Chapter 172 Lateral Lumbar Interbody Fusion
Indications and Techniques
The traditional lateral approach to the thoracic and lumbar spines was originally developed by Capener for the management of tuberculous spondylitis (Pott’s disease) in the 1950s.1 Through thoracotomy and retroperitoneal approaches, wide exposure of the lateral spinal column could be achieved for debridement and stabilization of the spine. In the 1970s, Larson et al. modified and popularized the lateral extracavitary (LEC) approach to the thoracic and lumbar spines to treat a variety of pathologies, effectively allowing for an entirely posterior approach to reach the anterior and lateral spine.2 However, aside from major trauma, infection, or tumor cases, which typically required removal of vertebral bodies, these approaches were not popular for interbody discectomy and stabilization. The risk of nerve injury and the extensive disruption of the psoas attachments were perceived as too great for benign conditions such as degenerative disc disease and back pain. Therefore, LLIF was relegated to rare use, namely, for anterior release in deformity/scoliosis cases.
The LLIF approach has gained increasing popularity with the development of minimally invasive surgery (MIS) techniques. Advantages of MIS techniques include smaller incisions, less tissue dissection, improved cosmesis, decreased blood loss, less postoperative pain, and thus, shorter recovery time and hospital stay. The use of an MIS technique in lateral approach allows access to the lumbar spine through the retroperitoneal fat and psoas major muscle via a small incision using a muscle splitting technique. Advances in electromyography (EMG) neuromonitoring have been incorporated into these techniques, which in turn have made them safer. Pioneering work in minimally invasive LLIF is credited to Bergey et al. for describing an endoscopic transpsoas discectomy in 20043 and to Ozgur et al. for describing the minimally invasive lateral interbody fusion technique in 2006.4 The minimally invasive LLIF technique has been trademarked under the proprietary name Extreme Lateral Interbody Fusion (XLIF, NuVasive, San Diego, CA) or Direct Lateral Interbody Fusion (DLIF, Medtronic, Memphis TN), but the procedure can be performed through a variety of tubular retractors outside of these proprietary formats.
Indications and Contraindications
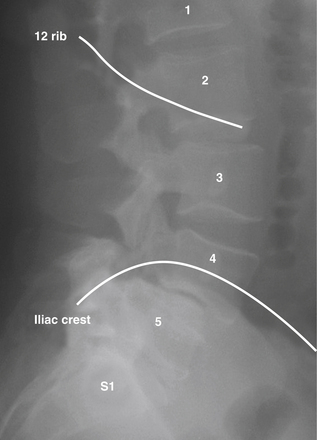
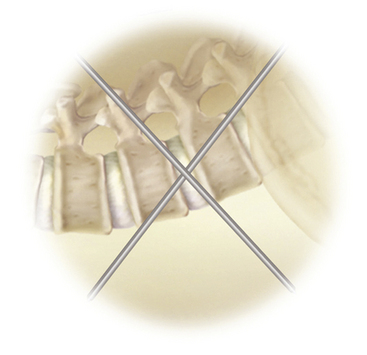
The goals of LLIF are to access the lumbar disc space safely, release the lateral annulus attachments, remove disc material, and place a structural graft. The results should be increased interbody height, restoration of collapse or deformity, and stabilization of interbody motion. LLIF is most suitable for interbody access from L2 to L4 for degenerative disc disease with or without instability, adjacent segmental disease, degenerative spondylolisthesis (grade I or II), and complex degenerative scoliotic deformity. LLIF can be performed at L1-2, but requires either removal of or maneuvering around the descending 12th rib (Fig. 172-1); it also can be performed at L4-5 but with a higher chance of nerve root injury. Also, the positions of the iliac crests determine whether L4-5 can be accessed. LLIF at L5-S1 is generally contraindicated due to obstruction by the iliac wing (Fig. 172-1). Other relative contraindications include grade III or greater degenerative spondylolisthesis, greater than 30-degree lumbar deformities, and bilateral retroperitoneal scarring. In addition, LLIF is generally not used alone when direct posterior decompression is necessary, such as with lumbar stenosis or disc rupture. It can, however, be combined with staged posterior decompression and posterior–lateral fusion if necessary. Patients with radicular symptoms and neuroforaminal stenosis can be considered for indirect decompression by restoring disc height and increasing foraminal diameter via LLIF, which is an active area of investigation.
Clinical Results
Unfortunately, there are limited clinical data concerning outcomes using LLIF techniques (including XLIF, DLIF, or other lateral lumbar interbody approaches). In a prospective series of 100 patients with adjacent segment degeneration after prior lumbar fusion, Rodgers et al. reported an average improvement of the visual analogue score (VAS) for pain from 8.6 to 2.8 within 6 months using the XLIF technique.5 Though this was not a comparison study, the mere improvement in pain scores in a patient who underwent prior lumbar fusion surgery with a minimally invasive XLIF is clinically meaningful.
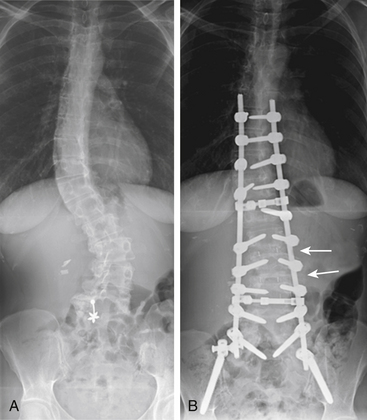
LLIF approaches may be especially beneficial in treating certain complex scoliotic deformities, as they provide excellent coronal and sagittal corrective ability (Fig. 172-2). Pimenta et al. reported 23 symptomatic adult scoliosis patients at levels between L2 and L5 using LLIF approaches and achieved significant changes in coronal and sagittal alignment, as well as improved pain score.6 Benglis et al. also presented favorable short-term outcomes with mid- to high-lumbar coronal deformities treated with LLIF techniques.7 All patients showed improvement in preoperative pain and solid arthrodesis at 6 months. Similarly, Diaz et al. reported a 3-year follow-up for 39 patients treated with LLIF for symptomatic degenerative scoliosis and showed consistent improvement of the VAS and scoliotic deformity improvement in 3-year follow-up.8 LLIF can also be combined with other minimally invasive techniques, such as trans–axial lumbar interbody fusion.9
Minimally invasive LLIF procedures might be also used for lumbar total disc replacement (TDR). Pimenta et al. described a series of 25 patients who has TDR placed using minimally invasive LLIF for degenerative disc disease with positive discography.10 The authors reported an improvement of the VAS from 7.5 to 2.6 and Oswestry Disability Index from 60 to 30. They also found this approach to be quick, to have minimal morbidity, and to avoid the need for anterior longitudinal ligament (ALL) removal; therefore, it has at least a theoretical advantage in segmental stability over the anterior approaches. Artificial discs placed in the lateral position have not yet been evaluated or approved by the Food and Drug Administration in the United States.
Surgical Anatomy
As in all surgical disciplines, a thorough understanding of the surgical anatomy involved in LLIF is crucial for maximal patient benefit and complication avoidance. For LLIF, the most critical anatomy is the distribution of the lumbar plexus within the psoas muscle, because the approach inevitably requires the use of a dilator or retractors to traverse the psoas muscle, which places the lumbar plexus at risk of injury.
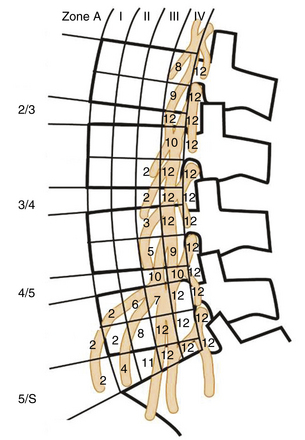
The lumbar plexus is embedded mainly in the posterior portion of the psoas, anterior to the lumbar transverse process (Fig. 172-3). It is composed of the ventral rami of the L1 through L4 roots. Major cutaneous branches include (1) the ilioinguinal and iliohypogastric nerves (L1), which supply the skin of the suprapubic and inguinal regions; (2) the genitofemoral nerve (L1 and L2), which supplies the cremaster muscle and the skin over femoral triangle; and (3) the lateral femoral cutaneous nerve (L2 and L3), which supplies the skin on the anterolateral surface of the thigh. The genitofemoral nerve pierces the anterior surface of the psoas muscle and runs inferiorly, deep to the psoas fascia. The two major motor branches of lumbar plexus are the obturator nerve (L2-L4), which emerges from the lower part of the medial border of psoas muscle and supplies the adductor muscles, and the femoral nerve (L2-L4), which emerges from the lower part of the lateral border of psoas muscle and supplies the hip flexors and knee extensors.
In 2003, Moro et al. defined the relationship between the psoas major muscle and the lumbar plexus using cadaveric dissection.11 Excluding the genitofemoral nerve, the roots and critical branches of the lumbar plexus were found to be overlapping with the dorsal half of the vertebral column above L4-5 in a lateral projection view. The genitofemoral nerve, however, traverses through the psoas to emerge on the ventral surface between the rostral third of the L3 and the L4 vertebral bodies. When genitofemoral nerve is taken into account, only the ventral half of the vertebral column above L2-3 is free of lumbar plexus. The safest corridor, then, for the minimally invasive LLIF approach is the ventral half of the vertebral body above L2-3. Damage to the genitofemoral nerve usually causes only a transient sensory disturbance to the ipsilateral scrotum and medial thigh, which rarely becomes a serious problem. The ventral half of the vertebral column above L4-5 is therefore considered safe if transient genitofemoral nerve dysfunction is acceptable.
In comparison, accessing the lateral vertebral body at or below L5 carries significant risk of damaging critical structures such as the L4 and L5 nerve roots, femoral nerve, and/or obturator nerve. Thus, although there is considerably more space between the psoas major muscle and the quadrates lumborum muscle at L5-S1 compared to at L4-5 and above, L5 and below may not be used for lateral approaches to the lumbar spine. Benglis et al. also found an obvious dorsal to ventral migration of the lumbar contribution to the lumbosacral plexus within the psoas muscle from L2 to L5 in their cadaver studies.12
Using the lateral transpsoas approach in cadaveric dissection to identify the structures at risk with transpsoas K-wire and dilator placement, Banagan et al. found a more serious potential anatomic problem13: The nerve roots and the genitofemoral nerve could be at risk in all their dissections in which the transpsoas approach is re-created. K-wire placement caused damage in 25% of cases at L3-4 and L4-5, including one direct L4 nerve root piercing. It was also found that the lumbar plexus was under tension after sequential dilator placement even when no direct injury happened during insertion. In addition to the lumbar plexus, the sympathetic chain was identified in the anterior third of the psoas over the disc spaces of L1 to L4, putting it at risk of potential damage with the transpsoas approach. Using a similar approach, Davis et al. found the femoral nerve is consistently at risk as it crosses the L4-5 interspace and can be compressed against the L5 transverse process when retractors are opened during the XLIF/DLIF procedures.14
Using a morphometric analysis of the ventral lumbar nerve roots and large vessels with the vertebral end plate, from hundreds of MRI studies, Regev et al. found the overlap of either roots or large vessels with the end plates gradually increased from L1-2 to L4-5. At the L4-5 level, the overlap can reach up to 87%, resulting in a very narrow corridor for potential LLIF procedures.15 Scoliosis was found to further decrease the potential safe corridor for LLIF. The preceding anatomic studies indicated the importance for neuromonitoring when establishing safe passage through the psoas muscle during LLIF procedures.
Surgical Technique
Open LLIF
Access to the lateral lumbar spine using an open approach allows several potential surgical dissection planes. In the LEC approach popularized by Larson et al., the lumbar spine is accessed with the plane of dissection posterior to the quadrates lumborum and anterolateral to the erector spinae muscles.2 The erector spinae muscle group is elevated and retracted medially to expose the lateral elements of the spine. In 2002, Wolfla et al. described a retroperitoneal L2 to L5 lumbar interbody fusion using a true lateral trajectory to treat symptomatic nonunion.16 The access to the lumbar spine was provided by retraction of the psoas and quadrates lumborum muscles posteriorly. In their series of 15 patients with painful pseudarthrosis from 1 or more (average 2.1) previous posterior lumbar operations, 87% had significant improvement after open LLIF, and a 90% radiographic fusion rate was reported.
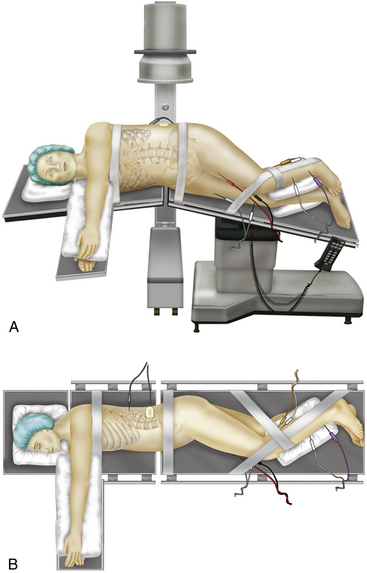
In open LLIF, the patient is placed in the lateral decubitus position in a plane perpendicular to the floor to facilitate obtaining lateral radiographs (Fig. 172-4). Generally, a left-sided approach is preferred for preferential retraction of the descending aorta as opposed to the inferior vena cava. In addition, for upper lumbar levels, the liver may prevent right-sided exposure. The ipsilateral lower extremity is preferably flexed at the hip to reduce tension on the psoas muscle.
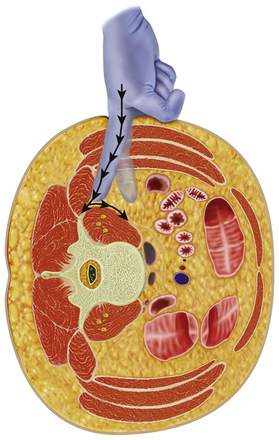
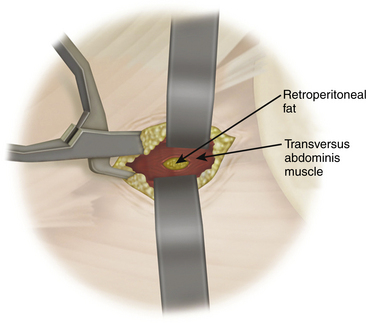
A standard left flank retroperitoneal exposure is performed based on the required level of exposure. Access can reliably be provided from L2 to L5, hindered above by the crura of the diaphragm and below by the ileum. After skin incision, the external oblique muscle and fascia are exposed and divided along its fibers. The underlying internal oblique and transverse abdominis muscles are then transected. After the deep fascia of the transverse abdominis is opened, the retroperitoneal space is entered. Blunt finger dissection is used to strip the peritoneum retroperitoneal contents anteriorly away from the quadratus lumborum and psoas muscles (Fig. 172-5), exposing the anterior spinal column and the great vessels. The psoas and quadratus lumborum muscles are then retracted dorsally, exposing the lumbar vertebrae. The ureter, genitofemoral nerve and sympathetic chain are identified and protected. The posterior border of the ALL is identified to serve as an important landmark later for the placement of the interbody fusion material. To perform interbody fusion, all disc spaces to be fitted with instrumentation are opened from the lateral border of the ALL to the base of the transverse process; the discs are then removed using angled curettes and rongeurs. During this step, the assistant needs to retract the psoas muscle out of the way manually. Retraction of the psoas should be from ventral to dorsal to protect the traversing nerve roots in the psoas muscles.
Minimally Invasive LLIF (XLIF/DLIF)
Minimally invasive LLIF involves anatomic exposure similar to that of open LLIF but incorporates multiple additional steps to increase safety and minimize exposure. The key steps include preoperative planning, needle electrode setup, patient positioning, fluoroscopic localization, dissection to the psoas muscle, neuromonitoring through the psoas muscle, sequential dilation and retractor placement, disc preparation, implant insertion, and closure.
Preoperative Planning
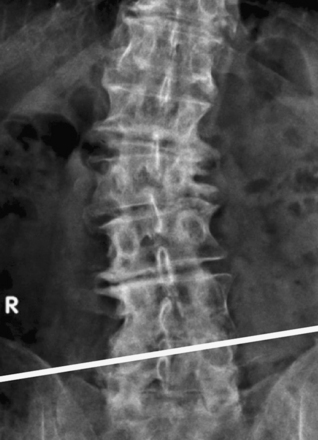
Preoperative planning also includes choosing the side of the approach. Usually, minimally invasive LLIF is approached from the left side. However, surgeons should also consider ease of access and surgeon preference in determining which side to approach. Preoperative AP and lateral x-ray films need to be examined to determine the side that appears to have easier access, for example, the side with a low iliac crest or shorter lower ribs (Fig. 172-6). In the case of degenerative scoliosis, the approach is usually from the side of the convexity.
Patient Positioning
The patient is placed on a radiolucent operating table in a true lateral position. The iliac crest is aligned with the break of the radiolucent surgical table. An axillary roll is placed to protect the neurovascular structures in the axilla. Padding is placed between the arms to ensure they remain suspended in the neutral position. The top leg of the patient should be flexed to relax the psoas muscle and prevent spreading of the nerves across the psoas muscle. Padding is also placed beneath and between the legs from the knees distally (see Fig. 172-4). The patient is then secured to the surgical table with tape. The patient is placed in a slight reverse Trendelenburg position, the head of the table is dropped, and a slight flexion is applied to the surgical table. This maneuver allows better access to the lumbar spine by increasing the distance between the iliac crest and the lower rib, as well as by opening the disc space to be entered.
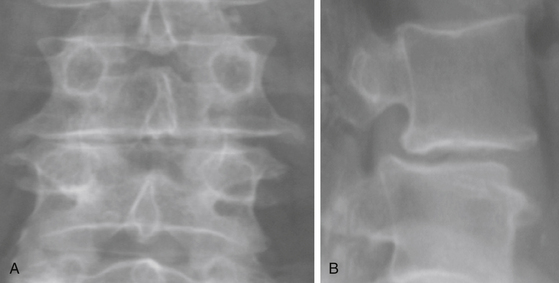
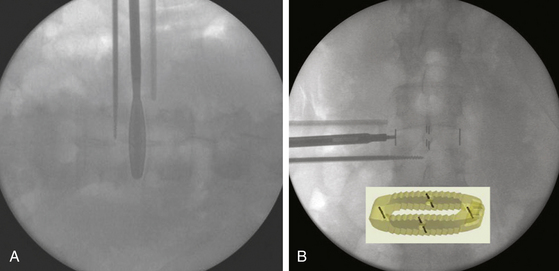
The patient must be placed in a plane perpendicular to the floor so that lateral fluoroscopy can provide good-quality, unobstructed images of the disc space of interest. First, a true AP image should be obtained to ensure the patient is positioned in a true lateral position. On the AP x-ray, clear and distinct pedicles that are equidistant from the spinous process should be visible (Fig. 172-7A). Then, a lateral x-ray is obtained, and clean, distinct end plates should be seen (Fig. 172-7B). It is critical that the C-arm remain in the 0- and 90-degree positions at all times to ensure a safe lateral working channel across the disc space. For multilevel cases, the surgical table is rotated independent of the C-arm to adjust images for each level, and biplanar fluoroscopy or equivalent is highly recommended.
Fluoroscopic Localization
Fluoroscopy is used to confirm the target segment and mark the initial incision by using bisecting K-wires laid on top of the skin (Fig. 172-8). For a single-level case, the skin is marked over the midsection of the target disc, and a 3-cm horizontal, vertical, or oblique incision is made (Fig. 172-9). For a two-level case, the skin is marked over the midsection of the intervening vertebral body. It may be possible to access multiple levels through one vertical skin incision, depending on the anatomy and curvature of the spine. Although it is possible to use a single incision for multiple levels, separate dilations through the psoas must be performed for each operative disc space.
Dissection to the Psoas Muscle
After a single skin incision, the subcutaneous fat layers are dissected until the abdominal musculature is reached. Hemostasis is achieved using monopolar cautery, and a small self-retaining retractor is used to facilitate initial dissection of the skin and subcutaneous layer. The first plane encountered is the external oblique fascia, the only layer that needs to be sharply incised. A Kelly clamp is then used to bluntly spread through the fibers of the external oblique, internal oblique, and transversalis muscles. All dissection should be parallel to the muscle fibers, which run in opposite directions in each plane. After bluntly penetrating the transversalis fascia, the yellow retroperitoneal fat is exposed. At this point, palpation of the quadratus muscle and the tip of the transverse process confirms the correct retroperitoneal plane. Palpation is then used to define the plane from the internal abdominal wall posteriorly down to the psoas muscle, which can then be visualized. The retroperitoneal fat and peritoneal contents are swept forward with the surgeon’s finger or a peanut elevator to allow direct access to the psoas muscle (see Fig. 172-5).
Dilation and Retractor Placement
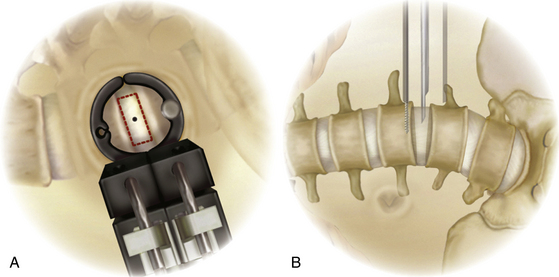
With the guidewire in place, sequential dilation spreads the fibers of the psoas up to a diameter of 22 mm, with free-running EMG active to detect any mechanical affect on the nerve roots. With the NeuroVision system (NuVasive), the dilators can continue to provide real-time EMG monitoring during each dilation, because they have stimulating electrodes at their tips. Care must be taken to ensure that each dilator reaches the disc space and to minimize the amount of residual muscle at the end of the dilators. If needed, fluoroscopy can be used to confirm that each dilator has reached the disc space. The first dilator may be extended slightly into the disc space to ensure complete dilation through the psoas muscle. After the largest dilator is placed, the appropriate retractor blades are selected based on the depth from skin to the disc. The retractor blades are placed onto the base, and the entire assembly is then placed over the dilators. The retractor is advanced with a back-and-forth twisting motion and only gentle downward pressure through the fascia and muscle. This technique helps ensure that the fascia and muscle fibers are not pulled down into the surgical corridor. After the retractor is docked on the lateral aspect of the disc space, the system is secured to the operating table and expanded (Fig. 172-10). The retractor should not be expanded past the midpoint of the vertebral body where the segmental vessels off the descending aorta typically course. In the Medtronic system, both retractor blades have a stability pin system to prevent retractor migration during the ensuing procedure. Prior to pin placement, it is prudent to use the stimulating probe to test both pin channels to ensure a nerve-free pathway. Afterward, the pin is threaded into the channel. With the stability pin in place, the dilator tubes are removed. A final lateral image is taken to confirm proper retractor placement over the lateral spine. With the retractor system in the correct position, an attached light source illuminates the surgical corridor.
Discectomy and End-Plate Preparation
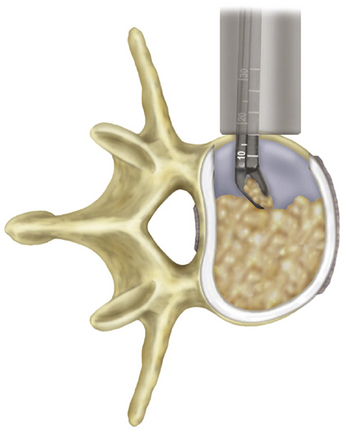
Typically, a thin layer of soft tissue remains at the base of the retractor blades. The stimulating ball-tip probe can be used again to stimulate all four quadrants at the retractor base and thus identify any nerve structures that may be present in this residual muscle. Once the annulus is visualized, a lateral annulotomy is performed using a bayoneted scalpel. The discectomy is completed using pituitary rongeurs and curettes (Fig. 172-11). A large Cobb is passed along both end plates to the annulus at the contralateral side to detach the disc. A mallet is then used to gently release both the superior and the inferior aspects of the contralateral annulus. This step is critical to ensure adequate distraction and coronal alignment. It is especially important for deformity correction. Both the ALL and the posterior longitudinal ligament are preserved in most circumstances. Attention is then turned to the end plates, which must be completely cleaned of cartilaginous disc without destruction of the cortical end plates. Shavers large enough to clean but small enough to avoid decortication are used to remove any residual disc material. Finally, care must always be taken to ensure the patient remains in a true lateral position and the instrument trajectory remains perpendicular to the floor to avoid potentially catastrophic injury to vessels or nerve structures.
Interbody Implant Placement
The disc space is sequentially distracted with trials until adequate disc space height is obtained and foraminal size is restored. Each trial is passed through the retractor and impacted into the disc space. A properly sized trial is centered on the spinous process and should span the ring apophysis to reach fully across the vertebral body end plate (Fig. 172-12A).
Once trialing is complete, the central cavity of the corresponding implant is filled with graft material. A mallet is then used to gently insert the implant under AP (or better, biplanar) fluoroscopic guidance. After the implant is positioned in the center of the disc space from a medial/lateral perspective, the inserter is unthreaded from the implant and removed. Placing the implant over the outer rim of the end plate on each side provides maximum support to the strength of the ring apophysis (Fig. 172-12B). After implant insertion, the stability pin is removed, the retractor system detached, and the retractor blades are carefully removed. The surgical site is irrigated, and the fascia over the external oblique is closed with interrupted Vicryl sutures. Finally, the subcutaneous layers and skin are closed routinely.
Supplemental Lateral Plate Fixation vs. Percutaneous Posterior Fixation
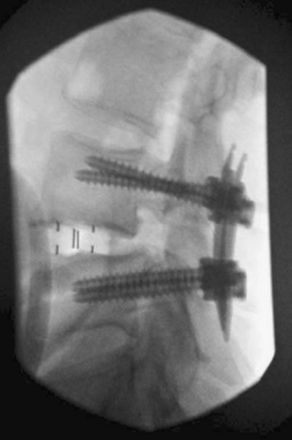
After a minimally invasive LLIF procedure, patient can be repositioned for posterior decompression and/or posterior fixation when deemed necessary. Commonly, percutaneous or open pedicle screw instrumentation is placed (Fig. 172-13). One alternative for supplemental reinforcement of the operative level is to use the lateral plating system such as the extreme lateral plate (XLP, NuVasive), in which case patient repositioning is no longer necessary, decreasing the operative time and the morbidity associated with a second operation. However, the effectiveness of the XLP system has not been compared with posterior pedicle screw placement in any systematic way.
Limitations and Potential Complications of LLIF
One of the major concerns of LLIF is the potential of damaging the lumbar plexus that traverse the psoas muscle. One recent anatomic study identified that the L4 nerve root, genitofemoral nerve, and sympathetic chains are often at risk of direct piercing or stretch injury during routine transpsoas K-wire and dilator placement, increasing concern over the safety of the direct transpsoas approach.13 Real-time EMG systems are designed to minimize this risk. To date, no studies have been done to validate the effectiveness of these systems in preventing lumbar plexus injury. Real-time EMG can only effectively identify a safe entry point to the intervertebral disc; however, the large dilators/retractors that allow passage of large lateral implants compress important nerve structures in the psoas muscle once it is fully opened. It is estimated that the femoral nerve is under compression 100% of the time during the lateral transpsoas approach.14 It is most at risk at the L4-5 interspace, with significant risk of being compressed against the L5 transverse process. As such, the actual retraction time must be monitored to minimize the risk of femoral nerve injury. An L4 neurogram might be able to show the trajectory of the L4 root/femoral nerve as it crosses the L4-5 intervertebral disc space, which might minimize L4 nerve root damage during the lateral transpsoas approach.17 Furthermore, trauma to the psoas muscle can produce hip pain and weakness.
Thus far, the complication profile associated with LLIF procedures has only been published in a limited number of series, and it appears that the approach-associated complications are generally quite benign. Temporary postoperative groin or thigh dysesthesias are among the most common complaints. Knight et al. published results from a series of 58 patients who underwent LLIF and found 8 approach-related complications (13.8%), the majority being transient meralgia paresthetica.18 Two patients (3.4%) in their series suffered from L4 nerve injury that showed residual motor deficits at 1 year postoperatively. In the series by Anand et al. with degenerative lumbar scoliosis, 3 of 12 patients (25%) experienced transient groin or thigh dysesthesias and 1 of 12 patients (8.3%) had quadriceps weakness that lasted 6 weeks.9 Transient hip flexor weakness can be seen up to 50% of the time.19 Reported complication rates and profiles appear to be comparable with anterior or posterior approaches. However, due to the limited systemic studies on LLIF-related complications, the benefit of LLIF must be carefully weighed against potential complications individually to provide the best clinical results for patients. The limitations of LLIF can occasionally be circumvented by combining it with other minimally invasive procedures. It was reported that by combining multiple minimally invasive procedures, multisegment deformity correction can be achieved9 with less blood loss and morbidity than in open procedures.
Anand N., Baron E.M., Thaiyananthan G., et al. Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis: a technique and feasibility study. J Spinal Disord Tech. 2008;21(7):459-467.
Banagan K., Gelb D., Poelstra K., Ludwig S. Anatomic mapping of lumbar nerve roots using a direct lateral transpsoas approach to the spine. Spine. 2009;9(10):59S-60S.
Benglis D.M., Elhammady M.S., Levi A.D., Vanni S. Minimally invasive anterolateral approaches for the treatment of back pain and adult degenerative deformity. Neurosurgery. 2008;63(Suppl 3):191-196.
Benglis D.M., Vanni S., Levi A.D. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10(2):139-144.
Bergey D.L., Villavicencio A.T., Goldstein T., Regan J.J. Endoscopic lateral transpsoas approach to the lumbar spine. Spine (Phila Pa 1976). 2004;29(15):1681-1688.
Capener N. The evolution of lateral rhachotomy. J Bone Joint Surg Br. 1954;36(2):173-179.
Davis T., Bae H., Rasouli A., Delamarter R.B. A Novel Approach to Predict the Femoral Nerve Course Prior to Transpsoas Spinal Access at L4-5 Intervertebral Disc Space. Spine. 2009;9(10):63s.
Davis T., Bae H., Rasouli A., Delamarter R.B. The lumbosacral plexus and the transpsoas approach to the intervertebral disc space: is there a “safe zone”? a cadaveric study. Spine. 2009;9(10):37s-38s.
Diaz R., Phillips F., Pimenta L., et al. XLIF for lumbar degenerative scoliosis: outcomes of minimally invasive surgical treatment out to 3 years postoperatively. Spine. 2006;6(5):75S.
Knight R.Q., Schwaegler P., Hanscom D., Roh J. Direct lateral lumbar interbody fusion for degenerative conditions: early complication profile. J Spinal Disord Tech. 2009;22(1):34-37.
Larson S.J., Holst R.A., Hemmy D.C., Sances A.Jr. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45(6):628-637.
Moro T., Kikuchi S., Konno S., Yaginuma H. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine (Phila Pa 1976). 2003;28(5):423-428. discussion 427-8
Ozgur B.M., Aryan H.E., Pimenta L., Taylor W.R. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435-443.
Pimenta L., Diaz R., Guerrero L. Lumbar total disc replacement from a minimally disruptive direct lateral approach: results of the first 25 patients. Spine. 2006;6(5):157S-158S.
Pimenta L., Lhamby J., Gharzeddine I., Coutinho E. XLIF approach for the treatment of adult scoliosis: 2-year follow-up. Spine. 2007;7(5):52s-53s.
Regev G.J., Chen L., Dhawan M., et al. Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine (Phila Pa 1976). 2009;34(12):1330-1335.
Rodgers W., Cox C.S., Gerber E. Minimally invasive treatment (XLIF) of adjacent segment disease after prior lumbar fusions. Internet Minimally Invasive Spinal Technol. 3(4), 2009.
Wolfla C.E., Maiman D.J., Coufal F.J., Wallace J.R. Retroperitoneal lateral lumbar interbody fusion with titanium threaded fusion cages. J Neurosurg. 2002;96(Suppl 1):50-55.
1. Capener N. The evolution of lateral rhachotomy. J Bone Joint Surg Br. 1954;36(2):173-179.
2. Larson S.J., Holst R.A., Hemmy D.C., Sances A.Jr. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45(6):628-637.
3. Bergey D.L., Villavicencio A.T., Goldstein T., Regan J.J. Endoscopic lateral transpsoas approach to the lumbar spine. Spine (Phila Pa 1976). 2004;29(15):1681-1688.
4. Ozgur B.M., Aryan H.E., Pimenta L., Taylor W.R. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435-443.
5. Rodgers W., Cox C.S., Gerber E. Minimally invasive treatment (XLIF) of adjacent segment disease after prior lumbar fusions. Internet Minimally Invasive Spinal Technol. 3(4), 2009.
6. Pimenta L., Lhamby J., Gharzeddine I., Coutinho E. XLIF Approach for the treatment of adult scoliosis: 2-year follow-up. Spine. 2007;7(5):52s-53s.
7. Benglis D.M., Elhammady M.S., Levi A.D., Vanni S. Minimally invasive anterolateral approaches for the treatment of back pain and adult degenerative deformity. Neurosurgery. 2008;63(Suppl 3):191-196.
8. Diaz R., Phillips F., Pimenta L. XLIF for lumbar degenerative scoliosis: outcomes of minimally invasive surgical treatment out to 3 years postoperatively. Spine. 2006;6(5):75S.
9. Anand N., Baron E.M., Thaiyananthan G., et al. Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis: a technique and feasibility study. J Spinal Disord Tech. 2008;21(7):459-467.
10. Pimenta L., Diaz R., Guerrero L. Lumbar total disc replacement from a minimally disruptive direct lateral approach: results of the first 25 patients. Spine. 2006;6(5):157S-158S.
11. Moro T., Kikuchi S., Konno S., Yaginuma H. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine (Phila Pa 1976). 2003;28(5):423-428. discussion 427-8
12. Benglis D.M., Vanni S., Levi A.D. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10(2):139-144.
13. Banagan K., Gelb D., Poelstra K., Ludwig S. Anatomic mapping of lumbar nerve roots using a direct lateral transpsoas approach to the spine. Spine. 2009;9(10):59s-60s.
14. Davis T., Bae H., Rasouli A., Delamarter R.B. The lumbosacral plexus and the transpsoas approach to the intervertebral disc space: is there a “safe zone”? a cadaveric study. Spine. 2009;9(10):37s-38s.
15. Regev G.J., Chen L., Dhawan M., et al. Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine (Phila Pa 1976). 2009;34(12):1330-1335.
16. Wolfla C.E., Maiman D.J., Coufal F.J., Wallace J.R. Retroperitoneal lateral lumbar interbody fusion with titanium threaded fusion cages. J Neurosurg. 2002;96(Suppl 1):50-55.
17. Davis T., Bae H., Rasouli A., Delamarter R.B. A novel approach to predict the femoral nerve course prior to transpsoas spinal access at L4-5 intervertebral disc space. Spine. 2009;9(10):63s.
18. Knight R.Q., Schwaegler P., Hanscom D., Roh J. Direct lateral lumbar interbody fusion for degenerative conditions: early complication profile. J Spinal Disord Tech. 2009;22(1):34-37.