Local and Topical Anesthesia
Background
The first local anesthetic was cocaine, an alkaloid in the leaves of the Erythroxylon coca shrub from the Andes Mountains. Early Incan society used cocaine for invasive procedures, including cranial trephination. In 1884, Koller1 used topical cocaine in the eye and was credited with the introduction of local anesthesia into clinical practice. In the same year, Zenfel used a topical solution of alcohol and cocaine to anesthetize the eardrum, and Hall2 introduced the drug into dentistry. In 1885 Halsted3 demonstrated that cocaine blocked nerve transmission, thereby laying the foundation for nerve block anesthesia. The search for alternatives to cocaine led to synthesis of the benzoic acid ester derivatives and the amide anesthetics used today. It was not until the 1960s that detailed understanding of the physiochemical properties, mechanism of action, pharmacokinetics, and toxicity of these agents emerged.
Pharmacology and Physiology
Chemical Structure and Physiochemical Properties
Most useful local anesthetic agents share a basic chemical structure:
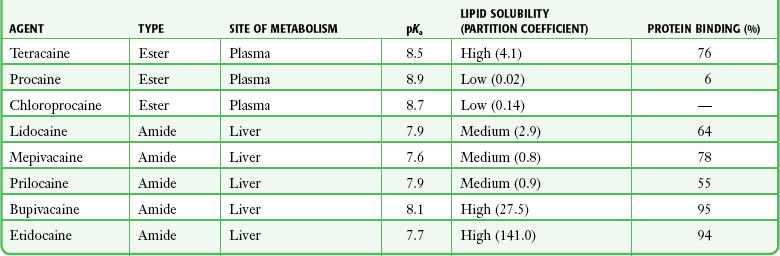
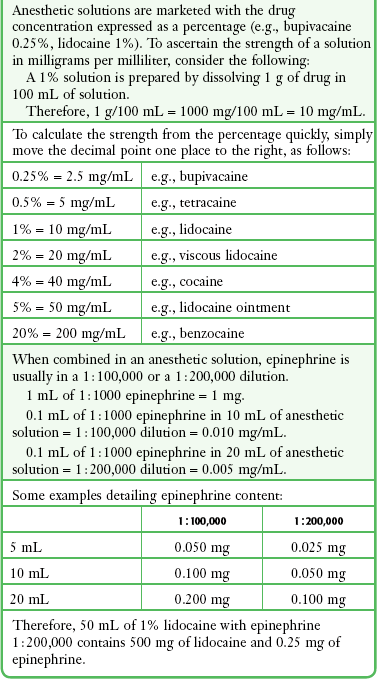
Subtle variations in this basic structure determine each agent’s main physiochemical properties: the negative log of dissociation constant (pKa), the partition coefficient (a measurement of lipid solubility), and the degree of protein binding. Each of these properties determines the drug’s potency, onset, and duration of action. However, physiochemical properties are not the sole determinant of clinical activity; other factors influence the drug’s effect. The intermediate chain between the aromatic and the hydrophilic segments is either an amino-ester or an amino-amide; these chemical structures form the basis for the two main classifications of local anesthetics. Common ester-type agents include procaine, chloroprocaine, cocaine, and tetracaine. Common amide-type agents include articaine, lidocaine, mepivacaine, prilocaine, bupivacaine, and etidocaine. Different biochemical pathways metabolize each class. Esters are hydrolyzed by plasma pseudocholinesterase. Cocaine, an ester, is also partly metabolized by N-demethylation and nonenzymatic hydrolysis. Individuals with pseudocholinesterase deficiency may have a greater potential for cocaine toxicity if large doses are used, although this has not been an issue when cocaine is used clinically as an anesthetic. Amides are metabolized in the liver by enzymatic degradation. Local anesthetics are poorly soluble weak bases combined with hydrogen chloride to produce the salt of a weak acid. In solution, the salt exists both as uncharged molecules (nonionized) and as positively charged cations (ionized). The nonionized form is lipid soluble, which enables it to diffuse through tissues and across nerve membranes. The ratio of nonionized to ionized forms depends on the pH of the medium (vial solution or tissue milieu) and on the pKa of the specific agent. The pKa is the pH in which 50% of the solution is in the uncharged form and 50% is in the charged form. When the pH of the solution or tissue is less than the pKa, more of the drug is ionized. When the pH increases, more of the drug is in the nonionized form. Because the nonionized form of drug can diffuse through tissues and nerves, manipulating the pH of the solution can alter a drug’s diffusion properties.
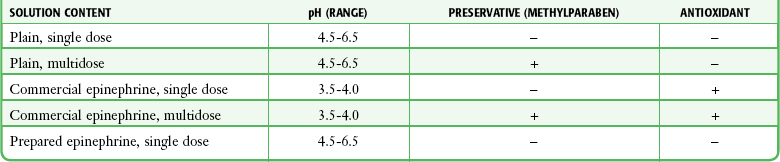
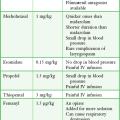
Local anesthetics are available in single-dose vials or ampules and in multidose vials, with and without epinephrine. Most solutions have a pH higher than 5. Multidose vials contain methylparaben, an antibacterial preservative. Local anesthetics premixed with epinephrine also contain an antioxidant (sodium bisulfite or sodium metabisulfite) to prevent deactivation of the vasoconstrictor. These solutions must be adjusted to a more acidic pH, approximately 3.5 to 4.0, to maintain the stability of epinephrine and its antioxidant. These properties as they relate to the amide group are depicted in Table 29-1.
Nerve Structure and Impulse Transmission
Functional and Structural Components of a Peripheral Nerve
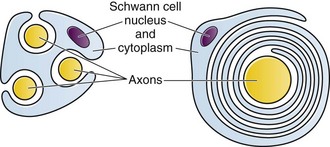
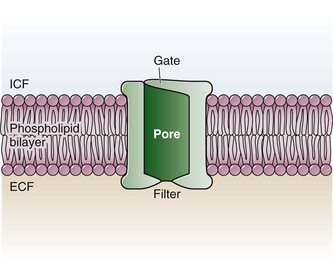
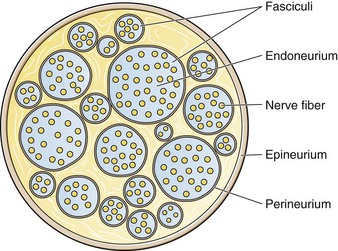
The functional nerve unit includes the nerve axon and its surrounding Schwann cell sheath. The Schwann cell (Fig. 29-1) may surround several unmyelinated axons or a single myelinated nerve fiber and form a myelin sheath. Junctions between sheaths along the axon called nodes of Ranvier contain sodium channels necessary for depolarization. As myelin sheath thickness increases from autonomic to sensory to motor fibers, the nodes of Ranvier are spaced farther apart. The most important structure affecting transmission of nerve impulses is the axon membrane (Fig. 29-2). The membrane consists of a double layer of phospholipids into which are embedded protein molecules that serve as channels containing pores for the movement of ions in and out of the cell. Most pores have a filter, or gate, that controls ion-specific movement and a sensory mechanism that opens or closes the gate. Bundles of nerve fibers (Fig. 29-3) are embedded in the endoneurium, which is made of collagen fibrils, and they are surrounded by a cellular layer, the perineurium. The perineurium functions as a diffusion barrier and maintains the composition of extracellular fluid around the nerve fibers. Surrounding the entire structure is the outer layer of a peripheral nerve, the epineurium, which is composed of areolar connective tissue.
Mechanism of Action
The Physiologic and Cellular Basis for Neuronal Blockade
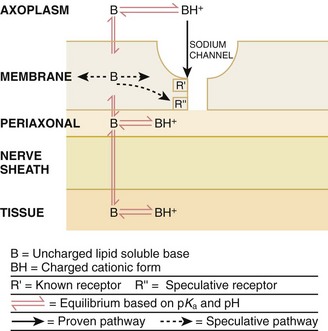
How anesthetic agents prevent sodium influx is still not completely understood. It is believed that the cationic charged form blocks the action potential from inside the membrane; the agent enters the sodium channel from the axoplasmic side and binds to a receptor.4,5 This “specific receptor” theory is well accepted and is considered the predominant mechanism in preventing influx of sodium. However, this theory cannot account for the action of benzocaine and other neutral compounds or the uncharged base forms of the common local anesthetics.
In summary (Fig. 29-4), when a local anesthetic (other than benzocaine) surrounds the perineurium, it equilibrates into its uncharged and charged forms based on tissue pH and pKa. In a more alkaline environment, a greater proportion of the uncharged form is present. The uncharged lipid-soluble form penetrates tissue, nerve sheath, and nerve membrane to gain access to the axoplasm and reequilibrates into both charged and uncharged forms. The charged form enters the sodium channel, decreases movement of sodium into the cell, and halts nerve transmission. The uncharged base is also involved in sodium channel blockade, but the exact nature of this mechanism is unknown.
Activity Profile during Neuronal Blockade
Onset of Action
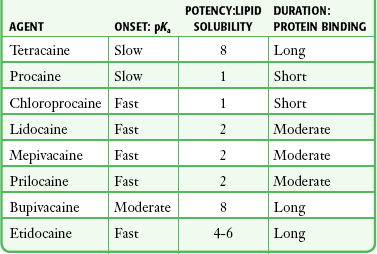
The pKa of an anesthetic is the primary physiochemical factor that determines its onset of action. Increased tissue penetration and a shortened onset of action are found in drugs with a lower pKa because more of the lipid-soluble uncharged form is present (Tables 29-2 and 29-3). Although in isolated nerve fibers the onset of action directly parallels pKa, other physiochemical factors also influence drug activity. For example, prilocaine and lidocaine have the same pKa, but lidocaine’s onset is faster because of its enhanced ability to penetrate through nonnerve tissue.
Potency
The lipid solubility of an anesthetic is a primary physicochemical factor determining potency. The drug’s partition coefficient, not the concentration of the lipid-soluble form determined by the pKa or pH, confers its lipid solubility. Because the nerve membrane is lipid, lipophilic anesthetics pass more easily into the cell and few molecules are needed to block conduction (see Tables 29-2 and 29-3).
Duration
The degree of protein binding of an anesthetic primarily determines its duration of action. Agents that bind more tightly to the protein receptor remain in the sodium channel longer (see Tables 29-2 and 29-3). Like potency, the duration of action is reduced by the vasodilation produced by local anesthetics. Prilocaine, which is less protein bound than lidocaine, has a longer duration of action because of its lesser degree of vasodilation. The duration of action also varies with the mode of administration. It is shorter when agents are applied topically.
The duration of action may be prolonged by several methods. Increasing the dose, usually by increasing the concentration, prolongs the duration to limits imposed by toxic effects. Raising the pH of the anesthetic solution has also been shown to prolong duration.6,7 The most practical way to increase duration is to use solutions that contain epinephrine.8 Epinephrine causes vasoconstriction, decreases systemic absorption, and allows more drug to reach the nerve. The effect of epinephrine varies according to the agent. Anesthetics that intrinsically produce more vasodilation (e.g., procaine, lidocaine, mepivacaine) benefit more from epinephrine’s vasoconstrictive action. The long-acting, highly lipid-soluble agents (e.g., bupivacaine, etidocaine) are less affected because they are substantially taken up by extradural fat and released slowly. In fact, lidocaine with epinephrine may be effective for as long as bupivacaine without epinephrine. Generally, most ED procedures can be accomplished quickly before the anesthesia wears off regardless of which drug is selected. Choose agents with a long duration of action when the procedure is lengthy or if postoperative analgesia is desired.
Topical Anesthesia
Local anesthetic agents may be applied topically to mucous membranes, intact skin, and lacerations. There are sufficient differences among these sites to merit a separate discussion of each one. Topical anesthesia of the eye is discussed in Chapter 62.
Mucous Membranes
Effective anesthesia of the intact mucous membranes (not intact skin) of the nose, mouth, throat, tracheobronchial tree, esophagus, and genitourinary tract may be provided by several anesthetics (Box 29-1). Tetracaine, lidocaine, and cocaine are the most effective commonly used agents (Table 29-4 and Box 29-1). Benzocaine (14% to 20%) is commonly used for intraoral or pharyngeal anesthesia (Fig. 29-5). The anesthesia produced is superficial and does not relieve pain that originates from submucosal structures. The onset of action may be slow, which limits its usefulness in urgent situations (such as passing a nasogastric tube). Agents applied topically can be absorbed systemically, and concentrated topical agents can cause toxicity.
TABLE 29-4
Practical Agents for ED Use: Mucosal Application
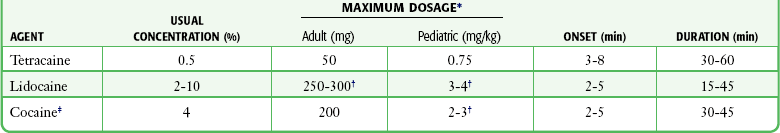
*These are conservative figures; see text for explanations.
†The lower dosage should be used for a maximum safe dose when feasible.
‡The 10% cocaine solution is best avoided because of minimal additional clinical benefit and the potential for coronary vasoconstriction in patients with coronary artery disease.
Cocaine is an effective, but potentially toxic topical agent that is applied to the mucous membranes of the upper respiratory tract. Although it is an ester, hepatic metabolism occurs, as does hydrolysis by plasma pseudocholinesterase. Absorption is enhanced in the presence of inflammation. Cocaine is the only anesthetic that produces vasoconstriction at clinically useful concentrations, hence its popularity for treating epistaxis. This major advantage is offset by its susceptibility to abuse and toxic potential. The toxic effects are due to direction stimulation of the CNS and blockade of norepinephrine reuptake in the peripheral nervous system. Cocaine should not be administered to patients who are sensitive to exogenous catecholamines or who are taking monoamine oxidase (MAO) inhibitor antidepressants. Clinical manifestations of toxicity include CNS excitement, seizures, and hyperthermia. Central and peripheral effects of hypertension, tachycardia, and ventricular arrhythmias may also be seen. Acute myocardial infarction has been reported after topical application.9 Cocaine is commonly used as a 4% solution with a maximum safe dose of 200 mg (2 to 3 mg/kg). A 10% solution is available, but this concentration adds little to the topical effect while enhancing the potential for toxicity. Coronary vasoconstriction may occur with doses as low as 2 mg/kg applied to the nasal mucosa. Although the clinical effect of this vasoconstriction is usually benign and without electrocardiographic changes, topical cocaine should be used cautiously in patients with known or suspected coronary artery disease. Dyclonine offers advantages over other topical anesthetic agents. It is a ketone derivative without an ester or amide linkage and may be used in patients who are allergic to the common anesthetics. Extensive experience with the topical preparation has shown it to be effective and safe.10 Dyclonine is marketed in 0.5% and 1% solutions, often in sore throat preparations, with a maximum adult recommended dose of 300 mg.
Benzocaine is an ester that is marketed in its neutral form in 14% to 20% preparations (Cetacaine, Americaine, Hurricaine) (see Fig. 29-5). Its low water solubility prevents significant penetration of the mucous membranes, thus reducing systemic toxicity if applied to intact mucosa. However, it is not a potent anesthetic and has a brief duration of action. It is more allergenic than other topical agents. Benzocaine is usually dispensed in an admixture with other therapeutic ingredients and is clinically effective only at relatively high (>14%) concentrations. Benzocaine is available as a nonprescription gel and liquid (6.3% to 20% Anbesol, for example) and is used for a variety of maladies, including ear pain, mouth pain, and teething. It is commonly used by dentists to produce mucosal anesthesia before intraoral nerve blocks (see Chapter 30). Adriani and Zepernick10 recommended this agent for lubricating catheters, airways, endotracheal tubes, and laryngoscopes and reported only one adverse reaction (methemoglobinemia) in their experience with approximately 150,000 patients. Methemoglobinemia occurs rarely after mucosal absorption of benzocaine used repeatedly for teething infants and after standard doses of benzocaine sprays used in endoscopic procedures.11
An excellent topical preparation is a combination of lidocaine, prilocaine, and tetracaine, especially useful for dental mucosa anesthesia (see Fig. 30-3). Topical gel mixtures of 2.5% lidocaine and 2.5% prilocaine (eutectic mixture of local anesthetics [EMLA]) are commonly used on intact skin but have also been used on mucous membranes. EMLA is more effective than 20% benzocaine when applied to the oral mucosa before needle injection for dental anesthesia. One study demonstrated that pain was reduced more quickly with EMLA than with benzocaine when applied to the buccal mucosa.12
As with infiltrated anesthesia, toxic reactions to topically applied anesthetics correlate with the peak blood levels achieved and not necessarily with the dose administered. Systemic absorption of a topical agent is more rapid, with a higher level being achieved than with the same dose given by infiltration (Fig. 29-6). The total dose of a topical anesthetic should be considerably less than that used for infiltration at a given site. Fractionating the total dose into three portions administered over a period of several minutes effectively reduces peak blood levels. Inadvertent suppression of the gag reflex, combined with difficulty swallowing, may lead to aspiration, an important potential adverse reaction to topical anesthesia of the nose, mouth, and pharynx. Infections from drug solutions in multidose vials for topical anesthesia of the larynx and trachea have not been substantiated.
Technique and Precautions
A commonly used “magic mouthwash” for the topical treatment of painful gingivostomatitis in children is often prescribed by emergency clinicians and pediatricians. There has been little scientific study of the preparation and it is not available commercially, but it has been used safely for decades. It consists of equal parts viscous lidocaine (2%), Maalox as a binder, and diphenhydramine elixir. Corticosteroids or nystatin is often added when the mixture is used to treat chemotherapy-induced mucositis.13 However, a recent review found no evidence that magic mouthwash is effective in treating this condition.14 The creamy mixture is swished around the mouth and expectorated or painted on specific lesions with a cotton swab. Packing an area with a cotton ball soaked with this mixture is one option. Repeated doses or swallowing of the elixir can produce systemic toxicity, so careful instruction should be given to limit use of the solution to every few hours. This combination would be theoretically less toxic than simply using topical lidocaine.
Emergency clinicians often prescribe 2% viscous lidocaine (20 mg/mL) for patients with pharyngitis, stomatitis, dental pain, or other inflammatory or irritative lesions in the oropharynx. Although this intervention is widely used and generally safe, the common misconception that topical anesthesia is totally innocuous may result in poor patient instructions and serious consequences. Topical lidocaine is helpful for painful mouth lesions but is of little practical value for acute pharyngitis, for which systemic analgesics are usually a better option. Seizures and death from topical lidocaine have been reported when excessive repeated doses have been administered.15,16 Toxic blood levels may occur because the anesthetic effect of viscous lidocaine lasts for only 30 to 60 minutes and patients with recurrent pain may either ignore or be ignorant of the safe dosing interval of 3 hours and medicate themselves more frequently. Patients tend to increase each dose to obtain greater relief, and inflammation may increase systemic absorption. In addition, painful oral lesions may last for several days.
Children are at higher risk for the rare toxicity of oral lidocaine. When compared with adults, children may exhibit increased lidocaine absorption, decreased clearance, and a longer half-life.17 Continued medication use allows lidocaine and its major metabolites monoethylglycinexylidide (MEGX) and glycinexylidide (GX) to accumulate. Both MEGX and GX are produced from the hepatic metabolism of lidocaine and are excreted in urine. They possess anesthetic and antiarrhythmic activity and have the potential for CNS toxicity. Although these metabolites are less potent than lidocaine, their elimination half-lives are considerably longer. Several investigators regard MEGX and GX to be the cause of CNS toxicity with prolonged topical use of lidocaine.16 The length of time that viscous lidocaine is retained in the mouth and whether the excess is expectorated or swallowed also affect the blood level produced. Expectorating the medication after swishing it in the mouth produces much lower blood levels than when it is swallowed. It seems logical that the most hazardous mode of administration would be to retain the solution in the mouth “until absorbed.”
Clearly explain the proper way to use viscous lidocaine and inform patients not to dose themselves ad libitum. Note that a 2% solution contains 20 mg/mL, or 100 mg per standard teaspoon (5 mL). The recommended maximum adult dose is 300 mg (15 mL of a 2% solution) no more frequently than every 3 hours. When possible, instruct the patient to decrease the dose by using direct cotton swab application. When gargled or swished in the mouth, limit application time to 1 to 2 minutes, and instruct the patient to expectorate excess solution. Limit use to 2 or 3 days, especially if swallowing the solution is necessary to obtain relief. Prescribe lower doses for patients at risk for decreased clearance (see “Systemic Toxic Reactions” later in this chapter). Doses for children are prescribed at 3 mg/kg. Because infants cannot expectorate well, do not use viscous lidocaine for minor oral irritation and teething. Recommend that no food be eaten for 1 hour after application because anesthesia of the oropharynx can interfere with swallowing and cause aspiration. Special note should be made of the over-the-counter availability of benzocaine, commonly used for toothaches and teething. A gel or liquid (Anbesol) is available in 6.3% to 20% formulations. When used repeatedly in the oral cavity on irritated tissue, systemic toxicity, including methemoglobinemia, may occur.
Lidocaine 4% solution can be atomized with a standard nebulizer device commonly used for delivering asthma medications and inhaled by the patient before insertion of a nasogastric tube. This method effectively anesthetizes the nasopharyngeal and oropharyngeal tissues, thereby easing the pain with tube insertion.18,19
Intact Skin
Lidocaine Cream: Lubens and coworkers20 used 30% lidocaine cream, saturated on a gauze pad and adherent to an elastic patch, for a myriad of procedures. Despite its effectiveness, safety, and painless application, the practicality of its use in an emergency setting is limited. In 1974, Lubens and coworkers20 reported an impressive list of uses, including minor operative procedures (e.g., excision of lesions, incision and drainage of abscesses), lumbar puncture, venipuncture, and allergy testing. Lidocaine remains one of the most commonly used topical compounds.
EMLA Cream, ELA-Max, and Tetracaine Base Patch: Various topical anesthetics have been suggested to decrease the pain of venipuncture or injections and to provide topical anesthesia for painful skin abrasions and lesions. These agents have been studied extensively and are safe, but they are not practical in many ED settings because of their slow onset of action and inadequate efficacy. However, their activity profiles make them more applicable than 30% lidocaine cream to emergency medicine. Tetracaine base is available as a solution, a gel, and a patch preparation. It is effective in crossing the lipid-rich barrier of the stratum corneum because it is highly lipophilic. EMLA was approved in the United States in 1992. It contains 2.5% lidocaine and 2.5% prilocaine in a unique oil-and-water emulsion yielding 5% EMLA. The mild lipophilic and hydrophilic properties of the component drugs are greatly increased when mixed together, thereby allowing absorption through intact skin. ELA-Max and ELA-Max5 are topical lidocaine anesthetic creams with a more rapid onset of action than EMLA cream. ELA-Max is a 4% concentration, and 5% ELA-Max is marketed as an anorectal cream that may benefit patients undergoing painful rectal procedures. Neither product has prilocaine, as is found in EMLA cream, and neither has U.S. Food and Drug Administration approval for pain relief before painful injections or intravenous (IV) insertion, but both have such potential.21
Tetracaine base seems to offer the advantage of being able to achieve effective anesthesia with a shorter application time and a longer duration.22 For tetracaine and EMLA preparations, onset, depth of anesthesia, duration, and blood levels vary directly with application time, use on thinner or inflamed skin, and larger doses.23 These preparations exhibit a reservoir effect.24 The drug is deposited in the stratum corneum and continues to diffuse along its concentration gradient, even after it is removed from the skin.
Tetracaine base, lidocaine cream, and EMLA can be useful in the ED for providing anesthesia for many procedures: venous cannulation, venipuncture, or any needle insertion, including preinfiltration anesthesia and lumbar puncture; a variety of minor surgical procedures; and anesthetizing the tympanic membrane and external auditory canal. EMLA has also been used effectively for débridement of ulcers.25
EMLA and 5% lidocaine cream are equally effective in reducing the pain of IV insertion.26 Luhmann and colleagues27 demonstrated that 5% lidocaine cream applied for 30 minutes under an occlusive dressing was as effective as infiltrated buffered lidocaine before IV catheter insertion in children. Obviously, infiltrative administration of buffered lidocaine requires skin puncture, but its onset of anesthesia is almost immediate. When time is not an issue, topical creams may be an acceptable alternative to infiltrated anesthesia or no anesthesia at all. About a 60-minute interval after application is required for these preparations to provide optimal topical analgesia to the intact skin for such procedures as venipuncture. Early cutaneous placement of these agents (e.g., over common IV sites while the patient is being triaged) is important for practical ED use.
Ethyl Chloride and Trichloromonofluoromethane and Dichlorodifluoromethane (Fluori-Methane) Sprays: These topical agents are often used for limited skin incisions (e.g., drainage of small abscesses), trigger point injections, joint aspiration, or injection of bursitis or tendonitis. These agents evaporate quickly from the skin and cool it to the point of freezing. Anesthesia is effective and immediate, but drawbacks include its short duration (only up to 1 minute), potential pain on thawing, and possibly lowered resistance to infection and delayed healing. Highly volatile ethyl chloride spray is flammable. Ethyl chloride has been studied in children to reduce the pain of venipuncture, but the results are mixed.28,29 Given their short duration of action and the time needed to perform pediatric venipuncture, these preparations have limited use for this purpose.
Technique
Lidocaine Cream: This 30% cream is saturated on a gauze pad that is adherent to an elastic patch and placed over the area to be injected or incised.20 The high concentration of anesthetic and an occlusive patch are needed to achieve effective skin penetration. The duration of action varies with the application time. A 45-minute application time is needed for most procedures. To achieve a topical anesthetic duration of 30 minutes, a 2-hour application is necessary.
Tetracaine Base Patch and EMLA Cream: Because both agents demonstrate a reservoir effect, anesthesia may increase or begin many minutes after removal of the drug. A precise description of application times and duration is not possible. Tetracaine base requires a minimum of 20 to 30 minutes of application time to produce several hours of anesthesia. EMLA requires an application time of 1 to 2 hours for a reported duration of 30 minutes to several hours. Occlusive dressings seem to increase penetration of EMLA whenever the cream is used. Patches are more convenient and cause no loss of effectiveness.
Ethyl Chloride and Fluori-Methane Sprays: Collect all the equipment required and make all preparations needed to immediately perform the desired procedure. Invert the bottle 25 cm from the skin and spray a stream along the proposed incision site until the area turns white and hard. Make the incision or local anesthetic injection immediately or during actual spraying of the agent for optimal results because the effect is fleeting. Some clinicians use these vapocoolant sprays to decrease the pain of injection of a more traditional local anesthetic such as lidocaine.
Iontophoresis: Anesthetic agents may be drawn into the skin without needles by electrical current applied through electrodes in a process called iontophoresis. Lidocaine with epinephrine administered via iontophoresis provides adequate anesthesia before venipuncture in pediatric patients and is superior to EMLA in providing cutaneous anesthesia.30–33 Iontophoresis is not widely used in emergency medicine but may be another alternative to applied anesthetic agents.
Microneedle Pretreatment: Recently developed technology known as microneedle pretreatment may improve the efficacy of cutaneously applied topical anesthetics. A functional microarray of fine needles painlessly perforates the stratum corneum to facilitate the penetration of applied medications without using traditional needle injections. A recent study demonstrated that perforation of the skin of healthy volunteers with a microneedle to which dyclonine was applied resulted in decreased time to anesthesia and a greater degree of pain reduction than did application of dyclonine to nonperforated skin.34 This emerging technology may improve the practical application of anesthetics to intact skin in the ED.
Jet Injection: Jet injection of anesthetic through intact skin may overcome some of the limitations imposed by the cutaneous application of anesthetics without needle infiltration. A jet injector is a device containing carbon dioxide gas that rapidly forces a plunger to expel drug through a small orifice applied over intact skin. Medication penetrates the epidermis to a depth of 5 to 8 mm in 0.2 second and causes the drug to be rapidly dispersed through the skin. Safety is improved because no needles are used to penetrate the skin. Several studies have demonstrated that jet injection of anesthetics provides more rapid anesthesia than does topical application of anesthetic agents and is preferred by patients over no anesthesia at all for painful procedures such as IV insertion and arterial blood gas sampling.35–37
Complications
General adverse reactions to anesthetic agents are discussed in the “Complications” section later in this chapter.
Tetracaine base is quite safe, with a low blood concentration after proper use. In one study, cutaneous erythema developed at the site of application in approximately 25% of patients.24 This vasodilatory effect may actually be an advantage when starting IV lines or performing venipuncture. EMLA is also quite safe. Although it has a high rate of local skin reactions, they are mild and transient, with disappearance 1 to 2 hours after removal of the cream. Despite the reported successful use of EMLA cream on skin ulcers, Hansson and associates25 and Powell and coworkers38 described an increase in bacterial growth, infection, and inflammation when used in experimental wounds. Methemoglobinemia resulting from the metabolites of prilocaine may occur with EMLA.23 The risk for clinically significant methemoglobinemia seems remote when EMLA is used properly. It is contraindicated in any infant younger than 3 months and in infants between 3 and 12 months of age who are currently taking methemoglobinemia-inducing drugs (nitrites, sulfonamides, antimalarials, phenobarbital, and acetaminophen). The risk for adverse effects is increased in patients with anemia, respiratory or cardiovascular disease, and deficiencies in glucose-6-phosphate dehydrogenase or methemoglobin reductase. Prolonged inhalation of ethyl chloride spray may produce general anesthesia, coma, or cardiorespiratory arrest. Ethyl chloride is also flammable, thus precluding its use with electrocautery.
Topically applied anesthetics are often covered with an occlusive dressing to contain the medication. Covering the medication with an occlusive dressing may dramatically increase drug absorption and the amount of metabolites. Oni and associates demonstrated that 4% lidocaine cream applied to volunteers with an occlusive dressing resulted in three times the level of serum lidocaine and double the level of MEGX when the same dose was applied to volunteers without occlusion.39 Use caution when occluding topical anesthetics over intact skin because markedly elevated serum level of drug may be reached.
Lacerations
In 1980, Pryor and colleagues40 reported their experience with a topical anesthetic solution (tetracaine-adrenaline-cocaine [TAC]) for wound repair. The original formula, used in most subsequent studies, consists of a solution of 0.5% tetracaine, 1 : 2,000 epinephrine (adrenaline), and 11.8% cocaine. Traditionally, anesthesia is produced by firmly applying a solution-saturated gauze pad or cotton ball directly to the laceration for 10 minutes. The resulting loss of cutaneous sensation is centered about the area of application. Gel formulations of topical wound anesthesia (TWA) with alternative mixtures of agents promise to improve the ease and safety of topical anesthetic solutions for wound repair in the ED. Advantages of TWA include painless application, no distortion of wound margins, good hemostasis, and good patient and parental acceptance in the pediatric age group.
Indications and Contraindications
Use of TWA is generally restricted to young children with wounds less than 5 cm in length in whom the delay in application of an anesthetic is acceptable and proper application can be ensured (Fig. 29-7). TWA containing vasoconstricting agents is not generally used in structures without a collateral blood supply (e.g., digits, tip of the nose, pinna of the ear, penis), although there is no evidence that this is unsafe. When some wound preparation is desired before anesthesia, remove large debris and clotted blood to allow the appropriate application of TWA and then finish wound preparation once the wound is properly anesthetized.
TWA appears to be less effective on the trunk and extremities than on the face and scalp and less effective than lidocaine infiltration in these areas. The 10- to 20-minute onset time may be unacceptable in a busy ED.41 Two other often mentioned drawbacks may be more theoretical than real. Vasoconstrictor-induced higher infection rates (see “Complications” later in this chapter) have not been clearly demonstrated. The argument that the necessary 10-minute application period is time-consuming and takes valuable nursing time is partially offset by using the child’s caretaker or adhesive paper tape alone to hold the solution in place. It is not necessary for anyone to “hold” the medicine in place when gel is used.
Some EDs still stock topical anesthetic gels that contain cocaine. Cocaine use is complex because of cost and federal regulations requiring storage in a locked cabinet and maintenance of separate written records of its use. In view of the evidence that mixtures without cocaine are efficacious, avoid the potential complications related to cocaine, and do not have the potential for abuse that cocaine does, the practicality of cocaine-containing TWA has been questioned.42
Agents and Effectiveness
TAC and Related Mixtures: Three clinical trials directly compared TAC with infiltrated lidocaine. Without specifying wound location, Pryor’s group40 found equal anesthetic effect. Complete anesthesia with TAC was achieved in 82% to 86% of patients as compared with 83% to 92% for subcutaneous lidocaine. The remaining patients obtained partial anesthesia. Hegenbarth and associates43 and Anderson and coworkers44 demonstrated results similar to those of Pryor’s group.40 Hegenbarth and associates found TAC to be equal to lidocaine only on the face and scalp and inferior to it at other locations. In contrast, other studies have confirmed excellent rates of effectiveness, especially on the face and scalp.45–49 TAC is more effective than its component drugs alone. On the face and scalp, TAC was found to be superior to tetracaine alone, although on nonfacial areas, both produced equally poor results.45 TAC was found to be more effective than cocaine alone and more effective than a tetracaine-epinephrine solution in the same dosage ratio.47,50
In 1990, Bonadio and Wagner48 showed that an epinephrine-cocaine solution (1 : 2,000 epinephrine and 11.8% cocaine) was equal to TAC in effectiveness. Bonadio and Wagner46 also found that half-strength TAC (0.25% tetracaine, 1 : 4000 epinephrine, 5.9% cocaine) achieved excellent results in patients with dermal lacerations of the face, lip, and scalp. Smith and Barry51 compared three strengths of TAC and found equal effectiveness among them; they recommended the lowest-strength cocaine formulation (1% tetracaine, 1 : 4000 epinephrine, 4% cocaine). Ernst and colleagues52 found similar effectiveness to TAC when a slightly different lidocaine-epinephrine-tetracaine (LET) solution (4% lidocaine, 1 : 2000 epinephrine, 1% tetracaine) was used.
TAC has also been compared with EMLA when placed in a wound for 60 minutes before wound repair. In a study of 32 wounds, supplemental anesthesia was required less often with EMLA.53 The non–cocaine-containing formulations are generally considered less toxic and have advantages in terms of reduced cost and avoidance of controlled substance precautions during storage.
LET and Related Solutions: Many variations of mixtures that do not contain cocaine have been studied; these include variations LET, lidocaine-epinephrine, tetracaine-phenylephrine, tetracaine-lidocaine-phenylephrine, bupivacaine-norepinephrine, and prilocaine-phenylephrine. Schilling and associates54 found that a LET solution (4% lidocaine, 1 : 1000 epinephrine, 0.5% tetracaine) was as effective as TAC. LET gel preparations are at least as effective as LET solutions.55 Singer and Stark56 determined that EMLA or LET gel placed in a wound on initial evaluation and before infiltration with lidocaine reduced the pain of infiltration, thereby allowing essentially painless injection of lidocaine.
Technique and Dosage
Gaufberg and coauthors57 described “sequential layer application” (SLA) of topical lidocaine with epinephrine (TLE) to deeper wounds that might not typically be amenable to the use of TWA. Cotton soaked with TLE was applied to the wound surface and 2 mm of surrounding skin for 10 to 15 minutes and then removed. A second similarly soaked piece of cotton was packed deeper into the wound for 10 to 15 minutes. Deep wounds were treated with a third piece of TLE-soaked cotton placed deeper into the wound. Anesthesia was presumed when 3 mm or more of pallor was seen on all wound margins. The group receiving lidocaine by SLA was compared with a group receiving infiltrated 2% buffered lidocaine with epinephrine. Both groups experienced a similar degree of anesthesia during suturing. Mean time to anesthesia with SLA was 29 minutes versus 5 minutes for the infiltration group. Despite the longer time in the SLA group, the authors argued that the additional time is worthwhile because the painful injection, risk for hollow-bore needle injury, and tissue distortion with infiltration were eliminated. If time permits, the SLA technique may be useful for wounds that would have otherwise required infiltrated anesthesia.
Hegenbarth and associates43 estimated the maximum safe dose of full-strength TAC to be 0.09 mL/kg based on the known maximal safe dose of infiltrated tetracaine, the mucosal application of cocaine, and an estimate of absorption of solution onto the applicator. The key to safety is to avoid TAC on mucosal surfaces or areas in which sniffing or swallowing may accidentally occur. Topical mucosal anesthesia is discussed elsewhere in this chapter.
Prepare an epinephrine-cocaine gel by adding 0.15 g of methylcellulose to 1.5 mL of epinephrine-cocaine solution. Stir the mixture thoroughly for a minute or two until a gel consistency is obtained. After sterile preparation, place the wound in a gravity-dependent position and apply the gel with a cotton-tipped swab to coat the entire wound cavity and margins. Allow the wound to stand for 15 to 20 minutes and thoroughly wash the wound cavity to remove the gel. In Bonadio and Wagner’s study,49 the average dose used was 0.35 mL of gel containing only 40 mg of cocaine. Again, other agents are equally efficacious and safer; select these over cocaine-containing mixtures whenever possible.
Complications
Most adverse events associated with TWA occur with mixtures that contain cocaine. Lidocaine toxicity is a well-recognized adverse event when higher doses are used (see “Systemic Toxic Reactions” later in this chapter), but lidocaine toxicity has not been reported with TWA mixtures.
Mucosal application may rarely lead to significant systemic toxicity; fatalities have been reported after application. Even after nonmucosal TAC use, cocaine levels appear in the blood and cocaine metabolites appear in the urine in the majority of patients,58,59 but tetracaine does not. Gel formulations of TAC tend to stay in the wound and thus reduce the risk of solution runoff onto mucosal surfaces. There is no need to use a gauze pad to apply the medication in a gel formulation or to hold it in place. Because the entire applied dose will stay in the wound, a lower dose can be used. Gel also provides more uniform application to tissues, which improves the anesthetic effect. In tissues containing end-arteries, ischemia caused by vasoconstrictors may occur. Avoid TAC on the digits, the tip of the nose, the penis, and the pinna of the ear. Use TAC with caution on patients with coronary artery disease, uncontrolled hypertension, seizures, or peripheral vascular disease. Patients with decreased plasma cholinesterase levels are theoretically at increased risk for systemic toxic effects, but this potential risk is of little clinical concern.
Infiltration Anesthesia
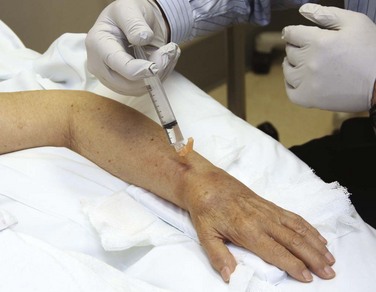
Injection of an anesthetic agent directly into tissue before surgical manipulation is known as infiltration anesthesia (Fig. 29-8). Field block anesthesia is also considered a form of infiltration anesthesia, particularly since the agents, concentrations, and recommended maximum dosages are the same. To create a field block, inject a field of anesthetic around the operative site. Make the injection proximal to or surrounding the area that you plan to manipulate. Combine infiltrative anesthesia with procedural sedation (see Chapter 33) to reduce anxiety or motion.
Indications and Contraindications
A disadvantage of local infiltration over nerve blocks is that a relatively large dose of the drug is needed to anesthetize a relatively small area. For extensive wounds, the amount of anesthetic required may risk systemic toxicity. The maximum allowable volume can be increased by adding epinephrine, using a lower concentration of the anesthetic agent, or both (Table 29-5). When large volumes are anticipated and a nerve block is anatomically feasible, the nerve block is preferred. Avoid using infiltration for large procedures in small children and in apprehensive patients, especially those with previous adverse reactions to the medications (whether vasovagal or otherwise). Local infiltration distorts the tissues that will be incised or repaired, which makes it undesirable in areas requiring precise anatomic alignment (e.g., some lip repairs).
Choice of Agent
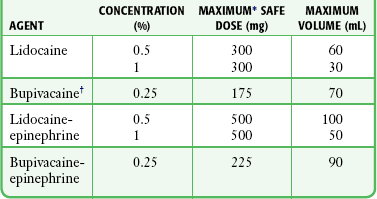
The local anesthetic agents most frequently used for infiltration are 0.5% to 1% lidocaine, 0.5% to 1% procaine, and 0.25% bupivacaine (Table 29-6). Higher concentrations are of no additional benefit. Lidocaine is most commonly used because of its excellent activity profile, low allergenicity, low toxicity, user familiarity, and ready availability. Procaine is useful for patients who are allergic to amide anesthetics. Some clinicians prefer bupivacaine because of its prolonged duration. Bupivacaine may also be preferred when postoperative analgesia is desired, for prolonged procedures, for dental anesthesia, or even for short procedures that may be interrupted in a busy ED.
TABLE 29-6
Practical Agents for ED Use: Local Infiltration
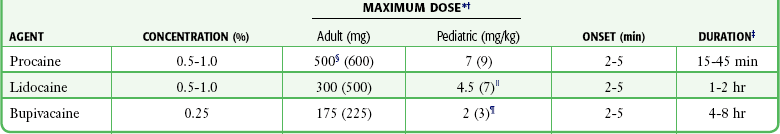
*These are quite conservative figures; see text for explanation.
†The higher maximum dose for solutions containing epinephrine appears in parentheses.
‡These values are for the agent alone; they can be extended considerably with the addition of epinephrine.
§Some authorities recommend up to 1000 mg or 14 mg/kg for procaine.
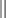 Some authorities recommend up to 7 mg/kg for plain lidocaine in children older than 1 year.
Some authorities recommend up to 7 mg/kg for plain lidocaine in children older than 1 year.
¶Because of lack of clinical trial experience, drug companies do not recommend the use of bupivacaine in children younger than 12 years, but bupivacaine is commonly used without problems in children.
A comparison of equianesthetic doses of lidocaine and bupivacaine for infiltration anesthesia (Table 29-7) revealed that the duration of action is the major difference between the two agents. For the majority of ED procedures it is not necessary to extend the duration of anesthesia beyond 1 hour; which makes plain lidocaine a logical anesthetic choice. Patients experience a moderate amount of pain after repair of a laceration when the lidocaine wears off in about 1 hour.60 Bupivacaine reduces the pain after repair of lacerations for at least 6 hours. This benefit of a prolonged duration of anesthesia must be weighed against the hazards of injury to the mucous membranes or an unprotected limb or the annoyance of prolonged numbness in patients who have undergone simple surgical procedures.
TABLE 29-7
Comparison of 1% Lidocaine and 0.25% Bupivacaine: Infiltration Anesthesia
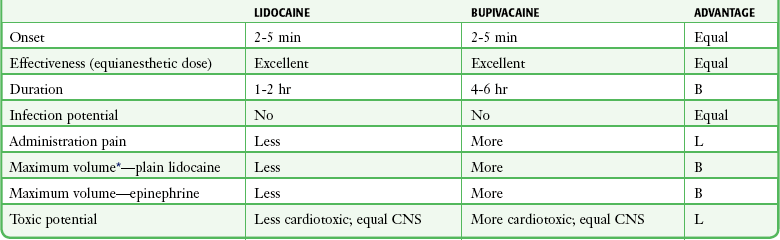
B, bupivacaine; CNS, central nervous system; L, lidocaine.
*See Table 29-6 for volume and concentration comparison.
A prolonged duration of anesthesia can also be achieved by adding epinephrine, sodium bicarbonate, or both to lidocaine. Epinephrine provides excellent wound hemostasis and slows systemic absorption. This latter property decreases the peak blood level, reduces the potential for a toxic reaction, and allows a greater volume of agent to be used for extensive lacerations. The major disadvantage of epinephrine is theoretical damage to host defenses, but it is generally clinically inconsequential (Box 29-2). Bicarbonate added to the anesthetic just before injection decreases the pain of administration. Bupivacaine, if used with due caution, is safe and easy to use. The deciding factors are many, but some logical choices are as follows:
Technique
Buffering
To alkalinize lidocaine, add 1 mL of sodium bicarbonate (8.4%, 1 mmol/mL) to every 10 mL of anesthetic solution. As the pH of the solution is raised, the anesthetic becomes unstable and has a decreased shelf life. It was initially recommended that buffered lidocaine be prepared just before use to avoid precipitation and degradation, but buffered lidocaine retains its effectiveness for 1 week and refrigeration may further increase its shelf life.61,62 Bicarbonate may be combined with plain lidocaine for both infiltrative anesthesia63,64 and digital nerve blocks.65 In one volunteer study, sodium bicarbonate was effectively combined with lidocaine and epinephrine.62
Sodium bicarbonate can be added to bupivacaine, but the solution tends to precipitate as the pH rises. The clinical effect of such precipitation is unclear, but if it occurs, it is probably prudent to use another solution prepared with less buffer. Precipitation varies directly with the concentration of bupivacaine and the time since mixture.66 Cheney and coworkers67 showed that 0.05 mL of 8.4% sodium bicarbonate (measured in a tuberculin syringe) could be mixed with 10 mL of 0.5% bupivacaine without precipitation. The goal of using bupivacaine is to prolong the duration of anesthesia; this effect can also be accomplished somewhat by using buffered lidocaine (plain or with epinephrine).
Temperature Manipulation
Warming an anesthetic to body temperature (37°C to 42°C) reduces the pain of infiltration,68,69 but warming may not reduce injection pain as much as buffering with sodium bicarbonate does. Bartfield and colleagues70 found that lidocaine warmed to 38.9°C was more painful than room-temperature buffered lidocaine during intradermal injection. Brogan and associates,71 using lidocaine warmed to 37°C, found the warmed lidocaine and room-temperature buffered lidocaine to be equivalent during wound infiltration. Neither study found a synergistic effect with combined warming and buffering. Martin and coworkers72 found that warmed (37°C) lidocaine was no less painful than buffered lidocaine. Anesthetic solutions can be warmed in a baby food warmer with thermostat temperature control or in an IV solution warmer. Warming is not believed to adversely affect the shelf life of the local anesthetic.
Locally cooling the area to be infiltrated may provide additional pain relief. Leff and colleagues73 demonstrated that patients receiving local infiltrative anesthesia for repair of an inguinal hernia had less pain when the incision area was cooled before infiltration. Patients were randomized to two groups and had a 1000-mL bag of saline placed over the inguinal area for 5 minutes. One group used saline bags at room temperature; the other group used saline bags cooled to 4°C. A significant decrease in pain perception was found in the group in which the inguinal area was cooled before lidocaine infiltration. Cooling of wounds in the ED before infiltration has not been studied.
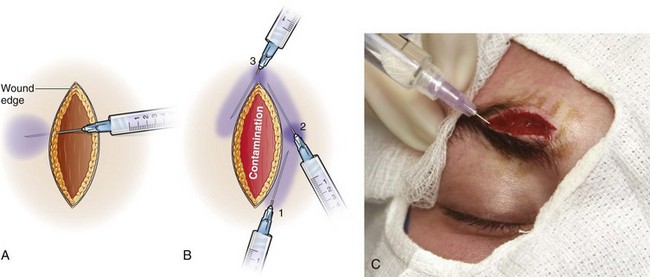
Injection
Because the patient barely feels a needle placed subcutaneously and skin puncture is often quite painful, make all wound injections through the wound edge and not through the skin (Fig. 29-9).74,75 Spread of infection beyond the wound margin has not been demonstrated clinically with this technique. Some clinicians choose to inject the anesthetic through intact skin in patients with a grossly contaminated wound. Bierman76 described a technique of patient distraction by applying light pressure to alternate sides of the wound with one’s fingers and repeated ambiguous questioning about feeling the light pressure rather than the ongoing wound injection. Ask school-aged children to count backward or to say the “ABCs” to distract them during injection. Inject 1% lidocaine through intact skin with the needle bevel facing upward because this is less painful than injecting with the needle bevel facing downward.77
Special Considerations
Hematoma block has been used for many years to provide anesthesia for fracture reduction, particularly of the distal end of the forearm and hand (Fig. 29-10). Its popularity has waned somewhat because of the fear (unproven and theoretical) of introducing infection at the fracture site and its limited efficacy. Although several studies have shown hematoma block to be safe, the anesthesia that it provides is not as good as that provided by a Bier block (see Chapter 32). Nevertheless, there are several reasons to consider this technique.78,79 The procedure is simple and quick to perform and does not require additional personnel. There is no need to wait for an anesthesiologist. A lower dose of the anesthetic agent is required for a hematoma block than for a Bier block (see Chapter 32). A hematoma block is particularly useful when a Bier block and general anesthesia are contraindicated.
Intraarticular Anesthesia and Analgesia
Findings from the history and physical examination of an acutely traumatized joint, such as the knee, often underestimate the severity of the injury. Instillation of 5 mL of 1% lidocaine after joint aspiration may help relieve pain and facilitate an examination, but its use is not routinely recommended.80 Since spasm and apprehension are often not relieved by local anesthesia, the information gained from this maneuver does usually not influence the ED treatment plan. Intraarticular anesthesia of the knee has no effect on gait pattern or joint proprioception.81 Postprocedure weight bearing may be allowed without fear of producing or increasing injury if otherwise indicated. Intraarticular anesthesia may enhance elbow use after aspiration of a hemarthrosis associated with a radial head fracture.82 The technique of administration is analogous to arthrocentesis (see Chapter 53). Intraarticular lidocaine has been effective in facilitating reduction of a shoulder dislocation. Animal experiments have demonstrated chondrotoxicity when local anesthetics are continuously infused into a joint.83,84 Although continuous joint infusion of anesthetic may be harmful when used in a postoperative setting, there is no evidence that a single injection of intraarticular anesthetic in the ED is harmful.
Morphine may be injected directly into joints for postoperative pain relief and potentially provide prolonged analgesia after reduction of fractures. Theoretically, there are local opioid receptors in joints that are capable of being stimulated by rather small doses of morphine to provide relief for up to 24 hours with a single dose. There may also be some systemic absorption of the intraarticular morphine that contributes to analgesia, but adverse systemic opioid effects are not seen with this technique. Data are sparse on the exact mechanism and optimal doses. Three to 6 hours may be required for analgesia to reach it maximum effect. In a systematic review, Gupta and colleagues concluded that postoperative intraarticular morphine injected into the knee joint at doses of 2 to 4 mg provides analgesia for up to 24 hours.84a There is a wide variability in efficacy, which may be dose related. Though studied primarily for postoperative use after knee surgery, a similar concept may be intuitively applied to traumatic joint pain, but this has not been studied adequately.
Intrapleural Anesthesia
Intrapleural anesthesia introduces a local anesthetic into the pleural space (i.e., between the parietal and visceral pleura) through an epidural catheter. The anesthetic can be introduced through a previously placed chest tube. The technique can provide relief for several conditions, primarily postthoracotomy pain, postcholecystectomy pain, and most importantly for emergency clinicians, posttraumatic chest pain (e.g., rib fractures, pneumothorax, hemothorax). This procedure is not only useful for pain relief but also facilitates turning, coughing, and deep breathing. Several studies have demonstrated improved respiratory mechanics when intrapleural anesthesia is used.85,86 Though not unanimous, most studies show that intrapleural anesthesia is effective in providing analgesia.87,88 Concern has been raised that intrapleural anesthesia may create a level of anesthesia below the umbilicus and make posttraumatic abdominal examinations unreliable. Until this issue is clarified, it seems prudent to rule out intraabdominal injury before intrapleural anesthesia is used.89
Technique
If a chest tube is in place, it is preferable to inject anesthetic into the pleural space through the chest tube. Clamp the tube for 10 to 15 minutes to allow the anesthetic to diffuse. When the tube cannot be taken off suction or if no tube is present, inject the local anesthetic percutaneously.90 With the patient in the lateral position (with the affected side up), place a 16-gauge Tuohy needle 8 to 10 cm from the posterior midline in the eighth intercostal space. Angle the needle 30 to 40 degrees with respect to the skin and aim medially, with the bevel up and directed just above the rib. After perforating the posterior intercostal membrane (felt as a distinct resistance), remove the stylet and attach a well-wetted, air-filled glass syringe to the Tuohy needle. Advance the needle until it enters the pleural space, which is denoted by the plunger being drawn down the syringe as a result of the negative pressure created during inspiration. Remove the syringe and introduce an epidural catheter 5 to 6 cm into the pleural space. Remove the Tuohy needle, obtain a chest radiograph to confirm proper position, and secure the catheter.
The most commonly used anesthetic and dose is 20 mL (0.3 mL/kg) of 0.5% bupivacaine. A dose repeated every 8 hours is safe and effective.91 Alternatively, infuse 0.25% bupivacaine at 0.1 to 0.2 mL/kg/hr after a bolus has been administered.85 The solution presumably diffuses from the pleural space “back” through the parietal pleura and the intercostal muscle to reach the intercostal spaces, where it blocks the intercostal nerves. The level of anesthesia can extend from T2 to T12 and involve the skin, chest, abdominal wall, and potentially the viscera if the visceral afferent fibers are blocked at the sympathetic chain in the paravertebral gutter.
Complications
Local Anesthetic Effect on Wounds
Wound Healing
Local anesthetics produce cytotoxic effects on cell structure and function in a dose- and time-related manner. These effects, at doses well below those used clinically, involve fibroblasts more than nervous tissue. Local anesthetics impair mitochondrial function and fibroblast proliferation and hasten apoptosis in vitro; the clinical relevance of these effects to the ED use of local anesthetics is likely to be minimal.92,93 Collagen synthesis is inhibited by lidocaine and bupivacaine.94 Morris and Tracey95 found that lidocaine in increasing concentrations progressively reduces the tensile strength of wounds. Epinephrine added to 1% and 2% concentrations of lidocaine further reduced tensile strength, but when epinephrine was added to distilled water or to 0.5% lidocaine, it had little effect. Several conclusions may be clinically relevant. Although it may delay the onset of anesthesia, 0.5% lidocaine solution, without epinephrine, may be best for maintaining wound strength.
Eriksson and associates96 found that lidocaine reduces the inflammatory response in wounds by decreasing the number of white cells and their metabolic activity. Although an inflammatory response may be beneficial in a contaminated wound, it can be detrimental in a sterile wound because of tissue toxicity created by the release of superoxide anions, lysosomal enzymes, thromboxanes, leukotrienes, and interleukins. The clinical relevance of this is unknown. None of the concerns mentioned earlier should prohibit the use of standard anesthetics or epinephrine when their use is otherwise indicated.
Wound Infection
Though not generally appreciated, it has long been known that local anesthetics possess antimicrobial activity in vitro. Lidocaine and procaine demonstrate concentration-dependent inhibition of culture growth of most gram-negative organisms.97–99 Gram-positive isolates are also significantly affected by lidocaine and, to a lesser extent, by procaine. Lidocaine inhibits the growth of common nosocomial pathogens, including Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, and several strains of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci.99 Administering anesthetics before obtaining material for culture, including injecting a joint before arthrocentesis, may give false-negative culture results. To avoid this problem, if possible inject the skin at the injection site and along the needle tract but not into the actual joint space until after a synovial fluid specimen has been obtained for culture. Berg and coworkers100 demonstrated that lidocaine administered before tissue biopsy of chronic wounds did not affect the culture results when the exposure time before culture was less than 2 hours. This effect is also significant when anesthetic ointments are applied before culture. EMLA cream applied before culture demonstrates powerful antimicrobial properties.100 Furthermore, it has been shown that adding sodium bicarbonate to lidocaine greatly enhances its inhibitory effect on bacteria.101 Although local anesthetics can interfere with culture testing, several studies have shown that local anesthetics, by themselves, do not appear to alter the incidence of wound infection.102,103
Epinephrine appears to exert a deleterious effect on host defenses, at least in animal models. Studies of infiltrated and topically applied epinephrine solutions in contaminated animal wounds show an increased potential for infection.102,103 Epinephrine-induced vasoconstriction may contribute to tissue hypoxia, retard the killing of S. aureus by leukocytes, and reduce leukocyte migration into the tissue.104 Most clinical studies using topical anesthetics with vasoconstrictor properties (e.g., TAC mixtures) do not demonstrate a significant rise in infection rates.40,43,44,49 The concerns mentioned earlier should not prohibit the use of epinephrine for wound preparation when its use is otherwise appropriate.
Local Injuries
Injury may result from the direct application of an anesthetic agent to a nerve or from passage of a needle through soft tissue structures. Factors implicated in transient or persistent neuropathy include acidic solutions, additives, the agent itself, needle trauma, compression from hematomas, and inadvertent injection of neurolytic agents. Born105 described a series of 49 wrist and metacarpal blocks with bupivacaine in which significant neuropathy developed in eight patients. He postulated that damage occurred from trapping of the drug in a confined space and recommended that whenever bupivacaine is used in this situation, it should be low in concentration and volume. Infection, hematomas, and broken needles are other local problems that can be avoided by using proper technique. Erroneous needle placement can also produce complications such as pneumothorax during a brachial plexus or intercostal block.
Use of Epinephrine with Local Anesthetics
Epinephrine in conjunction with local anesthetics prolongs the duration of anesthesia and produces a temporary hemostatic effect, but its inclusion in digital block solutions has traditionally been discouraged because of the belief that it can lead to ischemia and necrosis. Areas of special concern include the digits, penis, tip of the nose, and earlobe. Although tissue ischemia and sloughing have been reported with concentrations of 1 : 20,000, current practice involves concentrations in the range of 1 : 100,000 to 1 : 200,000 and the use of submaximal doses. Several authors suggest that epinephrine-containing solutions can be safely injected into the fingers without adverse sequelae.106–109
A study of more that 3000 cases of elective injection of low-dose epinephrine (≤1 : 100,000) into the hand and fingers failed to identify a single case of digital tissue loss, and phentolamine was not required to reverse vasoconstriction.106 Of 1111 procedures reported by Chowdhry and coauthors110 involving digital blocks with 1% lidocaine plus epinephrine (1 : 100,000) at a dose ranging between 0.5 and 10 mL (average, 4.3 mL), no patient in the epinephrine group exhibited digital ischemia or experienced nerve injuries or unusually delayed wound healing. Current data support the use of epinephrine, when correctly applied, for the performance of digital blocks of the fingers and toes.
The use of phentolamine (Regitine), which produces postsynaptic α-adrenergic blockade, is recommended for clinically significant vasoconstrictor-induced tissue ischemia. This medication is usually given by local infiltration, in the area where epinephrine has been injected, at a dose of 0.5 to 5.0 mg diluted 1 : 1 with saline. If local infiltration is ineffective because of tension within a tissue compartment or if the area of vasoconstriction is large, give phentolamine by the intraarterial route.111
Systemic Toxic Reactions
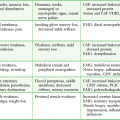
Although systemic toxic reactions occur in only 0.1% to 0.4% patients after the administration of local anesthetics, they are the most frequent serious adverse reactions encountered (Table 29-8).112 After the administration of a local anesthetic, some of the drug reaches its intended target and some is absorbed quickly into the systemic circulation. Peak blood levels are generally produced within 30 minutes. Many vagal reactions, nonspecific anxiety reactions, and sensitivity to preservatives have been attributed to “allergies” or to systemic toxicity to local anesthetics. Patients may also demonstrate systemic reactions to hidden allergens that may mimic a systemic reaction, such as anaphylactic reactions to the latex in surgical gloves.
TABLE 29-8
Differentiating Systemic Adverse Reactions
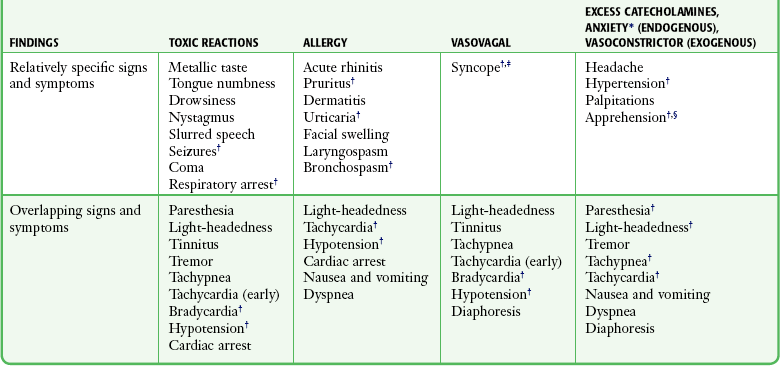
*Anxiety reaction, including hyperventilation syndrome.
†Denotes common and significant reactions.
‡Vasovagal syncope occurs with the patient upright; any loss of consciousness in the recumbent position implies a severe toxic or anaphylactic reaction.
§Although apprehension is classically associated with anxiety and vasoconstrictor reactions, milder toxic and allergic reactions may cause patient apprehension.
High Blood Levels
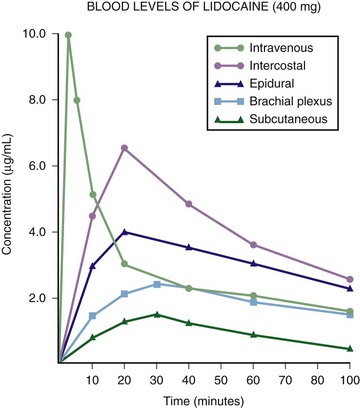
Site and Mode of Administration: In comparing the routes of administration for a given dose, the intravascular route produces the highest levels, followed by topical mucosal application and then infiltration (see Fig. 29-6). The more vascular the site, the more systemic absorption that occurs and the higher the level obtained. The following blocks are arranged in decreasing order of systemic absorption: intercostal, caudal, epidural, brachial plexus, and subcutaneous. It follows that the site of administration is an important variable in determining the safe dose of an anesthetic. For example, 400 mg of lidocaine may produce a nontoxic blood level with subcutaneous abdominal wall infiltration but produce a toxic level when used for an intercostal nerve block.
Rate: A more rapid IV injection will produce a higher blood level than a slower injection. A single topical application leads to a higher level than a dose that is fractionated over time.
Dose and Concentration: The larger the total dose, the higher the peak blood level. It is uncertain whether increasing the concentration while maintaining the total dose by decreasing the volume affects the serum level.
Addition of Epinephrine: Epinephrine produces vasoconstriction and reduces systemic absorption, thereby resulting in lower peak blood levels. Occasionally, the apprehension, tachycardia, or palpitations induced by epinephrine can be incorrectly interpreted by both the clinician and patient as an “allergic” reaction.
Specific Drug.: The more potent agents are more toxic on a milligram-to-milligram basis. Because anesthetics are used in equipotent doses (e.g., 1 mg of bupivacaine versus 4 mg of lidocaine), they are approximately equitoxic. The blood levels achieved by a particular agent depend on the agent’s absorption, distribution, and clearance from the circulation. Agents with high lipid solubility and lower protein binding (etidocaine > bupivacaine > lidocaine > mepivacaine) tend to become sequestered in tissue and have slower absorption and lower blood levels. Agents with a greater volume of distribution or faster clearance (etidocaine > lidocaine > mepivacaine > bupivacaine) also produce lower blood levels. Together, these effects produce margins of safety for each anesthetic, with etidocaine having the greatest safety margin, followed by bupivacaine, which is equal to or better than lidocaine.
Clearance: The liver metabolizes amides, with the clearance rate being a function of hepatic blood flow and the extraction capacity of the liver. Decreased hepatic blood flow, produced by norepinephrine, propranolol, or general anesthesia, slows clearance and potentially raises drug blood levels. Decreased drug extraction, associated with congestive heart failure, cirrhosis, or hypothermia, may produce a higher blood level. Hypovolemia, which decreases hepatic flow, does not raise blood levels because it causes an offsetting decrease in absorption.
Because lidocaine is metabolized in the liver by cytochrome P-450 enzymes, drugs that inhibit these enzymes may slow lidocaine clearance and increase the risk for lidocaine toxicity. Although the effect of ciprofloxacin and erythromycin on infiltrated lidocaine has not been studied, these drugs decrease the metabolism of lidocaine and increase the concentration of its major metabolites when lidocaine is injected intravenously.113–115 The clinical effect of this previously discussed phenomenon is unknown and probably of little consequence in ED wound care.
Maximum Safe Dosage: The maximum safe dose of a drug may be defined as the dose that produces a blood level of the drug just below the toxic level (Table 29-9). One maximum dose of an anesthetic agent appropriate for all patients and all conditions cannot be stated. A maximum safe dose cannot be based solely on the weight of a patient. In an adult, peak blood levels do not correlate well with weight because the volume of drug distribution is relatively constant.116,117 As an approximation, Arthur and McNicol118 recommended that maximum dosages for children be based on weight. Plain lidocaine may be used in doses of up to 4.5 mg/kg, and the addition of epinephrine allows a maximum dose of 7 mg/kg. Bupivacaine is not recommended for children younger than 12 years, although it is commonly used without adverse consequences. Furthermore, the dose should be modified according to the site and mode of administration.
Inadvertent Intravascular Injection: Most toxic reactions are caused by inadvertent intravascular injection of anesthetics whose doses were calculated for their intended extravascular sites. For example, lidocaine, 300 mg, is a safely infiltrated dose that would probably cause toxicity if directly injected into the bloodstream.
Host Factors
Hypoxia: It was initially thought that an overdose of a local anesthetic produces CNS stimulation and subsequent intracellular hypoxia, which then became the key precipitant for all toxic manifestations of the drug. It is now known that hypoxia may enhance anesthetic toxicity but is not the primary factor.
Acid-Base Status: Although studies of metabolic alkalosis have produced conflicting results, acidosis, particularly respiratory acidosis, can increase toxicity. The elevated CO2 produced by respiratory acidosis crosses the blood-brain barrier, where it may act directly on the receptor and indirectly by lowering intracellular pH. The lower pH causes more drug to ionize, thereby furthering the block in the sodium channel and increasing the potential for toxicity.
Protein Binding: The of concentration unbound drug relates more closely to toxic effects than does the total drug concentration (bound plus unbound) as measured in the blood. The amount of α-acid glycoprotein (AAG), the major plasma protein responsible for binding local anesthetics, is considerably decreased in neonates in comparison to adults. Arthur and McNicol118 implied that the low AAG levels in neonates are responsible for the increased toxic potential. Tucker and colleagues119 listed several disease states that alter AAG levels and protein binding but question whether they lead to changes in free drug concentration in vivo.
Concomitant Drugs.: For years, barbiturates were used to prevent and treat local anesthesia-induced seizures. Barbiturates were found to worsen anesthetic-induced apnea and cardiovascular depression. CNS depressants are used with caution when concern exists for local anesthetic toxicity. CNS stimulants have been shown to increase anesthetic-induced excitability and are avoided. Mixtures of local anesthetics have an additive effect on toxicity. If two drugs are used at half strength, they produce the same degree of toxicity as though each were used alone at normal strength. As discussed previously, drugs that slow metabolism by inhibiting hepatic enzymes may increase the risk for toxicity.
Prevention of Toxicity
Knowledge of factors contributing to toxicity guides preventive measures. Avoid esters in patients with an atypical form or a quantitative deficiency of pseudocholinesterase. Use amides with caution in patients with severe liver disease or congestive heart failure. Pay attention to maximum safe dosages based on the site, technique, use of epinephrine, and patient status. Add epinephrine when possible to decrease the rate of drug absorption at vascular sites. Reduce the drug concentration by saline dilution to increase the volume for administration when a large area must be infiltrated. Frequently aspirate in areas of high vascularity, even though a negative aspiration may not prevent IV administration.120 Slow infiltration is advised for safety and is also associated with less pain.
Treatment of Systemic Toxicity
Treat hypotension and bradycardia with fluids, leg elevation, α- and β-agonists (epinephrine, ephedrine, or dopamine), or atropine as the need dictates. Although lidocaine (with diazepam pretreatment) has been shown to be effective for bupivacaine-induced ventricular dysrhythmias, strong theoretical and experimental evidence indicates that bretylium is more effective.121,122 However, until bretylium becomes available again, amiodarone is a reasonable alternative. High doses of atropine and epinephrine can be successful in correcting pulseless idioventricular rhythm. Cardiopulmonary resuscitation is instituted when necessary.
Intravenous Lipid Emulsion
Animal studies, case reports/small series, and personal opinion have advocated the use of 20% lipid emulsion intravenously as a remarkable antidote to resuscitate bupivacaine- and mepivacaine-related cardiac arrest, a situation that is usually fatal (see http://lipidrescue.com). Rosenblatt and associates123 described the IV injection of 100 mL of 20% Intralipid (Baxter formulation used for hyperalimentation) followed by an infusion (0.5 mL/kg/min over a 2-hour period) and related this intervention to successful resuscitation in a scenario that appeared hopeless. Picard124 considers lipid emulsion a “crucial antidote” that should be available when local anesthetics are used for peripheral nerve blocks. It is unclear whether this intervention will prove useful for local anesthetic–related cardiac arrest, but the initial data are encouraging. Lipid emulsion therapy in otherwise hopeless situations of cardiac arrest secondary to local anesthetic overdose is supported (Box 29-3). It appears prudent and intuitive to initiate this antidote before cardiac arrest when significant local anesthetic toxicity is identified.
Allergic Reactions
Uncertainty often exists regarding the specific agent involved, and the clinician must choose an alternative approach to local anesthesia. If the wounds are extensive and the risk is acceptable, procedural sedation (see Chapter 33) or general anesthesia may be used. Conversely, if minimal pain is expected and the procedure is short (e.g., one or two sutures or staples in the scalp), no anesthesia may be required. These methods may be useful, but the degree of anesthesia produced is frequently not sufficient. Antihistamines injected into a wound have been used successfully for many years and represent a good alternative. Local anesthetic efficacy is found in varying degrees with all antihistamines. Ketamine anesthesia may be a useful alternative in some situations and is commonly used in children.
Diphenhydramine and Benzyl Alcohol
Several studies have demonstrated that 1% diphenhydramine (Benadryl) is as effective as 1% lidocaine for infiltrative anesthesia.125–127 As long as diphenhydramine is not used at concentrations greater than 1%, potential problems of skin necrosis or significant sedation are rare. Dilute the standard 5% parenteral form to a 1% concentration for subcutaneous injection (1 mL of drug to 4 mL of saline). The duration of action of diphenhydramine is shorter than that of lidocaine but appears to be adequate for most procedures. The injection pain of diphenhydramine exceeds that of lidocaine but can be diminished by reducing the concentration to 0.5%. At this lower concentration, the effectiveness of this agent on facial wounds is lost.128 The addition of epinephrine to 0.5% diphenhydramine results in a more painful solution with a shorter duration of action than a standard buffered lidocaine with epinephrine solution.129 Benzyl alcohol (0.9%) with epinephrine (1 : 100,000) compares favorably with diphenhydramine as an effective local anesthetic. This appears to be a useful alternative to diphenhydramine when lidocaine cannot be used but is of shorter duration than diphenhydramine.130–132
Skin Testing
Skin testing and progressive subcutaneous challenge doses deserve special mention because they appear to be logical and well-studied approaches. However, intradermal skin testing with local anesthetics is controversial and often of no practical benefit in the ED. False-positive results are frequently produced by local release of histamine in response to needle trauma, tissue distention, or preservatives in the solution.133 In addition, a high incidence of false-negative results can occur. It is questionable whether these low-molecular-weight drugs or their allergenic metabolites are ever capable of eliciting positive responses.134 Other disadvantages of skin testing include its time-consuming nature and potential hazard when even minute traces of an allergen may precipitate a serious reaction. Subcutaneous challenge testing in graduating doses has been advocated and may well eliminate many false responses, but it does not eliminate the problems of time and hazard. Swanson,135 recognizing that allergy to pure lidocaine is extremely rare, recommended 0.1 mL as a single intradermal skin test. Although his approach eliminates the time disadvantage, intradermal placement can still produce false responses. It would seem more reasonable to give this test dose subcutaneously while exercising due caution in the unlikely event that a patient exhibits a serious reaction.
Vasovagal Reactions
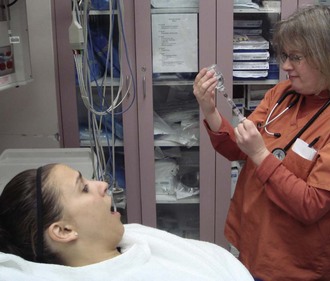
It is not standard to monitor patients (cardiac, pulse oximetry) during routine local anesthesia procedures. Vasovagal reactions are, however, common, especially in dental procedures (reported incidence, 2% to 3%), during which the patient is generally in an upright position. To limit vasovagal reactions related to local anesthesia in the ED, do not draw up medication in a syringe in front of the patient, and inject only when the patient is supine (Figs. 29-11 and 29-12). The patient initially experiences anxiety when a triggering event, commonly the sight or sensation of needle insertion, causes loss of sympathetic tone and an increase in vagal tone. The resultant hypotension and bradycardia may lead to syncope. Address the patient’s anxiety and administer the injections with the patient recumbent as useful preventive measures. Cardiac monitoring may help identify the onset of vagally induced bradycardia when suggested by the past history. Lay the patient supine and elevate the legs. Rarely, atropine is required. Should a patient lose consciousness while in a recumbent position, consider diagnoses other than vasovagal syncope, although significant bradycardia and even complete heart block may accompany a vagal reaction in a supine patient.
References
1. Koller, C. On the use of cocaine for producing anaesthesia on the eye. Lancet. 1884;2:990.
2. Hall, RJ. Hydrochlorate of cocaine. N Y Med J. 1884;40:643.
3. Halsted, WS. Practical comments on the use and abuse of cocaine; suggested by its invariably successful employment in more than a thousand minor surgical operations. N Y Med J. 1885;42:294.
4. Narahashi, T, Frazier, DT, Yamada, M. The site of action and active form of local anesthetics: I. Theory and pH experiments with tertiary compounds. J Pharmacol Exp Ther. 1970;171:32.
5. Frazier, DT, Narahashi, T, Yamada, M. The site of action and active form of local anesthetics: II. Experiments with quaternary compounds. J Pharmacol Exp Ther. 1970;171:45.
6. Galindo, A. pH-adjusted local anesthetics: Clinical experience. Reg Anesth. 1983;8:35.
7. Hilgier, M. Alkalinization of bupivacaine for brachial plexus block. Reg Anesth. 1985;10:59.
8. Todd, K, Berk, WA, Huang, R. Effect of body locale and addition of epinephrine on the duration of action of a local anesthetic agent. Ann Emerg Med. 1992;21:723.
9. Chiu, YC, Brecht, K, DasGupta, DS. Myocardial infarction with topical cocaine anesthesia for nasal surgery. Arch Otolaryngol Head Neck Surg. 1986;112:988.
10. Adriani, J, Zepernick, R. Clinical effectiveness of drugs used for topical anesthesia. JAMA. 1964;188:93.
11. O’Donohue, WJ, Jr., Moss, LM, Angelillo, VA. Acute methemoglobinemia induced by topical benzocaine and lidocaine. Arch Intern Med. 1980;140:1508.
12. Abu Al-Melh, M, Andersson, L, Behnehani, E. Reduction of pain from needle stick in the oral mucosa by topical anesthetics: a comparison study between lidocaine/prilocaine and benzocaine. J Clin Dent. 2005;6:53.
13. Chan, A, Ignoffo, RJ. Survey of topical oral solutions for the treatment of chemo-induced oral mucositis. J Oncol Pharm Pract. 2005;11:139.
14. Clarkson, JE, Worthington, HV, Eden, OB. Interventions for treating mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. (2):2004. [CD001973].
15. Hess, GP, Watson, PD. Seizures secondary to oral viscous lidocaine. Ann Emerg Med. 1988;17:725.
16. Mofenson, HC, Caraccio, TR, Miller, H, et al. Lidocaine toxicity from topical mucosal application. Clin Pediatr (Phila). 1983;22:190.
17. Gonzales, J. Lidocaine overdose: another preventable case? Pediatr Emerg Care. 1994;10:344.
18. Wolfe, TR, Fosnocht, DE, Linscott, MS. Atomized lidocaine as topical anesthesia for nasogastric tube placement: a randomized, double-blind, placebo-controlled trial. Ann Emerg Med. 2000;35:421.
19. Cullen, L, Taylor, D, Taylor, S, et al. Nebulized lidocaine decreases the discomfort of nasogastric tube insertion: a randomized, double-blind trial. Ann Emerg Med. 2004;44:131.
20. Lubens, HM, Ausdenmoore, RW, Shater, AD, et al. Anesthetic patch for painful procedures such as minor operations. Am J Dis Child. 1974;128:192.
21. Eichenfeld, LF, Funk, A, Fallon-Frienlander, S, et al. A clinical study to evaluate the efficacy of ELA-Max (4% liposomal lidocaine) as compared with eutectic mixture of local anesthetic cream for pain reduction of venipuncture in children. Pediatrics. 2002;109:1093–1099.
22. McCafferty, DF, Woolfson, AD. New patch delivery system for percutaneous local anaesthesia. Br J Anaesth. 1993;71:370.
23. Buckley, MM, Benfield, P. Eutectic lidocaine/prilocaine cream. Drugs. 1993;46:126.
24. Doyle, E, Freeman, J, Im, NT, et al. An evaluation of a new self-adhesive patch preparation of amethocaine for topical anaesthesia prior to venous cannulation in children. Anaesthesia. 1993;48:1050.
25. Hansson, C, Holm, J, Lillieborg, S, et al. Repeated treatment with lidocaine/prilocaine (EMLA) as a topical anaesthetic for the cleansing of venous leg ulcers. Acta Derm Venereol (Stockh). 1993;73:231.
26. Koh, JL, Harrison, D, Myers, R, et al. A randomized, double-blind comparison study of EMLA and ELA-Max for topical anesthesia in children undergoing intravenous insertion. Paediatr Anaesth. 2004;14:977.
27. Luhmann, J, Hurt, S, Shootman, M, et al. A comparison of buffered lidocaine versus ELA-Max before peripheral intravenous catheter insertions in children. Pediatrics. 2004;113:217.
28. Soueid, A, Richard, B. Ethyl chloride as a cryoanalgesic in pediatrics for venipuncture. Pediatr Emerg Care. 2007;23:380.
29. Costello, M, Ramundo, M, Christopher, NC, et al. Ethyl chloride vapocoolant spray fails to decrease pain associated with intravenous cannulation in children. Clin Pediatr (Phila). 2006;45:628.
30. DeCou, JM, Abrams, RS, Hammond, JH, et al. Iontophoresis: a needle-free, electrical system of local anesthesia delivery for pediatric surgical office procedures. J Pediatr Surg. 1999;34:946.
31. Squire, SJ, Kirchoff, KT, Hissong, K. Comparing two methods of topical anesthesia used before intravenous cannulation in pediatric patients. J Pediatr Health Care. 2000;14:68.
32. Zempsky, WT, Anand, KJ, Sullivan, KM, et al. Lidocaine iontophoresis for topical anesthesia before intravenous line placement in children. J Pediatr. 1998;132:1061.
33. Zempsky, WT, Parkinson, TM. Lidocaine iontophoresis for topical anesthesia before dermatologic procedures in children. Pediatr Dermatol. 2003;20:364.
34. Li, X, Zhao, R, Qin, Z, et al. Microneedle pretreatment improves efficacy of cutaneous topical anesthesia. Am J Emerg Med. 2010;28:130–134.
35. Auerbach, M, Tunik, M, Mojica, M. A randomized, double-blind controlled study of jet lidocaine compared to jet placebo for pain relief in children undergoing needle insertion in the emergency department. Acad Emerg Med. 2009;16:388–393.
36. Hajiseyedjavady, H, Saeedi, M, Eslami, V. Less painful arterial blood gas sampling using jet injection of 2% lidocaine: a randomized controlled clinical trial. Am J Emerg Med. 2012;30:1100–1104.
37. Booth, S, Koenig, H, Sikes, et al. Jet injection of 1% buffered lidocaine versus topical ELA-MAX for anesthesia before peripheral intravenous catheterization in children: a randomized controlled trial. Pediatr Emerg Care. 2008;24:511–515.
38. Powell, DM, Rodeheaver, GT, Foresman, PA, et al. Damage to tissue defenses by EMLA cream. J Emerg Med. 1991;9:205.
39. Oni, G, Brown, S, Burrus, C, et al. Effect of 4% topical lidocaine applied to the face on the serum levels of lidocaine and its metabolite, monoethylglycinexylidide. Aesthet Surg J. 2010;30:853–858.
40. Pryor, GJ, Kilpatrick, WR, Opp, DR. Local anesthesia in minor lacerations: topical TAC vs. lidocaine infiltration. Ann Emerg Med. 1980;9:568.
41. Ordog, GJ, Ordog, C. The efficacy of TAC (tetracaine, adrenaline, and cocaine) with various wound-application durations. Acad Emerg Med. 1994;1:360.
42. Eidelman, A, Weiss, JM, Enu, IK, et al. Comparative efficacy and costs of various topical anesthetics for repair of dermal lacerations: a systematic review of randomized, controlled trials. J Clin Anesth. 2005;17:106.
43. Hegenbarth, MA, Altieri, MF, Hawk, WH, et al. Comparison of topical tetracaine, adrenaline, and cocaine anesthesia with lidocaine infiltration for repair of lacerations in children. Ann Emerg Med. 1990;19:63.
44. Anderson, AB, Colecchi, C, Baronoski, R, et al. Local anesthesia in pediatric patients: topical TAC versus lidocaine. Ann Emerg Med. 1990;19:519.
45. White, WB, Iserson, KV, Criss, E. Topical anesthesia for laceration repair: tetracaine versus TAC (tetracaine, adrenaline, and cocaine). Am J Emerg Med. 1986;4:319.
46. Bonadio, WA, Wagner, V. Half-strength TAC topical anesthetic. Clin Pediatr (Phila). 1988;27:495.
47. Ernst, AA, Crabbe, LH, Winsemius, DK, et al. Comparison of tetracaine, adrenaline, and cocaine with cocaine alone for topical anesthesia. Ann Emerg Med. 1990;19:51.
48. Bonadio, WA, Wagner, V. Efficacy of tetracaine-adrenaline-cocaine topical anesthetic without tetracaine for facial laceration repair in children. Pediatrics. 1990;86:856.
49. Bonadio, WA, Wagner, V. Adrenaline-cocaine gel topical anesthetic for dermal laceration repair in children. Ann Emerg Med. 1992;21:1435.
50. Schaffer, D. Clinical comparison of TAC anesthetic solutions with and without cocaine. Ann Emerg Med. 1985;14:1077.
51. Smith, SM, Barry, RC. A comparison of three forms of TAC (tetracaine, Adrenalin, cocaine) for anesthesia of minor lacerations in children. Pediatr Emerg Care. 1990;6:266.
52. Ernst, AA, Marvez-Valls, E, Nick, TG, et al. LAT (lidocaine-adrenaline-tetracaine) versus TAC (tetracaine-adrenaline-cocaine) for topical anesthesia in face and scalp lacerations. Am J Emerg Med. 1995;13:151.
53. Zempsky, WT, Karasic, RB. EMLA versus TAC for topical anesthesia of extremity wounds in children. Ann Emerg Med. 1997;30:163.
54. Schilling, CG, Bank, DE, Borchert, BA, et al. Tetracaine, epinephrine (Adrenalin) and cocaine (TAC) versus lidocaine, epinephrine and tetracaine (LET) for anesthesia of lacerations in children. Ann Emerg Med. 1995;25:203.
55. Resch, K, Schilling, C, Borchert, BD, et al. Topical anesthesia for pediatric lacerations: a randomized trial of lidocaine-epinephrine-tetracaine solution versus gel. Ann Emerg Med. 1998;32:693.
56. Singer, AJ, Stark, MJ. LET versus EMLA for pretreating lacerations: a randomized trial. Acad Emerg Med. 2001;8:223.
57. Gaufberg, S, Walta, M, Workman, T. Expanding the use of topical anesthesia in wound management. Am J Emerg Med. 2007;25:379–384.
58. Altieri, M, Bogema, S, Schwartz, RH. TAC topical anesthesia produces positive urine tests for cocaine. Ann Emerg Med. 1990;19:577.
59. Terndrup, TE, Wall, HC, Mariani, PJ, et al. Plasma cocaine and tetracaine levels following application of topical anesthesia in children. Ann Emerg Med. 1992;21:162.
60. Spivey, WH, McNamura, RM, Mackenzie, RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752.
61. Bartfield, JM, Homer, PJ, Ford, DT, et al. Buffered lidocaine as a local anesthetic: an investigation of shelf life. Ann Emerg Med. 1992;21:24.
62. Larson, PO, Ragi, G, Swandby, M, et al. Stability of buffered lidocaine and epinephrine used for local anesthesia. J Dermatol Surg Oncol. 1991;17:411.
63. Bartfield, JM, Gennis, P, Barbera, J, et al. Buffered versus plain lidocaine as a local anesthetic for simple laceration repair. Ann Emerg Med. 1990;19:1387.
64. Orlinsky, M, Hudson, C, Chan, L, et al. Pain comparison of unbuffered versus buffered lidocaine in local wound infiltration. J Emerg Med. 1992;10:411.
65. Bartfield, JM, Ford, DT, Homer, PJ. Buffered versus plain lidocaine for digital nerve blocks. Ann Emerg Med. 1993;22:216.
66. Peterfreund, RA, Datta, S, Ostheimer, GW. pH Adjustment of local anesthetic solutions with sodium bicarbonate: laboratory evaluation of alkalinization and precipitation. Reg Anesth. 1989;14:265.
67. Cheney, PR, Molzen, G, Tandberg, D. The effect of pH buffering on reducing the pain associated with subcutaneous infiltration of bupivacaine. Am J Emerg Med. 1991;9:147.
68. Bainbridge, LC. Comparison of room temperature and body temperature local anaesthetic solutions. Br J Plast Surg. 1991;44:147.
69. Waldbillig, DK, Quinn, JV, Stiell, IG, et al. Randomized double-blind controlled trial comparing room temperature and heated lidocaine for digital nerve block. Ann Emerg Med. 1995;26:677.
70. Bartfield, JM, Crisafulli, KM, Raccio-Robak, N, et al. The effects of warming and buffering on pain of infiltration of lidocaine. Acad Emerg Med. 1995;2:254.
71. Brogan, GX, Jr., Giarrusso, E, Hollander, JE, et al. Comparison of plain, warmed, and buffered lidocaine for anesthesia of traumatic wounds. Ann Emerg Med. 1995;26:121.
72. Martin, S, Jones, JS, Wynn, BN. Does warming local anesthetic reduce the pain of subcutaneous injection? Am J Emerg Med. 1996;14:10.
73. Leff, DR, Nortley, M, Dang, V, et al. The effect of local cooling on pain perception during infiltration of local anaesthetic agents, a prospective randomised controlled trial. Anaesthesia. 2007;62:677.
74. Kelly, AM, Cohen, M, Richards, D. Minimizing the pain of local infiltration anesthesia for wounds by injection into the wound edges. J Emerg Med. 1994;12:593.
75. Bartfiled, J, Sokaris, S, Raccio-Robak, N. Local anesthesia for laceration: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100–104.
76. Bierman, SF. Painless wound injection through use of a two-finger confusion technique. Am J Emerg Med. 1988;6:266.
77. Candiotti, K, Rodriguez, Y, Koyyalamudi, P, et al. The effect of needle bevel position on pain for subcutaneous lidocaine injection. J Perianesth Nurs. 2009;24:241–243.
78. Case, RD. Haematoma block—a safe method of reducing Colles’ fractures. Injury. 1985;16:469.
79. Cobb, AG, Houghton, GR. Local anaesthetic infiltration versus Bier’s block for Colles’ fractures. BMJ. 1985;291:1683.
80. Newman, AP. Meniscal and ligamentous injuries of the knee. Top Emerg Med. 1988;10:1.
81. Barrack, RL, Skinner, HB, Brunet, ME, et al. Functional performance of the knee after intra-articular anesthesia. Am J Sports Med. 1983;11:258.
82. Holdsworth, BJ, Clement, DA, Rothwell, PN. Fractures of the radial head—the benefit of aspiration: a prospective controlled study. Injury. 1987;18:44.
83. Gomool, A, Kang, R, Williams, J, et al. Chondrolysis after continuous intra-articular bupivacaine infusion: an experimental model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy. 2006;22:813–819.
84. Dragoo, J, Korotkova, T, Kim, H, et al. Chondrotoxicity of low pH, epinephrine, and preservatives found in local anesthetics containing epinephrine. Am J Sports Med. 2010;38:1154–1159.
84a. Gupta, A, Bodin, L, Holmstrom, B, et al. A systematic review of the peripheral analgesic effects if intraarticular morphine. Anesth Analgesia. 2001;93:761.
85. Karmakar, MK, Critchley, LAH, Ho, AMH, et al. Continuous thoracic paravertebral infusion of bupivacaine for pain management in patients with multiple rib fractures. Chest. 2003;123:424.
86. Gabram, SG, Schwartz, RJ, Jacabs, LM, et al. Clinical management of blunt trauma patients with unilateral rib fractures: a randomized trial. World J Surg. 1995;19:388.
87. Knottenbelt, JD, James, MF, Bloomfield, M. Intrapleural bupivacaine analgesia in chest trauma: a randomized double-blind controlled trial. Injury. 1991;22:114.
88. Shinohara, K, Iwama, H, Akama, Y, et al. Interpleural block for patients with multiple rib fractures: comparison with epidural block. J Emerg Med. 1994;12:441.
89. Pond, WW, Somerville, GM, Thong, SH, et al. Traumatic splenic rupture masked by intrapleural lidocaine. Anesthesiology. 1989;70:154.
90. Stromskag, KE, Minor, B, Steen, PA. Side effects and complications related to interpleural analgesia: an update. Acta Anaesthesiol Scand. 1990;34:473.
91. Engdahl, O, Boe, J, Sandstedt, S. Plasma concentrations and hemodynamic changes after repeated interpleural injections of bupivacaine-epinephrine. Reg Anesth. 1993;18:374.
92. Kawasaki, C, Kawasaki, T, Ogata, M, et al. Lidocaine enhances apoptosis and suppresses mitochondrial functions of human neutrophil in vitro. J Trauma. 2010;68:401–408.
93. Fedder, C, Beck-Schimmer, B, Aguirre, J, et al. In vitro exposure of human fibroblasts to local anaesthetics impairs cell growth. Clin Exp Immunol. 2010;162:280–288.
94. Chvapil, M, Hameroff, SR, O’Den, K, et al. Local anesthetics and wound healing. J Surg Res. 1979;27:367.
95. Morris, T, Tracey, J. Lignocaine: its effect on wound healing. Br J Surg. 1977;64:902.
96. Eriksson, AS, Sinclair, R, Cassuto, J, et al. Influence of lidocaine on leukocyte function in the surgical wound. Anesthesiology. 1992;77:74.
97. Schmidt, RM, Rosenkranz, HS. Antimicrobial activity of local anesthetics: lidocaine and procaine. J Infect Dis. 1970;121:597.
98. Feldman, JM, Chapin-Robertson, K, Turner, J. Do agents used for epidural analgesia have antimicrobial properties? Reg Anesth. 1994;19:43.
99. Parr, AM, Zutman, DE, Davidson, JS. Antimicrobial activity of lidocaine against bacteria associated with nosocomial wound infection. Ann Plast Surg. 1999;43:239.
100. Berg, JO, Mossner, BK, Skov, MN, et al. Antibacterial properties of EMLA and lidocaine in wound tissue culture biopsies for culturing. Wound Repair Regen. 2006;14:581.
101. Thompson, KD, Welkyj, S, Massa, MC. Antibacterial activity of lidocaine in combination with a bicarbonate buffer. J Dermatol Surg Oncol. 1993;19:216.
102. Stevenson, TR, Rodeheaver, GT, Golden, GT, et al. Damage to tissue defenses by vasoconstrictors. JACEP. 1975;4:532.
103. Tran, DT, Miller, SH, Buck, D, et al. Potentiation of infection by epinephrine. Plast Reconstr Surg. 1985;76:933.
104. Hohn, DC, McKay, RD, Halliday, B, et al. Effect of O2 tension on microbicidal function of leukocytes in wounds and in vitro. Surg Forum. 1976;27:18.
105. Born, G. Neuropathy after bupivacaine (Marcaine) wrist and metacarpal nerve blocks. J Hand Surg [Am]. 1984;9:109.
106. Lalonde, D, Bell, M, Sparkes, G, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project Clinical Phase. J Hand Surg [Am]. 2005;30:1061.
107. Thomson, CJ, Lalonde, DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429.
108. Denkler, K. A comprehensive review of epinephrine in the finger: to do or not to do. Plast Reconstr Surg. 2001;108:114.
109. Wilhelmi, BJ, Blackwell, SJ, Miller, JH, et al. Do not use epinephrine in digital blocks: myth or truth? Plast Reconstr Surg. 2001;107:393.
110. Chowdhry, S, Seidenstricker, L, Cooney, DS, et al. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;125:2031.
111. McCauley, WA, Gerace, RV, Scilley, C. Treatment of accidental digital injection of epinephrine. Ann Emerg Med. 1991;20:665.
112. deJong, RH. Toxic effects of local anesthetics. JAMA. 1978;239:1166.
113. Isohanni, MH, Neuvonen, PJ, Palkama, VJ, et al. Effect of erythromycin and itraconazole on the pharmacokinetics of intravenous lignocaine. Eur J Clin Pharmocol. 1998;54:561.
114. Isohanni, MH, Ahonen, J, Neuvonen, PJ, et al. Effect of ciprofloxacin on the pharmacokinetics of intravenous lidocaine. Eur J Anaesthesiol. 2005;22:795.
115. Olkkola, KT, Isohanni, MH, Hamunen, K, et al. The effect of erythromycin and fluvoxamine on the pharmacokinetics of intravenous lidocaine. Anesth Analg. 2005;100:1352.
116. Scott, DB, Jebson, PJR, Braid, DB, et al. Factors affecting plasma levels of lignocaine and prilocaine. Br J Anaesth. 1972;44:1040.
117. Moore, DC, Mather, LE, Bridenbaugh, LD, et al. Arterial and venous plasma levels of bupivacaine following peripheral nerve blocks. Anesth Analg. 1976;55:763.
118. Arthur, DS, McNicol, LR. Local anesthetic techniques in paediatric surgery. Br J Anaesth. 1986;58:760.
119. Tucker, GT, Moore, DC, Bridenbaugh, PO, et al. Systemic absorption of mepivacaine in commonly used regional block procedures. Anesthesiology. 1972;37:277.
120. Moore, DC, Bridenbaugh, LD, Thompson, GE, et al. Bupivacaine: a review of 11,080 cases. Anesth Analg. 1978;57:42.
121. Kasten, GW, Martin, ST. Bupivacaine cardiovascular toxicity: comparison of treatment with bretylium and lidocaine. Anesth Analg. 1985;64:911.
122. Kasten, GW, Martin, ST. Successful cardiovascular resuscitation after massive intravenous bupivacaine overdosage in anesthetized dogs. Anesth Analg. 1985;64:491.
123. Rosenblatt, MA, Abel, M, Fischer, GW, et al. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology. 2006;105:217.
124. Picard, J. Lipid emulsion to treat bupivacaine toxicity [letter]. Anesthesia. 2005;60:1158.
125. Ernst, AA, Anand, P, Nick, T, et al. Lidocaine versus diphenhydramine for anesthesia in the repair of minor lacerations. J Trauma. 1993;34:354.
126. Dire, DJ, Hogan, DE. Double-blinded comparison of diphenhydramine versus lidocaine as a local anesthetic. Ann Emerg Med. 1993;22:1419.
127. Green, SM, Rothrock, SG, Gorchynski, J. Validation of diphenhydramine as a dermal local anesthetic. Ann Emerg Med. 1994;23:1284.
128. Ernst, AA, Marvez-Valls, E, Mall, G, et al. 1% Lidocaine versus 0.5% diphenhydramine for local anesthesia in minor laceration repair. Ann Emerg Med. 1994;23:1328.
129. Ernst, AA, Marvez-Valls, E, Nick, TG, et al. Comparison trial of four injectable anesthetics for laceration repair. Acad Emerg Med. 1996;3:228.
130. Bartfield, JM, May-Wheeling, HE, Raccio-Robak, N. Benzyl alcohol with epinephrine as an alternative to lidocaine with epinephrine. J Emerg Med. 2001;21:375.
131. Wilson, L, Martin, S. Benzyl alcohol as an alternative local anesthetic. Ann Emerg Med. 1999;33:495.
132. Bartfield, JM, Jandreau, SW, Raccio-Robak, N. Randomized trial of diphenhydramine versus benzyl alcohol with epinephrine as an alternative to lidocaine local anesthetic. Ann Emerg Med. 1998;32:650.
133. Aldrete, JA, Johnson, DA. Evaluation of intracutaneous testing for investigation of allergy to local anesthetic agents. Anesth Analg. 1970;49:173.
134. Covino, BG. Pharmacology of local anesthetic agents. Br J Anaesth. 1986;58:701.
135. Swanson, JG. Assessment of allergy to local anesthetics. Ann Emerg Med. 1983;12:316.