Chapter 12A Language and Speech Disorders
Aphasia and Aphasic Syndromes
Language Disorders: Overview
Definitions
Aphasia is defined as a disorder of language acquired secondary to brain damage. This definition, adapted from Alexander and Benson (1997), separates aphasia from several related disorders. First, aphasia is distinguished from congenital or developmental language disorders, called dysphasias. (In contrast with British usage, in the United States the term dysphasia applies to developmental language disorders rather than partial or incomplete aphasia.)
Third, aphasia is distinguished from disorders of thought. Thought involves the mental processing of images, memories, and perceptions, usually but not necessarily involving language symbols. Psychiatric disorders derange thought and alter the content of speech without affecting its linguistic structure. Schizophrenic patients, for example, may manifest bizarre and individualistic word choices, with loose associations and a loss of organization in discourse together with vague or unclear references and communication failures (Docherty et al., 1996). Elementary language and articulation, however, are intact. Abnormal language content in psychiatric disorders is therefore not considered to represent aphasia, because the disorder is more one of thought than of language. Language disorders associated with diffuse brain diseases such as encephalopathies and dementias do qualify as aphasias, but the involvement of other cognitive functions distinguishes them from aphasia secondary to focal brain lesions.
Relevant Neuroanatomy
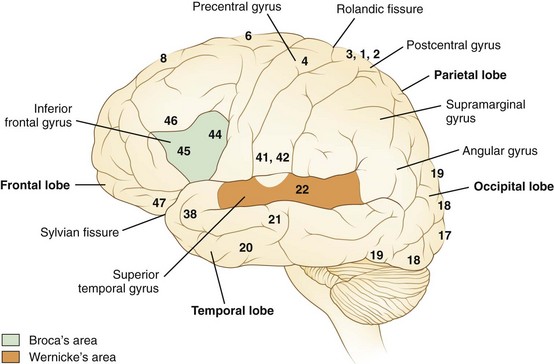
Language processes have a clear neuroanatomical basis. In simplest terms, the reception and processing of spoken language take place in the auditory system, beginning with the cochlea and proceeding through a series of way stations to the auditory cortex, the Heschl gyrus, in each superior temporal gyrus. Decoding sounds into linguistic information involves the posterior part of the left superior temporal gyrus, the Wernicke area or Brodmann area 22, which gives access to a network of cortical associations to assign word meanings. For both repetition and spontaneous speech, auditory information is transmitted to the Broca area in the posterior inferior frontal gyrus. This area of cortex “programs” the neurons in the adjacent motor cortex subserving the mouth and larynx, from which descending axons travel to the brainstem cranial nerve nuclei. The inferior parietal lobule, especially the supramarginal gyrus, also may be involved in phoneme processing in language comprehension and in phoneme production for repetition and speech (Hickok and Poeppel, 2000). These anatomical relationships are shown in Figs. 12A.1 and 12A.2. Reading requires perception of visual language stimuli by the occipital cortex, followed by processing into auditory language information via the heteromodal association cortex of the angular gyrus. Writing involves activation of motor neurons projecting to the arm and hand. A French study that used aphasia testing and magnetic resonance imaging (MRI) scans to evaluate 107 stroke patients confirmed the general themes of nearly 150 years of clinical aphasia research: that frontal lesions caused nonfluent aphasia, whereas posterior temporal lesions affected comprehension (Kreisler et al., 2000).
In right-handed people, and in a majority of left-handers as well, clinical syndromes of aphasia result from left hemisphere lesions. Rarely, aphasia may result from a right hemisphere lesion in a right-handed patient, a phenomenon called crossed aphasia (Bakar et al., 1996). In left-handed persons, language disorders are usually similar to those of right-handed persons with similar lesions, but occasional cases manifest with atypical syndromes that suggest a right hemisphere capability for at least some language functions. For example, a patient with a large left frontotemporoparietal lesion may have preserved comprehension, suggesting right hemisphere language comprehension. For the same reason, recovery from aphasia may be better in some left-handed than in right-handed patients with left hemisphere strokes.
Diagnostic Features
Muteness, a total loss of speech, may represent severe aphasia (see Aphemia later in the chapter). Muteness also can be a sign of dysarthria, frontal lobe dysfunction with akinetic mutism, severe extrapyramidal system dysfunction (as in Parkinson disease), non-neurological disorders of the larynx and pharynx, or even psychiatric syndromes such as catatonia. Caution must therefore be taken in diagnosing the mute patient as aphasic. A good rule of thumb is that if the patient can write or type and the language form and content appear normal, the disorder is probably not aphasic in origin. If the patient cannot speak or write but makes apparent effort to vocalize, and if there is also evidence of deficient comprehension, aphasic muteness is likely. Associated signs of a left hemisphere injury, such as right hemiparesis, also aid in diagnosis. Finally, if the patient gradually begins to make sounds containing paraphasic errors, aphasia can be identified with confidence.
Chronic encephalopathies, or dementias, pose a more difficult diagnostic problem because involvement of the language cortex produces readily detectable language deficits, especially involving naming, reading, and writing. These language disorders (see Language in Dementing Diseases later in this chapter) differ from aphasia secondary to focal lesions mainly by the involvement of other cognitive functions such as memory and visuospatial processes.
Bedside Language Examination
D. Frank Benson and Norman Geschwind popularized a bedside language examination of six parts, updated by Alexander and Benson (1997) (Box 12A.1). This examination provides useful localizing information about brain dysfunction and is well worth the few minutes it takes.
Auditory comprehension is tested first by asking the patient to follow a series of commands of one, two, and three steps. An example of a one-step command is “Stick out your tongue”; a two-step command is “Hold up your left thumb and close your eyes.” Successful following of commands ensures adequate comprehension, at least at this simple level, but failure to follow commands does not automatically establish a loss of comprehension. The patient must hear the command, understand the language the examiner speaks, and possess the motor ability to execute it, including the absence of apraxia. Apraxia (see Chapter 10 for full discussion) is defined operationally as the inability to carry out a motor command despite normal comprehension and normal ability to carry out the motor act in another context, such as for imitation or with use of a real object. Because apraxia is difficult to exclude with confidence, it is advisable to test comprehension by tasks that do not require a motor act, such as yes/no questions, or by commands that require only a pointing response. The responses to nonsense questions (e.g., “Do you vomit every day?”) quickly establish whether the patient comprehends. Nonsense questions often produce surprising results because of the tendency of some aphasics to cover up comprehension difficulty with social chatter.
Repetition of words and phrases should be deliberately tested. Dysarthric patients and those with apraxia of speech (see Chapter 12B) have difficulty with rapid sequences of consonants, such as in “Methodist Episcopal,” whereas aphasic persons have special difficulty with grammatically complex sentences. The phrase “no ifs, ands, or buts” is especially challenging for aphasics. Often, aphasics can repeat familiar or “high-probability” phrases much better than unfamiliar ones.
Aphasic Syndromes
Broca Aphasia
In 1861, the French physician Paul Broca described two patients, establishing the aphasia syndrome that now bears his name. The speech pattern is nonfluent; on bedside examination, the patient speaks hesitantly, often producing the principal meaning-containing nouns and verbs but omitting small grammatical words and morphemes. This pattern is called agrammatism or “telegraphic speech.” An example is “wife come hospital.” Patients with acute Broca aphasia may be mute or may produce only single words, often with dysarthria and apraxia of speech. They make many phonemic errors, inconsistent from utterance to utterance, with substitution of phonemes usually differing only slightly from the correct target (e.g., /p/ for /b/). Naming is deficient, but the patient often manifests a “tip-of-the-tongue” phenomenon, getting out the first letter or phoneme of the correct name. Paraphasic errors in naming more frequently are of literal than of verbal type. Auditory comprehension seems intact, but detailed testing usually reveals some deficiency, particularly in the comprehension of complex syntax. For example, for persons with Broca aphasia, sentences with embedded clauses involving prepositional relationships cause difficulty in comprehension as well as in expression (“The rug that Bill gave to Betty tripped the visitor”). A positron emission tomography (PET) study in normal persons (Caplan et al., 1998) showed activation of the Broca area in the frontal cortex during tests of syntactic comprehension; the Broca area thus appears to be involved in syntactical operations, both expressively and receptively. Repetition is hesitant in these patients, resembling their spontaneous speech. Reading often is impaired despite relatively preserved auditory comprehension. Benson termed this reading difficulty of Broca aphasics the “third alexia,” in contradistinction to the two classical types of alexia (see Aphasic Alexia later in the chapter). Patients with Broca aphasia may have difficulty with syntax in reading, just as in auditory comprehension and speech. Writing is virtually always deficient in Broca aphasics. Most patients have a right hemiparesis necessitating use of the nondominant left hand for writing, but this left-handed writing is far more abnormal than the awkward renditions of a normal right-handed person attempting to write left-handed. Many patients can scrawl only a few letters.
Associated neurological deficits of Broca aphasia include right hemiparesis, hemisensory loss, and apraxia of the oral apparatus and the nonparalyzed left limbs. Apraxia in response to motor commands is important to recognize because it may be mistaken for comprehension disturbance. As mentioned earlier, comprehension should also be tested by responses to yes/no questions or commands to point to an object. The common features of Broca aphasia are listed in Table 12A.1.
| Feature | Syndrome |
|---|---|
| Spontaneous speech | Nonfluent, mute or telegraphic, usually dysarthric |
| Naming | Impaired |
| Comprehension | Intact (mild difficulty with complex grammatical phrases) |
| Repetition | Impaired |
| Reading | Often impaired (“third alexia”) |
| Writing | Impaired (dysmorphic, dysgrammatical) |
| Associated signs | Right hemiparesis |
| Right hemisensory loss | |
| ± Apraxia of left limbs |
An important clinical feature of Broca aphasia is its frequent association with depression (Robinson, 1997). Patients with Broca aphasia typically are aware of and frustrated by their deficits. At times they become withdrawn and refuse help or therapy. Usually the depression lifts with recovery from the deficit, but it may be a limiting factor in rehabilitation.
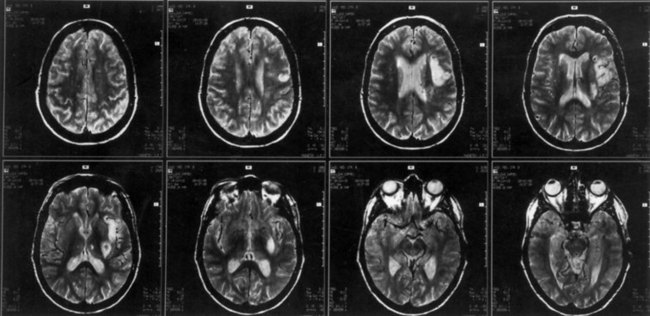
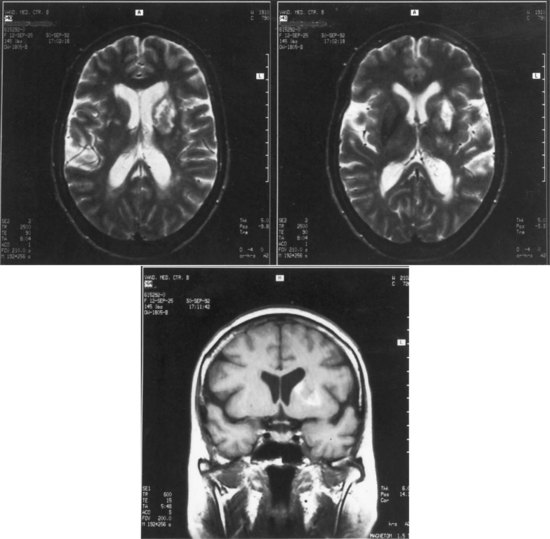
The lesions responsible for Broca aphasia usually include the traditional Broca area in the posterior part of the inferior frontal gyrus, along with damage to adjacent cortex and subcortical white matter. Most patients with lasting Broca aphasia, including Broca’s original cases, have much larger left frontoparietal lesions, including most of the territory of the upper division of the left middle cerebral artery. In such patients, the deficit typically evolves from global to Broca aphasia over weeks to months. Patients who manifest Broca aphasia immediately after their strokes, by contrast, have smaller lesions of the inferior frontal region, and their deficits generally resolve quickly. In computed tomography (CT) scan analyses at the Boston Veterans Administration Medical Center, lesions restricted to the lower precentral gyrus produced only dysarthria and mild expressive disturbance. Lesions involving the traditional Broca area (Brodmann areas 44 and 45) resulted in difficulty initiating speech, and lesions combining the Broca area, the lower precentral gyrus, and subcortical white matter yielded the full syndrome of Broca aphasia. In other studies at the center, damage to two subcortical white matter sites—the rostral subcallosal fasciculus deep to the Broca area and the periventricular white matter adjacent to the body of the left lateral ventricle—was required to cause permanent nonfluency. These concepts concerning the Broca area and its mainly temporary role in Broca aphasia have been confirmed by a recent MRI study, indicating that MRI lesions in the Broca area correlate with Broca or global aphasia in acute stroke, but not in the chronic period (Ochfeld et al., 2010). Fig. 12A.3 shows an MRI scan of the brain from a patient with Broca aphasia.
Wernicke Aphasia
Associated signs are limited in Wernicke aphasia; most patients have no elementary motor or sensory deficits, although a partial or complete right homonymous hemianopia may be present. The characteristic bedside examination findings in Wernicke aphasia are summarized in Table 12A.2.
| Feature | Syndrome |
|---|---|
| Spontaneous speech | Fluent with paraphasic errors; usually not dysarthric, sometimes logorrheic |
| Naming | Impaired (often bizarre paraphasic misnaming) |
| Comprehension | Impaired |
| Repetition | Impaired |
| Reading | Impaired for comprehension, reading aloud |
| Writing | Well formed, paragraphic |
| Associated signs | ± Right hemianopia |
| Motor, sensory signs usually absent |
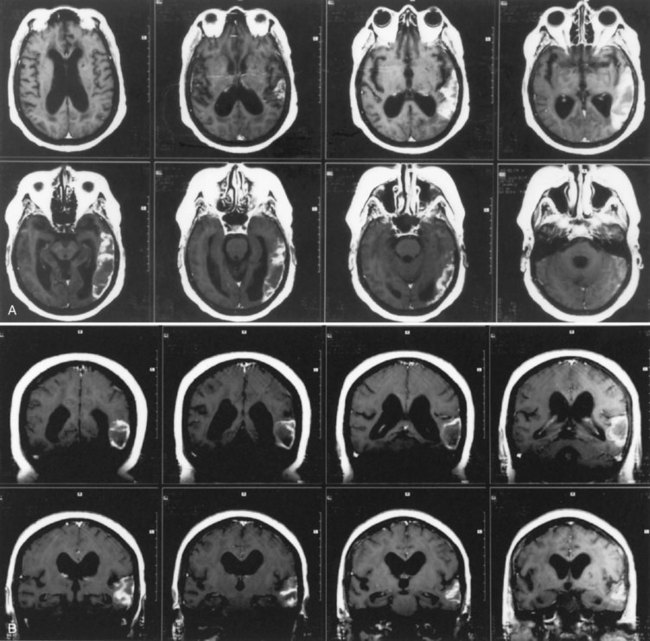
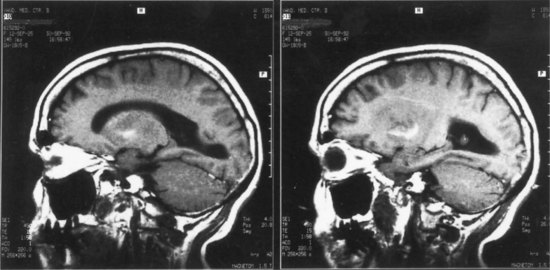
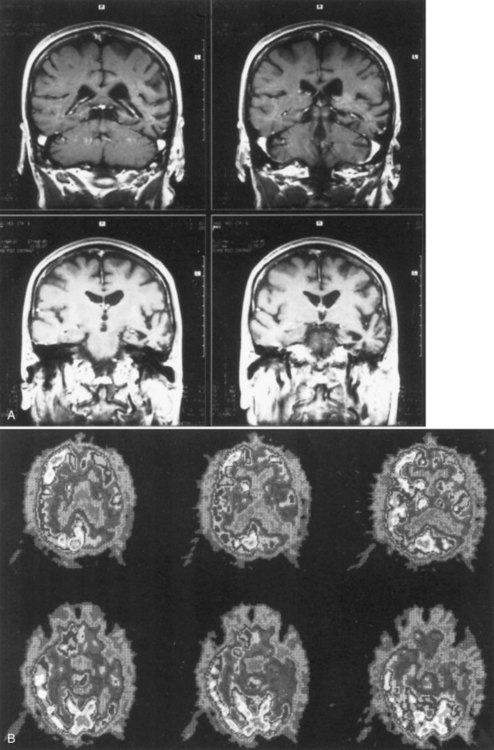
The lesions of patients with Wernicke aphasia usually involve the posterior portion of the superior temporal gyrus, sometimes extending into the inferior parietal lobule. Fig. 12A.4 shows a typical example. The exact confines of the Wernicke area have been much debated. Damage to this area (Brodmann area 22) has been reported to correlate most closely with persistent loss of comprehension of single words, although only larger temporoparietal lesions have been found in patients with lasting Wernicke aphasia. In the acute phase, the ability to match a spoken word to a picture is quantitatively related to decreased perfusion of the Wernicke area on perfusion-weighted MRI, indicating less variability during the acute phase than after recovery has taken place (Hillis et al., 2001). Electrical stimulation of the Wernicke area produces consistent interruption of auditory comprehension, supporting the importance of this region for decoding auditory language. A receptive speech area in the left inferior temporal gyrus has also been suggested by electrical stimulation studies and by a few descriptions of patients with seizures involving this area (Kirshner et al., 1995), but aphasia has not been recognized with destructive lesions of this area. Extension of the lesion of Wernicke aphasia into the inferior parietal region may predict greater involvement of reading comprehension. In terms of vascular anatomy, the Wernicke area lies within the territory of the inferior division of the left middle cerebral artery.
Global Aphasia
Global aphasia may be thought of as a summation of the deficits of Broca aphasia and Wernicke aphasia. Speech is nonfluent or mute, but comprehension also is poor, as are naming, repetition, reading, and writing. Most patients have dense right hemiparesis, hemisensory loss, and often hemianopia, although occasional patients have mild or no hemiparesis. Milder aphasic syndromes in which all modalities of language are affected often are called mixed aphasias. The lesions of patients with global aphasia are usually large, involving both the inferior frontal and superior temporal regions, and often much of the parietal lobe in between. This lesion represents most of the territory of the left middle cerebral artery. Patients in whom the superior temporal gyrus is spared tend to recover their auditory comprehension, with evolution of the deficit toward the syndrome of Broca aphasia. Recovery in global aphasia may be prolonged; in patients with global aphasia, more clinical improvement may occur during the second 6 months than in the first 6 months after a stroke. Characteristics of global aphasia are presented in Table 12A.3.
| Feature | Syndrome |
|---|---|
| Spontaneous speech | Mute or nonfluent |
| Naming | Impaired |
| Comprehension | Impaired |
| Repetition | Impaired |
| Reading | Impaired |
| Writing | Impaired |
| Associated signs | Right hemiparesis |
| Right hemisensory loss | |
| Right hemianopia |
Conduction Aphasia
Conduction aphasia is an uncommon but theoretically important syndrome that can be recognized by its striking deficit of repetition. Most patients have relatively normal spontaneous speech, although some make literal paraphasic errors and hesitate frequently for self-correction. Naming is impaired to varying degrees, but auditory comprehension is preserved. Repetition may be disturbed to seemingly ridiculous extremes such that a patient who is capable of self-expression at a sentence level and can comprehend conversation may be unable to repeat even single words. One such patient could not repeat the word “boy” but said “I like girls better.” Reading and writing are somewhat variable, but reading aloud may share some of the same difficulty as repeating. Associated deficits include hemianopia in some patients; right-sided sensory loss may be present, but right hemiparesis usually is mild or absent. Some patients have limb apraxia, creating a misimpression that comprehension is impaired. Bedside examination findings in conduction aphasia are summarized in Table 12A.4.
| Feature | Syndrome |
|---|---|
| Spontaneous speech | Fluent, some hesitancy, literal paraphasic errors |
| Naming | May be moderately impaired |
| Comprehension | Intact |
| Repetition | Severely impaired |
| Reading | ± Inability to read aloud; some reading comprehension |
| Writing | Variable deficits |
| Associated signs | ± Apraxia of left limbs |
| ± Right hemiparesis, usually mild | |
| ± Right hemisensory loss | |
| ± Right hemianopia |
Conduction aphasia has been advanced as a classical disconnection syndrome. Wernicke originally postulated that a lesion disconnecting the Wernicke and Broca areas would produce this syndrome; Geschwind later pointed to the arcuate fasciculus, a white-matter tract traveling from the deep temporal lobe, around the sylvian fissure, to the frontal lobe, as the site of disconnection. Anatomical involvement of the arcuate fasciculus is present in most if not all cases of conduction aphasia, but doubt has arisen as to the importance of the arcuate fasciculus to this syndrome. Bernal and Ardila (2009) cite evidence that the arcuate fasciculus terminates in the premotor/motor areas, and not in the Broca area. In addition, there is usually also cortical involvement of the supramarginal gyrus or temporal lobe. The supramarginal gyrus appears to be involved in auditory immediate memory and in phoneme perception related to word meaning as well as phoneme generation (Hickok and Poeppel, 2000). Lesions in this area are associated with conduction aphasia and phonemic paraphasic errors. Other investigators have pointed out that lesions of the arcuate fasciculus do not always produce conduction aphasia. Another theory of conduction aphasia suggests a defect in auditory verbal short-term memory.
Anomic Aphasia
Anomic aphasia refers to aphasic syndromes in which naming, or access to the internal lexicon, is the principal deficit. Spontaneous speech is normal except for the pauses and circumlocutions produced by the inability to name. Comprehension, repetition, reading, and writing are intact except for the same word-finding difficulty in written productions. Anomic aphasia is common but less specific in localization than the other aphasic syndromes. Isolated severe anomia may indicate focal left hemisphere pathology. Alexander and Benson (1997) refer to the angular gyrus as the site of lesions producing anomic aphasia, but lesions there usually produce other deficits as well, including alexia and the four elements of the Gerstmann syndrome: agraphia, right-left disorientation, acalculia, and finger agnosia, or inability to identify fingers. Isolated lesions of the temporal lobe can produce pure anomia, and PET studies of naming in normal persons also have shown consistent activation of the superior temporal lobe. Inability to produce nouns is characteristic of temporal lobe lesions, whereas inability to produce verbs occurs more often with frontal lesions. Even specific classes of nouns may be selectively affected in some cases of anomic aphasia. Anomia also is seen with mass lesions elsewhere in the brain, and in neurodegenerative disorders such as Alzheimer disease. Anomic aphasia also is a common stage in the recovery of many aphasic syndromes. Anomic aphasia thus serves as an indicator of left hemisphere or diffuse brain disease, but it has only limited localizing value. The typical features of anomic aphasia are presented in Table 12A.5.
| Feature | Syndrome |
|---|---|
| Spontaneous speech | Fluent, some word-finding pauses, circumlocution |
| Naming | Impaired |
| Comprehension | Intact |
| Repetition | Intact |
| Reading | Intact |
| Writing | Intact except for anomia |
| Associated signs | Variable or none |
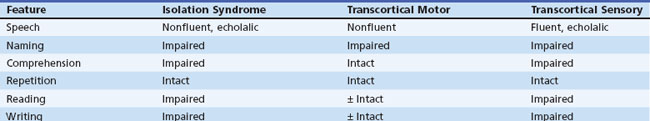
Transcortical Aphasias
The third transcortical syndrome, transcortical sensory aphasia, is an analog of Wernicke aphasia in which fluent paraphasic speech, paraphasic naming, impaired auditory and reading comprehension, and abnormal writing coexist with normal repetition. This syndrome is relatively uncommon, occurring with strokes of the left temporo-occipital area and in dementias. “Watershed” infarctions between the left middle and posterior cerebral artery territories may produce this syndrome. Bedside examination findings in the transcortical aphasias are summarized in Table 12A.6.
Subcortical Aphasias
Left thalamic hemorrhages frequently produce a Wernicke-like fluent aphasia with better comprehension than in cortical Wernicke aphasia. A fluctuating or “dichotomous” state has been described, alternating between an alert state with nearly normal language and a drowsy state in which the patient mumbles paraphasically and comprehends poorly. Luria has referred to this state as a “quasi-aphasic abnormality of vigilance.” One way of thinking of thalamic aphasia is that the thalamus plays a role in alerting the language cortex such that the language cortex, in effect, goes to sleep. Thalamic aphasia can occur even with a right thalamic lesion in a left-handed patient, indicating that hemispheric language dominance extends to the thalamic level. Although some skeptics have attributed thalamic aphasia to pressure on adjacent structures and secondary effects on the cortex, cases of thalamic aphasia have been described with small ischemic lesions, especially those involving the paramedian or anterior nuclei of the thalamus in the territory of the tuberothalamic artery. Because these lesions produce little or no mass effect, such cases indicate that the thalamus and its connections play a definite role in language function (Carrerra and Bogousslavsky, 2006).
Lesions of the left basal ganglia and deep white matter also cause aphasia. As in thalamic aphasia, the first syndromes described were in basal ganglia hemorrhages, especially those involving the putamen, the most common site of hypertensive intracerebral hemorrhage. Here the aphasic syndromes are more variable, but most commonly involve global or Broca-like aphasia. As in thalamic lesions, ischemic strokes have provided better localizing information than hemorrhage cases. The most common lesion is an infarct involving the anterior putamen, caudate nucleus, and anterior limb of the internal capsule. Patients with this lesion have an “anterior subcortical aphasia syndrome” involving dysarthria, decreased fluency, mildly impaired repetition, and mild comprehension disturbance. This syndrome most closely resembles Broca aphasia, but with greater dysarthria and less language dysfunction. Fig. 12A.5 shows imaging findings in an example of this syndrome. More restricted lesions of the anterior putamen, head of caudate, and periventricular white matter produce hesitancy or slow initiation of speech but little true language disturbance. More posterior lesions involving the putamen and deep temporal white matter, referred to as the temporal isthmus, are associated with fluent paraphasic speech and impaired comprehension, resembling the features of Wernicke aphasia. Small lesions in the posterior limb of the internal capsule and adjacent putamen cause mainly dysarthria, but mild aphasic deficits may occasionally occur. Finally, larger subcortical lesions involving both the anterior and posterior lesion sites produce global aphasia. A wide variety of aphasia syndromes can thus be seen with subcortical lesion sites. Nadeau and Crosson (1997) presented an anatomical model of basal ganglia involvement in speech and language, based on the known motor functions and fiber connections of these structures. Evidence from PET indicates that basal ganglia lesions affect language, both directly and indirectly, via decreased activation of cortical language areas.
The insula, a cortical structure that shares a deep location with the subcortical structures, may also be important to speech and language function. Dronkers (1996) reported that involvement of this area is closely associated with the presence of apraxia of speech in aphasic patients. Hillis and colleagues (2004), however, in MRI studies of brain in acute stroke patients, found that the left frontal cortex correlates more with speech apraxia than does the insula.
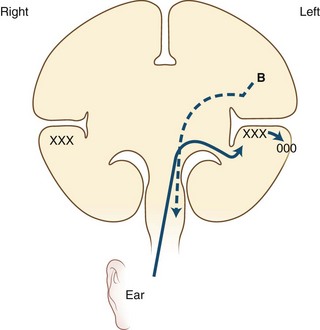
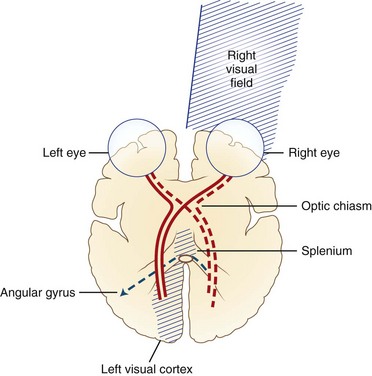
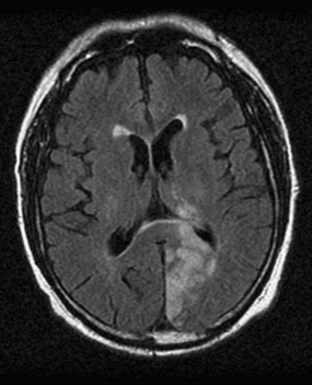
Pure Alexia without Agraphia
The causative lesion in pure alexia is nearly always a stroke in the territory of the left posterior cerebral artery, with infarction of the medial occipital lobe, often the splenium of the corpus callosum, and often the medial temporal lobe. Dejerine postulated a disconnection between the intact right visual cortex and left hemisphere language centers, particularly the angular gyrus. Fig. 12A.6 is an adaptation of Dejerine’s original diagram; Fig. 12A.7 shows an MRI of a patient with alexia without agraphia. Geschwind later rediscovered this “disconnection” hypothesis. Although Damasio and Damasio found splenial involvement in only 2 of 16 cases, they postulated a disconnection within the deep white matter of the left occipital lobe. As in the disconnection hypothesis for conduction aphasia, the theory fails to explain all the behavioral phenomena, such as the sparing of single-letter reading. A deficit in immediate memory span for visual language elements or an inability to perceive multiple letters at once (simultanagnosia) also can explain many features of the syndrome. Typical features of pure alexia without agraphia are presented in Table 12A.7.
Table 12A.7 Bedside Features of Pure Alexia without Agraphia
| Feature | Syndrome |
|---|---|
| Spontaneous speech | Intact |
| Naming | ± Impaired, especially colors |
| Comprehension | Intact |
| Repetition | Intact |
| Reading | Impaired (some sparing of single letters) |
| Writing | Intact |
| Associated signs | Right hemianopia or superior quadrantanopia |
| Short-term memory loss | |
| Motor, sensory signs usually absent |
Alexia with Agraphia
The second classic alexia syndrome, alexia with agraphia, described by Dejerine in 1891, may be thought of as an acquired illiteracy in which a previously literate patient is rendered unable to read or write. The oral language modalities of speech, naming, auditory comprehension, and repetition are largely intact, but in many cases the patient demonstrates a fluent paraphasic speech pattern with impaired naming. This syndrome thus overlaps Wernicke aphasia, especially in cases in which reading is more impaired than auditory comprehension. Associated deficits include right hemianopia and elements of the Gerstmann syndrome: agraphia, acalculia, right-left disorientation, and finger agnosia. The lesions typically involve the inferior parietal lobule, especially the angular gyrus. Etiologic disorders include strokes in the territory of the angular branch of the left middle cerebral artery and mass lesions in the same region. Characteristic features of the syndrome of alexia with agraphia are summarized in Table 12A.8.
| Feature | Syndrome |
|---|---|
| Spontaneous speech | Fluent, often some paraphasia |
| Naming | ± Impaired |
| Comprehension | Intact or less impaired than reading |
| Repetition | Intact |
| Reading | Severely impaired |
| Writing | Severely impaired |
| Associated signs | Right hemianopia |
| Motor, sensory signs usually absent |
Aphasic Alexia
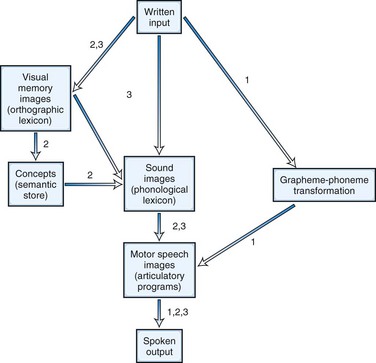
In addition to the two classic alexia syndromes, many patients with aphasia have associated reading disturbance. Examples already cited are the “third alexia” of Broca aphasia and the reading deficit of Wernicke aphasia. Neurolinguists and cognitive psychologists have divided alexias according to breakdown in specific stages of the reading process. The linguistic concepts of surface structure versus the deep meanings of words have been instrumental in these new classifications. Four patterns of alexia (or “dyslexia” in British usage) have been recognized: letter-by-letter, deep, phonological, and surface dyslexia. Fig. 12A.8 diagrams the steps in the reading process and the points of breakdown in the four syndromes. Letter-by-letter dyslexia is equivalent to pure alexia without agraphia. Deep dyslexia is a severe reading disorder in which patients recognize and read aloud only familiar words, especially concrete, imageable nouns and verbs. They make semantic or visual errors in reading and fail completely in reading nonsense syllables or nonwords. Word reading is not affected by word length or regularity of spelling; one patient, for example, could read “ambulance” but not “am.” Most cases are characterized by severe aphasia, with extensive left frontoparietal damage.
Agraphia
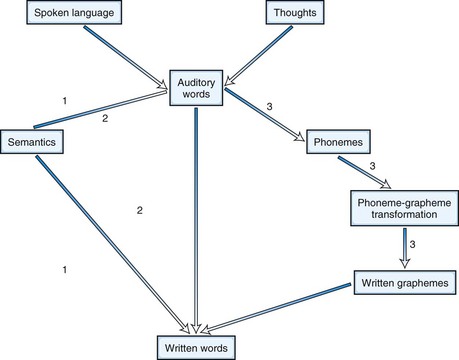
Agraphias can be analyzed in the same way as alexias (Fig. 12A.9). Thus, phonological agraphia involves the inability to convert phonemes into graphemes or to write pronounceable nonsense syllables in the presence of ability to write familiar words. Deep dysgraphia is similar to phonological agraphia, but the patient can read nouns and verbs better than articles, prepositions, adjectives, and adverbs. In lexical or surface dysgraphia, patients can write regularly spelled words and pronounceable nonsense words but not irregularly spelled words. These patients have intact phoneme-grapheme conversion but cannot write by a whole-word or “lexical” strategy.
Language in Right Hemisphere Disorders
Language and communication disorders are important even in patients with right hemisphere disease. First, left-handed patients may have right hemisphere language dominance and may acquire aphasic syndromes due to right hemisphere lesions. Second, right-handed patients occasionally become aphasic after right hemisphere strokes, a phenomenon called crossed aphasia (Bakar et al., 1996). These patients presumably have crossed or mixed dominance. Third, even right-handed persons with typical left hemisphere dominance for language have subtly altered language function after right hemisphere damage. Such patients are not aphasic in that the fundamental mechanisms of speech production, repetition, and comprehension are undisturbed. Affective aspects of language are impaired, however, such that the speech sounds flat and unemotional; the normal prosody or emotional intonation of speech is lost. Syndromes of loss of emotional aspects of speech are termed aprosodias. Motor aprosodia involves loss of expressive emotion with preservation of emotional comprehension; sensory aprosodia involves loss of comprehension of affective language, also called affective agnosia. In addition to emotional tone, stress and emphasis within a sentence are affected by right hemisphere dysfunction. Of greater importance, such vital aspects of human communication as metaphor, humor, sarcasm, irony, and related constituents of language that transcend the literal meaning of words are especially sensitive to right hemisphere dysfunction. These deficits significantly impair patients in the pragmatics of communication. In other words, right hemisphere–damaged patients understand what is said, but not how it is said. They may have difficulty following a complex story. Such higher-level language deficits are related to the right hemisphere disorders of inattention and neglect, discussed in Chapters 4 and 36.
Language in Dementing Diseases
The second pattern of language dissolution in dementia, considerably less common than the first, involves the gradual onset of a progressive aphasia, often without other cognitive deterioration. Auditory comprehension may be involved early in the illness, and specific aphasic symptoms are evident, such as paraphasic or nonfluent speech, misnaming, and errors of repetition. These deficits worsen gradually, mimicking the course of a brain tumor or mass lesion rather than that of a typical dementia (Grossman et al., 1996; Mesulam, 2001, 2003). The syndrome generally is referred to as primary progressive aphasia. CT scans may show focal atrophy in the left perisylvian region, whereas electroencephalographic studies may show focal slowing. PET has shown prominent areas of decreased metabolism in the left temporal region and adjacent cortical areas.
Primary progressive aphasia (PPA) is now considered a variant of a more general category of dementing illnesses called frontotemporal dementia (FTD) (Neary and Snowden, 1996; Neary et al., 1998; Josephs, 2008). There are several variants. Mesulam’s patients with PPA had largely nonfluent, Broca-like patterns of aphasia (2001, 2003). A progressive fluent aphasia with impaired naming and loss of understanding of words has been termed semantic dementia, often associated with surface alexia (Hodges and Patterson, 2007). A third type of progressive aphasia, the logopenic phonological type (Gorno-Tempini et al., 2008), is associated with AD. In general, patients with fluent aphasia who come to autopsy may have either AD or FTD, whereas those with nonfluent aphasia generally have non-Alzheimer disorders. The two most common subtypes in progressive nonfluent aphasia are those with tau staining and those with ubiquitin staining. Many familial cases of FTD have had genetic mutations in the tau gene on chromosome 17 (Heutink et al., 1997), whereas those with ubiquitin pathology may have mutations in the progranulin gene, related to the TAR-DNA binding protein (TDP-43) (Baker et al., 2006; Cruts et al., 2006; Hodges et al., 2004). Other variants include corticobasal degeneration and mixed FTD with motor neuron disease. In one study of 10 patients with PPA followed prospectively until they became nonfluent or mute, Kertesz and Munoz (2003) found that at autopsy, all had evidence of FTD: corticobasal degeneration in four, Pick body dementia in three, and tau and synuclein negative ubiquinated inclusions of the motor neuron disease in three. Kertesz and colleagues (2000) have proposed that Pick disease, FTD, corticobasal degeneration, and PPA should be linked together under the term Pick complex. Imaging studies have shown that primary progressive aphasia often is associated with atrophy in the left frontotemporal region, and other areas such as the fusiform and precentral gyri and intraparietal sulcus are activated, possibly as a compensatory neuronal strategy (Sonty et al., 2003). Whitwell and colleagues (2006) have used voxel-based MRI morphometry to delineate different patterns of atrophy in FTD associated with motor neuron disease versus ubiquitin pathology. Cases of isolated aphasia secondary to Creutzfeldt-Jakob disease have been reported, but these usually progress to dementia over a period of months.
Clinical Investigations in the Aphasic Patient
Other Useful Tests
For neurologists, the most helpful battery is the Boston Diagnostic Aphasia Examination or its Canadian adaptation, the Western Aphasia Battery. Both tests provide subtest information analogous to that obtained with the bedside examination, and therefore meaningful to neurologists, as well as aphasia syndrome classification. The Porch Index of Communicative Ability quantitates performance in many specific functions, allowing comparison over time. Other aphasia tests are designed to evaluate specific language areas. For example, the Boston Naming Test evaluates a wide variety of naming stimuli, whereas the Token Test evaluates higher-level comprehension deficits. Further information on neuropsychological tests can be found in Chapter 34.
More specific diagnosis in the aphasic patient rests on the confirmation of a brain lesion by neuroimaging (Fig. 12A.10). The CT brain scan (discussed in Chapter 33A) revolutionized the localization of aphasia by permitting “real-time” delineation of a focal lesion in a living patient; previously, the physician had to outlive the patient to obtain a clinical-pathological correlation at autopsy. MRI provides better resolution of areas difficult to see on CT images, such as the temporal cortex adjacent to the petrous bones, and more sensitive detection of tissue pathology, such as early changes of infarction. The anatomical distinction of cortical from subcortical aphasia is best made by MRI. Acute strokes are visualized early on diffusion-weighted MRI.
Cerebral arteriography is useful in the diagnosis of aneurysms, arteriovenous malformations (AVMs), arterial occlusions, vasculitis, and venous outflow obstructions. In preparation for epilepsy surgery, the Wada test, or infusion of amobarbital through an arterial catheter, is useful in the determination of language dominance. Other related studies using language activation with functional MRI (fMRI) or PET are beginning to rival the Wada test for the study of language dominance (Abou-Khalil and Schlaggar, 2002).
Single photon emission CT (SPECT), PET, and functional MRI (fMRI; see Chapter 33C) are contributing greatly to the study of language. Patterns of brain activation in response to language stimuli have been recorded, mainly in normal persons, and these studies have largely confirmed the localizations based on clinicopathological findings in disorders such as stroke over the past 140 years. In addition, these techniques can be used to map areas of the brain that activate during language functions after insults such as strokes, and the pattern of recovery can be studied. Some such studies have indicated right hemisphere activation in patients recovering from aphasia (Cappa et al., 1997), whereas others have found that only left hemisphere activation is associated with full recovery (Heiss et al., 1999). A recent fMRI study (Saur et al., 2006) has suggested hypometabolism in the language cortex shortly after an ischemic insult, followed by increased activation of homologous areas in the contralateral hemisphere, and then a shift back to the more normal pattern of left hemisphere activation. Subcortical contributions to aphasia and language in degenerative conditions have been studied with PET. These techniques provide the best correlation between brain structure and function currently available and should help advance our understanding of language disorders and their recovery.
Differential Diagnosis
Hemorrhagic strokes also are an important cause of aphasia, most commonly the basal ganglionic hemorrhages associated with hypertension. The deficits tend to worsen gradually over minutes to hours, in contrast with the sudden or stepwise onset of ischemic strokes. Headache, vomiting, and obtundation are more common with hemorrhages. Because hemorrhages compress cerebral tissue without necessarily destroying it, the ultimate recovery from aphasia often is better in hemorrhages than in ischemic strokes, although hemorrhages more often are fatal. Other potential causes of intracerebral hemorrhage include anticoagulants, head injury, blood dyscrasias, thrombocytopenia, and bleeding into structural lesions, such as infarctions, tumors, AVMs, and aneurysms. Hemorrhages from AVMs mimic strokes, with abrupt onset of focal neurological deficit. Ruptured aneurysms, on the other hand, manifest with severe headache and stiff neck or with coma; most patients have no focal deficits, but delayed deficits (e.g., aphasia) may develop secondary to vasospasm. Lobar hemorrhages may occur in elderly patients without hypertension. These hemorrhages occur near the cortical surface, sometimes extending into the subarachnoid space, and they may be recurrent. Histopathological studies have shown amyloid deposition in small arterioles, or amyloid angiopathy. A final vascular cause of aphasia is cerebral vasculitis (see Chapter 51E).
A final cause of aphasia is seizures. Seizures can be associated with aphasia in children as part of the Landau-Kleffner syndrome or in adults as either an ictal or postictal Todd phenomenon. Epileptic aphasia is important to recognize because anticonvulsant drug therapy can prevent the episodes, and unnecessary investigation or treatment for a suspected new lesion, such as a stroke, can be avoided. As mentioned earlier, localization of language areas in epileptic patients has contributed greatly to the knowledge of language organization in the brain. Greater than 15% of young epileptic patients have no Broca or Wernicke area. In addition, a new language area, the basal temporal language area (BTLA) has been discovered through epilepsy stimulation studies and only later confirmed in patients with spontaneous seizures (Kirshner et al., 1995).
Recovery and Rehabilitation of the Patient with Aphasia
Patients with aphasia from acute disorders such as stroke generally show spontaneous improvement over days, weeks, and months. In general, the greatest recovery occurs during the first 3 months, but improvement may continue over a prolonged period, especially in young patients and in persons with global aphasia. The aphasia type often changes during recovery: global aphasia evolves into Broca aphasia, and Wernicke aphasia into conduction or anomic aphasia. Language recovery may be mediated by shifting of functions to the right hemisphere or to adjacent left hemisphere regions. As mentioned earlier, studies of language activation PET and SPECT scanning techniques are advancing our understanding of the neuroanatomy of language recovery (Heiss et al., 1999). In addition, study of patients in the very acute phase of aphasia with techniques of diffusion and perfusion-weighted MRI has suggested less variability in the correlation of comprehension impairment with left temporal ischemia than has been suggested from testing of patients with chronic aphasia, after recovery and compensation have commenced (Hillis et al., 2001).
Speech therapy provided by speech/language pathologists attempts to facilitate language recovery by a variety of techniques and to help the patient compensate for lost functions (see Chapter 48). Repeated practice in articulation and comprehension tasks traditionally has been used to stimulate improvement. Other techniques include melodic intonation therapy, which uses melody to involve the right hemisphere in speech production; visual action therapy, which uses gestural expression; and treatment of aphasic perseveration, which aims to reduce repetitive utterances. Two other therapeutic techniques are functional communication therapy, which takes advantage of extralinguistic communication, and cVIC or Lingraphica, a computer program originally developed for primate communication. Patients who cannot speak can learn to produce simple sentences via computer. Augmentative devices make language expression possible through use of printers or voice simulators.
Speech therapy has remained controversial. Some studies have suggested that briefly trained volunteers can induce as much improvement as that achieved by speech/language pathologists, but large randomized trials have clearly indicated that patients who undergo formal speech therapy recover better than untreated patients (Robey, 1998). A recent Cochrane review also supports the efficacy of intensive speech/language therapy over conventional therapy (Kelly, Brady, and Enderby, 2010).
A new approach to language rehabilitation is the use of pharmacological agents to improve speech. In 1998, Albert and colleagues first reported that the dopaminergic drug, bromocriptine, promotes spontaneous speech output in transcortical motor aphasia. Several other studies have supported use of this drug in nonfluent aphasias, although a recent controlled study showed no benefit (Ashtary et al., 2006). Stimulant drugs also are being tested in aphasia rehabilitation. As new information accumulates on the neurochemistry of cognitive functions, other pharmacological therapies may be forthcoming.
Other new approaches to aphasia therapy include both transcranial magnetic stimulation (Martin, Naeser, and Ho, 2009) and transcranial direct-current stimulation (Baker et al., 2010). These are preliminary exploratory studies, and it remains to be seen from larger studies how effective these stimulation techniques will be.
Abou-Khalil B., Schlaggar B.L. Is it time to replace the Wada test? Neurology. 2002;59:160-161.
Alexander M.P., Benson D.F. The aphasias and related disturbances. In: Joynt R.J., editor. Clinical Neurology, vol. 1. Philadelphia: Lippincott; 1997:1-58.
Ashtary F., Janghorbani M., Chitsaz A., et al. A randomized, double-blind trial of bromocriptine efficacy in nonfluent aphasia after stroke. Neurology. 2006;28:914-916.
Bakar M., Kirshner H.S., Wertz R.T. Crossed aphasia: functional brain imaging with PET or SPECT. Arch Neurol. 1996;53:1026-1032.
Baker J.M., Rorden D., Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41:1229-1236.
Baker M., Mackenzie I.R., Pickering-Brown S.M., et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916-919.
Bernal B., Ardila A. The role of the arcuate fasciculus in conduction aphasia. Brain. 2009;132:2309-2316.
Caplan D., Alpert N., Waters G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. J Cogn Neurosci. 1998;10:541-552.
Cappa S.F., Perani D., Grassi F., et al. A PET follow-up study of recovery after stroke in acute aphasics. Brain Lang. 1997;56:55-67.
Carrerra E., Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. 2006;66:1817-1823.
Cruts M., Gijselinck I., van den Zee J., et al. Null mutations in progranulin cause ubiquitin positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920-924.
Docherty N.M., DeRosa M., Andreasen N.C. Communication disturbances in schizophrenia and mania. Arch Gen Psychiatry. 1996;53:358-364.
Dronkers N.F. A new brain region for controlling speech articulation. Nature. 1996;384:159-161.
Gorno-Tempini M.L., Brambati S.M., Ginex V., et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227-1234.
Grossman M., Mickanin J., Onishi K., et al. Progressive nonfluent aphasia: language, cognitive, and PET measures contrasted with probable Alzheimer’s disease. J Cogn Neurosci. 1996;8:135-154.
Heiss W.-D., Kessler J., Thiel A., et al. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45:430-438.
Heutink P., Stevens M., Rizzu P., et al. Hereditary frontotemporal dementia is linked to Chromosome 17q21-q22; a genetic and clinicopathological study of three Dutch families. Ann Neurol. 1997;41:150-159.
Hickok G., Poeppel D. Towards a functional neuroanatomy of speech perception. Trends Cogn Sci. 2000;4:131-138.
Hillis A.E., Wityk R.J., Tuffiash E., et al. Hypoperfusion of Wernicke’s area predicts severity of semantic deficit in acute stroke. Ann Neurol. 2001;50:561-566.
Hillis A.E., Work M., Barker P.B., et al. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479-1487.
Hodges J.R., Davies R.R., Xuereb J.H., et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399-406.
Hodges J.R., Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 2007;6:1004-1014.
Josephs K.A. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64:4-14.
Kelly, H., Brady, M.C., Enderby, P., 2010. Speech and language therapy for asphasia following stroke. Cochrane Database Syst Rev 12:CD000425.
Kertesz A., Martinez-Lage P., Davidson W., et al. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55:1368-1375.
Kertesz A., Munoz D.G. Primary progressive aphasia and Pick complex. J Neurol Sci. 2003;206:97-107.
Kirshner H.S., Hughes T., Fakhoury T., et al. Aphasia secondary to partial status epilepticus of the basal temporal language area. Neurology. 1995;45:1616-1618.
Kreisler A., Godefroy O., Delmaire C., et al. The anatomy of aphasia revisited. Neurology. 2000;54:1117-1123.
Martin P.I., Naeser M.A., Ho M. Research with transcranial magnetic stimulation in the treatment of aphasia. Curr Neurol Neurosci Rep. 2009;9:451-458.
Mesulam M.M. Primary progressive aphasia. Ann Neurol. 2001;49:425-432.
Mesulam M.M. Primary progressive aphasia—a language-based dementia. N Engl J Med. 2003;349:1535-1542.
Nadeau S.E., Crosson B. Subcortical aphasia. Brain Lang. 1997;58:355-402.
Neary D., Snowden J. Frontotemporal dementia: nosology, neuropsychology, and neuropathology. Brain Cogn. 1996;31:176-187.
Neary D., Snowden J., Gustafson L., et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546-1554.
Ochfeld E., Newhart M., Molitoris J., et al. Ischemia in Broca area is associated with Broca aphasia more reliably in acute than in chronic stroke. Stroke. 2010;41:325-330.
Robey R.R. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hearing Res. 1998;41:172-187.
Robinson R.G. Neuropsychiatric consequences of stroke. Ann Rev Med. 1997;48:217-229.
Saur D., Lange R., Baumgaertner A., et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1351-1356.
Sonty S.P., Mesulam M.M., Thompson C.K., et al. Primary progressive aphasia: PPA and the language network. Ann Neurol. 2003;53:35-49.
Whitwell J.L., Jack C.R., Senjem M.L., et al. Patterns of atrophy in pathologically confirmed FTLD with and without motor neuron degeneration. Neurology. 2006;66:102-104.