CHAPTER 26 LASIK for myopia, hyperopia, and astigmatism
Introduction
LASIK was developed about 20 years ago by Dr Pallikaris in Greece and by Dr Buratto in Italy evolving from previous lamellar refractive surgery performed by Dr Barraquer and Dr Ruiz1,2. This chapter will go through the different steps of LASIK, thus giving information about its role in refractive surgery.
Epidemiologic considerations and terminology
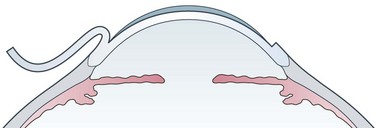
LASIK is the most performed refractive procedure in the world, with several millions of cases, and is overtaking surface ablations in most countries. LASIK stands for ‘Laser assisted in situ keratomileusis’. The purpose of LASIK is to change the refraction of the eye through modifying the corneal curvature by excimer laser ablation of corneal tissue. Different from surface ablations, the laser energy is applied within the corneal stroma under a 100–180 µm corneal flap cut with a microkeratome or with a femtosecond laser. A hinge is left to allow flap reposition without suturing (Fig. 26.1). The maximum amount of dioptric correction is determined by balancing the pupil diameter, the ablation optical zone, the cut depth, and the corneal thickness. The upper limit is around −10 D for myopia, and around +5 D for hyperopia and for astigmatism in most eyes3.
Clinical features, diagnosis, and differential diagnosis
Hyperopic eyes are treated by curving the central corneal surface. Frequently they have nasal decentration of fixation, and the relevant data from the topographer must be loaded into the laser to avoid decentration. Depth problems are rare with hyperopia, but the increase in negative spherical aberration induced by the treatment suggests one should not treat hyperopia above 5–6 D. High order aberrations are increased more by hyperopic than by myopic ablations4.
Recently, several LASIK procedures have been proposed to treat presbyopia. The most popular approach consists of increasing the spherical aberration of the central cornea, around a 2 mm central zone, obtained by superimposing hyperopic and myopic ablations. A second approach aims at curving the inferior central cornea. Although good results have been published5, these procedures are still controversial.
Anatomical considerations
The hinge position can be nasal or superior, because lateral or inferior positions favor flap displacement. Ideally, the LASIK cut should lie on a single lamellar plane within the corneal stroma. In practice, it crosses different planes and usually it is thinner in the center, impairing the corneal biomechanics and producing minor refractive changes6. The average cut depth is around 160 µm. Thin flaps below 130 µm can help, leaving more room to ablation, but are less stable in the first postoperative period, often requiring bandage soft contact lenses for one day. The laser ablation is similar to that of photorefractive keratectomy, but with lower regression and inflammation. Flap adhesion in the immediate postoperative is assured mainly by the endothelial pump and is usually excellent.
Fundamental principles
LASIK is based on a few assumptions: (i) the corneal curvature can be modified by cutting a flap, performing an excimer laser ablation and repositioning the flap; (ii) the cornea will remain transparent and mechanically stable in the postoperative; (iii) the optical properties of the cornea will remain of high quality. These assumptions proved true in uncomplicated cases, but corneal instability after surgery can develop especially if the procedure went too deep. Specifically in myopic LASIK care has to be taken to leave at least 250 µm of untreated tissue3.
Operation techniques
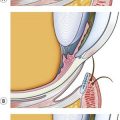
Femto-LASIK employs a femtosecond laser to obtain the corneal flap. Femtosecond lasers differ for energy, repetition rate, shape of the suction cup, required intraocular pressure, and delivery pattern. The disposable suction cup is applied to the eye, sometimes flattening the cornea according to the various models. The cut follows. A thin blunt spatula has to be employed to separate and to lift the flap because of the many small bridges remaining in the interface. Femtosecond flaps are considered to be more precise and consistent in depth as compared with microkeratomes7. In addition, the hinge position can be selected for each individual case, although only superior and nasal hinges offer postoperative stability. In contrast, flap manipulation is higher than with microkeratomes, and the cut surface is rougher.
Postoperative complications
Dry eye sensations are very common in the first postoperative period, depending on corneal nerve cutting. Flap striae are due to flap lifting and reposition, flap movement during the first postoperative hours, or high ablations. Thin striae are common and do not affect vision, but thick striae make it necessary to lift the flap and ‘iron’ it in the very first postoperative day. Flap displacement is rare, but involves careful cleaning of the debris and of the epithelial cells that immediately spread over the cut surfaces. Diffuse lamellar keratitis (0.2–0.5% of cases)8 takes place at the interface, and it is caused by material remaining there after ablation. If recognized early from patient pain, corneal flattening, and slit-lamp appearance, flap lifting and aggressive treatment by steroid and antibiotic eye drops can avoid corneal scarring and loss of visual acuity. Corneal infection is very rare, probably less than 0.02%, and can occur both as early infection due to common conjunctival contaminants, and as late infection due to slow growing bacteria. Epithelial ingrowth within the interface is also rare and seldom requires treatment.
The worst late complication is corneal ectasia (0.66% or less), a structural failure of the cornea that manifests with curvature steepening, tissue thinning, irregular astigmatism, and loss of vision. Corneal ectasia has been demonstrated to be associated with preoperative corneal irregularities, high myopia, and excessive thinning. Useful prevent on protocols have been published3, but we still do not know why some eyes develop ectasia while the majority do not. Glare and halos are perceived especially at night when the effective optical zone is much too small, or the pupil much too wide, although the relations among pupil diameter, optical zone, and visual symptoms have never been clarified. As for possible retinal breaks or detachment, after 20 years and several studies we can conclude that LASIK does not favor retinal detachment, leaving the risk typical of myopic eyes unchanged9.
Assessment of surgery: self-evaluation; results of surgery
The results of LASIK are assessed in terms of efficacy (% of eyes with visual acuity over 20/40, or within 0.5 D from intended refraction) and of safety (% of eyes with visual acuity equal to or better than the preoperative). Results are better for low refractive corrections: up to 98% of eyes with myopia <3 D attain full uncorrected vision10. Over-corrections and under-corrections may depend on incorrect preoperative assessment, or on ablation problems. In most eyes a re-operation can be carried out by simply lifting the flap and performing the required new ablation, but with increased risk of epithelial ingrowth. Flap re-cutting is preferred after 2 years, and mandatory after 3 years. In case, of insufficient corneal tissue, superficial ablation can be employed if the attempted correction is low.
1 Pallikaris IG, Papatznaki ME, Stathi EZ, et al. Laser in situ keratomileusis. Lasers Surg Med. 1990;10:463-468.
2 Buratto L, Ferrari M, Rama P. Excimer laser intrastromal keratomileusis. Am J Ophthalmol. 1992;113:291-295.
3 Randleman JB, Trattler WB, Stulting RD. Validation of the Ectasia Risk Score System for preoperative laser in situ keratomileusis screening. Am J Ophthalmol. 2008;145:813-818.
4 Kohnen T, Mahmoud K, Bühren J. Comparison of corneal higher-order aberrations induced by myopic and hyperopic LASIK. Ophthalmology. 112, 2005. 1692 e1–1692 e11
5 Alió JL, Chaubard JJ, Caliz A, et al. Correction of presbyopia by technovision central multifocal LASIK (presbyLASIK). J Refract Surg. 2006;22:453-460.
6 Waheed S, Chalita MR, Xu M, et al. Flap-induced and laser-induced ocular aberrations in a two-step LASIK procedure. J Refract Surg. 2005;21(4):346-352.
7 Chan A, Ou J, Manche EE. Comparison of the femtosecond laser and mechanical keratome for laser in situ keratomileusis. Arch Ophthalmol. 2008;126:1484-1490.
8 Gil-Cazorla R, Teus MA, de Benito-Llopis L, et al. Incidence of diffuse lamellar keratitis after laser in situ keratomileusis associated with the IntraLase 15 kHz femtosecond laser and Moria M2 microkeratome. J Cataract Refract Surg. 2008;34:28-31.
9 Arevalo JF, Ramirez E, Suarez E, et al. Retinal detachment in myopic eyes after laser in situ keratomileusis. J Refract Surg. 2001;18:708-714.
10 Schallhorn SC, Venter JA. One-month outcomes of wavefront-guided LASIK for low to moderate myopia with the VISX STAR S4 laser in 32,569 eyes. J Refract Surg. 2009;25:S634-S641.