Chapter 22 Laryngeal Mask Airway
III. Laryngeal Mask Airway Insertion Technique
IV. Classic Laryngeal Mask Airway
V. Flexible Laryngeal Mask Airway
VI. Laryngeal Mask Airway Use in Patients with Difficult-to-Manage Airways
VII. ProSeal Laryngeal Mask Airway
VIII. Supreme Laryngeal Mask Airway
IX. Disposable Laryngeal Mask Airways
X. Exchange Using the Aintree Intubation Catheter
I Introduction
The laryngeal mask airway (LMA, LMA Company, Henley, England) is a supraglottic airway device developed by Archie Brain, a physician and honorary consultant anesthetist of the Royal Berkshire Hospital, Reading, England. It was introduced into clinical practice in 1988. In the first paper on the LMA,1 Brain described the device as “an alternative to either the endotracheal tube (ETT) or the face-mask with either spontaneous or positive-pressure ventilation (PPV).” Twenty years and more than 200 million safe uses later, the LMA has significantly improved the comfort and safety of airway management worldwide. Many authorities in anesthesia consider the LMA to be the most important development in airway management in the past 50 years.
The LMA is a minimally invasive device designed for airway management in the unconscious patient. An inflatable mask is fitted with a tube that exits the mouth to permit ventilation of the lungs. The mask fits against the periglottic tissues, occupying the hypopharyngeal space and forming a seal above the glottis instead of within the trachea (Fig. 22-1). Originally produced as a single, general-purpose design in a range of sizes, it is currently made in various forms to satisfy different requirements.
The LMA is a supraglottic airway management device. A substantial body of literature, while supporting the wisdom of exercising caution when learning to use the supraglottic approach, provides ample evidence of a wide range of uses that go beyond those originally postulated.2 For example, the LMA is becoming increasingly popular outside the operating room, as evidenced by its endorsement by the European Resuscitation Council and the American Heart Association.3 Its more exotic uses include the adoption of the Fastrach ILMA, which is the intubating form of the LMA, by the National Aeronautics and Space Administration (NASA) as part of its emergency medical kit for space travel. A literature search indicates that the later varieties of the LMA, including the FLMA, ILMA,4 and PLMA,5 may be more appropriate tools for specific uses than the original LMA.
More than 2000 scientific articles have described the impact of the LMA on modern anesthesia, and a full review is beyond the scope of a single chapter. Our aim instead is to provide an overview of the LMA’s uses worldwide. After a historical review and comments regarding the correct LMA insertion technique, we explore the principal uses of each LMA model, discuss possible problems, and offer suggestions for getting the best results from each device. We describe the evolution of the LMA’s use in patients with difficult-to-manage airways and provide the current recommendations for LMA use from the American Society of Anesthesiologists (ASA) difficult airway algorithm.6
II History and Development
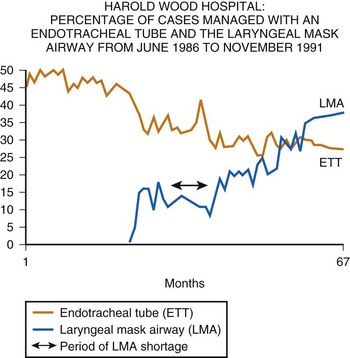
In 1988, the definitive device, now referred to as the LMA Classic, was released commercially in the United Kingdom. Although the LMA was rapidly adopted there, it was not until 1991 that the U.S. Food and Drug Administration permitted release of the device in the United States and then only with the stricture that “the LMA is not a replacement for the endotracheal tube.” Because the LMA was at that time a very unconventional way of managing the airway, this caution was understandable. Nevertheless, it resulted in a somewhat slower acceptance of the concept of “masking the larynx” in the United States than in the United Kingdom, where the LMA was purchased by every health authority within 2 years of its launch, resulting almost immediately in a reduction in ETT use (Fig. 22-2).
A From Idea to First Prototype
Brain is often asked how the idea of the LMA first arose. Like many before him, he thought that a supraglottic approach would be less traumatic than intubation and therefore more desirable, provided the method could be easier to control and more reliable than the face mask. Other inventors had mainly explored the oral and pharyngeal spaces above the larynx,7–12 but the LMA was the first device to encircle the periglottic tissues in the hypopharynx with a mask. The idea was conceived in early 1981, and later that year, Brain began providing dental anesthesia at the outpatient clinic of the Royal London Hospital. At that time, a device known as the Goldman dental nose piece was being used for airway maintenance during dental extractions in anesthetized patients. It was a reusable, vulcanized rubber mask that could be detached from its rigid base for cleaning (Fig. 22-3). The mask was designed to fit over the nose, leaving the mouth free for surgical access. Noticing a certain similarity between the contours around the nose and those around the glottis, Brain wondered if the Goldman mask could be modified to fit over the larynx. Placing a device over the larynx would short-circuit the upper airway passages, perhaps eliminating some of the problems associated with maintaining their patency under anesthesia.
Because the Goldman mask was scheduled to be discontinued in favor of a disposable version, Brain was able to obtain samples for experimentation. Using an acrylic adhesive, he glued a diagonally cut, 10-mm Portex ETT to the rubber mask’s attachment flange, which he had to draw across the mask aperture into the midline to form a base (Fig. 22-4). Figure 22-5 is a 1981 photograph of the inventor as he is about to insert one of the resulting prototypes into his anesthetized pharynx to test the validity of his hypothesis. In his diary, he recorded an absence of any complications, despite having repeated the experiment four times. This observation, combined with a broadly worded institutional review board approval, provided the justification for subsequent extensive clinical investigations, which occupied him for the next 7 years. During this time, he built many prototypes, which he used in approximately 7000 patients.13 He also kept extensive notes in private diaries to record his progress but published only a small number of cases, because he believed there was little point in presenting data concerning an invention that was not ready for production.
B Early Publications
The first article about the LMA, published in the British Journal of Anaesthesia in 1983, presented data from only 23 patients and received little attention.1 A second description 2 years later in Anaesthesia was subtitled “Development and Trials of a New Type of Airway” and detailed the LMA’s use in 118 patients, although it also reported that Brain and colleagues had gained experience in more than 500 cases.14 On the basis of this experience and a case report published the previous year in Archives of Emergency Medicine,15 Brain and colleagues felt justified in concluding that “the laryngeal mask may have a valuable role to play in all types of inhalational anesthesia, while its proven value in some cases of difficult intubation indicates that it may contribute to the safety of general anesthesia.”14
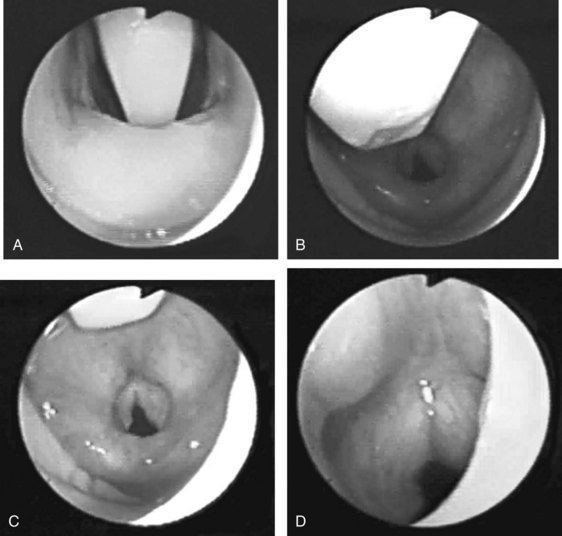
In the same issue of Anaesthesia, Brain presented further evidence of the potential usefulness of the device in cases of difficult intubation, but there was still no response to these claims from the profession, perhaps because no product was available for independent assessment.16 However, he had given one of his prototypes to a visiting American anesthesiologist, Ronald Katz, a physician and chairman of the anesthesiology department at the University of California–Los Angeles. Katz provided the first independent description of the LMA based on his experience with this prototype, writing in Wellcome Trends in Anesthesiology in October 1985 and again in February 1986.17,18 The second article provided the first published image—reproduced from one of the inventor’s transparencies—of the glottis seen fiberoptically through the LMA prototype and mentioned the potential of the device to overcome intubation problems. American awareness of the LMA preceded its availability in the United States by 6 years.
C From Handmade Models to Clinical Use
There were three reasons why the LMA took so long to become clinically available: the initial lack of commercial interest, which meant there were no models available for others to use; the solitary nature of the LMA’s development, which was carried out virtually single-handedly by a clinician who continued to work with largely homemade equipment until the first commercial prototypes were made in early 1988; and the complexity of the airway anatomy, which made it hard to design a device that was safe and effective. One advantage of the long development process was that the considerable clinical history that the inventor accumulated between 1981 and 1988 guided the development of the LMA, and the first factory-made models required little modification. They were produced in Gary, Indiana; tested by Brain; and then demonstrated to colleagues at the Royal East Sussex Hospital, Hastings, United Kingdom, in April 1988. Colin Alexander, the chairman of the anesthesiology department at the Royal East Sussex Hospital, immediately authorized the LMA’s use in his department and published his experience in a letter (“Use your Brain”) in Anaesthesia that year.19 He concluded that the device “should be considered whenever the indication for tracheal intubation does not include protection of the airway from gastric contents.” Meanwhile, a few colleagues who had been given prototypes by Brain (one person built his own) continued to explore the LMA’s use in known cases of difficult intubation, with encouraging results.13
Arguably, the most influential clinical study using Brain’s hand-built prototypes was not published until 1989, after the commercial form of the device had become available. Brodrick and colleagues studied 100 cases in which the patients breathed spontaneously.20 Eighteen anesthesiologists took part in the study and recorded a “clear and unobstructed airway in 98% of cases,” but obstruction on initial placement of the LMA occurred in 10 cases and “appeared to be as a result of downfolding of the epiglottis.” A stainless steel introducer tool with a handle similar to that of the Fastrach ILMA and a blade fitting into a slot in the distal anterior end of the LMA’s mask had been designed to overcome this problem. An aperture in the blade into which the epiglottis fit caused the epiglottis to be drawn upward as the tool was removed from the patient’s pharynx. This insertion tool was used successfully in these cases, but fears that it could cause trauma led to it being abandoned in the commercial form of the LMA. Brodrick is often quoted in support of limiting the use of the LMA to spontaneously breathing patients. However, because the LMAs used by Nunn’s group were prototypes, only a size 3 device was available for use in 72 men and 28 women (weight not recorded). Given the possibility that the LMA’s size was less than optimal, it is not surprising that eight patients could not be ventilated using positive pressure without unacceptable leaks and that the mean leak pressure was 17 cm H2O, similar to that recorded by Brain 6 years earlier.13
In addition to the information accumulated in his clinical work, study of the anatomy and physiology of the larynx and pharynx guided Brain throughout development of the LMA. As the work progressed, he struggled to achieve a balance in simplicity, efficacy, and safety. In designing a device that fit into the lower pharynx, measures favoring efficacy tended to counteract those favoring simplicity. Improving the seal, for example, required more complex construction to avoid potential trauma related to high mucosal pressures. Likewise, an effort to make the device easier to insert led to the development of an insertion tool, which was determined by Brain to be potentially unsafe and therefore abandoned, despite the efficacy it demonstrated.13 Ultimately, the solutions chosen were compromises. For example, Brain realized that seals with a leak pressure much higher than 20 cm H2O were rarely necessary in practice and that an inadequate seal often could be overcome by using a more appropriately sized device, using a better fixation technique, or giving a more appropriate anesthetic. To make insertion of the LMA maximally reliable and minimally traumatic, a technique gradually evolved that was based on the swallowing mechanism. Unfortunately, he underestimated the difficulty of teaching others to master such a subtle technique, which is virtually impossible to learn without direct, hands-on demonstration and guidance.21 As a result, many articles in the LMA literature suggest variant insertion methods, most of which had already been tried and rejected by Brain for being unreliable or traumatic.
Despite these difficulties, the history of the LMA since its first commercial launch is essentially a story of steady expansion in use around the world. Availability, cost, and user education have governed the speed at which the various forms of the LMA have been accepted. Figure 22-2 demonstrates data collected by the anesthesiology department of Harold Wood Hospital, a typical general hospital near London, on the influence of the LMA Classic in the first 3 years of its availability and on the use of the ETT and the face mask. The dip in the curve representing LMA use indicates a period when it was commercially unavailable. These data show that use of the ETT almost immediately declined in favor of the new method of airway management, and they illustrate the rapid growth of LMA use in the United Kingdom. They also cast some doubt on the widely held view that the popularity of the LMA in the United Kingdom has been due to the more frequent use of the face mask there than in the United States.
III Laryngeal Mask Airway Insertion Technique
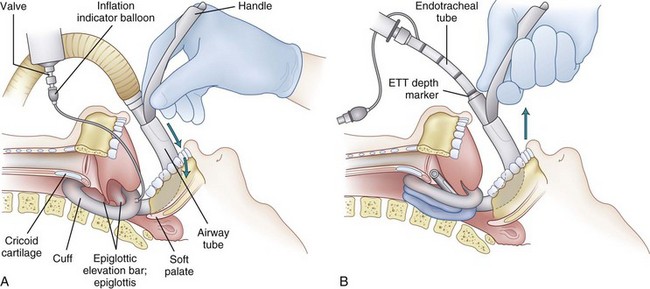
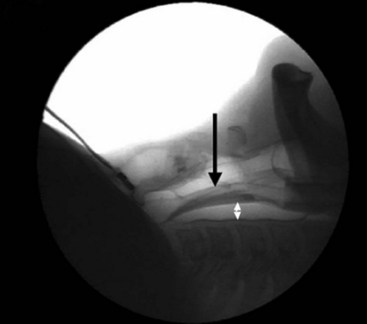
Investigations using magnetic resonance imaging (MRI) to assess the effect of the LMA on the anatomy of the airway may help to explain the reliability of the device. A study by Shorten and coworkers of 46 adults requiring sagittal MRI views of the head and neck compared the anatomic differences in awake, sedated, and anesthetized patients.22 With an LMA in place, the epiglottic angle was more than twice as great with respect to the posterior pharyngeal wall in the anesthetized group as it was in the sedated or awake group. This had no apparent effect on ventilatory function in most patients, probably due to the depth of the LMA bowl, which the inventor found to be a critical dimension when experimenting with different design ideas during the 1980s. The recommended standard insertion technique evolved slowly as Brain gained experience, and it was not until he had been inserting LMAs for almost 10 years that he realized that his technique was becoming more and more similar to the physiologic act of swallowing food. When he realized this, it was a simple matter to study this mechanism more closely and make allowances for the fact that in the anesthetized patient, this reflex is partially or completely abolished. The following key points emerged:
1. Correct mask deflation is important. The purpose of chewing food is to form a soft, atraumatic paste that can easily be passed through the pharynx and esophagus. At the onset of swallowing, this paste (i.e., food bolus) is pressed by the tongue into the hard palate. The pressure generated is distributed widely over the palatal surface, so there is no localized high-pressure point, which would give the sensation of a sharp object and lead to rejection instead of swallowing. Brain realized that to imitate the sensation of a soft food bolus, he needed to deflate the mask so that it presented an elastic, hollow shape. When this shape was pressed into the dome of the palate, the hollow form would need to be inverted. The pressure required by inserting the finger to achieve this would cause the outer rim of the mask to act like a gentle spring, producing the desired effect of spreading pressure smoothly over the entire posterior surface of the mask. This spring effect cannot be achieved without deflating the mask to a vacuum pressure of about −40 cm H2O. Only in this way was it possible to prevent the pointed distal end of the mask bowl from transmitting an irritating localized pressure point as it was pressed into the oropharyngeal curve. Partial mask inflation could not achieve this aim, however, because the soft distal end of the mask rolled backward, allowing the pointed tip of the mask bowl to scratch the palatal surface. Deflating the mask such that it followed the curvature of the palate had the same disadvantage, because it made the pointed end even more prominent. Inserting a fully inflated mask introduced excessive bulk, which created the possibility of tearing the cuff against the teeth and was physiologically equivalent to swallowing too large a bolus of food, which could lead to the rejection reflex.
2. The deflated mask must be lubricated if the oral cavity is not already wet. There is a parallel with swallowing because lubrication is a key part of deglutition. Because the mask is slid against the palate, it makes sense to apply a bolus of lubricant to the distal hollowed posterior surface of the mask immediately before insertion. Water-soluble jelly is a good substitute for oral secretions. It is not necessary or desirable to spread the lubricant over the whole surface of the mask before insertion.
3. Flatten the mask against the hard palate. Initially during swallowing, the tongue flattens the softened food bolus against the hard palate; during the first step in LMA insertion, the mask, correctly prepared to impart a sensation similar to that of a soft food bolus, is flattened by pressing it against the hard palate. Placing the index finger on the airway tube at its junction with the mask under the deflated proximal rim of the cuff is the best way to impart the necessary force.
4. Cranioposterior movement of the index finger. In swallowing, the bolus of food is advanced into the pharynx, esophagus, and stomach through precise coordination of several muscle groups, beginning with the tongue. During LMA insertion, the clinician must use her or his index finger to advance the mask in the cranioposterior direction, imitating the action of the tongue. This allows a completely deflated tip to slide smoothly along the hard palate, soft palate, and posterior pharyngeal wall while minimizing the contact of the mask with anterior structures such as the base of the tongue, epiglottis, and laryngeal inlet. The finger must continue to push in a cranioposterior direction even though the anatomy forces the mask and the finger to move caudally. The finger must never consciously be directed caudally and should be inserted to its fullest extent until resistance is felt as the mask tip enters the upper esophageal sphincter (UES). It is anatomically impossible to perform this action correctly without extending the proximal metacarpophalangeal joint of the index finger and flexing the wrist.
5. Widening the oropharyngeal angle is the first role of the nondominant hand. Cadaveric work demonstrates that if the head of the supine subject is pushed by the supinated hand in a caudal direction, head extension, neck flexion, and mouth opening are simultaneously achieved. This maneuver widens the oropharyngeal angle to greater than 90 degrees in the normal subject and draws the larynx away from the posterior pharyngeal wall. Both effects facilitate LMA insertion. The nondominant hand should therefore maintain firm caudal pressure on the occiput from the start of insertion until the mask has passed behind the tongue.
6. Removal of the index finger is the second role of the nondominant hand. To prevent the mask sliding out of position after it is fully inserted, the nondominant hand should move from behind the head to grasp the proximal end of the LMA before the index finger is removed. As the index finger is removed, the mask is held steady, or if it has not been fully inserted, it can be pressed further into position by the nondominant hand.
7. The mask is inflated. As the mask is inflated, its increased bulk and the relatively large radius of the airway tube cause it to slide cranially. It can be shown anatomically that this results in loss of contact between the mask tip and the UES. However, the LMA should not be held in place during inflation because this can result in the distal end of the mask stretching the UES. All LMAs should be inflated to a pressure of less than 60 cm H2O; pressures above this have been found to cause discomfort in awake volunteers.
8. Device fixation restores stability of the seal against the UES. The distal end of the tube is again pressed into the curve of the hard palate to reestablish firm contact between the proximal end of the device and the UES. While this pressure is maintained, adhesive tape is applied to the maxilla on one side of the patient’s face and passed over and under the tube in a single loop before fixing to the opposite maxilla. This form of fixation ensures stability of the device and is likely to afford maximum protection in the event of unexpected regurgitation, and it reduces the incidence of gastric insufflation during PPV.*
The basic insertion technique is identical for all LMA models. Unfortunately, misunderstanding of and a lack of commitment to mastering this technique are widespread, as reflected in the multiplicity of insertion methods advocated in the literature and the common belief that a failure rate of 10% is acceptable. When variant insertion techniques are used, the failure rate is about fivefold higher than it is with the standard insertion technique. The risk of complications, such as laryngeal or pharyngeal trauma and pulmonary aspiration, probably increases even more than the failure rate when the standard insertion technique is not used. The use of variant insertion techniques also may hinder or prevent the user from acquiring the skills necessary for advanced clinical applications of the LMA. As shown in several reports, use of the standard insertion technique results in a reliable airway, a minimal stress response, and an extremely low risk of complications, probably because the LMA’s position in relation to the respiratory and alimentary tracts is optimal when the standard insertion technique is used. The manuals for the LMA Classic, the FLMA, the Fastrach ILMA, and the PLMA offer step-by-step instructions on the recommended insertion technique.23
IV Classic Laryngeal Mask Airway
A Basic Uses
1 Indications for Use
Indications for LMA use have evolved since its invention. Although it has long been accepted that success rates for establishing an airway with the LMA tend to be high, even in relatively unskilled hands, it is equally clear that there is more to the art of using the LMA than getting it into place. Indications have steadily expanded as the device’s popularity has spread. Just as complications tend to diminish with increasing user experience, so the more confident and more adept user seems to find applications for the LMA that might previously have seemed inappropriate. Using the LMA for airway maintenance during atrial septal defect repair in children, for example, would no doubt strike many American anesthesiologists as highly unconventional, just as it would have alarmed the inventor had it been suggested he try this in 1988.24
2 Inherent Teaching Difficulties: A Vicious Circle
The learning curve for correct LMA insertion extends beyond the often cited 10 to 15 uses by one or two orders of magnitude.25 For the anesthesiologist who is just starting to get used to the LMA, an important advantage of confining its use to simple cases is that they represent the greater part of the surgical caseload in many locations, and there are many opportunities for learning and practice. Most anesthesiology residents, however, start out in a teaching hospital, where the caseload tends to be weighted more heavily toward long and complex procedures for which LMA use would be inappropriate in any but the most experienced hands. For this reason, it is common to hear experienced U.K. and U.S. consultant anesthesiologists complain that newly appointed colleagues, who have spent the greater part of their training years in teaching hospitals, appear to have only the most rudimentary concept of LMA use. It is, unfortunately, the same academic colleagues who carry out many of the studies that make up the core of evidence-based knowledge for the LMA. This represents a vicious circle that is not easy to break, particularly at a time when there is increasing pressure on the medical profession to justify all clinical activity by reference to proven techniques. A suggested way out of this impasse is outlined subsequently.
3 Graduating from Simple to Specialized Uses of the Laryngeal Mask Airway
The safety of the patient must be the guiding principle when deciding whether someone is qualified to perform specialized uses of the LMA. A clinician whose first-time insertion success rate is 90% or less should not be considered adequately trained to progress beyond the simplest procedures in ASA I patients. Davies and colleagues reported a success rate of 94% in the first 10 cases in which naval medical trainees used the LMA for the first time in ASA I anesthetized patients.26 The insertion success rate depends on the types of cases routinely encountered, and the preceding generalization applies to clinicians in an average peripheral hospital that performs a broad range of common procedures.
B Specialized Uses
1 Procedures Outside the Operating Room
a Radiology and Magnetic Resonance Imaging
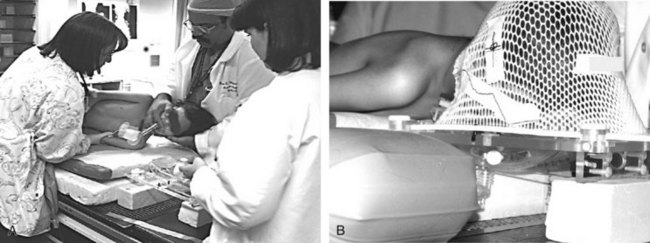
The potential advantages of the LMA in investigative imaging were first described in a letter to Anaesthesia from Glasgow, Scotland, in 1990.27 The investigators pointed out that the LMA permitted hands-free control of the airway in patients who needed to be kept immobile for prolonged periods, a situation often necessitating general anesthesia in restless or young patients. To improve its performance and durability, the LMA’s valve later was fitted with a small, stainless steel spring, which unfortunately interfered with MRI of the head and neck. However, LMAs equipped with valves made of nonferrous material were subsequently made available for use in this situation. Stevens and Burden, in a letter to Anaesthesia in 1994, commented that they had used the LMA Classic with the modified, nonmetallic valve in more than 500 small children undergoing MRI and that it had proved safe and reliable.28 They also presented an MRI image demonstrating that the FLMA could not be used for investigations involving the head and neck because the tube contained a wire, which obliterated the image of the surrounding area.
Goudsouzian and associates were the first Americans to report the efficacy of the LMA during MRI, using the images obtained during investigations in 28 children to comment on the position of the device when inserted by residents in training.29 Despite poor user skills (21% of attempts resulted in a failure to insert, 21% of the cases required more than one insertion attempt, 82% had a downward deflection of the epiglottis, and 7% had oropharyngeal misplacement), satisfactory ventilatory parameters were maintained in all of the children. The safety of the LMA, even in less than fully skilled hands, is a powerful argument for its use in areas remote from the operating room. Van Obbergh and colleagues, working in Brussels, Belgium, presented results from somewhat more experienced users.30 The LMA was used during MRI in 100 consecutive procedures in children that were carried out using propofol. The position of the LMA was not recorded, but only 16% of the cases required more than one insertion attempt, there were no failures to insert, and oxygen saturation values of 99.1% or above were maintained in all of the children. Ventilation was manually assisted using an Ayre T-piece.
b Radiation Therapy
Grebenik and coworkers were the first to describe the use of the LMA in pediatric radiation therapy in their study of 25 children who underwent a total of 312 courses of radiation therapy under anesthesia.31 The children were between 3 weeks and 3 years old, and eight of them were anesthetized once daily for 20 or more consecutive days. In each case, the LMA was left in place until the protective reflexes returned. The absence of complications suggests that the LMA may be appropriate for procedures requiring frequent, repeated use of anesthetics in children.
A major advantage of the LMA over the ETT in children receiving radiation therapy on a daily basis during a 4- to 6-week course of treatment is that the LMA does not invade the trachea. The risk of tracheal ulcerations, granulation tissue, and subsequent tracheal stenosis associated with repeated intubations with an ETT is eliminated. The LMA causes much less stimulation than does an ETT; anesthesia requirements for the LMA are significantly lower. For example, at the University of Texas M.D. Anderson Cancer Center in Houston, all children undergoing radiation therapy receive an intravenous infusion of propofol as the sole anesthetic agent during their treatment. The LMA is frequently used for airway management, and spontaneous respiration is preserved (Fig. 22-6). This allows more rapid turnover of patients and more efficient use of the radiation therapy suite.
c Diagnostic and Short Therapeutic Procedures in Children
The LMA can be very useful during short diagnostic, therapeutic, and minor surgical procedures performed in children in the hospital’s procedure room or in the operating room. These procedures include spinal puncture with or without intrathecal therapy, bone marrow aspirations, insertion or removal of a central line or a Port-a-Cath, and minor biopsies (Fig. 22-7). Most children tolerate these procedures well under deep intravenous sedation with propofol and local anesthesia with maintenance of spontaneous respiration. However, some children develop respiratory depression or airway obstruction during deep sedation. In those children, the LMA can be an excellent and much less invasive alternative to endotracheal intubation, resulting in a clear airway without resorting to general anesthesia and muscle relaxation.
d Cardiology
The LMA can be useful in patients undergoing transesophageal echocardiography (TEE). TEE is an invasive procedure, and many patients experience significant discomfort or are unable to tolerate TEE under topical anesthesia. In the cardiology clinic setting, TEE is usually performed with topical anesthesia consisting of spraying the oropharynx with a local anesthetic (e.g., 4% lidocaine or 20% benzocaine), with or without sedation.32,33 However, a significant number of patients experience discomfort during the procedure, and many cannot tolerate the insertion of the probe, even after sedation.34,35 Because topical anesthesia and light sedation do not completely abolish the gag reflex, deep sedation or general anesthesia may be necessary for patients with highly sensitive reflexes in the upper airway and pharynx. A possible solution for patients who cannot tolerate the insertion of the TEE probe with topical anesthesia and light sedation is deep sedation with propofol. The LMA can be used to maintain the airway in patients undergoing TEE examination during deep sedation with propofol.36 The LMA can easily be inserted with the TEE probe in place, and the presence of the LMA does not interfere with the manipulation of the probe (Fig. 22-8).
The LMA can be a better alternative than endotracheal intubation in patients undergoing cardioversion in whom face mask ventilation is difficult or inadequate. The minimal hemodynamic stimulation associated with insertion of the LMA, which contrasts with a significant hemodynamic response during endotracheal intubation, offers a great advantage in patients with cardiovascular disease.37
2 Head and Neck Surgery
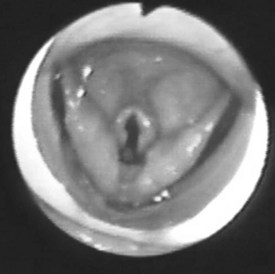
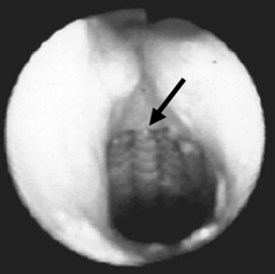
Most of the applications of the LMA in procedures involving the head and neck are done with the FLMA. However, a unique advantage of using the LMA Classic in these patients is the access to the larynx that the device allows (Fig. 22-9), which is particularly useful for diagnostic evaluation of the larynx and trachea and during neodymium:yttrium-aluminum-garnet (Nd:YAG) laser surgery. It is difficult to manage the airway using an ETT in patients who require Nd:YAG laser treatment of lesions located in the vocal cords or the proximal part of the trachea because the ETT limits access to these lesions. There also is a high risk of a laser-induced airway fire when an ETT is used. In contrast, the LMA provides an unobstructed view of the surgical field and virtually eliminates the risk of airway fire (Fig. 22-10).
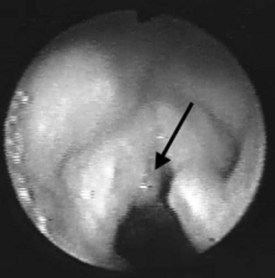
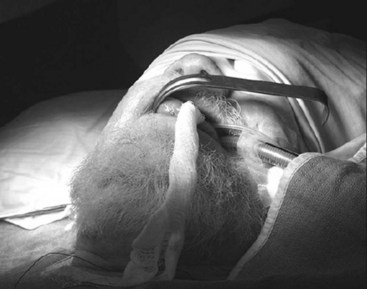
Head and neck surgery is associated with the risk of bruising or otherwise traumatizing nerves that control the motor functions of the larynx. The LMA can be useful in evaluating the function of the vocal cords at the conclusion of neck dissection and thyroid and parathyroid surgery.38 While the patient is still under general anesthesia, the LMA is inserted behind the ETT and inflated. The anesthesiologist then removes the ETT, and the patient is allowed to breathe spontaneously. As the patient emerges from general anesthesia, a fiberoptic bronchoscope (FOB) is inserted through the lumen of the LMA to observe the function of the vocal cords. At M.D. Anderson Cancer Center, this technique has become an important diagnostic tool to detect the functional status of the nerves providing motor function to the larynx and has allowed the anesthesiologists and surgeons to make more informed decisions about the postoperative airway management of their patients. Similarly, patients undergoing brainstem surgery in the area that involves the lower cranial nerves, which control the pharynx and larynx, can be evaluated fiberoptically through the LMA in the intensive care unit. This helps the intensivist and the surgeon determine whether the patient will be able to maintain her or his airway postoperatively (Fig. 22-11).
3 Pulmonary Medicine and Thoracic Surgery
a Bronchoscopy in Children and Adults
Physicians in the fields of thoracic surgery and pulmonary medicine have shown interest in the LMA because of the unique access it provides to the larynx and respiratory tree. Diagnostic fiberoptic laryngoscopy and FOB can be performed readily through the LMA in patients under general anesthesia or under topical anesthesia with sedation.39–41 Maekawa and colleagues, in a 1991 letter to Anesthesiology describing the use of a size 1 LMA as a conduit for FOB with a 3.6-mm flexible endoscope in two children (ages 2 and 8 months), listed four advantages. First, the LMA tube is much larger (5-mm internal diameter) than the corresponding ETT, permitting the use of a larger bronchoscope, which gives a better view than that obtained through the 2- to 2.5-mm bronchoscope normally required to fit through the ETT in this age group. Second, the use of the LMA permitted examination of the larynx, including vocal cord movement and the part of the trachea normally occupied by the ETT. Third, the larger-diameter LMA airway tube permits easy ventilation, which permits uninterrupted observation. Fourth, not using an ETT may be valuable in cases of laryngeal or tracheal stenosis that may be made worse by the passage of the ETT.42
In a letter to Anesthesiology, Theroux and associates described the use of the size 1 LMA as a conduit for intubation in a 2.5-kg baby with Schwarz-Jampel syndrome who could not be intubated by other means.43 The uncuffed ETT was advanced over a 2.2-mm bronchoscope, which was easily passed into the trachea through the LMA. In 1997, Mizikov and coworkers reported their experience of using FOB through the LMA in 45 children: 15 diagnostic cases, 22 cases of lavage, 7 cases of foreign body removal, and 1 case of electrocoagulation of an adenoma.44 Total intravenous anesthesia and PPV were used in all cases. Patients’ ages ranged from neonatal to 15 years. They used the Olympus BF3C20 bronchoscope (3.6-mm diameter) with the size 1 LMA, and the Olympus BFP30 (5-mm diameter) was used with the size 2 LMA. The epiglottis was within the mask in 96.5% of cases but was not associated with significant airway obstruction in any case.
b Laser Surgery of the Trachea
In 1992, Slinger and colleagues provided a detailed account of a difficult case of palliative laser resection of a severely obstructing distal tracheal mass.45 The airway was initially managed using a size 4 LMA, through which a 6-mm Olympus FOB was passed by a swivel connector to apply the laser. Spontaneous ventilation with propofol-isoflurane anesthesia was used. After 120 minutes, 50% of the tracheal lumen had been restored, at which point it was decided to convert to rigid bronchoscopy so that the surgeon could remove larger pieces of tissue by forceps, thereby facilitating the procedure. The physicians repeated this approach in two less severe cases without complications. They pointed out that the laser is not likely to burn the silicone LMA tube if it is switched on only when in the trachea. However, they stressed that use of the rigid bronchoscope remained the gold standard for such cases.
c Tracheobronchial Stent Placement
Another advantage of using the LMA in patients with pathology involving the tracheobronchial tree is evident during stent placement using fiberoptic guidance.46 The LMA’s shaft permits use of a 6-mm FOB and provides a larger cross-sectional area than does a 9-mm ETT, which is usually employed during stent placement. The LMA allows better ventilation during stent placement than does an ETT in patients who already have compromised respiratory function. In patients who have an obstruction high in the trachea, the LMA is much better than an ETT because it allows placement of the stent without the risk of extubating the patient (Fig. 22-12).
4 Neurosurgery
The hemodynamic stability associated with LMA use may be beneficial during induction in patients undergoing neurosurgical repair of an intracranial aneurysm and in patients with increased intracranial pressure. Silva and Brimacombe reported that hypertension, coughing, and bucking, all of which are common during emergence from general anesthesia with an ETT, were prevented in neurosurgical patients by replacing the ETT with the LMA at the end of the procedure.47 The insertion and use of the LMA in patients under general anesthesia have been associated with minimal changes in intracranial pressure.48 This characteristic may be particularly useful during ventriculoperitoneal shunt placement in children, adults, and patients who have suffered a traumatic brain injury.
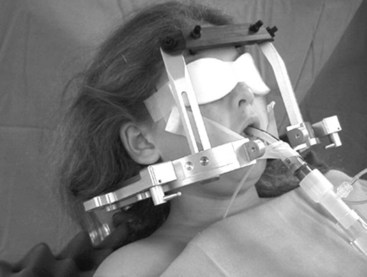
Other uses for the LMA in neurosurgical patients include awake craniotomy and stereotactic procedures in children and adults. In an awake craniotomy, eloquent areas of the brain are mapped and monitored intraoperatively by stimulating the cerebral cortex with an electrical current. This direct electrical stimulation of the cerebral cortex greatly increases the risk of focal or generalized seizures. Airway management using a face mask and endotracheal intubation during generalized seizures can be very difficult because the patient is usually lying on her or his side, giving the anesthesiologist limited access to the airway, and the patient’s head is fixed by metal pins and a frame to provide a stable surgical field and allow the use of modern intraoperative image guidance. However, an experienced and skilled anesthesiologist can easily insert the LMA into such patients, allowing ventilation and oxygenation in this potentially difficult clinical situation (Fig. 22-13).49
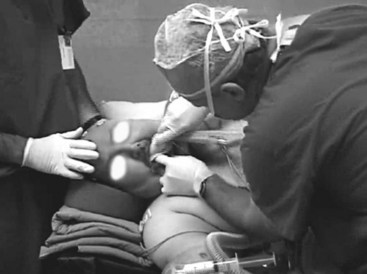
Stereotactic biopsies can be performed in children using the LMA. Anesthesia is induced with propofol in the radiology suite, and the LMA is inserted (Fig. 22-14). Local anesthetic is then injected to anesthetize the skin before pins for the stereotactic frame are applied. During continuous infusion of propofol, an MRI is obtained while the child breathes spontaneously through the LMA. While asleep, the child is transported to the operating room for the stereotactic biopsy. This technique provides optimal comfort for the children and excellent operating conditions for the radiologists and surgeons.
V Flexible Laryngeal Mask Airway
Many operations, particularly procedures performed on the head and neck, require the anesthesiologist and surgeon to share access to the airway. Traditionally, special ETTs that have a spiral coil built into them to increase the flexibility of the tube and to prevent kinking have been used to allow the surgeon to manipulate the ETT during surgery to gain access to the operating field. To make an LMA specifically for head and neck procedures, a similar spiral coil was incorporated into the LMA’s shaft, creating the FLMA (Fig. 22-15), which was introduced into clinical practice in 1994. The FLMA has since been used successfully in patients undergoing a variety of head and neck procedures.
A Tonsillectomy and Adenoidectomy
Several investigators have reported the successful use of the FLMA during tonsillectomies and adenoidectomies. The main advantages of using the FLMA for these procedures are better tolerance of the FLMA by the patients, fewer adverse airway events during emergence and on extubation, and less blood soiling of the airway because of the shielding of the larynx by the LMA’s cuff. Williams and Bailey reported that when the FLMA was used in children during adenotonsillectomies,50,51 there was little soiling of the trachea with blood. In contrast, when an uncuffed ETT was used, blood was almost always present in the trachea, causing coughing on emergence. At the end of surgery, there were also fewer episodes of airway obstruction in the FLMA group than in the ETT patients. This is probably because the FLMA was better tolerated by the patients, allowing extubation when the airway reflexes were less obtunded and the patients were able to maintain an open airway. Fiani and coworkers demonstrated in a randomized study of patients undergoing elective tonsillectomies that fewer complications such as laryngospasm, bleeding, bronchospasm, and desaturation occurred in the FLMA group than in the ETT group.52
The FLMA is useful during ear and nose surgery, such as tympanoplasty, myringoplasty, rhinoplasty, septoplasty, and nasal polypectomy.50,53–58 As reported by Watcha and colleagues, the LMA provided better oxygen saturation and better surgical conditions in pediatric patients undergoing myringoplasties than did the face mask.59 Although in other studies the FLMA and the ETT were equally effective in adult patients undergoing nasal surgery, there was less blood in the trachea and fewer airway events during emergence in the FLMA group.54,59
B Dental and Oral Surgery
The FLMA has been useful during dental procedures and oral surgery in children and adults (Fig. 22-16).60,61 For example, George and Sanders,62 in a comparison of the FLMA with the nasal mask during dental procedures in children, reported significantly fewer instances of airway obstruction, oxygen desaturation, and cardiac dysrhythmias in children in the FLMA group. Other oral surgeries in which the FLMA has been used successfully include repair of a cleft palate,63–65 glossopexy,66 removal of a tongue tumor,67 and fixation of mandibular fractures after failed endotracheal intubation.68
C Ophthalmologic Procedures
Patients with increased intraocular pressure who are undergoing intraocular procedures are frequently at higher risk for injury from sudden pressure changes during laryngoscopy and endotracheal intubation.69 Coughing and bucking on the ETT during emergence from general anesthesia can be detrimental and may cause suture rupture.70 The benefits of using the FLMA for eye surgery include less change in the intraocular pressure during induction and emergence from general anesthesia and less hemodynamic stimulation.71,72 The FLMA has been particularly useful during cataract surgery, trabeculectomy, and vitroretinal surgery.73 Use of the device has also benefited children undergoing strabismus repair, gonioscopy, nasolacrimal duct probing, eyelid repair, scleral and conjunctival procedures, and foreign body removal.74
VI Laryngeal Mask Airway Use in Patients with Difficult-to-Manage Airways
One of the most important tasks of an anesthesiologist is to provide ventilation and oxygenation for the patient during surgery. This requires establishing and maintaining a patent airway during the course of anesthesia. As reported in 1990 in a closed claims study published by the ASA, airway-related problems were among the leading causes of major morbidity and mortality directly related to anesthesia.75 This study laid the groundwork for the development of the ASA difficult airway algorithm, which established pathways for the care of patients with difficult-to-manage airways.76 Although the algorithm provides an organized approach to airway management based on preoperative findings, it does not constitute a foolproof system. Many patients still prove difficult to intubate by means of conventional laryngoscopy, even when the preoperative interview and physical examination do not yield any signs of potential difficulty. Airway swelling may ensue quickly after multiple intubation attempts. Although face mask ventilation in these patients might have been adequate initially, it may soon become difficult, creating the very dangerous and potentially life-threatening situation of inadequate oxygenation.
During the past 10 years, the LMA has received much attention because it can be effective in patients who are difficult to intubate by conventional laryngoscopy and difficult to ventilate with a face mask. The success of the LMA in these patients results from its design. The LMA fits into the potential space of the pharynx much like a hand fits into a glove. In contrast to rigid laryngoscopy, insertion of the LMA does not rely on direct visualization of the larynx. Many factors that are associated with difficult rigid laryngoscopy (e.g., Mallampati class III and IV, Cormack-Lehane grade 3 and 4 views) do not affect the insertion of and ventilation through the LMA.77,78
A Classic Laryngeal Mask Airway
The potential of the LMA for the emergency and nonemergency management of patients with difficult airways was appreciated shortly after its invention. In February 1983, an early prototype was used successfully by Brain in a 114-kg man undergoing laparotomy who could not be intubated. However, the first publication describing the LMA as a possible solution to airway management problems in emergency situations did not appear in the scientific literature until 1984.79 By 1985, the LMA had been used successfully in five adults in whom difficult intubation was anticipated, and in October 1987, it was used for the first time in cases of failed pediatric intubation.13 In 1989, Allison and McCrory used fiberoptic guidance for endotracheal intubations,80 and in 1991, McCrirrick and Pracilio first reported an awake intubation through the LMA.81 Numerous case reports appeared describing airway management with the LMA in several clinical situations, including adult and pediatric patients with cervical spine pathology, including cervical spine instability12,81–84; morbid obesity85; micrognathia and macrognathia86,87; Klippel-Feil syndrome88; Treacher Collins syndrome89; Pierre Robin syndrome90; Goldenhar’s syndrome91; Hurler’s syndrome92; Down syndrome93; or unexpected failed intubation associated with difficult face mask ventilation.94,95
A major step in recognizing the LMA’s potential in the management of airway emergencies took place in 1993, when it was incorporated into the Practice Guidelines for Management of the Difficult Airway by the ASA Task Force on Management of the Difficult Airway.76 Not enough data were available then to appreciate fully the LMA’s role in difficult airway situations. By 1996, the reported experience with the LMA had substantially increased, and Jonathan Benumof, who participated in the development of the ASA algorithm, assessed the LMA’s potential in a difficult airway scenario.96 His recommendations were that the LMA should be considered as originally recommended in the emergency pathway of the algorithm and in four additional situations: during awake intubation as an aid to endotracheal intubation, as a definite airway in nonemergency situations, as an aid to tracheal intubation in anesthetized patients, and as an aid to tracheal intubation after the airway is established in emergency situations. In his review, Benumof concluded, “With multiple uses and multiple places of use, the LMA is an important option within the ASA difficult airway algorithm. More importantly, the clinical record of LMA use in ‘cannot intubate, cannot ventilate’ situations has been excellent, and in patients whose lungs cannot be ventilated because of the supraglottic obstruction and whose trachea cannot be intubated due to unfavorable anatomy (but not periglottic pathology), the LMA should be immediately available and considered as the first treatment of choice.”96 In 2003, Benumof’s recommendations fully materialized in the Practice Guidelines for Management of the Difficult Airway that was updated by the ASA Task Force on Management of the Difficult Airway.6 Essentially, depending on the user’s level of expertise, the LMA can be used in any place in the algorithm.
B Fastrach Intubating Laryngeal Mask Airway
In 1983, while developing the LMA, Brain conducted a fiberoptic investigation that revealed the LMA’s potential as a guide for endotracheal intubation. The same year, he developed a prototype LMA and used it to intubate blindly three patients using a 9-mm ETT (Fig. 22-17). However, the development of an intubating LMA was not revisited by Brain until 1994. In response to clinicians’ growing demands for a device that had the same ventilatory properties as the LMA Classic but would serve as a better conduit for intubation, he designed the Fastrach ILMA (LMA North America, San Diego, CA), which was introduced in 1997 (Fig. 22-18).
The ILMA is designed to facilitate blind or fiberoptically guided endotracheal intubations without the need to move the cervical spine during intubation. Available only in adult sizes, the ILMA consists of an anatomically curved, stainless steel tube with a 13-mm internal diameter that is connected firmly at its distal end to the laryngeal mask. The angle of the metal shaft was carefully designed using measurements from sagittal MRI images to fit well into the oral and pharyngeal space while keeping the head and neck in a neutral position. Proximally, the metal shaft forms a standard 15-mm connector for the anesthesia circuit, and a rigid guiding handle serves to insert the device, eliminating the need to insert fingers into the mouth, and to stabilize and direct the device during intubation attempts. The two bars at the aperture of the LMA Classic have been replaced in the ILMA by a single, movable epiglottic elevating bar that pushes the epiglottis out of the way and allows smooth and unobstructed passage of the ETT as it emerges from the distal end of the ILMA’s metal shaft (Fig. 22-19). This shaft can accommodate an ETT up to 8.5 mm in diameter. The shaft of the ILMA is shorter than that of the LMA Classic, eliminating the need for longer ETTs in patients with long necks.
The ILMA was first described by Brain and coworkers in 1997 in the British Journal of Anaesthesia.97 In the same issue, there were two separate reports by Brain and Kapila and their colleagues98,99 of early clinical experience with the ILMA. Unfortunately, Kapila’s report described results obtained 3 years earlier using a more primitive prototype of the ILMA and created some confusion regarding the technique of intubation through the device. At the same time, early versions of the ILMA instruction manual included recommendations for use based on these early prototypes. Subsequently, the results from a large study that evaluated the effectiveness of the ILMA in patients with different types of difficult-to-manage airways rendered most of these earlier recommendations irrelevant by demonstrating that the only technique that improved the rate of blind intubation through the ILMA was Chandy’s maneuver (Chandy Verghese, Royal Berkshire Hospital, Reading, England, personal communication, January 1998).100
Chandy’s maneuver consists of two steps that are performed sequentially. The first step, which is important for establishing optimal ventilation, is to rotate the ILMA slightly in the sagittal plane using the metal handle until the least resistance to bag ventilation is achieved. The second step of Chandy’s maneuver is performed just before blind intubation and consists of using the metal handle to lift slightly (but not tilt) the ILMA away from the posterior pharyngeal wall. This facilitates the smooth passage of the ETT into the trachea (Fig. 22-20). Ferson and colleagues assessed the effectiveness of the ILMA in 254 patients, including patients with Cormack-Lehane grade 4 views, immobilized cervical spines, and airways distorted by tumors, surgery, or radiation therapy and patients wearing stereotactic frames.100 Insertion of the ILMA was accomplished in three attempts or fewer in all patients. The overall success rates for blind and fiberoptically guided intubations were 97% and 100%, respectively. This represents the largest analysis examining the use of the ILMA in patients with difficult-to-manage airways and demonstrates that the ILMA may be a particularly valuable tool in the emergency or elective airway management of patients in whom other techniques have failed and in the treatment of patients with immobilized cervical spines.
The ILMA can be used as an intubating tool in patients with normal airways. In a study conducted by Brain and colleagues, the hemodynamic response to the ILMA was similar to the response to the LMA Classic; in patients with compromised cardiovascular systems the ILMA could be a less stimulating alternative to rigid laryngoscopy for intubation of the trachea.98,101 Using the ILMA in patients with normal airways allows clinicians to develop good skills and familiarity with the device before using it in emergency situations.
VII ProSeal Laryngeal Mask Airway
The PLMA (LMA North America, San Diego, CA) (Fig. 22-21) was designed by Brain with the principal objective of providing a separate conduit to permit gastric fluids to bypass the glottis. He thought such a device was needed because even in fasted patients, the anesthesiologist can never be completely certain that the stomach is empty. The PLMA permits access to the stomach using standard gastric tubes, provides a more reliable seal around the glottis, reduces the leakage of inspired gases into the stomach, provides a more comfortable fit within the pharynx, facilitates the use of mechanical ventilation, and permits the diagnosis of incorrect LMA placement.
The LMA Classic may or may not block the esophagus, depending on how precisely it is positioned in the hypopharynx. Realizing this, Brain developed insertion and fixation techniques to ensure correct positioning, but these recommendations are widely ignored or unknown to most users, who continue to choose their own favored insertion techniques and methods of fixation. It therefore comes as no surprise that there are cases of aspiration in the LMA literature, although they are rare.102 In the PLMA, the drain tube opening at the distal tip of the mask ensures that if the tip is not correctly placed against the UES, inflation of the reservoir bag causes some of the gas to be diverted back up the drain tube, providing measurable evidence of inadequate device placement. The PLMA instruction manual further details the diagnostic functions made possible by the drain tube.103
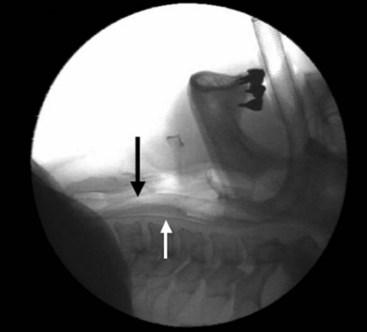
PLMA, which will soon be available in the same range of sizes as the LMA Classic (excluding size 6), has several important differences. The adult sizes are fitted with a posterior extension to the cuff that expands to a relatively small degree when inflated within the confined space of the pharynx, as demonstrated radiologically (Fig. 22-22). Similar images in which the cuff is inflated excessively demonstrate that the presence of the posterior cuff, although probably increasing the seal pressure by about 10%, introduces two dangers that must be guarded against. One is the possibility of herniating the cuff bowl forward toward the glottis, thereby causing partial or complete blockage of the delicate drain tube and compromising the airway (Fig. 22-23). Second, overinflation may cause the mask to slide proximally, reducing the efficacy of its seal against the UES. If the drain tube is blocked and its opening is simultaneously moved away from contact with the UES, the major advantages of the device are lost because there is no longer any guarantee that a seal exists against the UES, although the seal may appear to be excellent. The PLMA is therefore a more sophisticated device than the LMA Classic, and instructions for its use must be followed carefully.
Pediatric sizes of the PLMA feature the drain tube but no posterior cuff. The relatively larger airway and drain tube configuration required for optimal functioning in the pediatric anatomy result in a wider device angle when viewed laterally. If a posterior cuff were added, the angle would become critically large, causing the device to slide proximally out of the pharynx as it is inflated. Halaseh and colleagues reported their experience with the PLMA in 3000 elective cesarean sections in a single center and using a method of insertion that allowed a rapid establishment of a patent airway together with gastric drainage.104
VIII Supreme Laryngeal Mask Airway
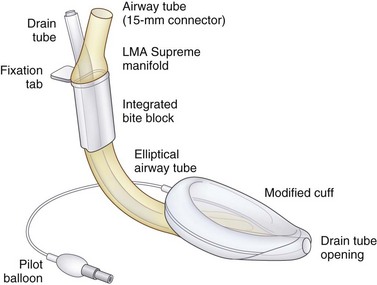
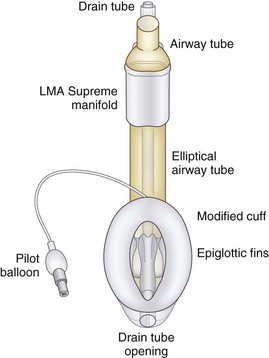
The LMA Supreme has a manifold with an integral bite block, an anatomically shaped airway tube enclosing a drain tube, a redesigned inflatable cuff (through which the drain tube passes), and a cuff inflation line with pilot balloon (Figs. 22-24 and 22-25). The LMA Supreme manifold and integral bite block is at the proximal end of the device. Two tubes project from the manifold. The standard 15-mm diameter airway tube is designed to be connected to the airway circuit. The narrower tube is the proximal end of the drain tube.104,105
The fixation tab is a rectangular structure molded onto the manifold at right angles, and it projects over the patient’s upper lip. It is designed to facilitate easy insertion and fixation of the LMA Supreme after insertion and inflation of its cuff. The fixation tab was found in early pilot studies to act as a visual guide to correct size selection. After fixation of the device and inflation of the cuff to a pressure of 60 cm H2O, the fixation tab should be 1.5 to 2 cm from the upper lip. If the fixation tab is less than this distance, the size chosen may be too small, and if it is more than 3.0 cm from the upper lip, the size chosen may be too large (see Figs. 22-24 and 22-25).
Extending distally from the manifold is the flattened, semirigid airway tube, which has an elliptical cross section and an integral bite block at its proximal end. The airway tube ends distally at the laryngeal inlet. The elliptical shape of the airway tube is intended to facilitate insertion in patients with reduced interdental space without requiring the insertion of fingers into the mouth. After it is in place, the flattened shape and stiffness of the airway tube also helps to minimize accidental rotation. The airway tube is much stiffer than that of the PLMA, but it is intended to bend with movements of the head and neck, unlike the completely rigid metallic airway tube of the ILMA. Patented lateral grooves on the airway tube of the LMA Supreme prevent it from kinking and occluding when bent, no matter how much it is flexed (see Figs. 22-24 and 22-25).
The drain tube runs from its rigid proximal end on the manifold through the middle of the airway tube and continues along the posterior surface of the cuff to the distal end of the cuff. At the distal end, it terminates as a centrally placed orifice designed to face into the opening of the UES, when the device is correctly inserted. The drain tube equalizes the pressure at the UES and atmosphere. In addition to venting gastrointestinal gases and liquids, the drain tube also serves as a conduit for the passage of a well-lubricated gastric tube (up to 16 F) into the stomach. The drain tube functions as a continuous clinical monitoring tool during PPV, indicating whether the LMA Supreme is correctly positioned immediately after insertion and warning the user if the device becomes displaced from its optimal position during use. Incorrect positioning of the LMA Supreme results in a poor airway seal and an audible leak of delivered gases through the drain tube (see Figs. 22-24 and 22-25).
The modified shape and enlarged surface area of the inflatable cuff enhances the anatomic fit of the device into the pharynx, permitting higher glottic seal pressures than the LMA Classic and LMA Unique. The distal cuff is over-molded to strengthen the tip and prevent it from folding over during insertion. Two fin-shaped structures extend on either side of the drain tube where it passes through the bowl of the mask, designed to prevent the epiglottis from obstructing the airway (see Figs. 22-1 and 22-2). The cuff has a conventionally placed inflation line terminating in a pilot balloon and one-way valve for mask inflation and deflation.
The technique for LMA Supreme insertion described in the study by Balog and coworkers is similar to that recommended in the instruction manual.74 The device was deflated to a vacuum using a 50-mL syringe and correctly shaped at the distal tip by compressing between finger and thumb during deflation to form a thin wedge. Insertion was performed with the head and neck in the semi-sniffing position. Complete extension is not required for successful insertion.105,106
In a prospective, descriptive study on the use of the LMA Supreme for airway management in 205 adult patients undergoing elective orthopedic surgery in the prone position, the investigators were able to demonstrate that first-pass success was achieved in 184 insertions.107 Regurgitation of gastric contents through the LMA drainage tube was observed in 205 patients. There were no cases of clinically relevant aspiration. Sharma and colleagues concluded that the LMA Supreme was a useful device for airway management in patients anesthetized in the prone position and for possible airway management with PPV, with or without neuromuscular block.107 No patient had to be turned to the supine position because of an airway problem during anesthesia. Brimacombe and coworkers demonstrated in 40 adult patients that maintenance of anesthesia is feasible in the prone position when the LMA Supreme was inserted by experienced users.108
The PLMA and LMA Supreme offer the additional advantages of higher glottic seal pressures than the LMA Classic, providing a separate conduit for passive passage of gastric contents and allowing the passage of a lubricated gastric tube through the drain tube to access liquid and gaseous gastric contents. The advantages of a higher glottic seal pressure, easy access to the stomach,105 ease of insertion in the supine, lateral, and prone positions, efficacy for PPV, and as a reliable and noninvasive airway during the recovery phase from general anesthesia support its use in this form of surgery.
In a well-designed, randomized, prospective study comparing the safety and efficacy of the LMA Supreme with the PLMA in elective ambulatory procedures, Seet and associates included 99 patients and concluded that although the LMA Supreme has lower oropharyngeal leak pressures than the PLMA, the first attempt insertion success rate was higher for the LMA Supreme.109 It is a safe, efficacious, and easy to use disposable supraglottic airway device suitable for elective ambulatory procedures.109 The higher first-attempt success rate may make the LMA Supreme a more suitable choice as an airway rescue device.
The higher intracuff pressures demonstrated for the PLMA may be related to the cuff material. The PLMA is made from silicone, and the LMA Supreme is constructed from polyvinyl chloride.106 Mucosal pressures are lower than pharyngeal perfusion pressure over the inflation range for the PLMA.110
Eschertzhuber and colleagues found a lower leak pressure with the size 4 LMA Supreme than with the size 4 PLMA in 93 healthy, female patients.106 However, there was no significant difference in leak pressure with the LMA Supreme and PLMA in two other studies by Verghese and Ramaswamy105 and Hosten and coworkers.111 A literature review of the oropharyngeal leak pressure of the supreme LMA and the PLMA showed conflicting results. A prospective, randomized, crossover study of 36 female patients using a size 4 Supreme or the PLMA showed no difference in the oropharyngeal leak pressure (28.6 versus 28.5 cm H2O, P = 0.91).105 Hosten and associates conducted a prospective, randomized, controlled trial enrolling 60 patients (men and women) and comparing the Supreme with the Proseal.111 Various LMA sizes (sizes 3 through 5) were used according to body weight. The leak pressures were also found to be similar for the Supreme and ProSeal groups at intracuff pressures of 60 cm H2O (26.1 versus 26.9 cm H2O).111 However, muscle relaxants were given to 13 of 60 patients, which might have confused the results. The application of neuromuscular blocking agents can result in a decreased leak pressure of the PLMA and may have influenced the accuracy of measurement.112 The lower oropharyngeal leak pressure for the LMA Supreme may be related to the less elastic properties of polyvinyl chloride.
Cuff pressures are not directly related to leak pressures. The materials play a major role in the compliance and elasticity of the device, as do the volume and elasticity of the patient’s oropharyngeal anatomy, but it is also essential that the device is correctly located in the hypopharynx and that it remains securely fixed in position. The fact that leak pressures have been found by some to be lower in the LMA Supreme may be due to material differences, but it could also reflect positioning and subsequent movement of the airway after insertion.113
IX Disposable Laryngeal Mask Airways
All pediatric and adult sizes of the disposable LMA (LMA Unique; LMA North America) have been subjected to a rigorous series of tests to confirm that their function is equivalent to that of the reusable silicone device.114,115 This has not been easy to accomplish because vinyl plastic materials lack the elasticity of silicone, and this tends to affect the insertion and seal characteristics of the LMA. It is likely that the plastic disposable LMAs will be found to be less appropriate for PPV than the LMA Classic. However, this limitation may be regarded as academic because the PLMA is specifically designed for use with positive pressure.
X Exchange Using the Aintree Intubation Catheter
When an LMA is placed after a difficult intubation or difficult ventilation by face mask, the anesthesiologist must determine whether the airway should be secured by an ETT for the surgical procedure. The Aintree intubating catheter (AIC, Cook Critical Care, Bloomington, IN) is a semirigid, 560-mm-long tube with internal and external diameters of 47 and 70 mm, respectively.116,117 It was designed to enable fiberoptically guided intubation of the trachea through a supraglottic airway device. The technique permits fiberoptic instrumentation of the airway while oxygenation and ventilation take place around the fiberscope through the airway device. Use of the AIC through the LMA Classic and the PLMA have been reported when conventional tracheal intubation has failed.118–122
The PLMA may offer additional benefits over the LMA Classic when using the AIC because of its larger airway bowl and the absence of aperture bars at the distal end of the airway tube. The deeper bowl of the PLMA and the absence of bars may allow improved access to the larynx compared with the LMA Classic and improve passage of the AIC during insertion.123,124 The PLMA is not as easy to insert as the LMA Classic, and it has a slightly smaller internal diameter through which an AIC can easily pass.
Clinical studies have reported less than 30 intubations with the LMA Classic or the cuffed oropharyngeal airway (COPA). Both reports were executed by the inventors of the device and between them involved only three operators. On a manikin study comparing the use of AIC over the LMA Classic and the PLMA using the fiberscope, Blair and colleagues found no significant difference between the devices with regard to ease of advancement of the fiberscope or the view of the vocal cords with the AIC.125 The study concluded that in a manikin, fiberoptically guided intubation through a PLMA was at least as easy and reliable as through the LMA Classic.125 The technique of exchanging an LMA over an AIC has only been described in humans during difficult airway situations and found as case reports in the literature.
XI Problems and Controversies
A Oropharyngolaryngeal Morbidity
Any foreign body that comes into contact with airway structures can cause complications. The LMA passes through and occupies anatomic areas from the oral cavity to the hypopharynx. Structures that are at high risk for injury from supraglottic airways, including the LMA, are mucous membranes, soft tissues of the pharynx and larynx, salivary glands, nerves and blood vessels of the neck, laryngeal cartilages, and bones of the neck. When injury occurs, it usually manifests in minor complaints such as a dry mouth or sore throat.126 These problems usually resolve quickly and have no long-term sequelae. However, more serious complications, such as hypoglossal and lingual nerve palsy, trauma to the epiglottis and larynx, dysarthria, dysphonia, and tongue cyanosis related to vascular compression, have been reported.127–133
1 Sore Throat
Sore throat, dry throat, pharyngeal erythema, and minor pharyngeal abrasions have been reported with the LMA. The published incidence of sore throat varies between 0% and 70%.126,132 Initially, it was not clear why the incidence of sore throat varied so much. Factors that may affect the incidence include the insertion technique, duration of the procedure, type of lubricant, type of ventilation (i.e., spontaneous or controlled), and intracuff pressure.134–137 Of these, the only variable that has been shown to reduce the incidence of sore throat reliably is lowering the intracuff pressure.138 Overinflation of the LMA’s cuff is a common practice when the size of the device is too small for a given patient and the practitioner encounters a significant leak while attempting assisted ventilation or PPV. The overinflated cuff becomes stiff and exerts high local pressure on tissues, especially those surrounding bony structures, such as the hyoid bone and cervical spine. Nitrous oxide diffuses through the silicone walls of the LMA, increasing the pressure inside the cuff.139 This can be remedied by monitoring the intracuff pressure and periodically withdrawing the gas from the LMA’s cuff. Based on Brain’s unpublished research in awake volunteers, the maximum pressure inside the LMA’s cuff should not exceed 60 cm H2O because higher pressure causes discomfort, presumably because of stretching of the constrictor muscles. Appropriate LMA size selection combined with a good insertion technique and low intracuff pressure reduces significantly the incidence of postoperative sore throat.
2 Vascular Compression and Nerve Damage
Although rare, compression of the blood vessels of the tongue that leads to tongue cyanosis has been observed after LMA insertion.130 This complication is likely to occur when the LMA is not inserted deeply enough or when the LMA’s cuff is overinflated. The blood vessels of the tongue or the lingual nerve can be compressed by malpositioning of the LMA’s shaft on the lateral side of the tongue, as found in cadaveric research. Similarly, if during LMA insertion the cuff is partially or fully inflated, the device frequently lies higher than when the correct insertion technique is used. Malpositioning of the LMA can compress the lingual or hypoglossal nerve, resulting in transient or prolonged nerve palsy. The hypoglossal nerve, which supplies all of the intrinsic and all but one of the extrinsic muscles of the tongue, is particularly vulnerable to injury at the point where the nerve loops anteriorly, close to the greater cornu of hyoid bone, and then runs along the lateral surface of the hyoglossal muscle and above the posterior border of the mylohyoid muscle before dividing into several branches that supply tongue muscles. To avoid these complications, the anesthesiologist should select the appropriate LMA size, use the standard insertion technique, and ensure that the intracuff pressure is no higher than 60 cm H2O.
B Risk of Aspiration
Aspiration during general anesthesia has an overall incidence of 1.4 to 6.5 per 10,000 cases and a mortality rate of 5%.140–143 In a study of 215,488 anesthesia cases, Warner and coworkers reported that the incidence of aspiration was 2.6 per 10,000 patients undergoing elective surgery and 11 per 10,000 patients undergoing emergency procedures.144 The mortality rate was 0.14 per 10,000 patients with an ASA physical status classifications III through V. The study by Warner and colleagues did not include patients whose airways were managed using the LMA.144
The LMA does not reliably protect against aspiration and should not be used electively in patients who are at high risk for this complication. However, most users do not adhere to the recommended insertion or fixation techniques when using the LMA, which are designed to optimize the seal of the distal end of the device against the UES. Nonetheless, a meta-analysis of the literature on the LMA by Brimacombe and Berry revealed that the overall incidence of pulmonary aspiration with the LMA is approximately 2 per 10,000 patients.145 Eighteen cases of suspected pulmonary aspiration during LMA use have been found by meta-analysis, and only 10 of these were confirmed radiographically. In only three patients did the aspiration warrant ventilatory support, which lasted 1 to 7 days. None of the patients who aspirated through the LMA suffered any long-term effects. Most of these patients had one or more factors predisposing them to aspiration, including emergency surgery in an unfasted patient, a difficult airway, obesity, a steep Trendelenburg position with intra-abdominal insufflation, and previous gastric surgery. Although the meta-analysis study contained only reports published through September 1993, a subsequent analysis of 11,910 LMA anesthesia cases by Verghese and Brimacombe yielded an even smaller incidence of aspiration (0.84 per 10,000).146
A study by Bernadini and Natalini compared the risk of pulmonary aspiration in patients whose lungs were mechanically ventilated through an LMA airway (35,630 procedures) or ETT (30,082 procedures).147 Three cases of pulmonary aspiration occurred with the LMA and seven with the ETT. No deaths were related to pulmonary aspiration. The incidence and outcome of pulmonary aspiration detected in this study were similar to those previously reported. The adjusted odds ratio (OR) for pulmonary aspiration with the LMA was determined to be 1.06 (95% confidence interval [CI], 0.20 to 5.62). Unplanned surgery (OR = 30.5; 95% CI, 8.6 to 108.9) and male sex (OR = 8.6, 95% CI, 1.1 to 68) were associated with an increased risk of aspiration and age younger than 14 years with a reduced risk (OR = 0.21; 95% CI, 0.07 to 0.64). There were contraindications and exclusions to the use of the LMA, but in this selected population, use of an LMA was not associated with an increased risk of pulmonary aspiration compared with an ETT.
1. Do not attempt to remove the LMA because a significant amount of regurgitant fluid may be trapped behind its cuff. The cuff shields and protects the larynx from the trapped fluid, and removing the LMA may worsen the situation.
2. Temporarily disconnect the circuit to allow drainage of the fluid while tilting the patient’s head down and to the side.
3. Suction the LMA, and administer 100% oxygen to the patient.
4. Ventilate the patient manually using low gas flows and small tidal volumes to minimize the risk of forcing fluid from the trachea into the small bronchi.
5. Use a large FOB to evaluate the tracheobronchial tree, and suction any remaining fluid.
6. If aspiration below the vocal cords is confirmed, consider intubating the patient with an ETT, and institute appropriate treatment protocols.
C Positive-Pressure Ventilation
When the first independent trial of the LMA was carried out, only a size 3 was available, and the correct fixation technique for ensuring an adequate seal against the UES had not been developed.148 When used in large patients, the device was not very reliable for applying PPV. Because the LMA concept was new, it was prudent for clinicians initially to limit the use of the LMA to patients who were breathing spontaneously. Even now, PPV should be considered an advanced use of the LMA, and clinicians should practice and gain experience using the LMA in patients who are breathing spontaneously before attempting controlled ventilation. However, after the LMA was introduced commercially in a range of sizes, first for adults and then for children, many LMA users found that after they became experienced, the LMA could be effective during PPV.73 Devitt and colleagues compared the effectiveness of the LMA with that of the ETT in 48 patients and demonstrated that PPV (range, 15 to 30 cm H2O) through the LMA was adequate and comparable to that achieved through the ETT.149 Epstein and associates studied the effectiveness of the LMA with controlled ventilation in children 3 months to 17 years old and concluded that the device performed effectively and reliably.150 In another study, Epstein and coworkers established that the airway seal pressures (25.9 to 31.2 cm H2O) were well maintained with the LMA in children.151
Two large clinical studies of more than 7000 patients led by Verghese and Van Damme established that the LMA was as effective as the ETT for controlled ventilation.114,152 Van Damme’s study also assessed the airway seal pressures and demonstrated that at 15 cm H2O or less pressure, leaks occurred with the LMA in only 2.7% of patients. Subsequently, Verghese reported his experience of using the LMA successfully in 5236 patients undergoing a variety of surgical procedures under general anesthesia and PPV.147
The following principles should be considered when using the LMA with PPV:
1. Selection of patients: Most patients with normal lung compliance who have fasted can be mechanically ventilated effectively with the LMA.
2. Size selection: Select the largest LMA size appropriate for the patient, which prevents a tendency to compensate for inadequate seal pressure by overinflating the cuff.
3. Insertion technique: Carefully follow the correct insertion technique to ensure optimal positioning of the LMA in the airway.
4. Fixation technique: Use the correct method of taping the LMA. This ensures proper contact between the LMA’s tip and the esophagus and prevents gastric insufflation.
5. Auscultation: Always auscultate over the stomach to ensure that gastric insufflation is not taking place.
6. Ventilatory parameters: Limit tidal volumes to 8 mL/kg, and control the end-tidal carbon dioxide by adjusting the respiratory rate.
7. Treatment of inadequate tidal volume: Maintain an adequate level of anesthesia for the particular surgical procedure.
D Use for Prolonged Procedures
The maximum duration for which the LMA can be safely used is not well established, but a limit of 2 hours was suggested soon after the LMA became widely available for clinical use.82 This recommendation was based on the possible increased risk of aspiration or pharyngeal morbidity. Subsequent reports demonstrated that in the hands of experienced users, the LMA can be safely used during procedures lasting up to 8 hours.153,154 In rare circumstances, the LMA has been used in the intensive care arena to provide effective respiratory support for 10 to 24 hours with no evidence of adverse effects.155 The PLMA, which is designed for PPV and has a gastric drainage tube to minimize the risk of aspiration, may be best suited for prolonged procedures.156 Regardless of the model used, clinicians must remain vigilant and provide adequate anesthesia to their patients, minimizing the risks of complications. If nitrous oxide is administered to the patient, the anesthesiologist should remember that this gas diffuses into the LMA’s cuff, increasing intracuff pressure. During prolonged LMA use, close monitoring of the intracuff pressure is recommended. To minimize the risk of mucosal ischemia associated with high intracuff pressures, hourly removal of a few milliliters of air from the cuff is considered a prudent practice.157
E Use in Nonsupine Positions
Use of the LMA in patients in other than the supine position should be considered an advanced technique.158–163 An alternative plan is necessary in case the device dislodges from its original position during the procedure. Because of the risk of LMA dislodgment in patients in nonsupine positions, consider inducing general anesthesia and inserting the LMA after the patient has been positioned for the procedure. This ensures the feasibility of inserting the LMA at any time during the procedure. If insertion cannot be performed successfully, reconsider whether the LMA should be used in that patient. The LMA insertion technique in patients in the lateral or prone position is significantly different from the insertion technique in supine patients. Using a thumb to insert the LMA may be the most useful method in patients positioned prone or laterally.164 An advantage of using the LMA in patients in the prone position is that they are able to position themselves comfortably before induction of general anesthesia, potentially reducing the incidence of back problems.165
F Device Problems
Before its clinical release, the LMA was subjected to rigorous testing to ensure compliance with the medical industry standards. However, some complications have been reported, including separation of the LMA cuff from the shaft,166,167 fragmentation of the device inside the patient,168 kinking of the LMA’s shaft,169 and failure to inflate or deflate the cuff.170,171 Most of these complications are related to use of the device beyond the manufacturer’s recommendations and to use of the wrong chemicals for sterilization and coating the device with silicone lubricants, which are not recommended for the LMA. Strict adherence to the manufacturer’s instructions for cleaning, sterilization, and use should always be followed, including not exceeding the recommended 40 uses per device. Instructions from the manufacturer’s manual should be followed if a malfunction occurs. With the introduction of the disposable LMA models, the number of device problems linked to overuse and wrong sterilization techniques may be eliminated, but all LMAs should be inspected and tested before use to ensure optimal performance and safety.
XII Conclusions
When the LMA was first introduced, it was considered to be an alternative to the face mask. However, its clinical applications have exceeded the original recommendations and benefit patients undergoing operations in all surgical and anesthetic subspecialties. Modern surgical techniques often are much less invasive than traditional operations, and many patients undergo procedures in day surgical centers. The LMA is much less stimulating to patients than the ETT and is considered the first choice for diagnostic and minimally invasive surgical procedures. The LMA has been effective and frequently life-saving in patients with difficult-to-manage airways. A study by Combes and colleagues demonstrated that the ILMA and gum elastic bougie were the most frequently used and most successful techniques in patients with unexpected difficult intubation in the operating room.172
XIII Clinical Pearls
• Learn and follow the correct maneuvers for precise insertion and fixation of each of the LMA devices as thoroughly described by Brain. Extensive research has demonstrated that using the proper insertion and fixation techniques leads to higher success rates for insertion and less troubleshooting.
• Optimal results on placement of all LMA types are achieved when the tip of the LMA is delivered to the upper esophageal sphincter (UES).
• Anesthesiologists must understand the advantages and disadvantages of using the LMA.
• Ventilation through the LMA when intubation and face mask ventilation have failed may be a life-saving procedure.
• Proper patient population selection, appropriate LMA selection, adequate size selection, proper attention to the adequate depth of anesthesia (especially when the patient is not paralyzed), and maintenance of an intracuff pressure no higher than 60 cm H2O (44 mm Hg) enhance the safety and efficacy of all LMAs.
All references can be found online at expertconsult.com.
105 Verghese C, Ramaswamy B. LMA Supreme—A new single-use LMA with gastric access: A report on its clinical efficacy. Br J Anaesth. 2008;101:405–410.
106 Eschertzhuber S, Brimacombe J, Hohlrieder M, Keller C. The Laryngeal Mask Airway Supreme—A single use laryngeal mask airway with an esophageal vent: A randomised, cross-over study with the Laryngeal Mask Airway ProSeal in paralysed, anaesthetised patients. Anaesthesia. 2009;64:79–83.
107 Sharma V, Verghese C, McKenna PJ. Prospective audit on the use of the LMA-Supreme for airway management of adult patients undergoing elective orthopedic surgery in the prone position. Br J Anaesth. 2010;105:228–232.
109 Seet E, Rajeev S, Firoz T, et al. Safety and efficacy of laryngeal mask airway Supreme versus laryngeal mask airway ProSeal: A randomised controlled trial. Eur J Anaesthesiol. 2010;27:602–607.
111 Hosten T, Gurkan Y, Ozdamar D, et al. A new supraglottic airway device: LMA-Supreme, comparison with LMA ProSeal. Acta Anaesthesiol Scand. 2009;53:852–857.
1 Brain AIJ. The laryngeal mask: A new concept in airway management. Br J Anaesth. 1983;55:801–805.
2 Asai T, Morris S. The laryngeal mask airway: Its features, effects, and role. Can J Anaesth. 1994;41:930–960.
3 Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. An International Consensus on Science. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102(Suppl):11–1384.
4 Baskett PJF, Parr MJA, Nolan JP. The intubating laryngeal mask: Results of a multicentre trial with experience of 500 cases. Anaesthesia. 1998;53:1174–1179.
5 Brain AI, Verghese C, Strube PJ. The LMA ProSeal: A laryngeal mask with an esophageal vent. Br J Anaesth. 2000;84:650–654.
6 Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277.
7 Fink BR. Roentgen ray studies of airway problems: I. The oropharyngeal airway. Anesthesiology. 1957;18:162–163.
8 Guedel AE. A nontraumatic pharyngeal airway. JAMA. 1933;100:1862.
9 Hewitt F. Clinical observation upon respiration during anesthesia. Proc R Med Chir Soc. 1981;3:31–38.
10 Leech BC. The pharyngeal bulb gasway: A new aid in cyclopropane anesthesia. Anesth Analg. 1937;16:22–25.
11 Northrup WP. Apparatus for artificial prolonged forcible respiration. Br Med J. 1984;ii:697.
12 Shipway F. Airway for intranasal operations. Br Med J. 1935;Apt 13:767.
13 Brain AIJ. The development of the laryngeal mask: A brief history of the invention, early clinical studies and experimental work from which the laryngeal mask evolved. Eur J Anaesthesiol. 1991;4:5–17.
14 Brain AIJ, McGhee TD, McAteer EJ, et al. The laryngeal mask airway: Development and preliminary trials of a new type of airway. Anaesthesia. 1985;40:356–361.
15 Brain AI. The laryngeal mask airway: A possible new solution to airway problems in the emergency situation. Arch Emerg Med. 1984;4:229–232.
16 Brain AIJ. Three cases of difficult intubation overcome by use of the laryngeal mask. Anaesthesia. 1985;40:353–355.
17 Katz R: Wellcome Trends Anesthesiology Oct 1985.
18 Katz R. Clinical update: The laryngeal mask airway. Wellcome Trends Anaesthesiology Feb. 1986.
19 Alexander CA, Leach AB, Thompson AR, Lister JB. Use your brain!. Anaesthesia. 1988;43:893–894.
20 Brodrick PM, Webster NR, Nunn JF. The laryngeal mask airway: A study of 100 patients during spontaneous breathing. Anaesthesia. 1989;44:238–241.
21 Ferson DZ, Bui TP, Arens JF. Evaluation of the effectiveness of two methods of training for the insertion of the laryngeal mask airway. Anesthesiology. 2001;95:A558.
22 Shorten GD, Opie NJ, Graziotti P, et al. Assessment of upper airway anatomy in awake, sedated, and anesthetised patients using magnetic resonance imaging. Anaesth Intensive Care. 1994;22:165–169.
23 Brain AIJ, Denman WT, Gousouzian NG. LMA instruction manual. San Diego: LMA North America, Inc; 1999.
24 Zerafa M, Baulch S, Elliott MJ, Petros AJ. Use of the laryngeal mask airway during repair of atrial septal defect in children. Paediatr Anaesth. 1999;9:257–259.
25 Lopez-Gil M, Brimacombe J, Cebrian J, Arranz J. The laryngeal mask airway in pediatric practice: A prospective study of skill acquisition by resident anesthesiologists. Anesthesiology. 1996;84:807–811.
26 Davies PR, Tighe SQ, Greenslade GL, Evans GH. Laryngeal mask airway and tracheal tube insertion by unskilled personnel. Lancet. 1990;336:977–979.
27 Rafferty C, Burke AM, Cossar DF, Farling PA. Laryngeal mask and magnetic resonance imaging. Anaesthesia. 1990;45:590–591.
28 Stevens JE, Burden G. Reinforced laryngeal mask airway and magnetic resonance imaging. Anaesthesia. 1994;49:79–80.
29 Goudsouzian NG, Denman W, Cleveland R, Shorten G. Radiologic localization of the laryngeal mask in children. Anesthesiology. 1992;77:1085–1089.
30 Van Obbergh LJ, Muller G, Zeippen B, Dooms G. Propofol infusion and laryngeal mask for magnetic resonance imaging in children. Anesthesiology. 1992;77:A1177.
31 Grebenik CR, Ferguson C, White A. The laryngeal mask airway in pediatric radiotherapy. Anaesthesiology. 1990;72:474–477.
32 De Belder MA, Leech G, Camm AJ. Transesophageal echocardiography in unsedated outpatients: Technique and patients’ tolerance. J Am Soc Echocardiogr. 1989;2:375–379.
33 Khandheria BK. The transesophageal echocardiographic examination: Is it safe? Echocardiography. 1994;11:55–63.
34 Chan KL, Cohen GI, Sochowski RA, Baird MG. Complications of transesophageal echocardiography in ambulatory adult patients: Analysis of 1500 consecutive examinations. J Am Soc Echocardiogr. 1991;4:577–582.
35 Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography: A multicenter survey of 10,419 examinations. Circulation. 1991;83:817–821.
36 Ferson D, Thakar D, Swafford J, et al. Use of deep intravenous sedation with propofol and the laryngeal mask airway during transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2003;17:443–446.
37 Imai M, Matsamura C, Hanaoka Y, et al. Comparison of cardiovascular responses to airway management: Fiberoptic intubation using a new adapter, laryngeal mask insertion, or conventional laryngoscopic intubation. J Clin Anesth. 1995;7:14–18.
38 Eltzschig HK, Posner M, Moore FD, Jr. The use of readily available equipment in a simple method for intraoperative monitoring of recurrent laryngeal nerve function during thyroid surgery: Initial experience with more than 300 cases. Arch Surg. 2002;137:452–456.
39 Brimacombe J, Tucker P, Simmons S. The laryngeal mask airway for awake diagnostic bronchoscopy: A retrospective study of 200 consecutive patients. Eur J Anaesthesiol. 1995;12:1017–1023.
40 Du Plessis M, Barr AM, Verghese C, Lyall JR. Fiberoptic bronchoscopy under general anesthesia using the laryngeal mask airway. Eur J Anaesthesiol. 1993;10:363–365.
41 Ferson DZ, Nesbitt JC, Nesbitt K, et al. The laryngeal mask airway: A new standard for airway evaluation in thoracic surgery. Ann Thorac Surg. 1997;63:768–772.
42 Maekawa N, Mikawa K, Tanaka O, et al. The laryngeal mask may be a useful device for fiberoptic airway endoscopy in pediatric anesthesia. Anesthesiology. 1991;75:169–170.
43 Theroux MC, Kettrick RG, Khine HH. Laryngeal mask airway and fiberoptic endoscopy in an infant with Schwartz-Jampel syndrome. Anesthesiology. 1995;82:605.
44 Mizikov VM, Variushina TV, Kirimov I. Fiberoptic bronchoscopy via laryngeal mask in children. Anesteziol Reanimatol. 1997;5:78–80.
45 Slinger P, Robinson R, Shennib H, et al. Alternative technique for laser resection of a carinal obstruction. J Cardiothorac Vasc Anesth. 1992;6:749–755.
46 Conacher ID. Anaesthesia and tracheobronchial stenting for central airway obstruction in adults. Br J Anaesth. 2003;90:367–374.
47 Silva L, Brimacombe JR. Tracheal tube/laryngeal mask exchange for emergence. Anesthesiology. 1996;85:218.
48 Ferson DZ. The effect of the laryngeal mask insertion on intracranial pressure in a patient with posterior fossa tumor: A case report. Internet J Anesthesiol. 1(3), 1997.
49 Sarang A, Dinsmore J. Anaesthesia for awake craniotomy: Evolution of a technique that facilitates awake neurological testing. Br J Anaesth. 2003;90:161–165.
50 Williams PJ, Bailey PM. Comparison of reinforced laryngeal mask airway and tracheal intubation for adenotonsillectomy. Br J Anaesth. 1993;70:30–33.
51 Williams PJ, Bailey PM. The reinforced laryngeal mask for adenotonsillectomy. Br J Anaesth. 1994;72:729.
52 Fiani N, Scandella C, Giolitto N, et al. Comparison of reinforced laryngeal mask airway vs endotracheal intubation in tonsillectomy. Anesthesiology. 1994;81:A491.
53 Bing J. Masque larynge pour réduction de fractura du nez. Ann Fr Anesth Reanim. 1991;10:494.
54 Dain SL, Webster AC, Morley-Foster P, et al. Propofol for insertion of the laryngeal mask airway for short ENT procedures in children. Anesth Analg. 1996;82:S83.
55 Daum RE, O’Reilly BJ. The laryngeal mask airway in ENT surgery. J Laryngol Otol. 1992;106:28–30.
56 Ebata T, Nishiki S, Masuda A, Amaha K. Anaesthesia for Treacher Collins syndrome using a laryngeal mask airway. Can J Anaesth. 1991;38:1043–1045.
57 Rheineck Leyssius AT, Vos RJ, Blommesteijn R, Kalkman CJ. Use of the laryngeal mask airway versus orotracheal intubation to secure a patient airway in rhinoplastic surgery. Anesthesiology. 1994;81:A1293.
58 Williams PJ, Thompsett C, Bailey PM. Comparison of reinforced laryngeal mask airway and tracheal intubation for nasal surgery. Anaesthesia. 1995;50:987–989.
59 Watcha MF, Garner FT, White PF, Lusk R. Laryngeal mask airway vs face mask and Guedel airway during pediatric myringotomy. Arch Otolaryngol Head Neck Surg. 1994;120:877–880.
60 Goodwin A, Ogg TW, Lamb W, Adlam D. The reinforced laryngeal mask airway in dental day surgery. Ambulatory Surg. 1993;1:31–35.
61 Quinn AC, Samaan A, McAteer EM, et al. The reinforced laryngeal mask for dento-alveolar surgery. Br J Anaesth. 1996;77:185–188.
62 George JM, Sanders GM. The reinforced laryngeal mask airway in paediatric outpatient dental surgery. Presented at the 11th World Congress of Anesthesiology, Sydney, April 14–20, 1996. Anaesthesia. 1999;54:546–551.
63 Beveridge ME. Laryngeal mask anaesthesia for repair of cleft palate. Anaesthesia. 1989;44:656–657.
64 Mason DG, Bingham RM. The laryngeal mask airway in children. Anaesthesia. 1990;45:760–763.
65 Zagnoev M, McCloskey J, Martin T. Fiberoptic intubation via the laryngeal mask airway. Anesth Analg. 1994;78:813–814.
66 Mecklem D, Brimacombe J, Yarker J. Glossopexy in Pierre Robin sequence using the laryngeal mask airway. J Clin Anesth. 1995;7:267–269.
67 Kadota Y, Oda T, Yoshimura N. Applications of a laryngeal mask to a fiberoptic bronchoscope–aided tracheal intubation. J Clin Anesth. 1992;4:503–504.
68 Allen JG, Flower EA. The Brain laryngeal mask: An alternative to difficult intubation. Br Dent J. 1990;168:202–204.
69 Barclay K, Wall T, Wareham K, Asai T. Intra-ocular pressure changes in patients with glaucoma: Comparison between laryngeal mask airway and tracheal tube. Anaesthesia. 1994;49:159–162.
70 Thomson KD. The effect of the laryngeal mask airway on coughing after eye surgery under general anaesthetic. Ophthalmic Surg. 1992;23:630–631.
71 Lamb K, James MFM, Janicki PK. The laryngeal mask airway for intraocular surgery: Effects on intraocular pressure and stress responses. Br J Anaesth. 1992;69:143–144.
72 Myint Y, Singh AK, Peacock JE, Padfield A. Changes in intra-ocular pressure during general anaesthesia: A comparison of spontaneous breathing through a laryngeal mask with positive pressure ventilation through a tracheal tube. Anaesthesia. 1995;50:126–129.
73 Wainwright AC. Positive pressure ventilation and the laryngeal mask airway in ophthalmic anaesthesia. Br J Anaesth. 1995;75:249–250.
74 Balog CC, Bogetz MS, Good WH, et al. The laryngeal mask is an ideal airway for many outpatient pediatric ophthalmologic procedures. Anesth Analg. 1994;78:S17.
75 Caplan RA, Posner KL, Ward RJ, Cheney FW. Adverse respiratory events in anesthesia: A closed claims analysis. Anesthesiology. 1990;72:828–833.
76 Caplan RA, Benumoff JL, Berry FA, et al. Practice guidelines for management of the difficult airway: A report by the ASA Task Force on Management of the Difficult Airway. Anesthesiology. 1993;78:597–602.
77 Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–1111.
78 Mallampati SR, Gatt SP, Gugino LD. A clinical study to predict difficult tracheal intubation. A prospective study. Can J Anaesth. 1985;32:429–434.
79 Brain AIJ. The laryngeal mask airway: A possible new solution to airway problems in the emergency situation. Arch Emerg Med. 1984;1:229–232.
80 Allison A, McCrory J. Tracheal placement of a gum elastic bougie using the laryngeal mask airway. Anaesthesia. 1990;45:419–420.
81 McCrirrick A, Pracilio JA. Awake intubation: A new technique. Anaesthesia. 1991;46:661–663.
82 Asai T. Fiberoptic tracheal intubation through the laryngeal mask airway in an awake patient with cervical spine instability. Anesth Analg. 1993;77:404.
83 Logan A. Use of the laryngeal mask in a patient with an unstable fracture of the cervical spine. Anaesthesia. 1991;46:987.
84 Smith BL. Brain airway in anesthesia for patients with juvenile chronic arthritis. Anaesthesia. 1988;43:421–422.
85 Godley M, Ramachandra AR. Use of LMA for awake intubation for caesarian section. Can J Anaesth. 1996;43:299–302.
86 Aye T, Milne B. Use of the laryngeal mask prior to definitive intubation in a difficult airway: A case report. J Emerg Med. 1995;13:711–714.
87 Thomson KD. A blind nasal intubation using a laryngeal mask airway. Anaesthesia. 1993;48:785–787.
88 Adejumo SWA, Davies MW. The laryngeal mask airway: Another trick. Anaesthesia. 1996;51:604.
89 Fuchs K, Kukule I, Knoch M, Wiegand W. Larynxmske versus Intubation bei erschwerten Intubationsbedingungen beim Franceschetti-Zwahlen-Klein-Syndrom (Treacher-Collins-Syndrome). Anasthesiol Intensivmed Noftalmed Schmerzther. 1993;28:190–192.
90 Baraka A. Laryngeal mask airway for resuscitation of a newborn with Pierre Robin syndrome. Anesthesiology. 1995;83:645–646.
91 Johnson CM, Sims C. Awake fiberoptic intubation via a laryngeal mask in an infant with Goldenhar’s syndrome. Anaesth Intensive Care. 1994;22:194–197.
92 Goldie AS, Hudson I. Fiberoptic tracheal intubation through a modified laryngeal mask. Paediatr Anaesth. 1992;2:344.
93 Gronert BJ. Laryngeal mask airway for management of airway and extracorporeal shock wave lithotripsy. Paediatr Anaesth. 1996;6:147–150.
94 McClune S, Regain M, Moore J. Laryngeal mask airway for caesarean section. Anaesthesia. 1990;45:227–228.
95 Silk JM, Hill HM, Calder I. Difficult intubation and the laryngeal mask. Eur J Anaesthesiol. 1991;4:47–51.
96 Benumof J. The laryngeal mask airway and the ASA difficult airway algorithm. Anesthesiology. 1996;84:686–699.
97 Brain AIJ, Verghese C, Kapila A, Brimacombe J. The intubating laryngeal mask: I. Development of a new device for intubation of the trachea. Br J Anaesth. 1997;79:699–703.
98 Brain AIJ, Verghese C, Addy EV, et al. The intubating laryngeal mask: II. A preliminary clinical report of a new means of intubating the trachea. Br J Anaesth. 1997;79:704–709.
99 Kapila A, Addy EV, Verghese C, Brain AIJ. The intubating laryngeal mask airway: An initial assessment of performance. Br J Anaesth. 1997;79:710–713.
100 Ferson DZ, Rosenblatt WH, Johansen MJ, et al. Use of the intubating LMA-Fastrach in 254 patients with difficult-to-manage airways. Anesthesiology. 2000;95:1175–1181.
101 Kahl M, Eberhart LHJ, Behnke H, et al. Stress response to tracheal intubation in patients undergoing coronary artery surgery: Direct laryngoscopy versus an intubating laryngeal mask airway. J Cardiothorac Vasc Anesth. 2004;18:275–280.
102 Nanji GM, Maltby JR. Vomiting and aspiration pneumonitis with the laryngeal mask airway. Can J Anaesth. 1992;39:69–70.
103 LMA-ProSeal instruction manual. San Diego: LMA North America, Inc, 2001.
104 Halaseh BK, Sukkar ZF, Hassan LH, et al. The use of ProSeal laryngeal mask airway in caesarean section: Experience in 3000 cases. Anaesth Intensive Care. 2010;38:1023–1028.
105 Verghese C, Ramaswamy B. LMA Supreme—A new single-use LMA with gastric access: A report on its clinical efficacy. Br J Anaesth. 2008;101:405–410.
106 Eschertzhuber S, Brimacombe J, Hohlrieder M, Keller C. The laryngeal mask airway Supreme—A single use laryngeal mask airway with an esophageal vent: A randomised, cross-over study with the laryngeal mask airway ProSeal in paralysed, anaesthetised patients. Anaesthesia. 2009;64:79–83.
107 Sharma V, Verghese C, McKenna PJ. Prospective audit on the use of the LMA-Supreme for airway management of adult patients undergoing elective orthopedic surgery in the prone position. Br J Anaesth. 2010;105:228–232.
108 Lopez AM, Valero R, Brimacombe J. Insertion and use of the LMA Supreme in the prone position. Anaesthesia. 2010;65:154–157.
109 Seet E, Rajeev S, Firoz T, et al. Safety and efficacy of laryngeal mask airway Supreme versus laryngeal mask airway ProSeal: A randomised controlled trial. Eur J Anaesthesiol. 2010;27:602–660.
110 Keller C, Brimacombe J. Mucosal pressure and oropharyngeal leak pressure with the ProSeal versus the classic laryngeal mask airway. Br J Anaesth. 2000;85:262–266.
111 Hosten T, Gurkan Y, Ozdamar D, et al. A new supraglottic airway device: LMA-Supreme, comparison with LMA ProSeal. Acta Anaesthesiol Scand. 2009;53:852–857.
112 Goldmann K, Hoch N, Wulf H. Influence of neuromuscular blockade on the airway leak pressure of the Proseal laryngeal mask airway. Anaesthesiol Intensivemed Notfallmed Schmerzther. 2006;41:228–232.
113 Strube PJ. The LMA Supreme and LMA ProSeal. Anaesthesia. 2009;64:691.
114 Verghese C, Berlet J, Kapila A, Pollard R. Clinical assessment of the single use laryngeal mask airway: The LMA-Unique. Br J Anaesth. 1998;80:677–679.
115 Verghese C, Smith TCG, Young E. Prospective survey of the use of the laryngeal mask airway in 2359 patients. Anaesthesia. 1993;48:58–60.
116 Charters P, O’Sullivan E. The dedicated airway: A review of the concept and update of current practice. Anaesthesia. 1999;54:778–786.
117 Hawkins M, O’Sullivan E, Charters P. Fibreoptic intubation using the cuffed oropharyngeal airway and Aintree intubation catheter. Anaesthesia. 1998;53:891–894.
118 Atherton DPL, O’Sullivan E, Lowe D, Charters P. A ventilation-exchange bougie for fibreoptic intubations with the laryngeal mask airway. Anaesthesia. 1996;51:1123–1126.
119 Zura A, Doyle DJ, Orlandi M. Use of the Aintree intubation catheter in a patient with an expected difficult airway. Can J Anaesthesia. 2005;52:646–649.
120 Higgs A, Clark E, Premraj K. Low-skill fibreoptic intubation: Use of the Aintree catheter with the Classic LMA. Anaesthesia. 2005;60:915–920.
121 Cook TM, Silsby J, Simpson TP. Airway rescue in acute upper airway obstruction using a Proseal Laryngeal mask airway and an Aintree catheter: A review of the ProSeal laryngeal mask airway in the management of the difficult airway. Anaesthesia. 2005;60:1129–1136.
122 Cook TM, Bigwood B, Cranshaw J. Complications of transtracheal jet ventilation and use of the Aintree Intubating Catheter for airway rescue. Anaesthesia. 2006;61:692–697.
123 Brimacombe J, Keller C, Judd DV. Gum elastic bougie-guided insertion of the ProSeal laryngeal mask airway is superior to the digital and introducer tool techniques. Anesthesiology. 2004;100:25–29.
124 Cook TM, Lee G, Nolan JP. The Proseal laryngeal mask airway: A review of the literature. Can J Anaesthesia. 2005;52:739–760.
125 Blair EJ, Mihai R, Cook TM. Tracheal intubation via the Classic and Proseal laryngeal mask airways: A manikin study using the Aintree Intubating Catheter. Anaesthesia. 2007;62:385–387.
126 McHardy FE, Chung F. Postoperative sore throat: Cause, prevention and treatment. Anaesthesia. 1999;54:444–453.
127 Asai T, Murao K, Yukawa H, Shingu K. Re-evaluation of appropriate size of the laryngeal mask airway. Br J Anaesth. 1999;83:478–479.
128 Figueredo E, Vivar-Diago M, Munoz-Blanco F. Laryngo-pharyngeal complaints after use of the laryngeal mask airway. Can J Anaesth. 1999;46:220–225.
129 Inomata S, Nishikiwa T, Suga A, Yamashita S. Transient bilateral vocal cord paralysis after insertion of a laryngeal mask airway. Anesthesiology. 1995;82:787–788.
130 Lloyd Jones FR, Hegab A. Recurrent laryngeal nerve palsy after laryngeal mask insertion. Anaesthesia. 1996;51:171–172.
131 Morikawa M. Vocal paralysis after use of the LM. J Clin Anesth. 1992;16:1194.
132 Rieger A, Brunne B, Hass I, et al. Laryngo-pharyngeal complaints following laryngeal mask airway and endotracheal intubation. J Clin Anesth. 1997;9:42–47.
133 Wynn JM, Jones KL. Tongue cyanosis after laryngeal mask insertion. Anesthesiology. 1994;80:1403–1404.
134 Brimacombe J, Berry A. Laryngeal mask airway cuff pressure and position during anaesthesia lasting one to two hours. Can J Anaesth. 1994;41:589–593.
135 Brimacombe J, Holyoake L, Keller C, et al. Pharyngolaryngeal, neck, and jaw discomfort after anesthesia with the face mask and laryngeal mask airway at high and low cuff volumes in males and females. Anesthesiology. 2000;93:26–31.
136 Grady DM, McHardy F, Wong J, et al. Pharyngolaryngeal morbidity with the laryngeal mask airway in spontaneously breathing patients: Does size matter? Anesthesiology. 2001;94:760–766.
137 Keller C, Sparr HJ, Brimacombe JR. Laryngeal mask lubrication: A comparative study of saline versus 2% lignocaine gel with cuff pressure control. Anaesthesia. 1997;52:592–597.
138 Brimacombe J, Holyoake L, Keller C, et al. Emergence characteristics and postoperative laryngopharyngeal morbidity with the laryngeal mask airway: A comparison of high versus low initial cuff volume. Anaesthesia. 2000;55:338–343.
139 Ouellette RG. The effect of nitrous oxide on laryngeal mask cuff pressure. AANA J. 2000;68:411–414.
140 Cohen MM, Duncan PG, Pope WDB, Wolkenstein C. A survey of 112000 anaesthetics at one teaching hospital. Can Anaesth Soc J. 1986;33:22–31.
141 Leigh JM, Tytler JA. Admissions to the intensive care unit after complications of anaesthetic techniques over 10 years. Anaesthesia. 1990;45:814–820.
142 Olsson GL, Hallen B, Hambraeus Jonzon K. Aspiration during anesthesia: A computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand. 1986:84–92.
143 Tiret L, Desmonts JM, Hatton F, Vourc’h G. Complications associated with anaesthesia: A prospective survey in France. Can Anaesth Soc J. 1986;33:336–344.
144 Warner MA, Warner WE, Webber JG. Clinical significance of pulmonary aspirations during the perioperative period. Anesthesiology. 1993;78:56–62.
145 Brimacombe J, Berry A. The incidence of aspiration associated with the laryngeal mask airway: A meta-analysis of published literature. J Clin Anesth. 1995;7:297–305.
146 Verghese C, Brimacombe J. Survey of laryngeal mask usage in 11910 patients: Safety and efficacy for conventional and nonconventional usage. Anesth Analg. 1996;82:129–133.
147 Bernardini A, Natalini G. Risk of pulmonary aspiration with laryngeal mask airway and tracheal tube: Analysis on 65 712 procedures with positive pressure ventilation. Anaesthesia. 2009;64:1289–1294.
148 Brain AIJ, Brimacombe JR. Alternative insertion techniques. In: Brimacombe JR, Brain AIJ, Berry AM. The laryngeal mask airway: A review and practical guide. Philadelphia: WB Saunders; 1997:83–85.
149 Devitt JH, Wenstone R, Noel AG, O’Donnell RRT. The laryngeal mask airway and positive-pressure ventilation. Anesthesiology. 1994;80:550–555.
150 Epstein RH, Ferouz F, Jenkins MA. Airway sealing pressures of the laryngeal mask airway in children. Anesthesiology. 1994;81:A1322.
151 Epstein RH, Ferouz F, Jenkins MA. Airway sealing pressures of the laryngeal mask in pediatric patients. J Clin Anesth. 1996;8:93–98.
152 Van Damme E. Die Kehlkopfmaske in der ambulanten Anasthesia: Eine Antwertung vom 5000 ambulanten Narkosen. Anasthesiol Intensivmed Notfallmed Schmerzther. 1994;29:284–286.
153 Brimacombe J, Archdeacon J. The laryngeal mask airway for unplanned prolonged procedures. Can J Anaesth. 1995;42:1176.
154 Brimacombe J, Shorney N. The laryngeal mask airway and prolonged balanced anesthesia. Can J Anaesth. 1993;40:360–364.
155 Arosio EM, Conci F. Use of the laryngeal mask airway for respiratory distress in the intensive care unit. Anaesthesia. 1995;50:635–636.
156 Braun U, Zerbst M, Fullekrug B, et al. A comparison of the Proseal laryngeal mask to the standard laryngeal mask on anesthesized, non-relaxed patients. Anasthesiol Intensivmed Notfallmed Schmerzther. 2002;37:727–733.
157 Hamakawa T. Sore throat after the use of the LM. J Clin Anesth. 1993;17:245–246.
158 Chen CH, Lin CC, Tan PP. Clinical experience of laryngeal mask airway in lateral position during anesthesia. Acta Anaesthesiol Sin. 1995;33:31–34.
159 Elias M. Laryngeal mask airway and radiotherapy in the prone position. Anaesthesia. 1992;47:1005.
160 Lim W, Cone AM. Laryngeal mask and the prone position. Anaesthesia. 1994;49:542.
161 Milligan KA. Laryngeal mask in the prone position. Anaesthesia. 1994;49:449.
162 Ngan Kee WD. Laryngeal mask airway for radiotherapy in the prone position. Anaesthesia. 1992;47:446–447.
163 Sidhu VS. Laryngeal mask airway for anesthesia in the prone position. Anaesth Intensive Care. 1992;20:119.
164 Brain A. Thumb insertion technique. In: Brimacombe JR, Brain AIJ, Berry AM. The laryngeal mask airway: A review and practical guide. Philadelphia: WB Saunders; 1997:287.
165 McCaughey W, Bhanumurthy S. Laryngeal mask placement in the prone position. Anaesthesia. 1993;48:1104–1105.
166 Khoo ST. The laryngeal mask airway: An unusual complication. Anaesth Intensive Care. 1993;21:249–250.
167 Vickers R, Springer A, Hindmarsh J. Problem with the laryngeal mask airway. Anaesthesia. 1994;45:892.
168 Squires SJ, Woods K. Fragmented laryngeal mask airway. Anaesthesia. 1992;47:274.
169 Martin DW. Kinking of the laryngeal mask airway in two children. Anaesthesia. 1990;45:488.
170 George A. Failed cuff inflation of a laryngeal mask. Anaesthesia. 1994;49:80.
171 Richards JT. Pilot tube of the laryngeal mask airway. Anaesthesia. 1994;49:450–451.
172 Combes X, Le Roux B, Suen P, et al. Unanticipated difficult airway in anesthetized patients: Prospective validation of a management algorithm. Anesthesiology. 2004;5:1146–1150.