20 Left Atrial Isomerism
I. CASE
A. Fetal echocardiography findings
1. The findings suggest a picture of left atrial isomerism (LAI) or polysplenia syndrome (Table 30-1).
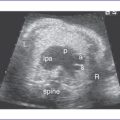
a. There is dextrocardia (cardiac axis = 60 degrees), and the stomach is on the right side of the fetal abdomen as well.
b. There is a midline liver and multilobed right-sided spleen.
c. The heart is mildly enlarged (cardiothoracic ratio = 0.47).
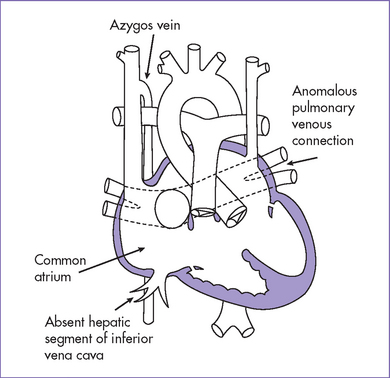
d. The inferior vena cava (IVC) is interrupted, with azygous continuation to a right superior vena cava (SVC) that connects directly to the right-sided atrium.
e. There is a left SVC to coronary sinus to right-sided atrium.
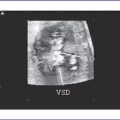
f. The four-chamber view reveals absence of the crux of the heart consistent with an atrioventricular septal defect (AVSD), with severe disproportion between the ventricles.
g. There is a dominant right-sided left ventricle (LV).
2. The outflow assessment reveals normally related great arteries with mild asymmetry. Aorta–to–pulmonary artery diameter ratio is 1:0.7.
3. The aortic valve is in continuity with the common atrioventricular (AV) valve and arises from the LV. The size is normal but the velocity through the aorta is increased (2 m/s). There is also mild aortic insufficiency.
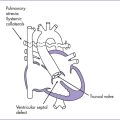
4. The pulmonary artery arises from a small right ventricle (RV) that is composed largely of an outlet chamber. The pulmonary valve is bicuspid, with mild flow acceleration by Doppler (2 m/s).
5. The branch pulmonary arteries are a good size for gestational age (right pulmonary artery = 1.9 mm, left pulmonary artery = 2.2 mm).
6. The aortic arch is leftward, and the ductal and aortic arches have antegrade flow.
7. Interatrial flow is unrestricted through a primum atrial septal defect (ASD) with a bidirectional shunt.
8. The pulmonary veins connect via a confluence that joins the posterior wall of the left-sided atrium, and they exhibit a normal flow pattern.
9. The hepatic veins connect to the right-sided atrium via a common trunk.
10. There is fetal bradycardia with an atrial heart rate of 115 bpm and a ventricular heart rate of 68 bpm, and complete AV block is suspected.
11. The LV Tei index (myocardial performance index) is normal.
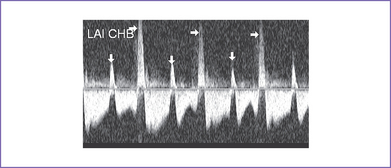
12. There was systolic a wave reversal at the atrial rate on each beat in the ductus venosus with intermittently larger a waves, suggesting cannon waves occurring during ventricular systole (Fig. 20-1).
13. There is no evidence of hydrops.
14. The peripheral arterial Doppler has a normal pulsatility index of 1.2, and the middle cerebral artery pulsatility index is 2.0.
TABLE 20-1 FEATURES CHARACTERIZING NORMAL ASYMMETRY

Adapted from Yoo SJ, Hornberger LK, Smallhorn J: Abnormal visceral and atrial situs and congenital heart disease. In Yagel S, Silverman NH, Gembruch U (eds): Fetal Cardiology: Embryology, Genetics, Physiology, Echocardiographic Evaluation, Diagnosis and Perinatal Management of Cardiac Diseases. London: Taylor & Francis Group, 2002.
D. Fetal management and counseling
a. Because the mother has already had chorionic villous sampling, there was no further discussion about fetal karyotype except that aneuploidy and 22q11.2 deletion are extremely rare in isomerism.
b. Diagnosis of tetralogy of Fallot (TOF) should prompt referral for the following:
E. Delivery
1. In LAI with complete heart block and complex congenital heart disease, delivery should be at term or as near as possible in a tertiary care center.
2. If the baby is developing fetal hydrops, earlier delivery may be necessary, as long as it is clearly possible to improve the hemodynamic condition of the fetus with delivery and intervention.
3. If delivery with postnatal intervention is not believed to be effective, aside from termination of pregnancy if desired by the patient, letting nature take its course with progressive hydrops may be the best option, with close follow-up of the mother to be certain there is no harmful consequence for her.
4. If there is no evolution of hydrops, cesarean section delivery may be considered given the difficulties with fetal monitoring in fetal bradycardia. Ideally, a planned cesarean section is performed at 36 to 38 weeks of gestation.
F. Neonatal management
b. Prostaglandin E1 (PGE1) infusion is indicated if there is evidence of critical pulmonary outflow tract obstruction with retrograde ductal flow in utero or unless the direction of ductal flow is not certain.
d. At times, volume and inotropic support may be indicated to improve ventricular function.
e. Assessment of heart rate in relation to the baby’s clinical condition.
G. Follow-up
1. Subacute bacterial endocarditis (SBE) prophylaxis is required for life.
2. For residual hemodynamic abnormalities, anticongestive medications and some restriction of activities may be required.
3. The patient should have one or two outpatient follow-up visits each year, with 12-lead electrocardiogram (ECG) and echocardiogram.
4. If there is any history of unexplained palpitations or abnormal heart rhythm, a metabolic stress test with 24-hour Holter monitoring is recommended.
5. Pacemaker checks are necessary to detect lead or battery failure.
I. Outcome of this case
a. At 33 weeks’ gestation, ventricular ectopy increased and a trial of terbutaline was made for 48 hours to determine whether the ectopy was due to terbutaline or myocardial failure. The ectopy continued and the myocardial function appeared worse.
b. Due to signs of fetal heart failure (cardiovascular profile score = 5) and a new pericardial effusion, a planned cesarean section was performed at 34 weeks. The mother was given steroids to ensure fetal lung maturity.
a. A girl was born with hydrops and with abnormal Apgar scores—5 at 1 minute and 7 at 5 minutes—and good weight.
b. Heart rate was 50 to 55 bpm, and mean blood pressure was 25 mm Hg.
c. Inotropic support in the form of IV milrinone and dopamine was started.
d. Moderate cyanosis was noted at birth; pulse oximeter reading was 77.
e. Postnatal echocardiography confirmed the diagnosis of LAI with dextrocardia, unbalanced atrioventricular septal defect (AVSD), normally related great arteries, severe valvar pulmonary stenosis, bicuspid aortic valve, and patent ductus arteriosus with mainly right-to-left shunt (Fig. 20-2). The pulmonary arteries were of good size, and transpulmonary Doppler velocity across the pulmonary outflow tract was 2.5 m/s in the presence of poor LV function.
f. Anticongestive therapy was begun, including brain natriuretic peptide (BNP) infusion.
g. PGE1 infusion was started because of persistent saturations less than 75%.
h. The baby had a permanent epicardial DDI (device–device interaction) pacemaker at 3 hours of age.
a. The original plan was to have a palliative aortopulmonary shunt at 1 week of age followed by a bilateral bidirectional Glenn (for the bilateral SVC) at 8 to 12 months of life followed by an extracardiac Fontan procedure. However, the ventricular function did not improve despite adequate inotropic support and pacemaker, and the baby was listed for heart transplant. After 4 weeks, a donor became available and transplantation was performed successfully.
b. Clear delineation of the hepatic veins as well as the pulmonary venous drainage was a prerequisite for heart transplantation.
c. Occasionally, the unusual systemic and pulmonary venous anatomy of affected infants can complicate the operative and postoperative outcomes of such infants with persistent jaundice.
II. YOUR HANDY REFERENCE
A. Prevalence
1. The incidence of heterotaxy or isomerism is approximately 1 per 10,000 total births. In the New England Infant Cardiac Program, heterotaxy was found in 4.2% of infants with congenital heart disease. In postnatal life, right atrial isomerism (RAI) is more common than LAI.
2. There is a tendency for LAI to affect female babies more than male babies.
3. Fetal series showed the predominance of LAI over RAI, which suggests a higher fetal death rate in LAI. In a fetal series, LAI accounted for 5.8% of all fetuses with a diagnosis of structural heart disease. In this series, LAI was twice as prevalent as RAI (Phoon, 1996).
B. Outcome
1. It has been suggested that the combination of LAI and congenital complete heart block (CCHB) is responsible for a higher fetal death rate. In the fetal series reported by Sharland and Cook (2000), out of 82 fetuses, there were only 9 survivors of 32 continuing pregnancies, 12 out of the 32 having died spontaneously in utero.
2. In fetuses with isomerism, there is often disconcordance among the positions of the heart, stomach, and descending aorta or aortic arch. In one fetal series, this concordance was found in 60% of fetuses.
a. The reported mortality with Fontan operations in LAI ranges from 0% to 29%.
b. The mortality for the extracardiac Fontan operation to connect the hepatic veins into the circuit is 50%.
c. It has been shown that isomerism has an adverse effect on the outcome of Fontan surgery. Mortality is increased, and overall mortality in 500 cases is 65% at 5 years.
d. Bilateral SVC without a bridging vein, which is common in isomerism in general, necessitates bilateral bidirectional Glenn, with the potential for increased morbidity.
e. Mortality in simple aortopulmonary shunt placement in LAI is reported to be low.
f. The mortality with cavopulmonary shunt placement in patients with interrupted IVC with azygous connection, the Kawashima operation, ranged between 0% and 33%.
4. There have been reports of the development of pulmonary arteriovenous fistulas, after the Kawashima operation, caused by the exclusion of hepatic venous blood from the pulmonary circulation. There have been other reports of subsequent resolution of intrapulmonary shunts after completion of the Fontan circulation.
5. The best chance for a better surgical outcome occurs in cases where a biventricular repair can be achieved. If there are not two good-sized ventricles, single-ventricle palliation is necessary.
6. Gilljam and colleagues (2000) reported on 163 patients with LAI.
a. In the biventricular repair group, the surgical mortality was 19%. When deaths that were unrelated to surgery were excluded, the mortality was 15%.
b. The total surgical mortality in the single ventricle group was 38%. When deaths that were unrelated to surgery were excluded, the mortality was 30%.
c. The relatively high mortality with the initial palliation (26%) can be explained by the fact that 32% of these operations involved complex procedures.
7. It has also been shown that the survival for this subgroup of patients is on a par with the 5-year survival data of 35% in patients with RAI. This is in contrast with previous suggestions that the prognosis is better for patients with LAI than RAI. Comparably better survival in the biventricular repair group may be attributed to a lower operative mortality.
8. Coarctation of the aorta in patients with LAI was also associated with less than optimal outcome, which may be expected because several of these patients had associated aortic outflow obstruction and hypoplastic LV.
9. The outcome in LAI with fetal AV block is reported to be poor, and a majority of the patients die before or shortly after birth The late outcome in the early survivors is reported to be poor in spite of pacing.
10. An association between atrial isomerism and maternal diabetes has been suggested by Slavotinek and colleagues (1996).
11. In a multivariate analysis of 163 patients with LAI, it was shown that six factors were independently associated with increased mortality.
b. Three extracardiac factors.
12. It has been reported that at least 10% to 13% of patients with LAI have a normal heart. LAI might not be diagnosed in these patients until they reach adulthood (and have a normal life span) unless they have associated extracardiac conditions.
C. Associated syndromes and extracardiac anomalies
1. Fetuses with LAI occasionally show the phenotype of Turner’s syndrome in the presence of a normal female karyotype. In male fetuses, anal atresia and sacral anomalies or cryptorchidism are found rarely.
2. Up to 25% of fetuses with LAI have nuchal swelling (without hygroma) or redundant nuchal skin folds, identifiable in the first trimester through nuchal translucency screening and also reported in second-trimester autopsies.
3. Bowel obstruction as a consequence of malrotation with midgut volvulus or Ladd’s bands and biliary atresia are major extracardiac pathologies that contribute to both the mortality and morbidity of the disease.
4. Some affected infants have poor or no splenic function.
5. The pulmonary anatomy could show bilateral hyparterial bronchi or bilobed lungs.
D. Clues to fetal sonographic diagnosis
1. Axis of the heart might be abnormal (more leftward [levocardia], dextrocardia, or more central [mesocardia]). Heart and stomach on opposite sides.
2. Hepatic veins connect directly to the atrial mass either as a single vessel or separately into the floor of the atria.
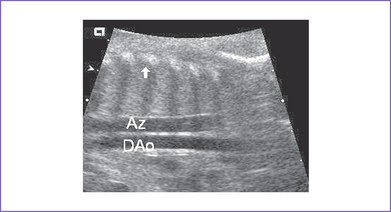
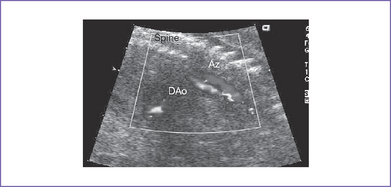
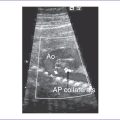
3. There may be two vascular channels behind the heart with flow in opposite directions, one pulsatile and one continuous (aorta and azygous, with the azygous sitting more posterior), and a dilated SVC into which the azygous connects (Figs. 20-3 and 20-4).
4. There may be a single SVC or bilateral SVC, often with one returning to the coronary sinus.
5. There may be an atrioventricular septal defect (AVSD), usually with two ventricles but often unbalanced.
6. The great arteries may be normally related or malposed in a double-outlet right ventricle configuration. There may be pulmonary stenosis, aortic outflow obstruction (often at least in part due to accessory AV valve tissue), bilateral outflow obstruction, or no outflow obstruction.
7. CCHB is a complete dissociation between atrial and ventricular rate. Both atrial and ventricular rates are often low; the former can show a picture of sick sinus syndrome with atrial bradycardia (rates often <120 bpm) and low ventricular escape rates (often <55 bpm).
8. The heart may be dilated and hypertrophied in the presence of CCHB as well as in the absence of a ductus venosus.
9. The atrial septum may be abnormally aligned, with anomalies of the pulmonary venous drainage.
10. The position of the stomach and heart on opposite sides is a strong clue to the presence of LAI.
E. Progression in utero
1. Progressive decrease in the ventricular rate in fetal CHB is likely, and serial weekly monitoring is mandatory.
2. Heart failure can develop due to valvar regurgitation, bilateral outflow tract obstruction, and myocardial dysfunction or slow rate (see Chapter 30).
3. The complex of CHD with LAI can progress in utero. For example, with a partial AV canal defect in LAI, the LV outflow tract can become progressively more narrowed, creating left-sided heart structure hypoplasia, and coarctation of the aorta.
F. Mechanism of fetal bradycardia
a. AV conduction is 1:1. It is an ominous sign and could be a manifestation of severe fetal compromise or distress.
b. Other arrhythmias can disguise themselves as sinus bradycardia. Although the incidence of these arrhythmias is low, their presence can have significant therapeutic implications. Accordingly, it is important to actively rule out these other arrhythmias before making a diagnosis of sinus bradycardia.
2. Multiple blocked atrial ectopic beats.
a. These multiple extrasystoles can lead to a subnormal ventricular rate.
b. The prognosis of such fetuses is generally good, although about 1% develop fetal tachycardia.
3. Complete congenital heart block (CCHB). The mechanism of CCHB depends on the underlying pathology.
b. Structurally abnormal heart.
G. Immediate postnatal management for LAI not diagnosed prenatally
1. Check pulse oximeter. An acceptable reading is greater than 92%; if it is lower, do a hyperoxia test.
a. Any ductus-dependent lesion is likely to be appreciated through a hyperoxia test.
b. If there is severe pulmonary outflow obstruction, the baby will have low PaO2 in both preductal and postductal areas; it is better not to rely on pulse oximeter readings but obtain an arterial blood gas.
c. If there is aortic outflow obstruction, there may be normal preductal PaO2 but abnormal postductal PaO2. If there is intracardiac mixing, however, the PaO2 in the preductal and postductal areas might not be so different.
2. Check the four-limb blood pressure.
a. If there is any discrepancy between upper and lower limb blood pressure (>20 mm Hg), suspect aortic coarctation.
b. This test will be of value only if the ductus arteriosus is closed or nearly closed.
c. Absence of the discrepancy between upper and lower limb blood pressure does not rule out coarctation if the ductus arteriosus is still patent.
3. A 12-lead ECG reading is important for confirming complete AV block and for documenting the atrial rate. Bradycardia may be present if the newborn’s heart rate is less than 80 bpm.
a. If the heart rate is regular and slow (<80 bpm) and the atrial and ventricular rate is 1:1, then sinus bradycardia may be present.
b. Transient bradycardia may be seen in normal neonates and requires no treatment.
c. Sustained sinus bradycardia needs to be evaluated further. It is important to understand the possible mechanism.
4. Transfer the patient to the neonatal or pediatric (cardiac if available) intensive care unit (ICU) for further assessment and management.
a. No treatment is required for asymptomatic newborn.
b. At times, volume and inotropic support (atropine or isoprenaline) are indicated to improve ventricular function.
c. Digoxin and diuretics could be used to treat CHF, if present.
5. If the baby is hydropic, management includes assisted ventilation, tapping of pleural or ascites fluid collections, albumin infusion, and support of the cardiac output.
6. A temporary transvenous ventricular pacemaker followed by permanent pacemaker is indicated if the patient is symptomatic and requires ongoing intravenous sympathomimetic and inotropic support. At times, a permanent pacemaker may be chosen as the initial procedure.
7. About 50% of patients with CCHB require pacing in the newborn period. Dual-chamber pacing is preferable.
8. Occasionally, patients with polysplenia syndrome (LAI) have no outflow tract obstruction with a ventricular-level communication and even double-outlet right ventricle and require pulmonary artery banding to protect the pulmonary vascular bed and balance the systemic and pulmonary blood flow.
a. For some babies, total correction with biventricular repair is possible.
b. If not, following pulmonary artery banding, staged single-ventricle palliation is necessary (bidirectional Glenn at 8-12 months and Fontan completion at 2-5 years).
c. The quoted mortality rate for Fontan operation in this scenario is slightly higher than in patients with similar anatomy but normal situs.
H. Pathophysiology of left atrial isomerism
1. There is failure of differentiation into right- and left-sided organs in the heterotaxy syndromes, with resulting congenital malformations of multiple organ systems.
a. The term situs is used to define the arrangement of the organs relative to the midline or sagittal plan.
b. Because abnormalities of the situs are the harbingers of congenital heart disease, it is important to determine the situs as the initial step of cardiac evaluation by echocardiography. This can be done by imaging the position of the spine, the descending aorta, and the vena cava and hepatic veins at the level of the diaphragm.
c. It is advisable to evaluate the situs in three anatomical locations independently.
2. Noncardiac malformations (Box 20-1). In LAI (polysplenia syndrome) the patient has multiple spleens associated with bilateral left-sidedness, including bilateral bilobed lungs (two left lungs), bilateral hyparterial bronchi, symmetrical liver (25%), occasional absence of gallbladder, and intestinal malrotation (80%) (see Table 20-1). Functional asplenia can occur later in life.
3. Cardiac malformations (Table 20-2).
a. The cardiovascular malformations involve all parts of the heart. A normal heart or only a minimal congenital malformation of the heart is present in 25% of patients with LAI.
b. Interruption of the IVC with azygous continuation (85%) is highly suggestive of polysplenia (LAI). Bilateral SVC and ASD are common in both right and left atrial isomerism.
c. The pulmonary veins from each lung tend to enter the posterior wall of the ipsilateral atrium separately (normal pulmonary venous return in 50%). Although there is the appearance of partial anomalous pulmonary venous return, the ispsilateral veins each connect to an LA.
d. The hepatic veins might not form a confluence but have separate openings in the floor of the atrium or atria.
e. The atrial septum is intact or deficient: ASD secundum (25%), ASD primum (60%), and common atrium. There are bilateral left atria (no sinus node). The coronary sinus is often absent.
f. The AV valve usually opens into both ventricles and may be a common AV valve that is partitioned or not partitioned in LAI. The AVSD may be balanced or unbalanced; unbalanced AVSD has a variable size discrepancy between the ventricles.
g. In up to 70% to 80% of patients, the ventriculoarterial connection is concordant.
h. Pulmonary stenosis or atresia is seen in 30% to 40%.
i. Left-sided obstructive lesions are seen in about 20% to 30%.
j. Bradycardia with AV dissociation is common in LAI, especially when there is AVSD.
k. The pattern of congenital complete heart block in cases with left isomerism is discontinuity between the AV node and the conduction axis, in contrast to the pattern of atrial-axis discontinuity produced in the normally structured heart when anti-Ro antibodies are found in the maternal serum.
l. There is usually complete mixing of systemic and pulmonary venous blood in the heart because of multiple cardiovascular malformations. When pulmonary blood flow is reduced, severe cyanosis results. When pulmonary blood flow is increased, cyanosis is not intense and congestive heart failure (CHF) often develops.
BOX 20-1 EXTRACARDIAC MALFORMATIONS IN RIGHT AND LEFT ATRIAL ISOMERISM
LEFT ATRIAL ISOMERISM
Intestinal malrotation in almost all
Biliary atresia and/or hypoplastic gallbladder in 20%
Note: Absence of ductus venosus is common in visceral heterotaxy.
Adapted from Yoo SJ, Hornberger LK, Smallhorn J: Abnormal visceral and atrial situs and congenital heart disease. In Yagel S, Silverman NH, Gembruch U (eds): Fetal Cardiology: Embryology, Genetics, Physiology, Echocardiographic Evaluation, Diagnosis and Perinatal Management of Cardiac Diseases. London: Taylor & Francis Group, 2002.
TABLE 20-2 COMMON CONGENITAL CARDIAC MALFORMATIONS IN RIGHT AND LEFT ISOMERISM

AVSD, atrioventricular septal defect; IVC, inferior vena cava; SVC, superior vena cava; TAPVC, total anomalous pulmonary venous connection.
From Yoo SJ, Hornberger LK, Smallhorn J: Abnormal visceral and atrial situs and congenital heart disease. In Yagel S, Silverman NH, Gembruch U (eds): Fetal Cardiology: Embryology, Genetics, Physiology, Echocardiographic Evaluation, Diagnosis and Perinatal Management of Cardiac Diseases. London: Taylor & Francis Group, 2002.
I. Risk of recurrence
1. Some cases of LAI are autosomal recessive.
2. Some cases are X-linked, such as those due to ZIC3 mutation (a single-gene problem).
a. In ZIC3 mutations (heterotaxy, visceral, X-linked, or HTX1), if the mother is a carrier of the mutation, her risk for having an affected son is 25%, or each of her sons has a 50% risk of being affected and each of her daughters has a 50% chance of being a carrier (unaffected in most cases, depending on the X inactivation).
b. ZIC3 mutation is associated with multiple abnormalities and not only heterotaxy.
c. ZIC3 mutation analysis is not necessary in all male fetuses with heterotaxy unless there are other abnormalities suggesting ZIC3 mutation.
III. TAKE-HOME MESSAGE
A. Diagnosis
1. LAI is the leading cause of fetal heart block occurring with structural heart disease, followed by congenitally corrected transposition of the great arteries (ccTGA), Ebstein’s anomaly, atrial and ventricular septal defects, TOF, and pulmonary stenosis.
2. The course of the coronary sinus posteroinferior to an atrial chamber, readily seen on the four-chamber view of the fetal heart, indicates left morphology of the atrium. The coronary sinus is always absent in the setting of right isomerism.
3. Given ideal imaging capabilities, the arrangement of the thoracic and abdominal organs and large vessels should be analyzed in a fetus so as not to miss the diagnosis of LAI.
4. The mechanism of CHB in LAI and AV discordance is due to discontinuity between the AV node and ventricular conduction tissue in such fetuses.
5. Chromosomal anomalies in the form of trisomies are extremely rare in atrial isomerism.
6. A normal heart or only minimal congenital heart disease is present in up to 25% of the patients with LAI polysplenia syndrome (PSS).
B. Prognosis
1. The combination of LAI and complete heart block in a fetus is an adverse prognostic indicator, and the outlook must be guarded in these cases, which have an additional risk of spontaneous intrauterine death.
2. In patients with congenital AV block and complex heart disease, the outlook may still be unfavorable, and in these patients cardiac transplantation may be an option.
3. Associated cardiac defects, such as single ventricle, aortic coarctation, congenital AV block, and extracardiac anomalies, such as biliary atresia, are significantly associated with increased mortality.
4. Recent advances in cardiac surgery and liver transplantation might offer improved prospects for survival in current patients with complex heart disease and biliary atresia.
5. Babies with isomerism are at increased risk of intestinal malrotation with obstruction (about 10%-12%).
Allan LD, Sharland GK, Milbum A, et al. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol. 1994;23(6):1452-1458.
Garcia OL, Mehta AV, Pickoff AS, et al. Left atrial isomerism and complete atrioventricular block: A report of six cases. Am J Cardiol. 1981;48:1103-1107.
Gentles TL, Mayer JEJr, Gauvreau K, et al. Fontan operation in five hundred consecutive patients: Factors influencing early and late outcome. J Thorac Cardiovasc Surg. 1997;114(3):376-391.
Gilljam T, McCrindle BW, Smallhorn JF, et al. Outcomes of left atrial isomerism over a 28-year period at a single institution. J Am Coll Cardiol. 2000;36(3):908-916.
Hashmi A, Abu-Sulaiman R, McCrindle BW, et al. Management and outcomes of right atrial isomerism: A 26-year experience. J Am Coll Cardiol. 1998;31(5):1120-1126.
Ho SY, Cook A, Anderson RH, et al. Isomerism of the atrial appendages in the fetus. Pediatr Pathol. 1991;11(4):589-608.
Huhta JC, Smallhorn JF, Macartney FJ. Two-dimensional echocardiographic diagnosis of situs. Br Heart J. 1982;48(2):97-108.
Kawashima Y, Kitamura S, Matsuda H, et al. Total cavopulmonary shunt operation in complex cardiac anomalies. J Thorac Cardiovasc Surg. 1984;87:74-81.
Knight WB, Mee RB. A cure for pulmonary arteriovenous fistulas? Ann Thorac Surg. 1995;59:999-1001.
Machado MV, Tynan MJ, Curry PV, Allan LD. Fetal complete heart block. Br Heart J. 1988;60:512-515.
Peoples WM, Moller JH, Edwards JE. Polysplenia: A review of 146 cases. Pediatr Cardiol. 1983;4(2):129-137.
Phoon CK, Neill CA. Asplenia syndrome: Insight into embryology through an analysis of cardiac and extracardiac anomalies. Am J Cardiol. 1994;73(8):581-587.
Phoon CK, Villegas MD, Ursell PC, Silverman NH. Left atrial isomerism detected in fetal life. Am J Cardiol. 1996;77(12):1083-1088.
Rubino M, Van Praagh S, Kadoba K, et al. Systemic and pulmonary venous connections in visceral heterotaxy with asplenia. Diagnostic and surgical considerations based on seventy-two autopsied cases. J Thorac Cardiovasc Surg. 1995;110(3):641-650.
Sharland G, Cook A. Hetrotaxy syndromes/isomerism of the atrial appendages. In: Allan L, Hornberger L, Sharland G, editors. Textbook of Fetal Cardiology. London: Greenwich Medical Media; 2000:333-346.
Slavotinek A, Hellen E, Gould S, et al. Three infants of diabetic mothers with malformations of left–right asymmetry—further evidence for the etiological role of diabetes in this malformation spectrum. Clin Dysmorphol. 1996;5:241-247.
Uemura H, Ho SY, Devine WA, et al. Atrial appendages and venoatrial connections in hearts from patients with visceral heterotaxy. Ann Thorac Surg. 1995;60(3):561-569.
van Mierop LHS, Gessner IH, Schiebler GL. Asplenia and polysplenia syndrome. Birth Defects: Original Article Series. 1972;8:74-82.