Chapter 12B Language and Speech Disorders
Motor Speech Disorders: Dysarthria and Apraxia of Speech
Motor Speech Disorders: Overview
Motor speech disorders are syndromes of abnormal articulation, the motor production of speech, without abnormalities of language. A patient with a motor speech disorder can produce normal expressive language in writing and can comprehend both spoken and written language. If a listener transcribes the speech of a patient with a motor speech disorder into print or type and then reads it, the text should sound normal. Motor speech disorders include dysarthrias, disorders of speech articulation, and apraxia of speech, a motor programming disorder for speech, as well as four rarer syndromes: aphemia, the foreign accent syndrome, acquired stuttering, and the opercular syndrome. Duffy (1995), in an analysis of speech and language disorders at the Mayo Clinic, reported that 46.3% of the patients had dysarthria, 27.1% aphasia, 4.6% apraxia of speech, 9% other speech disorders (such as stuttering), and 13% other cognitive or linguistic disorders.
Dysarthrias
Dysarthrias involve the abnormal articulation of sounds or phonemes. The pathogenic mechanism in dysarthria is abnormal neuromuscular activation of the speech muscles, affecting the speed, strength, timing, range, or accuracy of movements involving speech (Duffy, 1995). The most consistent finding in dysarthria is the distortion of consonant sounds. Speech disorders can be mechanical, as in patients with physical disorders of the pharynx and larynx, but dysarthrias are neurogenic, related to dysfunction of the central nervous system, nerves, neuromuscular junction, or muscle, with a contribution of sensory deficits in some cases. Dysarthria can affect not only articulation but also phonation, breathing, and prosody (emotional tone) of speech. Total loss of ability to articulate is called anarthria.
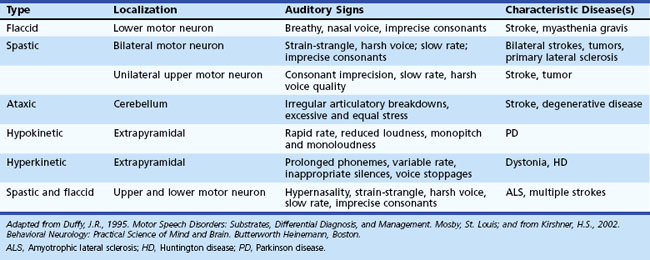
The Mayo Clinic classification of dysarthria (Duffy, 1995), widely used in the United States, includes six categories: (1) flaccid, (2) spastic and “unilateral upper motor neuron,” (3) ataxic, (4) hypokinetic, (5) hyperkinetic, and (6) mixed dysarthria. These types of dysarthria are summarized in Table 12B.1.
A milder variant of spastic dysarthria, unilateral upper motor neuron (UUMN) dysarthria, is associated with unilateral upper motor neuron lesions, such as strokes (Duffy, 1995). This type of dysarthria has similar features to spastic dysarthria, but UUMN dysarthria is less severe. Unilateral upper motor neuron dysarthria is one of the most common types of dysarthria, since it occurs in patients with unilateral strokes. In a study by Urban and colleagues (2006), left hemisphere strokes were more likely than right hemisphere strokes to be associated with dysarthria.
Ataxic dysarthria, associated with cerebellar disorders, is characterized by one of two patterns: irregular breakdowns of speech with explosions of syllables interrupted by pauses, or a slow cadence of speech with excessively equal stress on every syllable. The second pattern of ataxic dysarthria is referred to as scanning speech. A patient with ataxic dysarthria, attempting to repeat the phoneme /p/ as rapidly as possible, for example, produces either an irregular rhythm, resembling popcorn popping, or a very slow rhythm. Causes of ataxic dysarthria include cerebellar strokes, tumors, multiple sclerosis, and cerebellar and spinocerebellar degenerations. A recent report localized ataxic dysarthria to the upper cerebellar loci of the quadrangularis and simplex lobules by magnetic resonance imaging (MRI) (Ogawa et al., 2010).
Hypokinetic dysarthria, the typical speech pattern in Parkinson disease (PD), is notable for decreased and monotonous loudness and pitch, rapid rate, and occasional consonant errors. In a study of brain activation by positron emission tomography (PET) methodology, Liotti and colleagues (2003) described premotor and supplementary motor area activation in patients with PD and untreated hypokinetic dysarthria, but not in normal subjects. After completion of a voice treatment protocol, these premotor and motor activations diminished, whereas right-sided basal ganglia activations increased.
The management of dysarthria includes speech therapy techniques to strengthen muscles, train more precise articulations, slow the rate of speech to increase intelligibility, or teach the patient to stress specific phonemes. Devices such as pacing boards to slow articulation, palatal lifts to reduce hypernasality, amplifiers to increase voice volume, communication boards for subjects to point to pictures, and augmentative communication devices and computer techniques can be used when the patient is unable to communicate in speech. Visual cues may help dysarthric speakers become more intelligible (Hustad and Garcia, 2005). Surgical procedures such as a pharyngeal flap to reduce hypernasality or vocal fold Teflon injection or transposition surgery to increase loudness may help the patient speak more intelligibly. Treatment of PD can improve dysarthria in terms of both speech therapy and pharmacological treatments (DeLetter et al., 2005; Pinto et al., 2004; Trail et al., 2005), but surgical or deep brain stimulation procedures occasionally result in worsened intelligibility (Farrell et al., 2005; Guehl et al., 2006). In general, few randomized trials have examined the efficacy of speech therapy techniques for dysarthria (Sellars et al., 2005). A more detailed discussion of dysarthria can be found in Kirshner (2002).
Apraxia of Speech
Apraxia of speech is a disorder of the programming of articulation of sequences of phonemes, especially consonants. The motor speech system makes errors in selection of consonant phonemes in the absence of any weakness, slowness, or incoordination of the muscles of speech articulation. The term apraxia of speech implies that the disorder is one of a skilled, sequential motor activity (as in other apraxias) rather than a primary motor disorder. Consonants frequently are substituted rather than distorted, as in dysarthria. Patients have special difficulty with polysyllabic words and consonant shifts as well as in initiating articulation of a word. Errors are inconsistent from one attempt to the next, in contrast with the consistent distortion of phonemes in dysarthria (Ogar et al., 2005).
The four cardinal features of apraxia of speech are (1) effortful, groping, or trial-and-error attempts at speech with efforts at self-correction, (2) dysprosody, (3) inconsistencies in articulation errors, and (4) difficulty with initiating utterances. Usually the patient has the most difficulty with the first phoneme of a polysyllabic utterance. The patient may make an error in attempting to produce a word on one trial, a different error the next time, and a normal utterance the third time. Apraxia of speech involves primarily consonant transitions; vowels are relatively unaffected (Jacks et al., 2010). A study of the diagnosis of apraxia of speech by speech pathologists viewing videoclips of patients showed very good reliability and consistency of diagnosis (Mumby et al., 2007).
Apraxia of speech is rare in isolated form, but it frequently contributes to the speech and language deficit of Broca aphasia. A patient with apraxia of speech in addition to aphasia often can write better than he or she can speak, and comprehension is relatively preserved. Dronkers (1996) presented evidence from CT and MRI scans indicating that although the anatomical lesions vary, patients with apraxia of speech virtually always have damage in the left hemisphere insula, whereas patients without apraxia of speech do not. More recent MRI correlations of apraxia of speech in acute stroke patients by Hillis and colleagues (2004), however, have pointed to the traditional Broca area in the left frontal cortex as the site of damage in apraxia of speech.
Apraxia of speech also occurs in neurodegenerative disorders. In a recent review of 17 cases of progressive aphasia or apraxia of speech, Josephs and colleagues (2006) found apraxia of speech in 11 cases, all with conditions associated with tau pathology, such as progressive supranuclear palsy, corticobasal degeneration, and frontotemporal dementia or Pick disease. Voxel-based morphometric studies of the MRIs from these patients showed predominant atrophy in the precentral and supplementary cortices with apraxia of speech and in the perisylvian cortex in primary progressive aphasia.
Other Motor Speech Disorders
Aphemia
Another consideration in the differential diagnosis with both apraxia of speech and dysarthria is the syndrome of aphemia. Broca first used this term (aphemie in French) to designate the syndrome later called Broca aphasia, but in recent years, aphemia has been reserved for a syndrome of near-muteness, with normal comprehension, reading, and writing. Aphemia clearly is a motor speech disorder rather than an aphasia if written language and comprehension are indeed intact. Patients often are anarthric with no speech whatever, and then effortful, nonfluent speech emerges. Some patients have persisting dysarthria with dysphonia and sometimes distortions of articulation that sound similar to foreign accents (as described next). Pure anarthria may be associated with lesions of the face area of the motor cortex. Functional imaging studies also suggest that articulation is mediated at the level of the primary motor face area (Riecker et al., 2000), and disruption of speech articulation can be produced by transcranial magnetic stimulation over the motor face area (Epstein et al., 1999). Controversy remains as to whether aphemia is equivalent to apraxia of speech. In general, aphemia is likely to involve lesions in the vicinity of the primary motor cortex and perhaps the Broca area, whereas apraxia of speech may be localized to the insula (although, see Hillis et al., 2004).
Foreign Accent Syndrome
The foreign accent syndrome is an acquired form of motor speech disorder related to the dysarthrias, in which the patient acquires a dysfluency resembling a foreign accent, usually after a unilateral stroke (Kurowski et al., 1996). The syndrome has also been reported in multiple sclerosis (Chanson et al., 2009), traumatic brain injury (Lippert-Gruener et al., 2005), and in the degenerative disorder known as primary progressive aphasia or frontotemporal dementia (Luzzi et al., 2008). Lesions may involve the motor cortex of the left hemisphere. The dysfluency also can be mixed with aphasia.
Acquired Stuttering
Another uncommon motor speech disorder associated with acquired brain lesions is a pattern resembling developmental stuttering, referred to as acquired stuttering or cortical stuttering. Both developmental and acquired stuttering are associated with hesitancy in producing initial phonemes, pauses in speech, contortions of the face, and sometimes repetition of phonemes and associated dysrhythmia of speech. Acquired stuttering clearly overlaps with apraxia of speech but may lack the other features of apraxia of speech. Acquired stuttering has been described most often in patients with left hemisphere cortical strokes (Franco et al., 2000; Sahin et al., 2005; Turgut et al., 2002), but the syndrome also has been reported with subcortical lesions including infarctions of the pons, basal ganglia, and subcortical white matter (Ciabarra et al., 2000). Acquired stuttering also follows traumatic brain injury (Yeoh et al., 2006) and seizures, especially involving the supplementary motor area (Chung et al., 2004).
The neurobiology of developmental stuttering, a much more common disorder than acquired stuttering, is poorly understood. Stuttering appears to be a genetic disorder involving mutations in genes governing lysosomal metabolism (Kang et al., 2010). A functional MRI study suggested that patterns of cerebral activation during articulation in persons who stutter are different from those in normal persons, with greater right hemisphere activation in stutterers (Van Borsel et al., 2003). PET imaging studies have revealed either reduced left perisylvian hypometabolism (Wu et al., 1998), or increased right hemisphere activation (Fox et al., 2000). Abnormalities in the basal ganglia and in dopamine metabolism have also been suggested (Wu et al., 1997). MRI studies with diffusion anisotropy have suggested delayed or impaired myelination (Cykowski et al., 2010).
The treatment of stuttering involves behavioral techniques (reviewed in Prins and Ingram, 2009); altered auditory feedback techniques (Antipova et al., 2008; Lincoln et al., 2010); and pharmacological therapies with dopamine-blocking drugs such as risperidone, olanzapine (Maguire et al., 2004), or pimozide (Stager et al., 2005), or selective serotonin reuptake inhibitors such as paroxetine (Busan et al., 2009). A new antagonist at the γ-aminobutyric acid (GABA)-A receptor, pagoclone, currently under testing for anxiety disorders, has shown positive results in stuttering (Maguire et al., 2010). Overall, however, pharmacological therapies for stuttering have had limited success (Prasse and Kikano, 2008). In very refractory cases, deep brain stimulation in the vicinity of the centromedial thalamus may be of help (Bhatnagar and Andy, 1989, Bhatnagar and Mandybur, 2005).
Opercular Syndrome
The opercular syndrome, also called Foix-Chavany-Marie syndrome or cheiro-oral syndrome (Bakar et al., 1998), is a severe form of pseudobulbar palsy in which patients with bilateral lesions of the perisylvian cortex or subcortical connections become completely mute. These patients can follow commands involving the extremities but not those mediated by the cranial nerves. For example, they may be unable to open or close the eyes or mouth or to smile voluntarily, yet they smile when amused, yawn spontaneously, and even utter cries in response to emotional stimuli. The ability to follow limb commands shows that the disorder is not an aphasic disorder of comprehension. The discrepancy between automatic activation of the cranial musculature and inability to perform the same actions voluntarily has been called an automatic-voluntary dissociation.
Antipova E.A., Purdy S.C., Blakeley M., et al. Effects of altered auditory feedback (AAF) on stuttering frequency during monologue speech production. J Fluency Disord. 2008;33:274-290.
Bakar M., Kirshner H.S., Niaz F. The opercular-subopercular syndrome: four cases with review of the literature. Behav Neurol. 1998;11:97-103.
Bhatnagar S., Andy O. Alleviation of acquired stuttering with human centromedian thalamic stimulation. J Neurol Neurosurg Psychiatry. 1989;52:1182-1184.
Bhatnagar S., Mandybur G. Effects of intralaminar thalamic stimulation on language functions. Brain Lang. 2005;92:1-11.
Busan P., Battaglini P.P., Borelli M., et al. Investigating the efficacy of paroxetine in developmental stuttering. Clin Neuropharmacol. 2009;32:183-188.
Chanson J.B., Kremer S., Blanc F., et al. Foreign accent syndromes as a first sign of multiple sclerosis. Mult Scler. 2009;15:1123-1125.
Chung S.J., Im J.H., Lee J.H., et al. Stuttering and gait disturbance after supplementary motor seizure. Mov Disord. 2004;19:1106-1109.
Ciabarra A.M., Elkind M.S., Roberts J.K., et al. Subcortical infarction resulting in acquired stuttering. J Neurol Neurosurg Psychiatry. 2000;69:546-549.
Cykowski M.D., Fox P.T., Ingham R.J., et al. A study of the reproducibility and etiology of diffusion anisotropy difference in developmental stuttering: A potential role for impaired myelination. Neuroimage. 2010. May 13 (Epub ahead of print)
DeLetter M., Santens P., van Borsel J. The effects of levodopa on word intelligibility in Parkinson’s disease. J Commun Disord. 2005;38:187-196.
Dronkers N.F. A new brain region for coordinating speech articulation. Nature. 1996;384:159-161.
Duffy J.R. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management. St. Louis: Mosby; 1995.
Epstein C.M., Meador K.J., Loring D.W., et al. Localization and characterization of speech arrest during transcranial magnetic stimulation. Clin Neurophysiol. 1999;110:1073-1079.
Farrell A., Theodoros D., Ward E., et al. Effects of neurosurgical management of Parkinson’s disease on speech characteristics and oromotor function. J Speech Hear Res. 2005;48:5-20.
Fox P., Ingham R., Ingham J., et al. Brain correlates of stuttering and syllable production: a PET performance-correlational analysis. Brain. 2000;123:1985-2004.
Franco E., Casado J.L., Lopez Dominguez J.M., et al. Stuttering as the only manifestation of a cerebral infarct. Neurologia. 2000;15:414-416.
Guehl D., Cuny E., Benazzouz A., et al. Side-effects of subthalamic stimulation in Parkinson’s disease: clinical evolution and predictive factors. Eur J Neurol. 2006;13:963-971.
Hillis A.E., Work M., Barker P.B., et al. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479-1487.
Hustad K.C., Garcia J.M. Aided and unaided speech supplementation strategies: effect of alphabet cues and iconic hand gestures on dysarthric speech. J Speech Lang Hear Res. 2005;48:996-1012.
Jacks A., Mathes K.A., Marquardt T.P. Vowel acoustics in adults with apraxia of speech. J Speech Lang Hear Res. 2010;53:61-74.
Josephs K.A., Duffy J.R., Strand E.A., et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385-1398.
Kang C., Riazuddin S., Mundorff J., et al. Mutations in the lysosomal enzyme-targeting pathway and persistent stuttering. New Engl J Med. 2010;362:677-685.
Kirshner H.S. Behavioral Neurology: Practical Science of Mind and Brain. Boston: Butterworth Heinemann; 2002.
Kurowski K.M., Blumstein S.E., Alexander M. The foreign accent syndrome: a reconsideration. Brain Lang. 1996;54:1-25.
Lincoln M., Packman A., Onslow M., et al. An experimental investigation of the effect of AAF on the conversational speech of adults who stutter. J Speech Lang Hear Res. 2010;53:1122-1131.
Liotti M., Ramig L.O., Vogel D., et al. Hypophonia in Parkinson’s disease. Neural correlates of voice treatment revealed by PET. Neurology. 2003;60:432-440.
Lippert-Gruener M., Weinert U., Greisbach T., et al. Foreign accent syndrome following traumatic brain injury. Brain Inj. 2005;19:955-958.
Luzzi S., Viticchi G., Piccirilli M., et al. Foreign accent syndrome as the initial sign of primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2008;79:79-81.
Maguire G.A., Yu B.P., Franklin D.L., et al. Alleviating stuttering with pharmacological interventions. Expert Opin Pharmacother. 2004;5:1565-1571.
Maguire G., Franklin D., Vatakis N.G., et al. Exploratory randomized clinical study of pagoclone in persistent developmental stuttering: the Examining Pagoclone for Persistent Developmental Stuttering Study. J Clin Psychopharmacol. 2010;30:48-56.
Mumby K., Bowen A., Hesketh A. Apraxia of speech: how reliable are speech and language therapists’ diagnoses? Clin Rehabil. 2007;21:760-767.
Ogar J., Slama H., Dronkers N., et al. Apraxia of speech: an overview. Neurocase. 2005;11:427-432.
Ogawa K., Yoshihashi H., Suzuki Y., et al. Clinical study of the responsible lesion for dysarthria in the cerebellum. Intern Med. 2010;49:861-864.
Pinto S., Ozsancak C., Tripoliti E., et al. Treatments for dysarthria in Parkinson’s disease. Lancet Neurol. 2004;3:547-556.
Prasse J.E., Kikano G.E. Stuttering: an overview. Am Fam Physician. 2008;77:1271-1276.
Prins D., Ingham R.J. Evidence-based treatment and stuttering—historical perspective. J Speech Lang Hear Res. 2009;52:254-263.
Riecker A., Ackermann H., Wildgruber D., et al. Articulatory/phonetic sequencing at the level of the anterior perisylvian cortex: a functional magnetic resonance imaging (fMRI) study. Brain Lang. 2000;75:259-276.
Sahin H.A., Krespi Y., Yilmaz A., et al. Stuttering due to ischemic stroke. Behav Neurol. 2005;16:37-39.
Sellars, C., Hughes, T., Langhorne, P., 2005. Speech and language therapy for dysarthria due to non-progressive brain damage. Cochrane Database Syst Rev, vol. 3, CD002088.
Stager S.V., Calis K., Grothe D., et al. Treatment with medications affecting dopaminergic and serotonergic mechanisms: effects on fluency and anxiety in persons who stutter. J Fluency Disord. 2005;30:319-335.
Trail M., Fox C., Ramig L.O., et al. Speech treatment for Parkinson’s disease. Neuro Rehabil. 2005;20:205-221.
Turgut N., Utku U., Balci K. A case of acquired stuttering resulting from left parietal infarction. Acta Neurol Scand. 2002;105:408-410.
Urban P.P., Rolke R., Wicht S., et al. Left-hemispheric dominance for articulation: a prospective study on acute ischaemic dysarthria at different localizations. Brain. 2006;129:767-777.
Van Borsel J., Achten E., Santens P., et al. fMRI of developmental stuttering: a pilot study. Brain Lang. 2003;85:369-376.
Wu J., Maguire G., Riley G., et al. Increased dopamine activity associated with stuttering. Neuroreport. 1997;8:767-770.
Wu J., Maguire G., Riley G., et al. A positron emission tomography [F18] deoxyglucose study of developmental stuttering. Neuroreport. 1998;6:501-505.
Yeoh H.K., Lind C.R., Law A.J. Acute transient cerebellar dysfunction and stuttering following mild closed head injury. Child’s Nerv Syst. 2006;22:310-313.