Legionella
1. Identify the causative agent of Legionnaires’ disease.
2. List sources for Legionella in the environment, including those that are both man-made and naturally occurring.
3. Describe how Legionella infections are acquired.
4. Describe how Legionella avoids destruction by the host, including where the organisms survive and replicate.
5. Compare and contrast the three primary clinical manifestations of Legionella, including signs and symptoms.
6. List the specimens acceptable for Legionella testing, including storage and transportation of specimens.
7. Describe the different types of testing for Legionella, including sensitivity and specificity.
8. Explain the chemical principle for buffered charcoal-yeast extract (BCYE) with and without inhibitory agents and the proper use for each.
9. Describe the morphology of the Legionella when grown under optimal growth conditions, including oxygenation, temperature, and length of incubation.
General Characteristics
All Legionella spp. are mesophilic (20° to 45° C), obligately aerobic, faintly staining, thin, gram-negative fastidious bacilli that require a medium supplemented with iron and L-cysteine, and buffered to pH 6.9 for optimum growth. The organisms utilize protein for energy generation rather than carbohydrates. The overwhelming majority of Legionella spp. are motile. As of this writing, more than 52 species belong to this genus. Nevertheless, the organism Legionella pneumophila predominates as a human pathogen within the genus and consists of 16 serotypes. In approximately decreasing order of clinical importance are L. pneumophila serotype 1 (about 70% to 90% of the cases of Legionnaires’ disease), L. pneumophila serotype 6, L. micdadei, L. dumoffii, L. anisa, and L. feeleii. Of note, many species of Legionella have only been isolated from the environment or recorded as individual cases. To date, 20 species of Legionella are documented as human pathogens in addition to L. pneumophila. Box 35-1 is an abbreviated list of some of the species of Legionella.
Pathogenesis and Spectrum of Disease
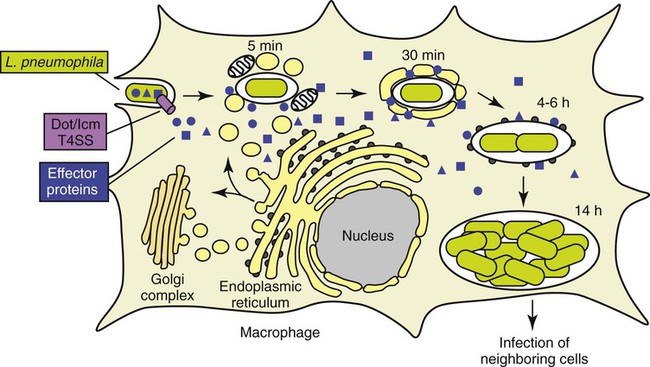
In eukaryotic cells, most proteins secreted or transported inside vesicles to other cellular compartments are synthesized at the endoplasmic reticulum (ER) (Figure 35-1). Many bacterial pathogens use secretion systems as a part of how they cause disease. L. pneumophila possesses genes that are able to “trick” eukaryotic cells into transporting them to the endoplasmic reticulum; these virulence genes are called dot (defective organelle trafficking) or icm (intracellular multiplication). This dot/icm secretion system in L. pneumophila consists of 23 genes and is a type IV secretion system. Bacterial type IV secretion systems are bacterial devices that deliver macromolecules such as proteins across and into cells. After entry but before bacterial replication, L. pneumophila, residing in a membrane-bound vacuole, is surrounded by a ribosome-studded membrane derived from the host cell’s ER and mitochondria. Thus, by exploiting host cell functions, L. pneumophila is able to gain access to the lumen of the ER, which supports its survival and replication where the environment is rich in peptides. A second type II secretion system has also been implicated in the virulence of some strains of Legionella. The type II secretion system carries numerous genes for enzymatic degradation including lipases, proteinases, and a number of novel proteins. Mutations within the type II secretion system results in decreased infectivity of the organism. A number of additional bacterial factors have also been identified as crucial for intracellular infection; some of these are listed in Box 35-2.
(Modified from 2009annualreport.nichd.nih.gov/ump.html.)
Legionella spp. are associated with a spectrum of clinical presentations, ranging from asymptomatic infection to severe, life-threatening diseases. Serologic evidence exists for the presence of asymptomatic disease, because many healthy people surveyed possess antibodies to Legionella spp. Table 35-1 provides a more detailed description of the following three primary clinical manifestations:
TABLE 35-1
Disease Spectrum Associated with Legionella sp.
| Epidemiology | Disease | |
| Pneumonia (Legionnaires’ Disease) | Community and nosocomial transmission (inhalation of aerosolized particles); immunocompromised patients, particularly in cell-mediated immunity; rarely occurs in children | Acute pneumonia indistinguishable from other bacterial pneumonias; clinical syndrome may include nonproductive cough, myalgia, diarrhea, hyponatremia, hypophosphatemia, and elevated liver enzymes |
| Pontiac Fever | Community setting associated with employment (industrial or recreational) or other group | Self-limiting, febrile illness; symptoms may include cough, dyspnea, abdominal pain, fever, and myalgia; pneumonia does not occur |
| Extrapulmonary | Rare, metastatic complications from underlying pneumonia; incidents of inoculation into sites via punctures have been identified or therapeutic bathing; highly associated with immunocompromised patients | Abscesses have been identified in the brain, spleen, lymph nodes, muscles, surgical wounds, and a variety of tissues and organs |
From Mandell GL, Bennett JE, Dolin R: Principles and practices of infectious diseases, ed 7, Philadelphia, 2010, Elsevier.
Laboratory Diagnosis
Specimen Collection and Transport
Direct Detection Methods
Several laboratory methods are used to detect Legionella spp. directly in clinical specimens.
Antigens
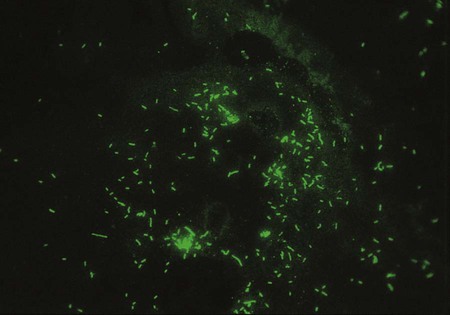
One approach to direct detection of legionellae in clinical specimens is the direct immunofluorescent antibody (DFA) test of respiratory secretions. Polyclonal and monoclonal antisera conjugated with fluorescein are available from several commercial suppliers. Specimens are first tested with pools of antisera containing antibodies to several serotypes of L. pneumophila or several Legionella spp. Those that exhibit positive results are then reexamined with specific conjugated antisera. One reagent made by Genetic Systems Corporation (Seattle, Washington) is a monoclonal antibody directed against a cell wall protein common to L. pneumophila. The manufacturer’s directions should be followed explicitly, and material from commercial systems should never be divided and used separately. Laboratories should decide which serotypes to test for routinely based on the prevalence of isolates in their geographic area. The sensitivity of the DFA test ranges from 25% to 75%, and its specificity is greater than 95%. If positive, organisms appear as brightly fluorescent rods (Figure 35-2). Of importance, cultures always must be performed, because Legionella spp. or serotypes not included in the antisera pool can be recovered. In addition, even in the hands of an experienced microbiologist, false positives may occur. The high complexity of the test and lack of high reproducible sensitivity has reduced the number of laboratories offering DFA testing for Legionella sp.
Cultivation
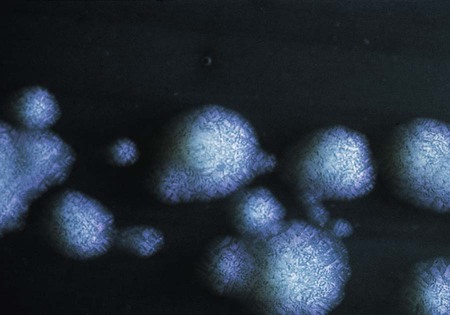
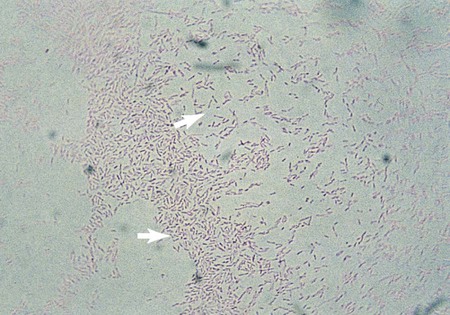
L. pneumophila grows at a temperature range from approximately 20° to 42° C. Plates are typically incubated in a candle jar at the optimal temperature of 35° to 37° C in a humid atmosphere. Some Legionella spp. may be stimulated by increased 2% to 5% concentration of CO2, including L. sainthelensi and L. oakridgensis. The low level of CO2 will not prevent the growth of L. pneumophila. If this concentration is not possible, incubation in air is preferable to 5% to 10% CO2, which may inhibit some legionellae, specifically L. pneumophila. Within 3 to 4 days, colonies should be visible. Plates are held for a maximum of 2 weeks before they are discarded. Blood cultures in biphasic media should be held for 1 month. At 5 days, colonies are 3 to 4 mm in diameter, gray-white to blue-green, glistening, convex, and circular and may exhibit a cut-glass type of internal granular speckling (Figure 35-3). A Gram stain yields thin, gram-negative bacilli (Figure 35-4).
1. Legionella can be spread by all of the following except?
3. Which of the following agars should be used for culturing Legionella?
4. What is the specimen of choice for isolating Legionella?
5. Which of the following is acceptable for therapy?
6. Legionella injects proteins into the host cell by:
7. Legionella can be definitively diagnosed by a:
a. twofold rise in anti-Legionella antibody with an IFA
b. single serum with a titer of 128
8. Which of the following is not a characteristic of Legionella?
a. faintly staining, thin, gram-negative bacilli
b. requires iron and L-cysteine supplements
9. All of the following are true of Legionella except:
a. thrives at warmer temperatures
b. can survive up to 5 years in water
_____ Humoral immunity plays an important role in the defense against Legionella.
_____ Sputum specimens should be treated with sulfuric acid before culturing.
_____ Respiratory secretions may be held for 24 hours at room temperature before culturing.
_____ Specimens collected by bronchial alveolar lavage should be concentrated tenfold before culturing.