CHAPTER 63 Surgical strategies for AMD
Introduction
The retinal pigment epithelium (RPE) holds the strategic position of the metabolic gatekeeper between photoreceptors and the choriocapillaries and hence plays a key role in maintaining retinal function. The RPE/photoreceptor complex suffers cumulative damage over a lifetime, which is thought to induce age-related macular degeneration (AMD) in susceptible individuals with a genetic predisposition (including dysregulation of the complement system). Two main routes of the disease occur: a slower progressing atrophic form where most patients currently have limited therapeutic options, and a potentially rapidly blinding exudative/neovascular form1,2. For the latter, intravitreal injections with anti-angiogenic drugs have now replaced laser photocoagulation or photodynamic therapies, offering a highly effective, yet palliative treatment for neovascular AMD. Still, long-term effects remain to be elucidated, and a proportion of patients lose vision despite pharmacologic therapy. A thorough review of intravitreal anti-angiogenic treatments is beyond the scope of this article; the interested reader is therefore referred to comprehensive articles on this matter (see for example, refs 3,4).
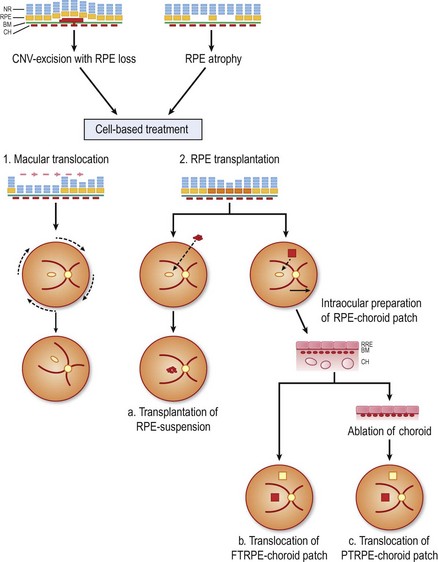
This chapter describes the current state for surgical approaches to AMD, and touches on future perspectives. Within this context, the use of the RPE cell as a ‘therapeutic agent’ is a potential strategy, as healthy RPE cells would, in theory, restore the functions of their degenerated counterparts (Fig. 63.1). The ultimate success of these cell-based therapies, however, clearly depends on delivery and maintenance of cells in a properly polarized state, capable to perform most, if not all, complex functions of the RPE. While in dire need for patients with advanced AMD, it is unlikely, in the authors’ opinion, that clinically feasible photoreceptor replacement will be achieved in the mid-term, and this aspect of retinal tissue engineering is therefore not discussed.
Epidemiologic considerations and terminology
Age-related macular degeneration is the leading cause of visual impairment in industrialized countries in patients over 50 years of age5,6. The prevalence of AMD increases with age so that up to one-third of individuals aged 75 and older suffer from some form of AMD7,8. Currently, it is estimated that 1.75 million individuals suffer from this disease in the United States and about 7 million are reported to be ‘at risk’9. Higher expectations on quality of life demanded by the expanding aging population hence result in increasingly significant morbidity from AMD10.
Clinical features
AMD is believed to be caused by progressive deterioration of RPE, Bruch’s membrane, and the choriocapillaris–choroid complex, which consequently leads to subsequent damage of the photoreceptor cells1,2,11. The hallmark of the disease and first visible changes are drusen, which present clinically on funduscopy as subretinal yellowish-white dots. According to their size and margin they are divided into hard and soft drusen. The risk for progression to advanced disease stages correlates to number, size, and density of drusen12. Soft drusen can coalesce and form pigment epithelial detachments (PED). Of these PEDs, 30–50% will become vascularized and may cause severe visual loss within the following years13. In patients with bilateral drusen and good vision in both eyes, the annual incidence of new atrophic or exudative lesions is approximately 8% over 3 years14.
In AMD, the RPE cells may become dysfunctional, which is seen in pigment irregularities with either hyper- or hypopigmentation often accompanied by drusen, which results either in the non-neovascular form with progression to geographic atrophy (‘atrophic AMD’) or in neovascular AMD (‘exudative, wet AMD’) with choroidal neovascularization (CNV) accompanied by exudation and hemorrhage, and subsequent cell death and scar formation10,15. Neovascular membranes secondary to AMD, particularly in patients on anti-coagulants, are prone to extensive bleeding into the subretinal space or vitreous. The hemorrhage in turn prevents metabolic exchange between the RPE and outer neural retina, and is retinotoxic16.
Fundamental principles
Dysfunction of the RPE may alter the extracellular environment for photoreceptors and Bruch’s membrane and thereby contribute to a variety of sight-threatening diseases including AMD. A thorough discussion of its underlying fundamental principles is beyond the scope of this article, as it encompasses factors such as, genetic polymorphisms of the complement system17,18, accumulation of intralysosomal degradation products affecting RPE function19, autoimmune mechanisms20, and Bruch’s membrane aging21.
Medical treatments
CCR3, an eosinophil/mast cell chemokine receptor, has been specifically shown to be expressed in choroidal neovascular endothelial cells in patients with CNV due to AMD. Its suppression in mouse models with induced CNV is more effective, yet less toxic than anti-VEGF treatments and it therefore represents a new, very promising therapeutic strategy in exudative AMD patients22.
Indications for surgery
Most AMD patients suffer from the slowly progressive early dry form, for which there currently are no efficient therapies. Some patients will convert into neovascular AMD, which can now be successfully treated by intravitreal injection of anti-VEGF drugs. However, with long-standing disease and/or associated structural damage to the RPE and surrounding structures, treatment responses may be disappointing and warrant the search for alternative strategies. Surgical approaches in the past have included excision of CNV, and macular translocation as well removal of massive subretrinal hemorrhages. In these instances, a reconstruction of the submacular architecture or maculoplasty would represent a curative treatment21,23. Currently discussed AMD subforms for which a surgical intervention may be considered include:
Patient selection and assessment
The most crucial factor for successful postoperative visual rehabilitation is preop macular function. Da Cruz and associates recommend, besides obtaining a history of duration of visual loss and BCVA, performing microperimetry, fixation analysis, and multifocal ERG24. High resolution, spectral domain OCT with 3D rendering allows for better assessment of subretinal pathology. The prognostic value of pre- and postop RPE autofluorescence imaging has recently been addressed, whereby a normal autofluorescence signal would indicate a functioning photoreceptor–RPE complex 24,25. Particularly for patients with primary GA of the RPE, it may serve as an important adjunct to determine remaining subfoveal RPE coverage.
Surgical techniques
Macular surgery for massive hemorrhages
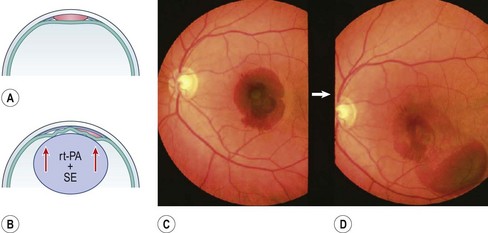
Circumscribed submacular hemorrhages can sometimes be liquified by intravitreal injection of 25–75 µg recombinant tissue plasminogen activator (rt-PA) and gas, followed by face-down positioning for several days to displace the lesion peripherally. The optimal therapeutic window for this intervention is within 2 weeks after the initial event; early treatments are clearly preferable. After this time, the blood clot may begin fibrotic remodeling which can induce strong adhesions within the adjacent neural retina. All subsequent therapies become difficult and fibrinolysis no longer works26.
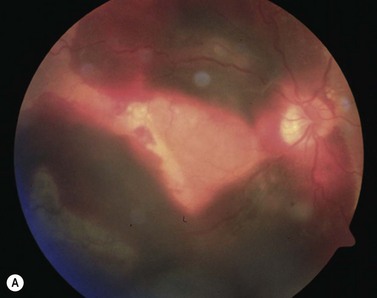
With extensive bleeding more invasive measures may become necessary. These may encompass, depending on the extent of the hemorrhage, a vitrectomy with a paramacular retinotomy, or 180° retinotomy, removal of the hemorrhage and with subsequent gas, or silicone-oil fill of the eye; see Figure 63.227.
Macular surgery for choroidal neovascularization
Surgical interventions in exudative AMD had their beginnings in excisions of subfoveal CNV28,29, which, however, usually resulted in simultaneous removal of the RPE ‘trapped’ within the neovascular complex30. Extensive loss of subfoveal RPE leads inadvertently to irreversible loss of vision30. In a retrospective meta-analysis evaluating 26 different studies and a total of 647 cases of subretinal membrane excision in AMD patients, it was shown that improvement was achieved in about 33% and deterioration occurred in 27%. A mean recurrence rate of the CNV in 25% (0–55%) was found in addition, which added to further visual loss in primary successful cases31. In a prospective multicenter study comparing submacular surgery with laser photocoagulation, the two treatment options were found to be equivalent. After 2 years, 65% of laser treated cases versus 50% of surgically treated eyes had a visual acuity that was better than or no more than one line worse than baseline32. Therefore submacular membrane extraction alone nowadays no longer represents a promising functional outcome31. Photoreceptor survival requires an intact functional RPE, therefore counteracting the bystander damage during CNV extraction poses two options: (1) rotate the still functional neurosensory retina away from the RPE defect; or (2) replace the RPE defect with cell transplantation (see Fig. 63.1).
Macular translocation (MT360)
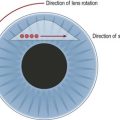
The concept of macular translocation was initially proposed by Machemer and Steinhorst33. Improvements of the technique have enabled some patients to retain their reading vision. Aisenbrey and associates found for a mean follow-up of 38 months, a stabilization or improvement in half of the patients34. This technique involves a pars plana vitrectomy followed by 360° retinotomy and a complete retinal detachment by subretinal injection of calcium magnesium free BSS35. The retina is then rotated about 45° (typically upward) around its attachment at the optic disc. Retinal reattachment is achieved by a silicone oil tamponade with subsequent circular laser photocoagulation at the margin of the retinotomy. A counter-rotation of the globe is recommended, either during the primary procedure or at the time of silicone oil removal, to avoid diplopia from cyclorotation.
Shortcomings of this procedure include a relatively elaborate surgery, secondary procedures (silicone oil removal, counter-rotation of the globe, etc.), as well as potential complications such as proliferative vitreoretinopathy (PVR), rhegmatogenic retinal detachment, macular edema, etc. Postoperative double vision is not always treated satisfactorily. The initially high complication rates of about 30% could be reduced to 8–18% in centers with more experience36–39.
Cellular replacement strategies of the RPE
The concept of RPE transplantation evolved out of promising results in animal experiments with RPE gene mutations40,41. Peyman et al. were the first group to transplant a patch of RPE and choroid in a patient with CNV due to AMD42. Since then many techniques for homologous and autologous RPE replacement have been developed21,24,43,44.
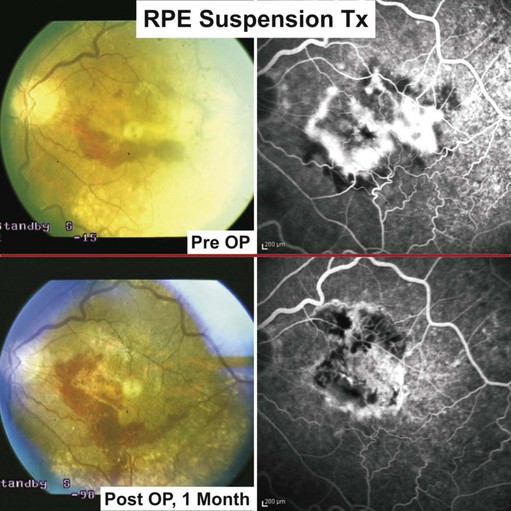
RPE-cell transplantation using an injection of cells injected into the subretinal space
Cell suspensions of homologous fetal RPE were first transplanted in humans by Algvere and associates45–47. Central retinal function, however, could not be retained in the long term, for which immune rejection was blamed. The immune privilege of the subretinal space may be compromised through pathologic changes in neovascular AMD and iatrogenic damage incurred during the transplantation48,49.
To avoid rejection, autologous RPE replacement strategies were pursued. Binder and coworkers were able to show, in a prospective case–control study in patients with CNV secondary to AMD, a statistically significant improvement (near vision and multifocal ERG) in the transplanted group50. Eighty percent of patients achieved stabilization or improved visual acuity within the follow-up of 1 year.
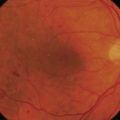
Following vitrectomy, RPE cells were obtained from the retinal periphery in the affected eye during the surgical procedure and injected underneath the macula; see Figures 63.1 and 63.451.
In vitro studies pointed out that age-related changes in Bruch’s membrane compromise attachment and/or function of RPE52,53. To address the latter situation, van Meurs and colleagues used poly-L lysine (a common adhesive used in cell culture) in their suspension transplants. They were not able to demonstrate an advantage of this method and observed high PVR rates54.
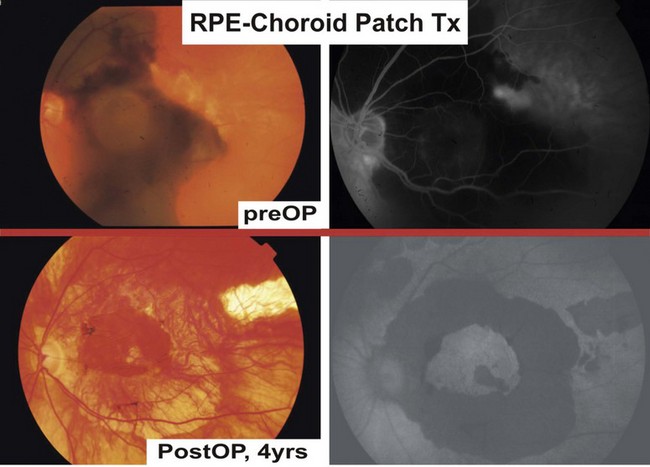
RPE/choroid patch transplantation
Clinical trials with RPE/choroid patch translocations have elegantly bypassed the dilemma of cell attachment and distribution in RPE suspension therapies; potential advantages include the preservation of the physiologic monolayer, the simultaneous provision of a substrate, as well as minimizing trauma to the transplanted cells. After few weeks, choroidal vessels of most patches will anastomose with the underlying choroidal vasculature55.
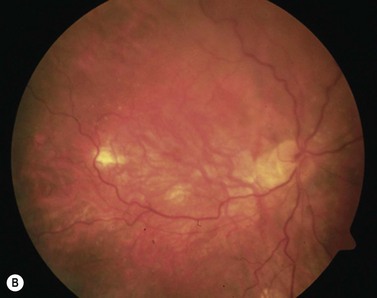
Adapted from Stanga et al., van Meurs et al. suggested a technique where a free graft/patch of RPE/choroid is harvested from the midperiphery, and then translocated underneath the macula; see Figures 63.1 and 63.556,57,63. Many centers worldwide have now adopted this technique and confirmed fixation over the patch graft, as well as its revascularization in a high proportion of patients. It appears that preoperative macular function correlates with postoperative visual recovery (Fig. 63.5).
Holz et al. suggested in a small case series a modified technique59, whereby choroidal vessels of the RPE/choroid-sheets are ablated with a 308 nm excimer laser. The rationale was to reduce diffusion distance from the choroidal wound bed to the graft and outer retinal layers, as well as untoward cellular reactions that would limit subsequent visual recovery. In vitro studies have demonstrated safety of the laser irradiation60. Ma and associates then used a mechanical intraocular ablation technique61, and achieved promising results in a small case series62.
Visual rehabilitation in RPE patch therapies, however, suffers from frequent complication rates, of which the most common include postoperative subretinal bleeds, macular holes, PVR, fibrosis of the patch24. Overall, a mean decline of visual loss is reported if postoperative complications are included. If these cases are excluded from visual analysis, the mean gain in visual acuity is 2–3 lines24. Van Meurs’ group underscored the latter correlation in a prospective statistical analysis of 48 cases63. It is noteworthy that, in a single center, multiple surgeon comparison of macular translocation with patch translocation, the former yielded better long-term results64. As long as the complication rates remain high, the possible gain of vision limits this surgical technique to cases otherwise not treatable with pharmacologic treatments65. It is therefore encouraging that the mere mechanical ablation in analogy to Holz’ technique by Ma and associates was able to demonstrate low PVR rates (14.3%) at 1-year follow-up and good functional outcomes in exudative AMD cases complicated by subretinal hemorrhage66.
Macular surgery for non-exudative AMD
Advanced dry AMD, i.e. geographic atrophy (GA), is the second most common cause for irreversible visual loss67. Effective cell-based RPE therapy would need to intervene early, ideally when the GA has yet spared the foveal region (see Fig. 63.1). In a pilot study, Algvere et al. implanted a sheet of homologous fetal RPE in four patients with GA; however, no visual improvement was reported47. A number of surgeons performed full macular translocation (MT360) in patients with GA secondary to AMD, but only eyes with relatively short-term visual loss and preserved preoperative foveal function showed stabilization or gain of visual acuity, particularly in near vision68,69. A puzzling, ‘copy-paste phenomenon’ was observed after a few months in roughly 40% of all accumulated (thus far published) cases with MT360 in GA due to AMD: a new atrophic zone appeared underneath the neofovea which resembled the preoperative lesion68.
In a case series with autologous free RPE/choroid patch grafts by Kirchhof and coworkers, the majority of GA patients with retained preop central fixation achieved stabilization or gain of visual acuity25. In patients where BM had been intentionally ruptured intraoperatively, the patch showed revascularization, which also correlated with better functional results. This series, however, was compromised by postop complications. It is noteworthy though that even in long-term follow-up (beyond 12 months) no copy-paste phenomenon had been observed, perhaps suggesting that the peripheral RPE differs in disease potential (personal communication, B. Kirchhof 9/2009).
The group around R. Aramant and M. Seiler has been perfecting a technique for co-transplantation of fetal sheets of neural retina together with RPE over the past two decades70. The Phase II studies have included patients with non-exudative (late stage) AMD71; none had experienced rejection of the transplants. None of the patients had improved sensitivity in postop microperimetry, which was perhaps related to bad VA at baseline71.
Upcoming trends
Stem cell derived RPE-like cells
Reports are accumulating about cells derived from mammalian stem cells which acquire RPE-like phenotypes. Six different sources (embryonic stem cells, ciliary margin stem cells, adipose mesenchymal stromal cells, neural stem cells, bone marrow derived cells, and induced pluripotent stem cells) have been reported to date, of which induced pluripotent cells (iPSC-s) hold the greatest promise72,73. These cells behave like fetal RPE and are derived from the patient (=autologous), thus eliminating immune rejection. Virtually any somatic cell can be reprogrammed by gene transfection into a pluripotent stage to then yield cells of all tissue types upon redifferentiation74.
Tissue engineering to reconstruct the RPE/Bruch’s membrane complex
Based on the successful proof of principle from various animal studies and some promising cases treated with macular translocation, it seems there is still more potential for surgical therapy of the macula. Future cell-based RPE therapies will be aided by the field of tissue engineering, where carrier matrices for cell transport and/or reconstruction of defective organs are already in clinical trials (see, for example, ref. 75). The concept of a prosthetic Bruch’s membrane is likewise inspired by this vastly expanding research area21. RPE (-like) cells are placed in vitro on a carrier matrix (i.e. Bruch’s membrane prosthesis), which encourages physiologic RPE function, facilitates implantation, and protects the graft from deleterious influences of aged submacular Bruch’s membrane. Meanwhile, over 20 different biologic as well as artificial substrates were proposed (for an overview see ref. 21). It is possible that, with optimized instrumentation, techniques, and cell sources, RPE replacement therapy will one day become comparable to current treatment standards in cataract surgery.
1 Holz FG, Pauleikhoff D, Spaide RF, et al. Age-related Macular Degeneration. Berlin: Springer; 2007.
2 Holz FG, Pauleikhoff D, Klein R, et al. Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol. 2004;137(3):504-510.
3 Ciulla TA, Rosenfeld PJ. Antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2009;20(3):158-165.
4 Eter N, Krohne TU, Holz FG. New pharmacologic approaches to therapy for age-related macular degeneration. BioDrugs. 2006;20(3):167-179.
5 Ambati J, Ambati BK, Yoo SH, et al. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257-293.
6 Fine SL, Berger JW, Maguire MG, et al. Age-related macular degeneration. N Engl J Med. 2000;342(7):483-492.
7 Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122(4):598-614.
8 Bird AC. The Bowman lecture. Towards an understanding of age-related macular disease. Eye. 2003;17(4):457-466.
9 The Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-572.
10 Klein R, Klein BE, Jensen SC, et al. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104(1):7-21.
11 Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24(Suppl):335-610.
12 Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol. 1997;115(6):741-747.
13 Casswell AG, Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. Br J Ophthalmol. 1985;69(6):397-403.
14 Holz FG, Wolfensberger TJ, Piguet B, et al. Bilateral macular drusen in age-related macular degeneration. Prognosis and risk factors. Ophthalmology. 1994;101(9):1522-1528.
15 Freund KB, Yannuzzi LA, Sorenson JA. Age-related macular degeneration and choroidal neovascularization. Am J Ophthalmol. 1993;115(6):786-791.
16 Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 1982;94(6):762-773.
17 Scholl HP, Fleckenstein M, Charbel Issa P, et al. An update on the genetics of age-related macular degeneration. Mol Vis. 2007;13:196-205.
18 Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28(1):1-18.
19 Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80(5):595-606.
20 Hollyfield JG, Bonilha VL, Rayborn ME, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14(2):194-198.
21 Binder S, Stanzel BV, Krebs I, et al. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007;26(5):516-554.
22 Takeda A, Baffi JZ, Kleinman ME, et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460(7252):225-230.
23 Del Priore LV, Tezel TH, Kaplan HJ. Maculoplasty for age-related macular degeneration: reengineering Bruch’s membrane and the human macula. Prog Retin Eye Res. 2006;25(6):539-562.
24 da Cruz L, Chen FK, Ahmado A, et al. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26(6):598-635.
25 Joussen AM, Joeres S, Fawzy N, et al. Autologous translocation of the choroid and retinal pigment epithelium in patients with geographic atrophy. Ophthalmology. 2007;114(3):551-560.
26 Hesse L. Treating subretinal hemorrhage with tissue plasminogen activator. Arch Ophthalmol. 2002;120(1):102-103. author reply 203
27 Haupert CL, McCuen BW2nd, Jaffe GJ, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid–gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol. 2001;131(2):208-215.
28 Thomas MA, Dickinson JD, Melberg NS, et al. Visual results after surgical removal of subfoveal choroidal neovascular membranes. Ophthalmology. 1994;101(8):1384-1396.
29 de Juan EJr, Machemer R. Vitreous surgery for hemorrhagic and fibrous complications of age-related macular degeneration. Am J Ophthalmol. 1988;105(1):25-29.
30 Ormerod LD, Puklin JE, Frank RN. Long-term outcomes after the surgical removal of advanced subfoveal neovascular membranes in age-related macular degeneration. Ophthalmology. 1994;101(7):1201-1210.
31 Falkner CI, Leitich H, Frommlet F, et al. The end of submacular surgery for age-related macular degeneration? A meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2007;245(4):490-501.
32 Bressler NM, Bressler SB, Hawkins BS, et al. Submacular surgery trials randomized pilot trial of laser photocoagulation versus surgery for recurrent choroidal neovascularization secondary to age-related macular degeneration: I. Ophthalmic outcomes submacular surgery trials pilot study report number 1. Am J Ophthalmol. 2000;130(4):387-407.
33 Machemer R, Steinhorst UH. Retinal separation, retinotomy, and macular relocation: II. A surgical approach for age-related macular degeneration? Graefes Arch Clin Exp Ophthalmol. 1993;231(11):635-641.
34 Aisenbrey S, Bartz-Schmidt KU, Walter P, et al. Long-term follow-up of macular translocation with 360 degrees retinotomy for exudative age-related macular degeneration. Arch Ophthalmol. 2007;125(10):1367-1372.
35 Szurman P, Roters S, Grisanti S, et al. Ultrastructural changes after artificial retinal detachment with modified retinal adhesion. Invest Ophthalmol Vis Sci. 2006;47(11):4983-4989.
36 Aisenbrey S, Lafaut BA, Szurman P, et al. Macular translocation with 360 degrees retinotomy for exudative age-related macular degeneration. Arch Ophthalmol. 2002;120(4):451-459.
37 Mruthyunjaya P, Stinnett SS, Toth CA. Change in visual function after macular translocation with 360 degrees retinectomy for neovascular age-related macular degeneration. Ophthalmology. 2004;111(9):1715-1724.
38 Ohji M, Fujikado T, Kusaka S, et al. Comparison of three techniques of foveal translocation in patients with subfoveal choroidal neovascularization resulting from age-related macular degeneration. Am J Ophthalmol. 2001;132(6):888-896.
39 Pertile G, Claes C. Macular translocation with 360 degree retinotomy for management of age-related macular degeneration with subfoveal choroidal neovascularization. Am J Ophthalmol. 2002;134(4):560-565.
40 Lopez R, Gouras P, Kjeldbye H, et al. Transplanted retinal pigment epithelium modifies the retinal degeneration in the RCS rat. Invest Ophthalmol Vis Sci. 1989;30(3):586-588.
41 Sheedlo HJ, Li LX, Turner JE. Functional and structural characteristics of photoreceptor cells rescued in RPE-cell grafted retinas of RCS dystrophic rats. Exp Eye Res. 1989;48(6):841-854.
42 Peyman GA, Blinder KJ, Paris CL, et al. A technique for retinal pigment epithelium transplantation for age-related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surg. 1991;22(2):102-108.
43 Gouras P, Algvere P. Retinal cell transplantation in the macula: new techniques. Vision Res. 1996;36(24):4121-4125.
44 Thumann G, Walter P. [Non-pharmacological interventional perspectives in AMD]. Klin Monatsbl Augenheilkd. 2008;225(8):699-702.
45 Algvere PV, Gouras P, Dafgard Kopp E. Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur J Ophthalmol. 1999;9(3):217-230.
46 Algvere PV, Berglin L, Gouras P, et al. Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1994;232(12):707-716.
47 Algvere PV, Berglin L, Gouras P, et al. Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefes Arch Clin Exp Ophthalmol. 1997;235(3):149-158.
48 Enzmann V, Faude F, Wiedemann P, et al. Immunological problems of transplantation into the subretinal space. Acta Anat (Basel). 1998;162(2–3):178-183.
49 Streilein JW, Ma N, Wenkel H, et al. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res. 2002;42(4):487-495.
50 Binder S, Krebs I, Hilgers RD, et al. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: a prospective trial. Invest Ophthalmol Vis Sci. 2004;45(11):4151-4160.
51 Binder S, Stolba U, Krebs I, et al. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: a pilot study. Am J Ophthalmol. 2002;133(2):215-225.
52 Tezel TH, Del Priore LV, Kaplan HJ. Reengineering of aged Bruch’s membrane to enhance retinal pigment epithelium repopulation. Invest Ophthalmol Vis Sci. 2004;45(9):3337-3348.
53 Gullapalli VK, Sugino IK, Van Patten Y, et al. Retinal pigment epithelium resurfacing of aged submacular human Bruch’s membrane. Trans Am Ophthalmol Soc. 2004;102:123-137. discussion 137–8
54 van Meurs JC, ter Averst E, Hofland LJ, et al. Autologous peripheral retinal pigment epithelium translocation in patients with subfoveal neovascular membranes. Br J Ophthalmol. 2004;88(1):110-113.
55 Maaijwee K, Van Den Biesen PR, Missotten T, et al. Angiographic evidence for revascularization of an RPE-choroid graft in patients with age-related macular degeneration. Retina. 2008;28(3):498-503.
56 Stanga PE, Kychenthal A, Fitzke FW, et al. Retinal pigment epithelium translocation and central visual function in age related macular degeneration: preliminary results. Internat Ophthalmol. 2001;23(4–6):297-307.
57 van Meurs JC, Van Den Biesen PR. Autologous retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: short-term follow-up. Am J Ophthalmol. 2003;136(4):688-695.
58 Maaijwee K, Joussen AM, Kirchhof B, et al. Retinal pigment epithelium (RPE)-choroid graft translocation in the treatment of an RPE tear: preliminary results. Br J Ophthalmol. 2008;92(4):526-529.
59 Bindewald A, Roth F, Van Meurs J, et al. [Transplantation of retinal pigment epithelium (RPE) following CNV removal in patients with AMD Techniques, results, outlook]. Ophthalmologe. 2004;101(9):886-894.
60 Krohne TU, Hunt S, Holz FG. Effect of 308 nm excimer laser irradiation on retinal pigment epithelium cell viability in vitro. Br J Ophthalmol. 2009;93(1):91-95.
61 Zhang T, Hu Y, Li Y, et al. Photoreceptor repair by autologous transplantation of retinal pigment epithelium and partial-thickness choroid graft in rabbits. Invest Ophthalmol Vis Sci. 2009;50(6):2982-2988.
62 Ma Z, Han L, Wang C, et al. Autologous transplantation of retinal pigment epithelium–Bruch’s membrane complex for hemorrhagic age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(6):2975-2981.
63 Maaijwee K, Missotten T, Mulder P, et al. Influence of intraoperative course on visual outcome after an RPE-choroid translocation. Invest Ophthalmol Vis Sci. 2008;49(2):758-761.
64 Chen FK, Patel PJ, Uppal GS, et al. A comparison of macular translocation with patch graft in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(4):1848-1855.
65 Stanzel BV, Bindewald-Wittich A, Holz FG, et al. Vitreoretinale Eingriffe bei fortgeschrittener altersabhängiger Makuladegeneration. Spektrum der Augenheilkunde. 2008;22(6):348.
66 Ma Z, Han L, Wang C, et al. Autologous Transplantation of retinal pigment epithelium–Bruch’s membrane complex for hemorrhagic age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(6):2975-2981.
67 Owen CG, Fletcher AE, Donoghue M, et al. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom? Br J Ophthalmol. 2003;87(3):312-317.
68 Eckardt C, Eckardt U. Macular translocation in nonexudative age-related macular degeneration. Retina. 2002;22(6):786-794.
69 Cahill MT, Mruthyunjaya P, Bowes Rickman C, et al. Recurrence of retinal pigment epithelial changes after macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch Ophthalmol. 2005;123(7):935-938.
70 Aramant RB, Seiler MJ. Progress in retinal sheet transplantation. Prog Retin Eye Res. 2004;23(5):475-494.
71 Radtke ND, Aramant RB, Petry HM, et al. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008;146(2):172-182.
72 Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27(10):2427-2434.
73 Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458(3):126-131.
74 Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797-801.
75 Boccaccini AR, Gough JE. Tissue engineering using ceramics and polymers. Cambridge (England): Woodhead Publishing Limited; 2007.