Laboratory Safety
1. Define and differentiate sterilization, disinfection, and antiseptic.
2. List the factors that influence the effectiveness of disinfectants in the microbiology laboratory.
3. Describe the methods used for the disposal of hazardous waste, including physical and chemical methods, and the material and/or organisms effectively eliminated by each method.
4. Define a chemical hygiene plan and describe the purpose of the methods and items that are elements of the plan, including proper labeling of hazardous materials, training programs, and material safety data sheets.
5. Name the four types of fire extinguishers and the specific flammables that each is effective in controlling.
6. Describe the process of Universal or Standard Precautions in the microbiology laboratory, including handling of infectious materials, personal hygiene, use of personal protective equipment, handling of sharp objects, and hand-washing procedures.
7. Define Biosafety Levels 1 through 4, including the precautions required for each, and identify a representative organism for each.
8. Outline the basic guidelines for packing and shipping infectious substances.
9. Describe the management and response required during a biologic or chemical exposure incident in the laboratory.
These guidelines are still incorporated into safety programs in the diagnostic microbiology laboratory. Safety programs also have been expanded to include not only the proper handling of biologic hazards encountered in processing patient specimens and handling infectious microorganisms, but also fire safety; electrical safety; the safe handling, storage, and disposal of chemicals and radioactive substances; and techniques for safely lifting or moving heavy objects. In areas of the country prone to natural disasters (e.g., earthquakes, hurricanes, snowstorms), safety programs include disaster preparedness plans that outline the steps to take in an emergency. Although all microbiologists are responsible for their own health and safety, the institution and supervising personnel are required to provide safety training to familiarize microbiologists with known hazards in the workplace and to prevent exposure. Laboratory safety is considered an integral part of overall laboratory services, and federal law in the United States mandates pre-employment safety training, followed by quarterly safety in-services. Safety training regulations are enforced by the United States Department of Labor Occupational Safety and Health Administration (OSHA). Regulations and requirements may vary based on the type of laboratory and updated regulations. It is recommended that the laboratory review these requirements as provided by OSHA (www.osha.gov).
Sterilization and Disinfection
Methods of Sterilization
The physical methods of sterilization include:
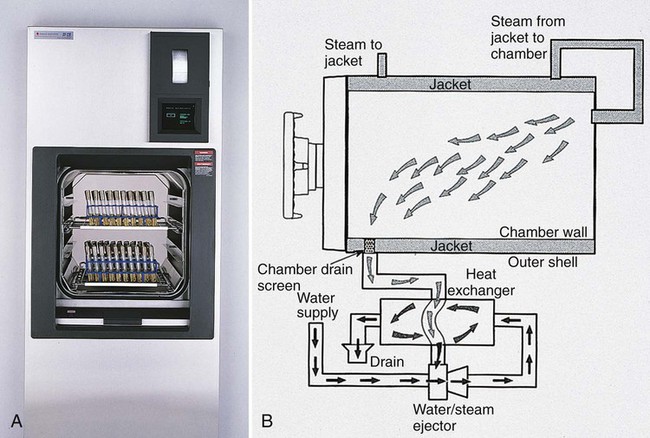
Moist heat (steam under pressure) is used to sterilize biohazardous trash and heat-stable objects; an autoclave is used for this purpose. An autoclave is essentially a large pressure cooker. Moist heat in the form of saturated steam under 1 atmosphere (15 psi [pounds per square inch]) of pressure causes the irreversible denaturation of enzymes and structural proteins. The most commonly used steam sterilizer in the microbiology laboratory is the gravity displacement type (Figure 4-1). Steam enters at the top of the sterilizing chamber; because steam is lighter than air, it displaces the air in the chamber and forces it out the bottom through the drain vent. The two common sterilization temperatures are 121°C (250°F) and 132°C (270°F). Items such as media, liquids, and instruments are usually autoclaved for 15 minutes at 121°C. Infectious medical waste, on the other hand, is often sterilized at 132°C for 30 to 60 minutes to allow penetration of the steam throughout the waste and the displacement of air trapped inside the autoclave bag. Moist heat is the fastest and simplest physical method of sterilization.
Methods of Disinfection
Physical Methods of Disinfection
The three physical methods of disinfection are:
• Boiling at 100°C for 15 minutes, which kills vegetative bacteria
• Pasteurizing at 63°C for 30 minutes or 72°C for 15 seconds, which kills food pathogens without damaging the nutritional value or flavor
• Using nonionizing radiation such as ultraviolet (UV) light
Chemical Methods of Disinfection
Chemical disinfectants comprise many classes, including:
A number of factors influence the activity of disinfectants, including:
Chemical Safety
In 1987, the U.S. Occupational Safety and Health Administration (OSHA) published the Hazard Communication Standard, which provides for certain institutional educational practices to ensure that all laboratory personnel have a thorough working knowledge of the hazards of the chemicals with which they work. This standard has also been called the “employee right to know.” It mandates that all hazardous chemicals in the workplace be identified and clearly marked with a National Fire Protection Association (NFPA) label stating the health risks, such as carcinogen (cause of cancer), mutagen (cause of mutations in deoxyribonucleic acid [DNA] or ribonucleic acid [RNA]), or teratogen (cause of birth defects), and the hazard class, for example, corrosive (harmful to mucous membranes, skin, eyes, or tissues), poison, flammable, or oxidizing (Figure 4-2).
• Name, address, and telephone number of manufacturer
• Physical and chemical properties
• Health effects and first aid
• Spill, leak, and disposal procedures
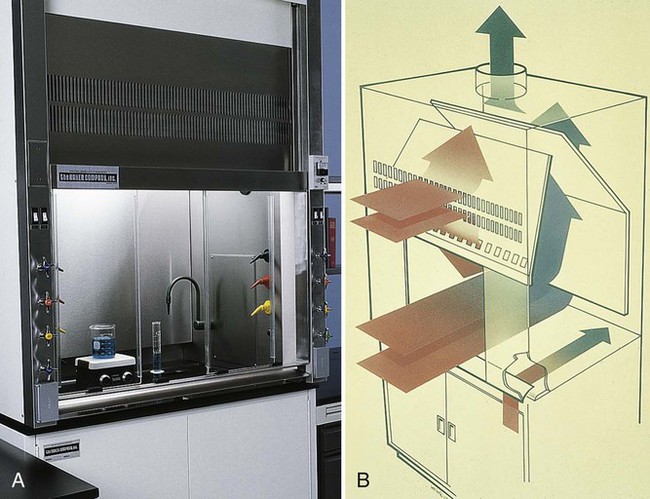
Fume hoods (Figure 4-3) are provided in the laboratory to prevent inhalation of toxic fumes. Fume hoods protect against chemical odor by exhausting air to the outside, but they are not HEPA-filtered to trap pathogenic microorganisms. It is important to remember that a BSC (discussed later in the chapter) is not a fume hood.
Handling of Compressed Gases
Compressed gas cylinders (CO2, anaerobic gas mixture) contain pressurized gases and must be properly handled and secured. When leaking cylinders have fallen, tanks have become missiles, resulting in loss of life and destruction of property. Therefore, gas tanks should be properly chained (Figure 4-4, A) and stored in well-ventilated areas. The metal cap, which is removed when the regulator is installed, should always be in place when a gas cylinder is not in use. Cylinders should be transported chained to special dollies (Figure 4-4, B).
Biosafety
• Rubbing the eyes or nose with contaminated hands
• Inhaling aerosols produced during centrifugation, mixing with a vortex or spills of liquid cultures
• Accidentally ingesting microorganisms by putting pens or fingers in the mouth
• Receiving percutaneous inoculation (i.e., through puncture from an accidental needle stick)
• Manipulating and/or opening bacterial cultures in liquid media or on plates, creating potentially hazardous aerosols outside of a biosafety hood
Risks from a microbiology laboratory may extend to adjacent laboratories and to the families of those who work in the microbiology laboratory. For example, Blaser and Feldman1 noted that 5 of 31 individuals who contracted typhoid fever from proficiency testing specimens did not work in a microbiology laboratory. Two patients were family members of a microbiologist who had worked with S. enterica subsp. Typhi; two were students whose afternoon class was in the laboratory where the organism had been cultured that morning; and one worked in an adjacent chemistry laboratory.
Exposure Control Plan
• Employee education and orientation
• Appropriate disposal of hazardous waste
• Standard (formerly Universal) Precautions
• Engineering controls and safe work practices, as well as appropriate waste disposal and use of BSCs
• Personal protective equipment (PPE), such as laboratory coats, shoe covers, gowns, gloves, and eye protection (goggles, face shields)
• Postexposure plan for investigating all accidents and a plan to prevent recurrences
Disposal of Hazardous Waste
• Substituting less hazardous chemicals when possible; for example, substituting ethyl acetate for ether in ova and parasite concentrations and Hemo-de in place of xylene for trichrome stains
• Developing procedures that use less of a hazardous chemical; for example, substituting infrared technology for radioisotopes in blood culture instruments
• Segregating infectious waste from uncontaminated (paper) trash
• Substituting miniaturized systems for identification and antimicrobial susceptibility testing of potential pathogens to reduce the volume of chemical reagents and infectious waste
Infectious waste (agar plates, tubes, reagent bottles) should be placed into two leak-proof, plastic bags for sturdiness (Figure 4-5); this is known as double bagging. Pipettes, swabs, and other glass objects should be placed into rigid cardboard containers (Figure 4-6) before disposal. Broken glass is placed in thick boxes lined with plastic biohazard bags (Figure 4-7); when full, the box is incinerated or autoclaved. Sharp objects, including scalpels and needles, are placed in sharps containers (Figure 4-8) and then autoclaved or incinerated.
Standard Precautions
• Do not eat, drink, smoke, or apply cosmetics (including lip balm).
• Do not insert or remove contact lenses.
• Do not bite nails or chew on pens.
• Limit access to the laboratory to trained personnel only.
• Assume all patients are infectious for all blood-borne pathogens.
• Use appropriate barrier precautions to prevent skin and mucous membrane exposure, including wearing gloves at all times and masks, goggles, gowns, or aprons if splash or droplet formation is a risk.
• Thoroughly wash hands and other skin surfaces after removing gloves and immediately after any contamination.
• Take special care to prevent injuries with sharp objects, such as needles and scalpels.
Mouth-pipetting is strictly prohibited. Mechanical devices must be used for drawing all liquids into pipettes. Eating, drinking, smoking, and applying cosmetics are strictly forbidden in work areas. Food and drink must be stored in refrigerators in areas separate from the work area. All personnel should wash their hands with soap and water after removing gloves, after handling infectious material, and before leaving the laboratory area. In addition, it is good practice to store sera collected periodically from all health care workers so that, in the event of an accident, a seroconversion (acquisition of antibodies to an infectious agent) can be documented (see Chapter 10).
• Wear gloves and a laboratory coat.
• Deal carefully with needles and lancets.
• Discard sharps in an appropriate, puncture-resistant container.
• Never recap needles by hand; if necessary, special safety devices are available. (Manufacturers are now producing needles with built in safety devices to prevent accidental needle sticks).
Engineering Controls
Laboratory Environment
Biologic Safety Cabinet
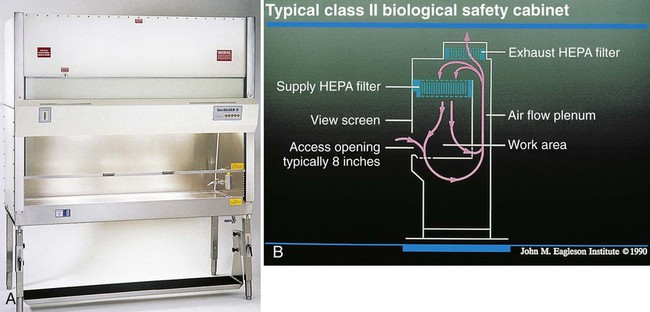
A BSC is a device that encloses a workspace in such a way as to protect workers from aerosol exposure to infectious disease agents. Air that contains the infectious material is sterilized, either by heat, ultraviolet light or, most commonly, by passage through a HEPA filter that removes most particles larger than 0.3 µm in diameter. These cabinets are designated as class I through 3, according to the effective level of biologic containment. Class I cabinets allow room (unsterilized) air to pass into the cabinet and around the area and material within, sterilizing only the air to be exhausted (Figure 4-9). They have negative pressure, are ventilated to the outside, and are usually operated with an open front.
Class II cabinets sterilize air that flows over the infectious material, as well as air to be exhausted. The air flows in “sheets,” which serve as barriers to particles from outside the cabinet and direct the flow of contaminated air into the filters (Figure 4-10). Such cabinets are called vertical laminar flow BSCs. Class II cabinets have a variable sash opening through which the operator gains access to the work surface. Depending on their inlet flow velocity and the percent of air that is HEPA filtered and recirculated, class II cabinets are further differentiated into type A or B. A class IIA cabinet is self-contained, and 70% of the air is recirculated. The exhaust air in class IIB cabinets is discharged outside the building. A class IIB cabinet is selected if radioisotopes, toxic chemicals, or carcinogens will be used.
Because they are completely enclosed and have negative pressure, class III cabinets afford the most protection to the worker. Air coming into and going out of the cabinet is filter sterilized, and the infectious material within is handled with rubber gloves that are attached and sealed to the cabinet (Figure 4-11).
Personal Protective Equipment
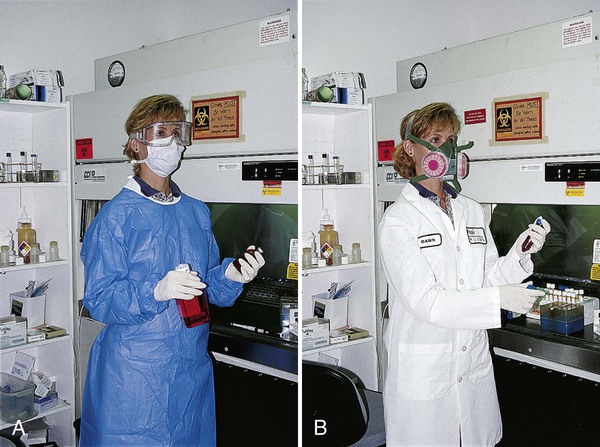
OSHA regulations require that health care facilities provide employees with all personal protective equipment necessary to protect them from hazards encountered during the course of work (Figure 4-12). PPE usually includes plastic shields or goggles to protect workers from droplets, disposal containers for sharp objects, holders for glass bottles, trays in which to carry smaller hazardous items (e.g., blood culture bottles), handheld pipetting devices, impervious gowns, laboratory coats, disposable gloves, masks, safety carriers for centrifuges (especially those used in the Acid Fast Bacteriology (AFB) laboratory), and HEPA respirators.
HEPA respirators are required for all health care workers, including phlebotomists, who enter the rooms of patients with tuberculosis, as well as workers who clean up spills of pathogenic microorganisms (see Chapter 80). All respirators should be fit-tested for each individual so that each person is assured that his or hers is working properly. Males must shave their facial hair to achieve a tight fit. Respirators are evaluated according to guidelines of the National Institute for Occupational Safety and Health (NIOSH), a branch of the CDC. N95 or P100 disposable masks (available from 3M, St. Paul, Minnesota) are commonly used in the clinical laboratory.
Classification of Biologic Agents Based on Hazard
Classification of Etiological Agents on the Basis of Hazard, from the CDC, served as a reference for assessing the relative risks of working with various biologic agents until the CDC, together with the National Institutes of Health (NIH), produced the manual Biosafety in Microbiological and Biomedical Laboratories. The fifth edition of this manual is currently available on the CDC website (www.cdc.gov/biosafety/publications/bmbl5/BMBL.pdf). In general, patient specimens pose a greater risk to laboratory workers than do microorganisms in culture, because the nature of etiologic agents in patient specimens is initially unknown.
Mailing Biohazardous Materials
In March 2005, the requirements for packaging and shipping of biologic material were significantly revised in response to an international desire to ensure reasonable yet safe and trouble-free shipment practices for infectious material. Before this date, clinical specimens submitted for infectious disease diagnosis, as well as isolates of any microorganism, were considered an “infectious substance” and packaged and labeled under UN 6.2 dangerous goods regulations. Infectious substances now are classified as category A, B, or C organisms. A category A specimen is an infectious substance capable of causing disease in healthy humans and animals; it is assigned to division UN 6.2, UN 2814, UN 2900, or UN 3373. Category B includes infectious substances that are not included in category A and are assigned to UN 3373. Only the category A organisms or specimens listed in Table 4-1 must be shipped as dangerous goods. The UN created the designation UN 3373 so that non−category A specimens or cultures can be packed and shipped as diagnostic or clinical specimens. The proper shipping name for a UN 3371 specimen is “biological substance, category B.”
TABLE 4-1
Examples of Infectious Substances Included in Category A
African swine fever virus (cultures only)*
Avian paramyxovirus type 1—Velogenic Newcastle disease virus (cultures only)
Classical swine fever virus (cultures only)
Foot and mouth disease virus (cultures only)
Lumpy skin disease virus (cultures only)
Mycoplasma mycoides—Contagious bovine pleuropneumonia (cultures only)*
Peste des petits ruminants virus (cultures only)*
Rinderpest virus (cultures only)*
Sheep-pox virus (cultures only)*
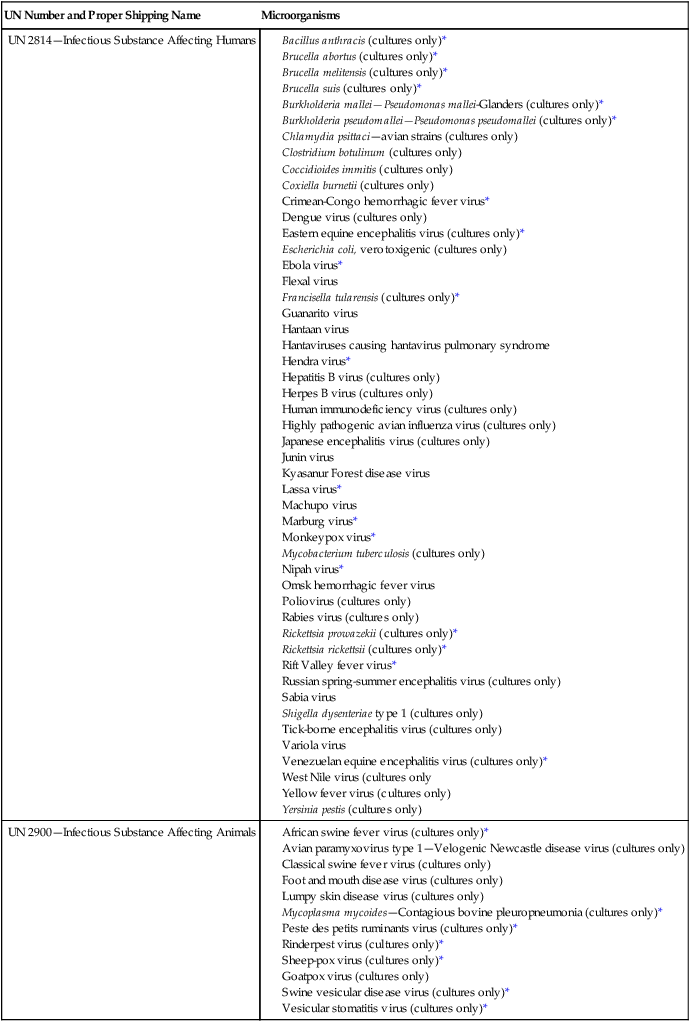
This table is not exhaustive. Infectious substances, including new or emerging pathogens, that do not appear in the table but that meet the same criteria must be assigned to category A. In addition, if doubt exists as to whether a substance meets the criteria, it must be included in category A.
*An infectious agent also designated as a “select agent” that has the potential to pose a severe threat to public health and safety.
The use of the former shipping names for diagnostic or clinical specimens is no longer permitted. If the laboratory director is unsure whether a patient has symptoms of a category A agent, it is prudent to ship the specimen as an infectious substance rather than a biologic substance. Figure 4-13, A, shows triple packaging for both diagnostic, clinical or infectious substances in a pouch; Figure 14-13, B, shows triple packaging for diagnostic, clinical, or infectious substances in a rigid bottle.
Infectious specimens or isolates should be wrapped with absorbent material and placed inside a plastic biohazard bag, called a primary receptacle. The primary receptacle is then inserted into a secondary container, most often a watertight, hard plastic mailer. The secondary container is capped and placed inside an outer, tertiary container that protects it from physical and water damage (see Figure 4-13, B). A UN class 6 label on the outer box confirms that the packaging meets all the required standards. The package must display the UN Packaging Specification Marking and must be labeled with a specific hazard label as an infectious substance. A packing list and a Shippers Declaration for Dangerous Goods Form must accompany the air bill or ground form. Diagnostic or clinical specimens are packaged similarly, but a UN specification marking is not required and it is not necessary to fill out a shippers declaration.
Shipping and packaging regulations from the Code of Federal Regulations can be found at the website www.gpoaccess.gov/cfr/. IATA regulations can be found at the website www.iata.org. International importation or exportation of biologic agents requires a permit from the CDC. Information on importing and exporting a variety of materials may be found at www.cdc.gov/ncidod/srp/specimens/shipping-packing.html.