Chapter 97E Liver transplantation for cholangiocarcinoma and other neoplastic diseases
Hilar Cholangiocarcinoma (See Chapter 50B)
CCA, the second most common primary malignant tumor of the liver, arises from the cholangiocytes of the intrahepatic and extrahepatic bile ducts. The incidence of this tumor has been estimated at 3000 to 5000 cases per year (de Groen et al, 1999; Olnes & Erlich, 2004), and the prevalence of intrahepatic disease appears to be on the rise (Shaib et al, 2004). CCA can arise within the liver, in the perihilar location, or along the extrahepatic bile duct (see Chapters 50A and 50B). CCA has three growth patterns: 1) mass-forming tumors are usually intrahepatic, 2) sclerosing tumors arise in the perihilar and extrahepatic bile ducts, and 3) polypoid tumors grow within the major intrahepatic and extrahepatic ducts. Surgical extirpation has been the standard treatment for all three tumor types.
The treatment of hilar CCA has been most troublesome because it is impossible to achieve a tumor-free (R0) margin of resection. Radical resection with partial hepatectomy was shown to improve survival (Launois et al, 1979) for patients with hilar CCA, but few patients come to medical attention with disease amenable to complete resection. Indeed, fewer than 30% of patients are candidates for resection at diagnosis because of either bilateral liver involvement, encasement of hilar vascular structures, involvement of sectoral bile ducts, and/or underlying liver disease, such as primary sclerosing cholangitis (PSC; see Chapter 41). Liver transplantation appears promising for the treatment of intrahepatic and hilar CCA, because the procedure affords a radical resection, is not limited by bilateral ductal or vascular involvement, and treats underlying liver disease.
Early Experience with Liver Transplantation
Unfortunately, early experiences with liver transplantation for the treatment of CCA were uniformly poor. Liver transplantation for both intrahepatic and hilar CCA was fraught with high recurrence rates and poor patient survival. The Cincinnati Tumor Registry reported a large multicenter analysis for patients transplanted from 1968 to 1997, wherein 1-, 3-, and 5-year patient survival rates were only 72%, 48%, and 23%, respectively (Meyer et al, 2000). The recurrence rate was 51% with a median time to recurrence of only 9.7 months. Local recurrence within the allograft was the most common initial site of recurrence (47%) followed by distant metastases to the lung (30%). Recurrence of tumor portended an extremely poor prognosis, with a median survival of only 2 months. Adjuvant therapy was not found to be beneficial, and no difference was reported in the survival rate of known tumors versus incidental tumors found at the time of orthotopic liver transplantation (OLT); results were poor for both intrahepatic and hilar tumors.
A multitude of retrospective studies have confirmed these findings. A Spanish multicenter study reported similar results for 59 patients who underwent OLT for CCA from 1988 to 2001 (Robles et al, 2004) and found 5-year survival was 30% with a 53% recurrence rate for 39 patients with hilar CCA. Results were equally poor for 23 patients with intrahepatic CCA, for which 5-year survival was 42%, and the recurrence rate was 35%. Similarly, a Scandinavian study reported a 5-year survival of 30% following OLT in a primary sclerosing cholangitis (PSC) population with early-stage CCA (Brandsaeter et al, 2004).
Several centers have reported their outcomes with incidental tumors discovered in patients undergoing transplantation for chronic liver disease. Ghali and colleagues (2005) reviewed the Canadian experience from 1996 through June 2003 and identified 10 cases, 8 arising in patients with PSC. Most of these tumors had favorable characteristics that included small size (<1 cm) and absence of perihepatic lymph node involvement; 90% were well differentiated, and 60% arose in the extrahepatic or hilar ducts. Despite the favorable characteristics, 3-year survival was only 30%. Only the University of California–Los Angeles has reported reasonable survival outcomes in incidental CCA detected in the explant after OLT (Goss et al, 1997). Ten patients with incidental CCA had a 5-year survival of 87%, which was comparable to PSC patients without CCA, although pathologic characteristics were not included in the paper. As with all other experiences, the 4 patients transplanted with known CCA had poor outcomes, and none were alive at 5 years.
A more radical approach with cluster abdominal transplantation reported by the University of Pittsburgh had equally poor results: a 3-year survival of 20% and a 57% recurrence rate (Alessiani et al, 1995). A similar experience was recently reported by Neuhaus’ team in Berlin (Seehofer et al, 2009). Sixteen patients with CCA were treated by combined liver transplantation and pancreatoduodenectomy (PD) between 1992 and 1998, and results were compared to those achieved for 8 patients who did not undergo PD, which at the time of liver transplantation was associated with significantly higher morbidity than transplantation alone. Long-term survival (>4 years) was achieved in only 3 lymph node–negative patients of 20 patients who survived the perioperative period. Neuhaus and colleagues concluded that “there is no good evidence that more radical resections alone are able to markedly improve long-term results.”
Neoadjuvant Therapy and Liver Transplantation
Despite the poor results with liver transplantation alone, some patients with favorable hilar CCA—that is, those with negative margins and no regional lymph node metastases—did benefit from transplantation (Shimoda et al, 2001). In addition, a small group of patients at the Mayo Clinic treated with primary radiotherapy and chemosensitization alone, without resection, had a 5-year survival of 22% (Foo et al, 1997). Based on the known palliative efficacy of radiotherapy for CCA—and knowledge that CCA resection failures are usually due to locoregional recurrence, rather than distant metastases (Jarnagin et al, 2003)—the transplant team at the University of Nebraska pioneered a strategy of high-dose neoadjuvant brachytherapy and chemotherapy followed by liver transplantation (Sudan et al, 2002) for patients with unresectable hilar CCA.
Mayo Clinic Experience
Inclusion and Exclusion Criteria
Criteria for protocol enrollment are designed to select those patients least likely to develop metastatic disease, most likely to respond to neoadjuvant therapy, and who have a high probability for survival after transplantation. Appropriate patients include those with early-stage hilar CCA determined to be unresectable or those who have underlying PSC, because CCA arising in the setting of PSC has a very poor natural history following standard resection (Rosen et al, 1991).
Staging Operation
Prior to 2003, 30% to 40% of patients had findings during the staging operation that precluded transplantation. EUS-guided aspiration of regional nodes (not the primary tumor) was implemented to exclude patients with lymph node metastases prior to initiation of neoadjuvant therapy. Initial findings from 47 patients identified eight (17%) with metastases (Gleeson et al, 2008). No morphologic features of the lymph nodes were found at EUS that predicted microscopic disease. Since routine use of EUS was implemented in 2003, the percentage of patients with a positive staging exploration has been reduced to 15%. EUS avoids the morbidity and mortality of high-dose neoadjuvant therapy and prevents an unnecessary operation for patients destined to fall out at operative staging.
Results
One hundred ninety-six patients were enrolled in the Mayo Clinic protocol from 1993 until April 10, 2010 (Fig. 97E.1). Sixteen patients died, became too debilitated for transplantation, or had disease progression prior to operative staging. Three patients underwent transplantation at other centers, and five were receiving neoadjuvant therapy and/or awaiting operative staging. One hundred seventy-two patients underwent operative staging, and 38 patients (22%) had findings precluding transplantation. After staging, 1 patient died from hepatic decompensation, 1 developed intrahepatic metastases, and 3 patients underwent transplantation at other centers. The remaining 126 patients underwent transplantation, 84 with deceased-donor grafts, 41 with living-donor grafts, and one with a domino familial amyloid donor graft.
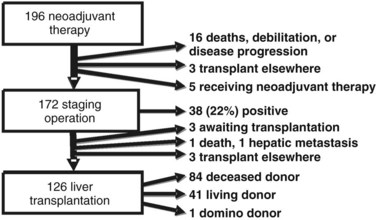
FIGURE 97E.1 Mayo Clinic neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma, 1993 through 2010.
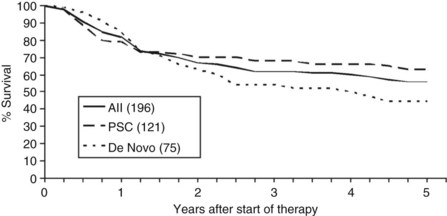
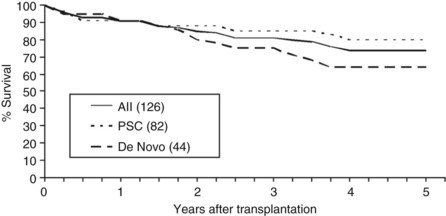
Overall patient survival from enrollment in the protocol (intention-to-treat analysis) was 56% ± 4% at 5 years after the start of neoadjuvant therapy (Fig. 97E.2), 63% ± 5% for patients with underlying PSC, and 44% ± 7% for patients with de novo CCA. Five-year survival after transplantation was 74% ± 5%; 80% ± 5% for patients with underlying PSC and 64% ± 8% for patients with de novo CCA (Fig. 97E.3). No difference in survival was reported among patients undergoing LDLT versus DDLT.
Recurrence and Prognostic Factors
Twenty-one of the 125 patients (17%) who underwent OLT developed recurrent disease at a mean of 25 months (range, 7 to 64 months) after transplantation, and 10 (48%) of the 21 recurrences were distant metastases. The initial sites for recurrent CCA are shown in Table 97E.1.
Table 97E.1 Cholangiocarcinoma Recurrence After Transplantation
| Site | Time (mo) | Status (mo) |
|---|---|---|
| Regional | 7, 10, 14 | Dead at 10, 17, 18 |
| 14, 15, 17, 65 | Alive at 21, 16, 24, 67 | |
| Peritoneal | 15 | Alive at 23 |
| 22, 25, 27 | Dead at 29, 41, 43 | |
| Chest | 18, 24 | Alive at 36, 48 |
| 22, 30, 40 | Dead at 24, 30, 64 | |
| Bone | 7, 8, 55 | Dead at 37, 10, 84 |
| 47 | Dead at 48 | |
| Miscellaneous | 34 | Alive at 37 |
| 47 | Dead at 48 |
Mean time to recurrence was 25 months.
The Mayo group reviewed their experience in 2006 with an aim to identify clinical and pathologic predictors of recurrence (Heimbach et al, 2006). At that time, 65 patients had undergone transplantation; the mean follow-up was 32 months, and 11 patients (17%) had documented recurrences after transplantation. Clinical factors identified as predictors of recurrence included older patient age, prior cholecystectomy, CA19-9 greater than 100 at the time of transplantation, a visible mass on cross-sectional imaging, and an extended waiting time between conclusion of neoadjuvant therapy and OLT. Pathologic findings identified as predictors of recurrence included residual CCA larger than 2 cm, high-grade histology, and perineural invasion.
Pancreatoduodenectomy and Transplantation
Vascular Complications
Late vascular complications are much more common after transplantation for CCA than after other indications because of the neoadjuvant therapy (Mantel et al, 2007). Use of the irradiated native common hepatic artery is avoided during DDLT in lieu of a deceased-donor iliac artery graft from the infrarenal aorta. This strategy was initially implemented in LDLT but resulted in an unacceptable rate of acute hepatic artery thrombosis. Subsequently, the native hepatic artery has been used for arterial inflow in LDLT; this strategy reduced early hepatic artery thrombosis but it is associated with a 20% late stenosis/thrombosis rate. Likewise, there is also a 20% rate of late portal vein stenosis (with or without thrombosis) seen with both living- and deceased-donor transplantation. A deceased donor iliac vein is used as an interposition graft during LDLT, and the stenosis has always involved the native portal vein and its anastomosis with the interposition graft.
Medical and Neoadjuvant Complications
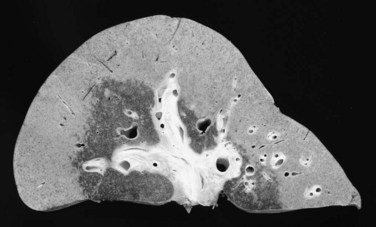
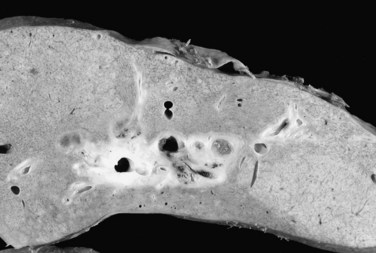
The effect of neoadjuvant radiotherapy on the liver is profound (Fig. 97E.4). Widespread necrosis is found in the hilus with necrotic debris in the major ducts (Fig. 97E.5); thus pretransplant infectious complications related to neoadjuvant therapy are very common and require continuous care and frequent hospitalization for patients awaiting transplantation. Nearly all patients have one or more episodes of cholangitis with occasional development of intrahepatic abscesses. Cholecystitis is treated nonoperatively with antibiotics, although severe cases may require endoscopic placement of a cystic duct stent or percutaneous cholecystostomy. Several patients have developed gallbladder necrosis with rupture that required emergency operations. Gastritis, duodenitis, and poor gastric emptying are also common and can persist after transplantation, which can lead to difficulties with nutrition. Several patients have required operative intervention for duodenal perforation or life-threatening hemorrhage from duodenitis and peptic ulceration.
Key Questions
Efficacy
The Mayo protocol’s efficacy is self-evident: all of the patients had either CCA arising in the setting of PSC or unresectable CCA arising de novo. Resection was not possible, and the results with the Mayo protocol are clearly better than the natural history of untreated CCA, which has a 50% to 70% mortality rate at 1 year (Burak et al, 2004; Farley et al, 1995). The standard surgical treatment for hilar CCA is resection with partial hepatectomy, en bloc excision of the extrahepatic duct, and radical lymphadenectomy. After complete extirpation of tumor, 5-year survival following an R0 resection ranges from 30% to 45%.
Appropriate Use of Donor Organs
The results achieved with liver transplantation for CCA after neoadjuvant therapy and operative staging are comparable to results achieved with liver transplantation for other malignant, chronic, and acute liver diseases such as PSC, hepatitis C, and HCC (Heimbach et al, 2004). Because patients with CCA arising in the setting of PSC and those with unresectable CCA arising de novo have no other potentially curative treatment options, liver transplantation is an appropriate use of both deceased-donor and living-donor livers.
Deceased-Donor Allocation and Prioritization
A group of transplant surgeons and physicians met as an ad hoc MELD Exception Study Group in March 2006 to review the available data for the treatment of hilar CCA with neoadjuvant therapy followed by OLT (Gores et al, 2006). The group concluded that sufficient data existed to justify priority for patients enrolled in clinical trials that used neoadjuvant therapy provided that 1) transplant centers submit formal patient care protocols to the UNOS Liver and Intestinal Committee; 2) candidates satisfy accepted diagnostic criteria for CCA and are considered to be unresectable on the basis of technical considerations or underlying liver disease, such as PSC; 3) tumor mass, when visible on cross-sectional imaging studies, is less than 3 cm in radial diameter; 4) imaging studies to assess patients for intrahepatic and extrahepatic metastases are repeated prior to interval score increases; 5) regional hepatic lymph node involvement and the peritoneal cavity is assessed by operative staging after completion of neoadjuvant therapy and prior to transplantation; and 6) transperitoneal aspiration or biopsy of the primary tumor is avoided because of the high risk of tumor seeding associated with these procedures. The group also concluded that prioritization of patients with biliary dysplasia to avoid progression to CCA was unjustified. In 2009, UNOS adopted these MELD exception criteria, and the score adjustment is identical to that for HCC, with score increases at 3-month intervals.
Resection Versus Transplantation
Because results with the Mayo protocol equal or exceed the results achieved with resection, it would seem reasonable to consider neoadjuvant therapy and liver transplantation in lieu of resection for patients with potentially resectable disease. Rea and colleagues (2005) retrospectively compared results for patients treated by potentially curative resection and neoadjuvant therapy and liver transplantation at the Mayo Clinic between 1993 and 2004. Although no statistically significant difference in survival was found between the two groups, a strong trend was seen toward better survival in the group treated with neoadjuvant therapy and liver transplantation.
Since that time, the Mayo group has accrued a larger experience with liver transplantation for patients with unresectable de novo CCA, and the results with the protocol show an overall survival of 44% at 5 years after the start of neoadjuvant therapy, a result not too dissimilar from those achieved with resection. A prospective trial of resection versus transplantation is impractical because of the large number of patients that would be necessary to show a significant difference in survival. Furthermore, a crossover would not be possible. Patients treated with neoadjuvant therapy would not be amenable to resection. The effect of high-dose neoadjuvant therapy on the underlying liver and bile duct is profound (see Figs. 97E.4 and 97E.5) and precludes biliary reconstruction because of widespread intrahepatic duct necrosis. Patients found to be unresectable at operation would not be appropriate for subsequent neoadjuvant therapy and transplantation. Results with resection are also improving with increasing experience and technical innovation; Neuhaus and colleagues (1999, 2003) demonstrated excellent 5-year survival using vascular reconstruction with extended hepatectomy (Jonas et al, 2008).
Neuroendocrine Cancer (See Chapter 81B)
Gastroenteropancreatic neuroendocrine cancers (NECs) are a rare, diverse group of tumors with an incidence of one to two cases per 100,000 per year with a slight female predominance (Taal & Visser, 2004). These tumors are characteristically indolent in nature and are frequently found to be metastatic at the time of diagnosis. The liver is the most common site for metastases, and widespread hepatic dissemination is the leading cause of death in these patients (Frilling et al, 2009). Fortunately, these cancers are often confined to the liver for protracted periods of time with 5-year survival of 13% to 54% even without treatment (Godwin, 1975; Thompson et al, 1988; Que et al, 1995).
Various therapeutic options have been utilized for NEC with hepatic metastases; such treatments include transarterial chemoembolization (TACE), radiofrequency and cryoablation, systemic chemotherapy, peptide receptor radionuclide therapy (see Chapters 84A, 84B, 85A and 85B), cytoreductive resection, surgical resection (see Chapter 81B), and liver transplantation. Surgical extirpation with an R0 resection is the most effective therapy for metastatic NEC confined to the liver; however, only 10% to 57% of these tumors are amenable to compete resection (Chen et al, 1998; Elias et al, 2003; Frilling et al, 2009; Grazi et al, 2000; Mazzaferro et al, 2007; Musunuru et al, 2006; Yao et al, 2001).
Liver transplantation is an option for treatment of patients with a low probability of achieving an R0 resection. The goals of liver transplantation are to 1) achieve palliation by alleviating symptoms for patients with functioning NEC; 2) achieve palliation by delaying death as a result of tumor replacement of the liver, taking advantage of the slow progression and the time it will take to replace the new liver with tumor, “resetting the clock”; and 3) achieve a cure. Liver transplantation is highly effective at relieving symptoms for patients with functioning NEC. There are several single-center (Florman et al, 2004; Frilling et al, 2006; Mazzaferro et al, 2007; Rosenau et al, 2002) and multicenter (Le Treut et al, 2008) studies reporting 36% to 90% patient survival at 5 years. The possibility of achieving a cure, however, is very low. Disease-free survival is only 20% to 77% at 5 years, and tumor recurrence after 5 years is common.
The Mayo Clinic developed a protocol in 2002 designed to assess efficacy of liver transplantation for patients with metastatic NEC confined to the liver and regional hepatic lymph nodes (van Vilsteren et al, 2006). The clinical inclusion/exclusion criteria are summarized in Box 97E.1. Each patient was screened for locally recurrent and extrahepatic disease with chest, abdomen, and pelvic CT scans, bone scan, and somatostatin receptor scintigraphy within 60 days of preliminary registration for transplantation. Prior treatment modalities did not exclude patients, as long as they did not technically prohibit OLT. Patients were observed for 6 months following resection of the primary NEC to exclude extrahepatic disease progression. Reevaluation for metastatic disease prior to liver transplantation was performed every 4 months via laboratory tests, tumor marker assays, and abdominal imaging studies. Extrahepatic metastases were excluded via operative staging with laparoscopy prior to OLT.
Box 97E.1 Mayo Clinic Transplantation Selection Criteria for Patients with Metastatic Neuroendocrine Carcinoma
Inclusion Criteria
1 Histologic confirmation of NEC (liver primary vs. metastatic disease confined to the liver)
2 Unresectable hepatic disease involving both lobes
3 Complete resection of the primary NEC or single dominant liver NEC believed to be a primary lesion
4 Absence of extrahepatic disease with the exception of regional lymph nodes resectable as part of standard liver transplantation
5 At least a 6-month interval from resection of primary NEC and transplantation without extrahepatic disease progression
6 Patient deemed suitable for liver transplantation, meeting all United Network for Organ Sharing listing criteria
Exclusion Criteria
1 Prior nonselective hepatic artery embolization unless approved by the transplant surgeon (only for living-donor transplantation)
3 Anaplastic or poorly differentiated (grade 3 or 4) tumor
4 Right atrial pressure >15 mm Hg
6 Other factors that preclude transplantation, including severe comorbidities, infection, or other malignancies
Other
1 Patients are staged with computed tomographic scan of the chest, abdomen, and pelvis; bone scan; and somatostatin receptor scintigraphy scan
2 All patients undergo open or laparoscopic staging celiotomy
3 Patients are reevaluated every 4 months with abdominal imaging to evaluate for extrahepatic disease progression
Hepatic Epithelioid Hemangioendothelioma (See Chapter 78B)
HEHE, first described in 1982, is a rare neoplasm that originates from vascular endothelium, and it has unpredictable malignant potential (Weiss & Enzinger, 1982). Ishak and colleagues (1984) first reported liver involvement in a series of 32 patients with HEHE, which arises between the second and ninth decades with a 3 : 2 female/male predominance (Mehrabi et al, 2006). The etiology of HEHE remains elusive, although a multitude of conditions have been proposed to contribute to its development; these include viral hepatitis, liver trauma, oral contraceptives, primary biliary cirrhosis, alcohol consumption, and exposure to vinyl chloride, asbestos, and Thorotrast (Darras et al, 1988; Dean et al, 1985; Lauffer et al, 1996; Makhlouf et al, 1999; Mehrabi et al, 2006; Soslow et al, 1997).
The most common clinical manifestations of HEHE are right upper quadrant pain, hepatomegaly, and weight loss. Weakness, anorexia, epigastric mass, ascites, nausea, jaundice, and fatigue are additional symptoms. Up to 25% of these lesions are detected as incidental findings (Lauffer et al, 1996; Mehrabi et al, 2006).
Two variants of HEHE have been described, nodular and diffuse. In actuality, the variants represent early- and late-stage disease. The nodular type is seen early with a highly variable number of 1- to 12-cm lesions arising throughout the liver. The lesions are typically hypodense on CT scan with high-density contrast uptake in the periphery. Individual nodules increase in size by spreading along the hepatic or portal veins, and they eventually coalesce to form diffuse peripheral lesions. The CT appearance of the later diffuse type is typically a large, slow-growing lesion resulting from coalescence of smaller lesions. Flattening of the capsule may occur because of fibrosis, peripheral enhancement of contrast may be seen with many hypervascular central lesions, and hypertrophy of the unaffected segments of the liver may be apparent (Fulcher & Sterling, 2002; Lyburn et al, 2003; Miller et al, 1992).
Definitive radiographic diagnosis of the HEHE is usually not possible, especially in the nodular form, because of the similarity of this tumor with primary epithelial liver tumors and hepatic metastases. In a review of 434 cases of HEHE, the majority of patients (87%) had multifocal disease involving both lobes of the liver at the time of presentation. Extrahepatic disease at diagnosis was common (36.6%) with involvement of the lung (8.5%), regional lymph nodes (7.7%), peritoneum (6.1%), spleen (3.2%), and diaphragm (1.6%) (Mehrabi et al, 2006).
Histologically, HEHE may resemble other conditions such as venoocclusive disease, CCA, hemangioma, angiosarcoma, and metastatic signet ring cell carcinoma (Corrin et al, 1979). Furthermore, immunohistochemical staining may suggest a tumor of neuroendocrine origin. Histologic findings are consistent with epithelioid or histiocytoid morphology and intracytoplasmic lumina containing red blood cells. Immunohistochemical staining is positive for factor VIII–related antigen and endothelial cell markers (CD31, CD34) and negative for mucin, bile, carcinoembryonic antigen, and α-fetoprotein. Ultrastructural findings are characterized by a well-developed basal lamina, pinocytotic vesicles, and Weibel-Palade bodies (Ishak et al, 1984; Lauffer et al, 1996; Mehrabi et al, 2006).
Treatment of HEHE is difficult. Resection is often not possible because of the presence of multifocal bilobar disease at the time of diagnosis. In the review by Mehrabi and colleagues (2006), only 9.4% of patients were amenable to resection. Patient survival was 100% at 1 year and 75% at 5 years. For many patients, observation alone is reasonable, because progression may be extremely slow, if it occurs at all. Others have reported poorer results (Ben-Haim et al, 1999) and have suggested that resection should be limited to patients presenting with unilobar, liver-confined disease (Mosoia et al, 2008).
Liver transplantation is an option for patients with extensive intrahepatic disease. Several series have reported outcomes equivalent to those for liver transplantation for other diagnoses. In a report from the European Liver Transplant Registry (ELTR), patient survival rates at 1, 5, and 10 years were 93%, 82%, and 72% with 90%, 82%, and 64% disease-free survival, respectively. Neither disease-free survival nor patient survival was influenced by neoadjuvant therapy, lymph node status, or the presence of extrahepatic disease; however, vascular invasion had a negative impact on patient survival (Lerut et al, 2007). A multicenter Canadian experience with 11 patients reported 5-year survival of 82% with a 36.4% recurrence rate (Nudo et al, 2008). A recent query of the UNOS database identified 110 patients who underwent OLT for HEHE in the United States between 1987 and 2005 (Rodriguez et al, 2008). Patient survival was 80% and 64% at 1 and 5 years. In their survey of the available literature, Mehrabi and colleagues (2006) reported 1- and 5-year patient survival of 96% and 54.5%, respectively, after OLT.
The Mayo Clinic recently reviewed its experience with 30 patients treated for HEHE between 1984 and 2007 (Grotz et al, 2010) and found a 2 : 1 female predominance with a mean age at diagnosis of 46 years (range, 21 to 79 years). The diagnosis of HEHE was confirmed in all cases by immunohistochemical staining for factor VIII–related antigen, CD31, and CD34. Patients were divided into groups based on the number and size of nodules present within the liver: half had 10 or fewer nodules, and half had more than 10 nodules; the largest lesion was smaller than 5 cm in 43%, it was 5 to 10 cm in 30%, and it was larger than 10 cm in 27% of cases. Extrahepatic disease was present at diagnosis in 37% of patients (n = 11). Sites of metastases included lung (n = 8), peritoneum (n = 2), bone (n = 2), brain (n = 1), and skin (n = 1), and one third of the patients had multiple sites of extrahepatic involvement.
Alessiani M, et al. Assessment of five-year experience with abdominal organ cluster transplantation. J Am Coll Surg. 1995;180:1-9.
Ben-Haim M, et al. Hepatic epithelioid hemangioendothelioma: resection or transplantation, which and when? Liver Transpl Surg. 1999;5:526-531.
Brandsaeter B, et al. Liver transplantation for primary sclerosing cholangitis; predictors and consequences of hepatobiliary malignancy. J Hepatol. 2004;40:815-822.
Burak K, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523-526.
Chen H, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88-92. discussion 92-83
Corrin B, et al. Histogenesis of the so-called “intravascular bronchioloalveolar tumour.”. J Pathol. 1979;128:163-167.
Darras T, Moisse R, Colette JM. Epithelioid hemangioendothelioma of the liver. J Belge Radiol. 1988;71:722-723.
de Groen PC, et al. Biliary tract cancers. N Engl J Med. 1999;341:1368-1378.
Dean PJ, Haggitt RC, O’Hara CJ. Malignant epithelioid hemangioendothelioma of the liver in young women: relationship to oral contraceptive use. Am J Surg Pathol. 1985;9:695-704.
Elias D, et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery. 2003;133:375-382.
Farley DR, Weaver AL, Nagorney DM. “Natural history” of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70:425-429.
Florman S, et al. Liver transplantation for neuroendocrine tumors. J Gastrointest Surg. 2004;8:208-212.
Foo ML, et al. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:929-935.
Frilling A, et al. Treatment with (90)Y- and (177)Lu-DOTATOC in patients with metastatic neuroendocrine tumors. Surgery. 2006;140:968-976. discussion 976-967
Frilling A, et al. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br J Surg. 2009;96:175-184.
Fulcher AS, Sterling RK. Hepatic neoplasms: computed tomography and magnetic resonance features. J Clin Gastroenterol. 2002;34:463-471.
Ghali P, et al. Liver transplantation for incidental cholangiocarcinoma: analysis of the Canadian experience. Liver Transpl. 2005;11:1412-1416.
Gleeson FC, et al. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67:438-443.
Godwin JD2nd. Carcinoid tumors: an analysis of 2,837 cases. Cancer. 1975;36:560-569.
Gores GJGR, Sudan D, Rosen CB, the MELD Exception Study Group. Model for End-Stage Liver Disease (MELD) exception for cholangiocarcinoma or biliary dysplasia. Liver Transpl. 2006;12:S95-S97.
Goss JA, et al. Orthotopic liver transplantation for primary sclerosing cholangitis: a 12-year single center experience. Ann Surg. 1997;225:472-481. discussion 481-473
Grazi GL, et al. Highly aggressive policy of hepatic resections for neuroendocrine liver metastases. Hepatogastroenterology. 2000;47:481-486.
Grotz TE, et al. Hepatic epitheliod hemangioendothelioma: is transplant the only treatment option? HPB. 2010;12:546-553.
Heimbach JK, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24:201-207.
Heimbach JK, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703-1707.
Ishak KG, et al. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol. 1984;15:839-852.
Jarnagin WR, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689-1700.
Jonas S, et al. Radical surgery for hilar cholangiocarcinoma. Eur J Surg Oncol. 2008;34:263-271.
Lauffer JM, et al. Epithelioid hemangioendothelioma of the liver: a rare hepatic tumor. Cancer. 1996;78:2318-2327.
Launois B, et al. Carcinoma of the hepatic hilus: surgical management and the case for resection. Ann Surg. 1979;190:151-157.
Lerut JP, et al. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg. 2007;246:949-957. discussion 957
Le Treut YP, et al. Predictors of long-term survival after liver transplantation for metastatic endocrine tumors: an 85-case French multicentric report. Am J Transplant. 2008;8:1205-1213.
Lyburn ID, et al. Hepatic epithelioid hemangioendothelioma: sonographic, CT, and MR imaging appearances. AJR Am J Roentgenol. 2003;180:1359-1364.
Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer. 1999;85:562-582.
Mantel HT, et al. Vascular complications after orthotopic liver transplantation after neoadjuvant therapy for hilar cholangiocarcinoma. Liver Transpl. 2007;13:1372-1381.
Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol. 2007;47:460-466.
Mehrabi A, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108-2121.
Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633-1637.
Miller WJ, et al. Epithelioid hemangioendothelioma of the liver: imaging findings with pathologic correlation. AJR Am J Roentgenol. 1992;159:53-57.
Mosoia L, et al. Hepatic epithelioid hemangioendothelioma: long-term results of surgical management. J Surg Oncol. 2008;98:432-437.
Musunuru S, et al. Metastatic neuroendocrine hepatic tumors: resection improves survival. Arch Surg. 2006;141:1000-1004. discussion 1005
Neuhaus P, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808-818. discussion 819
Neuhaus P, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg. 2003;388:194-200.
Nudo CG, et al. Liver transplantation for hepatic epithelioid hemangioendothelioma: the Canadian multicentre experience. Can J Gastroenterol. 2008;22:821-824.
Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167-179.
Que FG, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169:36-42. discussion 42-33
Rea DJ, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451-458. discussion 458-461
Robles R, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265-271.
Rodriguez JA, et al. Long-term outcomes following liver transplantation for hepatic hemangioendothelioma: the UNOS experience from 1987 to 2005. J Gastrointest Surg. 2008;12:110-116.
Rosen CB, et al. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991;213:21-25.
Rosenau J, et al. Ki67, E-cadherin, and p53 as prognostic indicators of long-term outcome after liver transplantation for metastatic neuroendocrine tumors. Transplantation. 2002;73:386-394.
Seehofer D, et al. Extended bile duct resection and [corrected] liver and transplantation in patients with hilar cholangiocarcinoma: long-term results. Liver Transpl. 2009;15:1499-1507.
Shaib YH, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472-477.
Shimoda M, et al. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl. 2001;7:1023-1033.
Soslow RA, et al. Cytopathologic features of hepatic epithelioid hemangioendothelioma. Diagn Cytopathol. 1997;17:50-53.
Sudan D, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774-779.
Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80(Suppl 1):3-7.
Thompson GB, et al. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery. 1988;104:1011-1017.
van Vilsteren FG, et al. Liver transplantation for gastroenteropancreatic neuroendocrine cancers: defining selection criteria to improve survival. Liver Transpl. 2006;12:448-456.
Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970-981.
Yao KA, et al. Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery. 2001;130:677-682. discussion 682-675