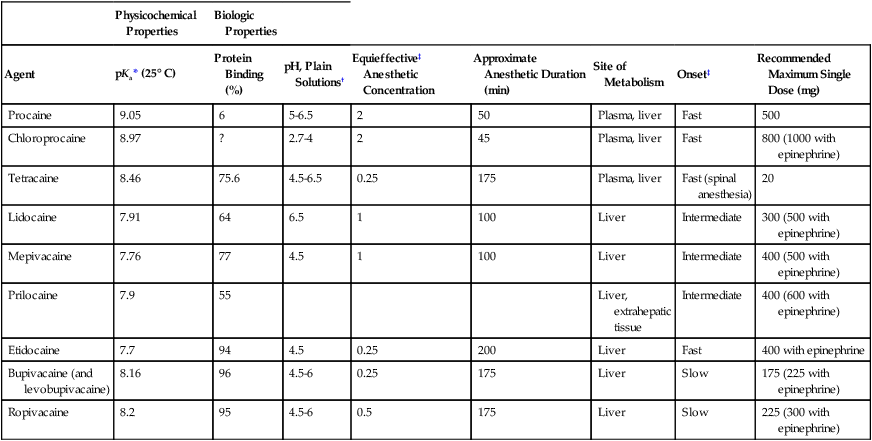
Table 116-1
Physicochemical/Biologic Properties of Local Anesthetic Agents
| Physicochemical Properties | Biologic Properties | |||||||
| Agent | pKa* (25° C) | Protein Binding (%) | pH, Plain Solutions† | Equieffective‡ Anesthetic Concentration | Approximate Anesthetic Duration (min) | Site of Metabolism | Onset‡ | Recommended Maximum Single Dose (mg) |
| Procaine | 9.05 | 6 | 5-6.5 | 2 | 50 | Plasma, liver | Fast | 500 |
| Chloroprocaine | 8.97 | ? | 2.7-4 | 2 | 45 | Plasma, liver | Fast | 800 (1000 with epinephrine) |
| Tetracaine | 8.46 | 75.6 | 4.5-6.5 | 0.25 | 175 | Plasma, liver | Fast (spinal anesthesia) | 20 |
| Lidocaine | 7.91 | 64 | 6.5 | 1 | 100 | Liver | Intermediate | 300 (500 with epinephrine) |
| Mepivacaine | 7.76 | 77 | 4.5 | 1 | 100 | Liver | Intermediate | 400 (500 with epinephrine) |
| Prilocaine | 7.9 | 55 | Liver, extrahepatic tissue | Intermediate | 400 (600 with epinephrine) | |||
| Etidocaine | 7.7 | 94 | 4.5 | 0.25 | 200 | Liver | Fast | 400 with epinephrine |
| Bupivacaine (and levobupivacaine) | 8.16 | 96 | 4.5-6 | 0.25 | 175 | Liver | Slow | 175 (225 with epinephrine) |
| Ropivacaine | 8.2 | 95 | 4.5-6 | 0.5 | 175 | Liver | Slow | 225 (300 with epinephrine) |
*pH corresponds with 50% ionization.
†Epinephrine-containing solutions have a pH that is 1-1.5 units lower than the pH of plain solutions.
‡When used for a brachial plexus block.
Adapted from Mather LE, Tucker GT. Properties, absorption, and disposition of local anesthetic agents. In: Cousins MJ, Carr DB, Horlocker TT, Bridenbaugh PO, eds. Neural Blockade in Clinical Anesthesia and Management of Pain. 4th ed. Philadelphia, Lippincott Williams & Williams, 2009:48-95; and Rosenberg PH, Veering BT, Urmey WF. Recommended doses of local anesthetics: A multifactorial concept. Reg Anesth Pain Med. 2004;29:264-275.
Physiochemical properties
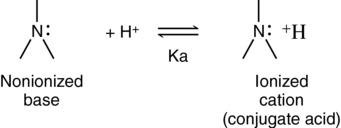
In solution, the local anesthetics exist in equilibrium as ionized and nonionized forms (Figure 116-2). The nonionized (lipid-soluble) base crosses the axonal membrane and, once intracellular, in a more acidic environment, ionizes. The ionized (water-soluble) cation is responsible for neural blockade.
Physiologic disposition
Esters
Ester local anesthetic agents are metabolized by pseudocholinesterase (plasma cholinesterase) and partially by red blood cell esterases. Hydrolysis occurs at the ester linkage and yields an alcohol and para-aminobenzoic acid (or a PABA) derivative. Because ester local anesthetics are metabolized by pseudocholinesterase, toxicity and duration of blockade may be prolonged in patients with liver disease, in neonates, or in atypical cholinesterase carriers (Table 116-2).
Table 116-2
| Study Population/Sample | No. of Patients/Specimens | Half-life (sec)* |
| Mothers | 7 | 20.9 ± 5.8 |
| Umbilical cords | 7 | 42.6 ± 11.2 |
| Male control subjects | 6 | 20.6 ± 4.1 |
| Female control subjects | 5 | 25.2 ± 3.7 |
| Homozygous–atypical cholinesterase carriers | 10 | 106.0 ± 45.0 |
*Data are presented as means ± SD.
Results are from O’Brien JE, Abbey V, Hinsvark O, et al. Metabolism and measurement of chloroprocaine, an ester-type anesthetic. J Pharm Sci. 1979;68:75-78.
Amides
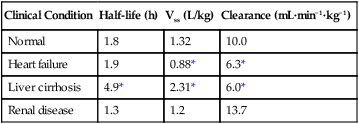
Because these drugs have neurologic and cardiac toxicity, which is related to blood concentration, epinephrine in concentrations of 2.5 to 5 μg/mL should be added to the local anesthetic solution when large doses are administered, providing there are no contraindications to the use of epinephrine. The vasoconstriction caused by epinephrine decreases perfusion at the site of injection, thereby slowing systemic uptake and decreasing toxicity but also prolonging the duration of action. As a rule, conditions (e.g., end-stage pregnancy, increased age for epidural or spinal anesthesia or analgesia) or diseases (uremia) that may increase the rate of the initial uptake of the local anesthetic agent are indications to reduce the dose in comparison to the dose used for young, healthy, and nonpregnant adults. On the other hand, the reduced clearance of local anesthetic agents associated with renal, hepatic, and cardiac diseases is the most important reason to reduce the dose for repeated or continuous administration (Table 116-3). The magnitude of the reduction should be related to the expected influence of the pharmacodynamic or pharmacokinetic change.
Table 116-3
Lidocaine Disposition in Healthy Patients and Those With Various Diseases
| Clinical Condition | Half-life (h) | Vss (L/kg) | Clearance (mL·min−1·kg−1) |
| Normal | 1.8 | 1.32 | 10.0 |
| Heart failure | 1.9 | 0.88* | 6.3* |
| Liver cirrhosis | 4.9* | 2.31* | 6.0* |
| Renal disease | 1.3 | 1.2 | 13.7 |
Vss, the volume of distribution at steady state.
*Values differ significantly in comparison with normal subjects.
From Thompson P, Melmon KL, Richardson JA, Rowland M. Lidocaine pharmacokinetics in advanced heart failure, liver disease and renal failure in humans. Ann Intern Med. 1973; 78:499-508.