Chapter 148 Laser Treatment of Choroidal Melanoma
Introduction
Photocoagulation, as introduced by Meyer-Schwickerath in 1949,1 was among the first eye-salvaging treatment methods for choroidal melanomas and the first to make use of a light source. The armamentarium of eye salvaging treatment modalities for choroidal melanomas has significantly enlarged since then, including radiation techniques (brachytherapy, teletherapy with protons, and stereotactic conformal radiation with photons) and surgical excision (transscleral or transretinal). Techniques and light sources for laser treatment of choroidal melanomas have changed as well. Transpupillary thermotherapy making use of infrared lasers at a subcoagulation level was introduced in 1992 by Journée-de Korver and colleagues2 and utilized for primary treatment of small uveal melanomas. Other laser techniques like photodynamic treatment of uveal melanomas were evaluated but still have to be considered as experimental treatment. Although the initial enthusiasm for laser treatment of uveal melanomas has been dampened, laser treatment in all its varieties is a powerful tool in the management of choroidal melanomas when used in selected cases and as an adjunct to other treatment methods. This chapter discusses the current role of laser treatment in the management of choroidal melanomas in relation to the alternative treatment methods currently available.
Laser techniques available for the treatment of intraocular tumors
By convention, photocoagulation is considered to be laser treatment at a temperature level of >75°C. Photocoagulation attempts to destroy a uveal tumor with light from a high-intensity light source, which originally was not a laser but a xenon arc light.3
Non-coagulative laser treatment techniques include low-power long-exposure treatment techniques making use of a conventional argon blue-green laser4 or infrared laser.5 Depending on the wavelength of the laser used the lesions are located deeper in the choroid when infrared or near infrared lasers are used and lesions created by argon blue-green lasers do not extend 1 mm in size while infrared lasers may result in a depth of tumor necrosis of several millimeters.5
Photocoagulation
Photocoagulation treatment is started by circumvallating the tumor with an intensive chorioretinal scar followed by direct treatment of the tumor with high-intensity burns including disruption of the tumor tissue and occasionally appearance of gas bubbles inside the target tissue. As expected, this technique may result in numerous treatment-related retinal complications including retinal breaks, epiretinal membrane formation vascular occlusion and tractional retinal detachment. Moreover, only small tumors can be treated and even in this subset of small tumors a relatively high recurrence rate of uveal melanomas after photocoagulation treatment has been reported. In particular, extrascleral extension of the melanoma may occur growing underneath the fibrous scar overlying the tumor remnants. Consequently, photocoagulation treatment as sole treatment of a uveal melanoma has been abandoned in most centers.6
Transpupillary thermotherapy
Technique
The term “transpupillary thermotherapy” (TTT) was introduced by Journée-de Korver et al.5 to describe a technique in which a near-infrared or infrared light source for long-term exposure laser treatment of uveal melanomas is used. There is controversy in the literature on whether the word “thermotherapy” is appropriate, or whether this treatment technique should be regarded as long-exposure subthreshold photocoagulation using a long wavelength light source, since a whitish-gray discoloration of the target tissue at the end of the procedure is recommended.7 As published by Journée-de Korver et al.5 thermotherapy is different from hyperthermia, which by definition is heating the tumor to a temperature of 42–44°C to enhance the cytotoxic effect of ionizing radiation on tumor cells. In TTT, temperatures of approximately 45–60°C within the tumor are reached with irreversible cytotoxic effects so that additional radiotherapy may be not required.8
Infrared or near-infrared light penetrates deeper into the choroidal tissue than light from argon blue-green lasers theoretically avoiding undesirable coagulation effects in the retina. In contrast to other wavelengths, the absorption of ocular media for infrared is very low (approximately 4–7%).5 Disadvantages include the invisibility of the actual laser beam resulting in the need for an aiming beam and an increased risk of choroidal hemorrhage because of the deeper penetration of the laser light. Numerous light sources based on a semiconductor diode laser are commercially available which may be used for TTT.
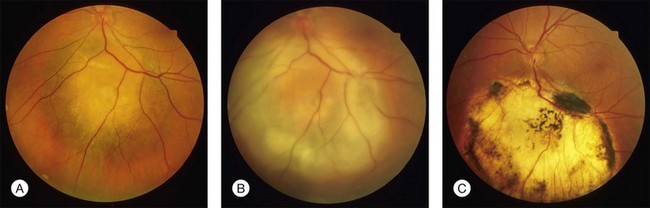
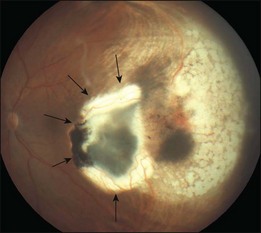
In clinical practice, TTT is performed with retrobulbar anesthesia using a slit lamp and a contact lens. A laser beam with a spot size of 3 mm and a maximal power density of 12 W/cm2 is used. At the second half of the exposure time of at least 1 minute, a grayish discoloration of the tumor tissue should be visible, indicating a temperature of the target tissue of 60–65°C. Ideally, no occlusion of retinal vessels and no coagulation effects in the overlying retina should occur. Three to four treatment sessions are needed which should result in an atrophic scar with central pigment and visible sclera at the treatment site (Fig. 148.1).
Potential complications of TTT are related to the heat delivered to ocular structures and include accidental combustion of the anterior segment, retinal vascular occlusions, retinal and vitreous hemorrhages, macular edema, nerve fiber bundle defects with subsequent visual fields defects and epiretinal membrane formation with traction on the retina.9,10
Experimental data provided evidence that the absorption of heat is enhanced in particular in amelanotic tumors by systemic administration of a chromophore like indocyanine green immediately before TTT.11,12 Although a prospective randomized trial did not find any beneficial effect in supplemental use of indocyanine green in TTT of choroidal melanomas,13 other authors did find enhanced local tumor control when indocyanine was used in combination with TTT.14
TTT as primary treatment of choroidal melanoma
When TTT was introduced in the treatment of choroidal melanoma, short-term follow-up data impressively showed that in appropriate cases tumor regression may be achieved in more than 90%. Tumors considered being appropriate for TTT included melanomas with a largest tumor diameter of <12 mm and not more than 4 mm thickness located posterior to the equator where the clinical diagnosis of a malignant melanoma was established.8,15
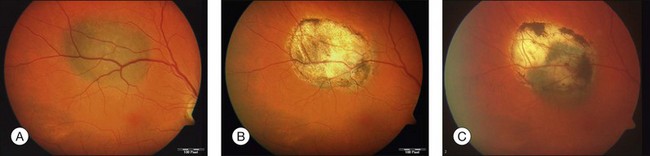
Studies with a longer follow-up, however, have dampened the initial enthusiasm showing that conventional photocoagulation and TTT may be not as different as previously thought.16 Shields and associates were among the first to find that 9% of the tumors developed recurrent growth (Figs 148.2, 148.3).9 Patients with tumors abutting or overhanging the optic disc or those requiring more than three sessions for tumor control were more likely to develop tumor recurrence. Other groups have published similar results in particular in juxtapapillary tumors10,17–23 The pattern of recurrence has been evaluated in a histopathological series of seven eyes enucleated after TTT.24 The authors found a lateral growth of the tumor and an extrascleral extension in five out of seven cases, which were detected only in a single case ultrasonographically. Other groups also reported extrascleral extension after TTT.6,24,25 These findings correlate to similar histologic findings after conventional photocoagulation, making it highly questionable that TTT is advantageous.
Progressive choroidal vascular remodeling and retinochoroidal anastomosis in sequential indocyanine angiograms may indicate recurrent tumor growth after TTT.26,27 These findings suggest that vascular occlusion after TTT is not complete as previously thought, which may explain the high recurrence rate after stand-alone TTT.
These controversial aspects of TTT as sole treatment of choroidal melanomas have recently been reviewed by a group of ocular oncologists.16 They found recurrence rates as high as 56%.19 They concluded that the potential risk for visual loss is lower for TTT compared to local radiation. Local tumor control, however, is insufficient, making TTT not effective for the treatment of small choroidal melanomas.
TTT as ancillary treatment of choroidal melanoma
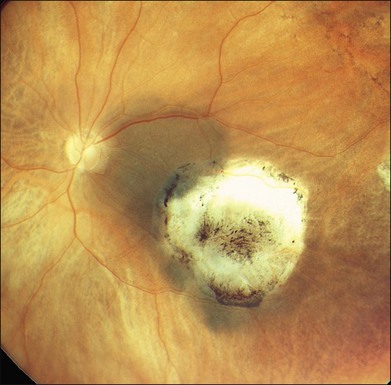
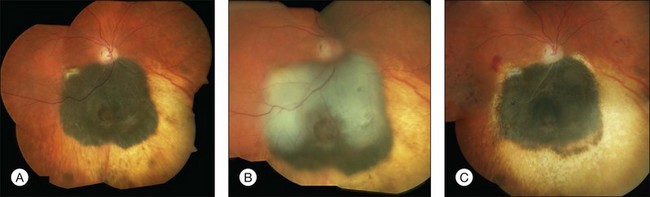
While stand-alone treatment of choroidal melanomas with TTT is disappointing combined treatment offers promising options.28 TTT as ancillary treatment may be used for several indications including so-called “sandwich therapy,” geographical misses after radiotherapy or surgical excision and insufficient regression or recurrent growth of the tumor (Figs 148.4, 148.5).
The Leiden Group suggested “sandwich therapy” (brachytherapy of the tumor base and TTT of the tumor apex) for the treatment of choroidal melanoma, as both treatment modalities may be synergistic.12 The rationale for “sandwich therapy” is the enhanced cytotoxic effect of radiation and the potential reduction of radiation dose when thermotherapy and radiation are combined. Another complementary effect is the efficacy of TTT at the tumor apex and the maximal radiation dose at the tumor base in brachytherapy.
In contrast to the insufficient tumor control after stand-alone TTT, numerous groups have evaluated the combination of thermotherapy and radiation reporting promising results.29–34 Although it is difficult to determine whether the visual outcome is more favorable when using “sandwich therapy” instead of brachytherapy alone,35 and although some authors could not detect that “sandwich therapy” is beneficial,36 there is significant evidence that TTT in combination with brachytherapy is effective and may enhance local tumor control even in relatively thick tumors which may not be sufficiently treated with brachytherapy alone.30 In particular, in tumors overhanging or encircling the optic disc31,37 more eyes can be preserved in the group of patients with combined treatment. A better visual outcome, however, could not be achieved.32
TTT may be applied prior to or during treatment30 or in multiple sessions after brachytherapy.33 The chromosome status of the tumor may correlate to the regression of the tumor after combined treatment. Monosomy 3 in the tumor is highly significantly correlated to increased mortality and interestingly is significantly correlated to a faster regression after brachytherapy combined with TTT.34
TTT as ancillary treatment was evaluated in radiotherapy modalities other than brachytherapy like proton radiation.38 In this series large uveal melanomas between 7 and 15 mm thickness were randomized for proton treatment alone and for combined treatment with three sessions of TTT after radiation with a significant lower secondary enucleation rate and less exudative retinal detachment in the latter group.
Geographical misses may complicate radiotherapy of choroidal melanoma, in particular when the tumor is located at the posterior pole and the scleral base is hard to be covered by a radioactive plaque. Geographical misses may be intentional when the tumor is located close to the optic disc or partially surrounding the optic disc margin requiring additional TTT to destroy the tumor. In contrast, the unfavorable results in primary TTT of juxtapapillary tumors TTT is effective in ancillary treatment of these conditions,39 as well as in choroidal melanomas with insufficient tumor regression or recurrent tumor growth after local radiation. Additionally, TTT may be used after transscleral or transretinal surgical excision to prevent recurrent tumor growth at the edges of the pseudocoloboma.
Laser photocoagulation as ancillary treatment for uveal melanoma
Radiation retinopathy, radiation-induced optic neuropathy
Radiation techniques are well established in eye salvaging treatment of uveal melanoma but may be complicated by radiation-related complications like radiation retinopathy and radiation optic neuropathy. Incidence and severity of these complications are related to the radiation dose used, the dose distribution of the radiation source (β-ray plaques, γ-ray plaques or external beam sources like a proton beam), and the location of the tumor (posterior or anterior to he equator). Radiation retinopathy is a slowly progressive, delayed-onset disease of retinal blood vessels due to changes in the structure and permeability of the retinal vessels.40 Characteristic clinical findings are macular edema, capillary non-perfusion, cotton-wool spots, capillary telangiectasia, retinal neovascularization, microaneurysms, retinal hemorrhages, intraretinal exudation, and neuronal changes such as disc edema, disc pallor, optic nerve atrophy, and neovascularization of the disc.41
If proliferative retinopathy occurs and remains untreated, rubeosis of the iris and secondary neovascular glaucoma may occur. There is no effective treatment to cure vision loss due to radiation retinopathy. Panretinal photocoagulation, however, is effective in preventing complications related to radiation-induced ocular ischemia.42,43 Radiation-induced macular edema may benefit from focal laser treatment.44,45 New options include intravitreal VEGF inhibitors in analogy to the treatment of diabetic macular edema or the combination of both treatment options.46
Surgical removal of uveal melanoma
The benefit of photocoagulation prior to surgical excision of a uveal melanoma is unclear and remains controversial. An ora-to-ora coagulation at the central margin of the tumor before “block excision” as advocated by Naumann and Rummelt47 is no longer recommended.48 TTT or conventional photocoagulation, however, is effective in reducing the risk of continuous or recurrent tumor growth after surgical excision. Damato and colleagues49 have recommended the use of photocoagulation at the edges of the pseudocoloboma as part of the routine management of patients following transscleral local resection. When endoresection is used to remove a choroidal melanoma, endolaser coagulation of the tumor site, particularly of the tumor edges, is mandatory because of the high risk of incomplete removal of tumor tissue.50
Exudative retinal detachment
Choroidal melanomas are frequently associated with exudative retinal detachment due to tumor-related vascular leakage. Moreover, extensive exudative retinal detachment is a well-known complication of brachytherapy of uveal melanoma51,52 and may lead to loss of vision and eventually enucleation. Several authors have reported that scatter photocoagulation on the surface of the tumor may be helpful in the treatment of melanoma-associated retinal detachment, which may be combined with vitreoretinal surgery, drainage of subretinal fluid, and fluid–air exchange.53 In charged particle therapy of uveal melanomas the ancillary use of laser thermotherapy significantly reduced the incidence and duration of exudative retinal detachment after radiation treatment.38,54
Photodynamic therapy of uveal melanomas
The effect of photodynamic therapy (PDT) (photoradiation) is based on the cytotoxic effect of singlet oxygen radicals by photosensitizers when exposed to visible light. The first generation of photosensitizers used was hematoporphyrin derivatives (HpD).55 HpD-based photodynamic therapy, however, had numerous disadvantages, among which was a high rate of recurrences (probably related to the poor tissue penetration of 630 nm laser light into tumor tissue), secondary glaucoma,56 and severe systemic side-effects (patients had to avoid sunlight for weeks to prevent deleterious phototoxic effects on the skin) which led to rejection of this therapeutic approach.57
Benzoporphyrin-derived photosensitizers like verteporfin, developed for the treatment of nonmalignant diseases, such as subretinal neovascular membranes in age-related macular degeneration have been evaluated experimentally58 and clinically in the treatment of choroidal melanomas.57 Verteporfin offers numerous advantages over first-generation photosensitizing drugs including better tissue penetration and fewer systemic side effects with regard to skin toxicity and has been proven to be effective in the treatment of choroidal hemangioma.59 Initial data from a small series of patients showed that PDT with verteporfin is basically capable of destroying a choroidal melanoma using a light dose of 100 J/cm2.57 Histopathologic data from eyes where PDT with verteporfin was performed on an experimental basis 2–3 days prior to planned enucleation because of a large uveal melanoma, confirmed these results.60 In this series, tumor necrosis was induced in a depth up to 2.5 mm using a light dose ≥100 J/cm2 demonstrating the potential of PDT with verteporfin to induce tumor necrosis. These results, however, lack clinical confirmation, as only single case reports on verteporfin PDT have been published since then,61 so the potential of PDT with verteporfin in primary or ancillary treatment has yet to be evaluated.
Experimental techniques
Photoablation of ocular melanoma has been reported so far only in an animal model.62 The technique makes use of a 15-W argon blue-green laser as an endophotocoagulator with a vitreous cutter used simultaneously to remove liberated tumor debris. To date, this approach has not been used clinically. Some authors have reported anecdotally on the use of the Nd:YAG laser to resect a uveal melanoma63; but larger series using this technique have not yet been reported. A Dutch group presented experimental results in an animal model after transscleral laser thermotherapy of choroidal melanoma using an infrared diode laser, which was able to destroy the tumor and preserve the stability of the sclera.64 To date, no clinical results have been published. New laser sources introduced in the treatment of experimental intraocular melanoma included a focused, raster-scanned beam from an Nd:yttrium-lanthanum-fluoride laser (1047 nm).65
1 Meyer-Schwickerath G. The preservation of vision by treatment of intraocular tumors with light coagulation. Arch Ophthalmol. 1961;66:458–466.
2 Journée-de Korver JG, Oosterhuis JA, Kakebeeke-Kemme HM, et al. Transpupillary thermotherapy (TTT) by infrared irradiation of choroidal melanoma. Doc Ophthalmol. 1992;82:185–191.
3 Shields JA, Glazer LC, Mieler WF, et al. Comparison of xenon arc and argon laser photocoagulation in the treatment of choroidal melanomas. Am J Ophthalmol. 1990;109:647–655.
4 Foulds WS, Damato BE. Low-energy long-exposure laser therapy in the management of choroidal melanoma. Graefes Arch Clin Exp Ophthalmol. 1986;224:26–31.
5 Journée-de Korver HG, Midena E, Singh AD. Infrared thermotherapy: from laboratory to clinic. Ophthalmol Clin North Am. 2005;18:99–110.
6 Tsai T, O’Brien JM, Engstrom R, et al. Extrascleral extension of a choroidal melanoma after argon photocoagulation and transpupillary thermotherapy. Br J Ophthalmol. 2002;86:358–359.
7 Oosterhuis JA, Journée-de Korver HG, Kakebeeke-Kemme HM, et al. Transpupillary thermotherapy in choroidal melanomas. Arch Ophthalmol. 1995;113:315–321.
8 Oosterhuis JA, Journée-de Korver HG, Keunen JE. Transpupillary thermotherapy: results in 50 patients with choroidal melanoma. Arch Ophthalmol. 1998;116:157–162.
9 Shields CL, Shields JA, Perez N, et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002;109:225–234.
10 Parrozzani R, Boccassini B, De Belvis V, et al. Long-term outcome of transpupillary thermotherapy as primary treatment of selected choroidal melanoma. Acta Ophthalmol. 2009;87:789–792.
11 Chong LP, Ozler SA, de Queiroz JMJ, et al. Indocyanine green-enhanced diode laser treatment of melanoma in a rabbit model. Retina. 1993;13:251–259.
12 Journée-de Korver JG, Keunen JE. Thermotherapy in the management of choroidal melanoma. Prog Retin Eye Res. 2002;21:303–317.
13 De Potter P, Jamart J. Adjuvant indocyanine green in transpupillary thermotherapy for choroidal melanoma. Ophthalmology. 2003;110:406–413.
14 Liggett PE, Lavaque AJ, Chaudhry NA, et al. Preliminary results of combined simultaneous transpupillary thermotherapy and ICG-based photodynamic therapy for choroidal melanoma. Ophthalmic Surg Lasers Imaging. 2005;36:463–470.
15 Shields CL, Shields JA, Cater J, et al. Transpupillary thermotherapy for choroidal melanoma: tumor control and visual results in 100 consecutive cases. Ophthalmology. 1998;105:581–590.
16 Singh AD, Kivela T, Seregard S, et al. Primary transpupillary thermotherapy of “small” choroidal melanoma: is it safe? Br J Ophthalmol. 2008;92:727–728.
17 Pan Y, Diddie K, Lim JI. Primary transpupillary thermotherapy for small choroidal melanomas. Br J Ophthalmol. 2008;92:747–750.
18 Win PH, Robertson DM, Buettner H, et al. Extended follow-up of small melanocytic choroidal tumors treated with transpupillary thermotherapy. Arch Ophthalmol. 2006;124:503–506.
19 Spire M, Devouassoux MS, Kodjikian L, et al. Primary transpupillary thermotherapy for 18 small posterior pole uveal melanomas. Am J Ophthalmol. 2006;141:840–849.
20 Singh AD, Eagle RC, Jr., Shields CL, et al. Clinicopathologic reports, case reports, and small case series: enucleation following transpupillary thermotherapy of choroidal melanoma: clinicopathologic correlations. Arch Ophthalmol. 2003;121:397–400.
21 Finger PT, Lipka AC, Lipkowitz JL, et al. Failure of transpupillary thermotherapy (TTT) for choroidal melanoma: two cases with histopathological correlation. Br J Ophthalmol. 2000;84:1075–1076.
22 Diaz CE, Capone A, Jr., Grossniklaus HE. Clinicopathologic findings in recurrent choroidal melanoma after transpupillary thermotherapy. Ophthalmology. 1998;105:1419–1424.
23 Aaberg TM, Jr., Bergstrom CS, Hickner ZJ, et al. Long-term results of primary transpupillary thermal therapy for the treatment of choroidal malignant melanoma. Br J Ophthalmol. 2008;92:741–746.
24 Zaldivar RA, Aaberg TM, Sternberg P, Jr., et al. Clinicopathologic findings in choroidal melanomas after failed transpupillary thermotherapy. Am J Ophthalmol. 2003;135:657–663.
25 Singh AD, Rundle PA, Berry-Brincat A, et al. Extrascleral extension of choroidal malignant melanoma following transpupillary thermotherapy. Eye. 2004;18:91–93.
26 Midena E, Pilotto E, de Belvis V, et al. Choroidal vascular changes after transpupillary thermotherapy for choroidal melanoma. Ophthalmology. 2003;110:2216–2222.
27 Pilotto E, Vujosevic S, De Belvis V, et al. Long-term choroidal vascular changes after iodine brachytherapy versus transpupillary thermotherapy for choroidal melanoma. Eur J Ophthalmol. 2009;19:646–653.
28 Harbour JW, Meredith TA, Thompson PA, et al. Transpupillary thermotherapy versus plaque radiotherapy for suspected choroidal melanomas. Ophthalmology. 2003;110:2207–2214.
29 Bartlema YM, Oosterhuis JA, Journee-De Korver JG, et al. Combined plaque radiotherapy and transpupillary thermotherapy in choroidal melanoma: 5 years’ experience. Br J Ophthalmol. 2003;87:1370–1373.
30 Kreusel KM, Bechrakis N, Riese J, et al. Combined brachytherapy and transpupillary thermotherapy for large choroidal melanoma: tumor regression and early complications. Graefes Arch Clin Exp Ophthalmol. 2006;244:1575–1580.
31 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma overhanging the optic disc in 141 consecutive patients. Arch Ophthalmol. 2008;126:1515–1522.
32 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma: tumor control in 650 consecutive cases. Ophthalmology. 2011;118:402–407.
33 Shields CL, Cater J, Shields JA, et al. Combined plaque radiotherapy and transpupillary thermotherapy for choroidal melanoma: tumor control and treatment complications in 270 consecutive patients. Arch Ophthalmol. 2002;120:933–940.
34 Shields CL, Bianciotto C, Rudich D, et al. Regression of uveal melanoma after plaque radiotherapy and thermotherapy based on chromosome 3 status. Retina. 2008;28:1289–1295.
35 Keunen JE, Journee-de Korver JG, Oosterhuis JA. Transpupillary thermotherapy of choroidal melanoma with or without brachytherapy: a dilemma. Br J Ophthalmol. 1999;83:987–988.
36 Gunduz K, Kurt RA, Akmese HE, et al. Ruthenium-106 plaque radiotherapy alone or in combination with transpupillary thermotherapy in the management of choroidal melanoma. Jpn J Ophthalmol. 2010;54:338–343.
37 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for choroidal melanoma encircling the optic disc (circumpapillary choroidal melanoma). Arch Ophthalmol. 2007;125:1202–1209.
38 Desjardins L, Lumbroso-Le Rouic L, Levy-Gabriel C, et al. Combined proton beam radiotherapy and transpupillary thermotherapy for large uveal melanomas: a randomized study of 151 patients. Ophthalmic Res. 2006;38:255–260.
39 Seregard S, Landau I. Transpupillary thermotherapy as an adjunct to ruthenium plaque radiotherapy for choroidal melanoma. Acta Ophthalmol Scand. 2001;79:19–22.
40 Archer DB, Gardiner TA. Ionizing radiation and the retina. Curr Opin Ophthalmol. 1994;5:59–65.
41 Gunduz K, Shields CL, Shields JA, et al. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol. 1999;117:609–614.
42 Augsburger JJ, Roth SE, Magargal LE, et al. Panretinal photocoagulation for radiation-induced ocular ischemia. Ophthalmic Surg. 1987;18:589–593.
43 Bianciotto C, Shields CL, Pirondini C, et al. Proliferative radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology. 2010;117:1005–1012.
44 Horgan N, Shields CL, Mashayekhi A, et al. Classification and treatment of radiation maculopathy. Curr Opin Ophthalmol. 2010;21:233–238.
45 Hykin PG, Shields CL, Shields JA, et al. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology. 1998;105:1425–1429.
46 Wen JC, McCannel TA. Treatment of radiation retinopathy following plaque brachytherapy for choroidal melanoma. Curr Opin Ophthalmol. 2009;20:200–204.
47 Naumann GO, Rummelt V. Block excision of tumors of the anterior uvea. Report on 68 consecutive patients. Ophthalmology. 1996;103:2017–2027.
48 Damato B, Groenewald CP, McGalliard JN, et al. Rhegmatogenous retinal detachment after transscleral local resection of choroidal melanoma. Ophthalmology. 2002;109:2137–2143.
49 Damato BE, Paul J, Foulds WS. Risk factors for residual and recurrent uveal melanoma after trans-scleral local resection. Br J Ophthalmol. 1996;80:102–108.
50 Schilling H, Bornfeld N, Talies S, et al. Endoresektion grosser Melanome der Uvea nach stereotaktischer Single-dose-Vorbestrahlung mit dem Leksell Gamma-Knife – Erste Erfahrungen an 46 Fällen. Klin Monatsbl Augenheilkd. 2006;223:513–520.
51 Puusaari I, Heikkonen J, Kivela T. Ocular complications after iodine brachytherapy for large uveal melanomas. Ophthalmology. 2004;111:1768–1777.
52 Kivela T, Eskelin S, Makitie T, et al. Exudative retinal detachment from malignant uveal melanoma: predictors and prognostic significance. Invest Ophthalmol Vis Sci. 2001;42:2085–2093.
53 Folk JC, Weingeist TA, Coonan P, et al. The treatment of serous macular detachment secondary to choroidal melanomas and nevi. Ophthalmology. 1989;96:547–551.
54 Char DH, Bove R, Phillips TL. Laser and proton radiation to reduce uveal melanoma-associated exudative retinal detachments. Am J Ophthalmol. 2003;136:180–182.
55 Favilla I, Favilla ML, Gosbell AD, et al. Photodynamic therapy: a 5-year study of its effectiveness in the treatment of posterior uveal melanoma, and evaluation of haematoporphyrin uptake and photocytotoxicity of melanoma cells in tissue culture. Melanoma Res. 1995;5:355–364.
56 Lewis RA, Tse DT, Phelps CD, et al. Neovascular glaucoma after photoradiation therapy for uveal melanoma. Arch Ophthalmol. 1984;102:839–842.
57 Barbazetto IA, Lee TC, Rollins IS, et al. Treatment of choroidal melanoma using photodynamic therapy. Am J Ophthalmol. 2003;135:898–899.
58 Kim RY, Hu LK, Foster BS, et al. Photodynamic therapy of pigmented choroidal melanomas of greater than 3-mm thickness. Ophthalmology. 1996;103:2029–2036.
59 Jurklies B, Anastassiou G, Ortmans S, et al. Photodynamic therapy using verteporfin in circumscribed choroidal haemangioma. Br J Ophthalmol. 2003;87:84–89.
60 Wachtlin J, Bechrakis NE, Foerster MH. Photodynamische Therapie mit Verteporfin beim Aderhautmelanom Eine klinisch-histopathologische Pilotstudie. Ophthalmologe. 2005;102:241–246.
61 Donaldson MJ, Lim L, Harper CA, et al. Primary treatment of choroidal amelanotic melanoma with photodynamic therapy. Clin Experiment Ophthalmol. 2005;33:548–549.
62 Jaffe GJ, Mieler WF, Burke JM, et al. Photoablation of ocular melanoma with a high-powered argon endolaser. Arch Ophthalmol. 1989;107:113–118.
63 Peyman GA, Alghadyan A, Peace JH. A contact Nd:YAG laser to resect large ciliary body and choroidal tumors. Int Ophthalmol. 1987;11:55–61.
64 Rem AI, Oosterhuis JA, Journee-de Korver HG, et al. Transscleral thermotherapy: short- and long-term effects of transscleral conductive heating in rabbit eyes. Arch Ophthalmol. 2003;121:510–516.
65 Krause MH, Xiong J, Gragoudas ES, et al. Treatment of experimental choroidal melanoma with an Nd:yttrium-lanthanum-fluoride laser at 1047 nm. Arch Ophthalmol. 2003;121:357–363.