Labor and Delivery and Their Complications
Perspective
Emergency department (ED) births are rare. In most cases, patients in labor are triaged directly to the obstetric suite for urgent management, maintaining a continuum of care with their primary providers. Because some births are precipitous and obstetric resources may not be immediately available, the emergency physician must possess the basic skills for intrapartum management of both normal and abnormal deliveries. In addition, a general knowledge of postpartum care is required in case of the occasional out-of-hospital delivery.1,2
Epidemiology of Emergency Delivery
In 2004 the perinatal mortality rate in the United States was 6.2 per 1000 live births and fetal deaths (<20 weeks of gestation).3 Delivery complications and mortalities do occur with greater frequency in the ED, where the perinatal mortality rate is approximately 8 to 10%.4 There are multiple causes of the “high-risk” ED delivery profile. The ED is often selected by an obstetric population with unexpected complications. Antepartum hemorrhage, premature rupture of membranes (PROM), eclampsia, premature labor, abruptio placentae, precipitous delivery, malpresentation, and umbilical cord emergencies are overrepresented in the ED population.4
Psychosocial factors further skew the epidemiology of ED deliveries. Women who present with precipitous deliveries often have had little or no prenatal care.5 Pregnant women who have drug or alcohol problems or are victims of domestic violence represent a disproportionate number of patients who deliver in the ED. Women who are unaware or in denial of their pregnancies or immigrants without access to other medical care also present to the ED when labor begins.6–8
Patient Transfer Considerations
Because of the high risk associated with ED delivery, patients should be transported to a facility that has obstetric and neonatal resources whenever possible. The management of a premature infant may require highly specialized intensive care that is unavailable at many community hospitals. The desire to transfer a woman with an impending high-risk delivery to such a facility should be tempered, however, by clinical and medicolegal judgment.9,10
Medicolegal Considerations
Transfer, with resultant en route delivery, can be disastrous for the mother and fetus. Such a transfer also violates federal law. The Consolidated Omnibus Reconciliation Budget Act (COBRA) of 1989 was based on an “inappropriate” obstetric transfer.11 Federal law has identified labor as a condition unsuitable for transfer because of its unstable nature. Although the intent of this legislation is to protect women from medical abandonment because of financial reasons, COBRA runs the risk of forcing emergency physicians to occasionally perform difficult high-risk deliveries that might have better outcomes with transfer.12
Nursery Requirements
For many ED deliveries, labor will have progressed to a point at which tocolysis is contraindicated and delivery is imminent. In general, this is when the mother feels the urge to push or the head is crowning. Whenever possible, a neonatologist or pediatrician should attend high-risk preterm (<36 weeks of gestation) deliveries, and preparations for neonatal resuscitation and high-level nursery care should be initiated (Box 181-1).
Normal Delivery
Stages of Labor
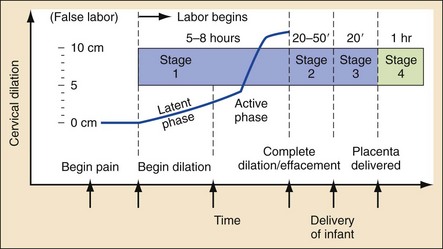
The first stage of labor is the cervical stage, ending with a completely dilated, fully effaced cervix. It is divided into a latent phase, with slow cervical dilation, and an active phase, with more rapid dilation. The active phase begins once the cervix is dilated 3 cm.13 In multiparous women, the active phase can progress rapidly into stage 2 of labor (delivery of the fetus). Most women who deliver in the ED arrive while in the active phase of stage 1 or early stage 2 labor (Fig. 181-1).4
The duration of the first stage of labor averages 8 hours in nulliparous women and 5 hours in multiparous women. During this time, frequent assessment of fetal well-being is important. For low-risk pregnancies, fetal heart tones should be auscultated approximately every 15 minutes. For higher risk pregnancies, continuous external electrical monitoring may help identify fetal distress, allowing appropriate intervention.14
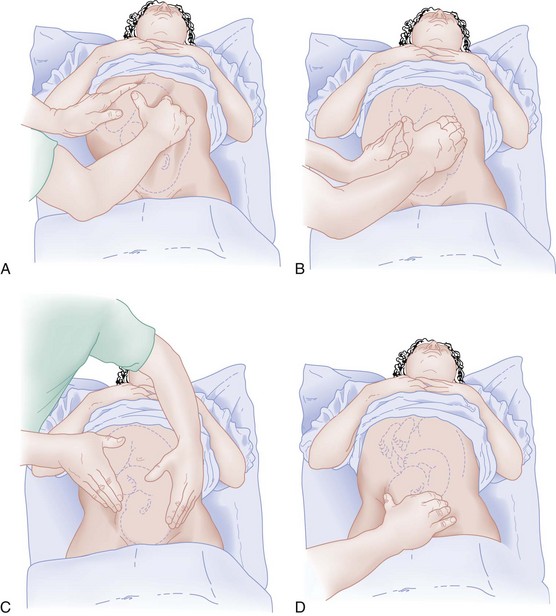
Abdominal examination with Leopold’s maneuvers may confirm the lie of the fetus (Fig. 181-2). After labor has begun, particularly during the active phase of stage 1, Leopold’s maneuvers are difficult to use. The firm contractions of the uterus prevent the identification of fetal “small parts.” Other modalities of assessing the lie, such as ultrasonography, may be necessary if presentation remains in question.15
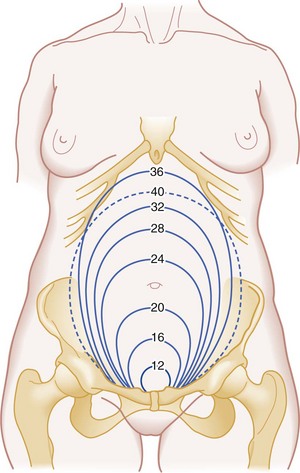
Maternal examination also provides a rough guide to gestational age. At 20 weeks’ gestation, the uterine fundus reaches the umbilicus. Approximately 1 cm of fundal height is added per week of gestation until 36 weeks. At that time, the fundal height decreases as the fetus “drops” into the pelvis (Fig. 181-3). These estimates help establish gestational age rapidly.
Effacement refers to the thickness of the cervix. A paper-thin cervix is 100% effaced.
Dilation indicates the diameter of the cervical opening in centimeters. Complete, or maximum, dilation is 10 cm.
Position describes the relationship of the fetal presenting part to the birth canal. The most common position of the head is occiput anterior.
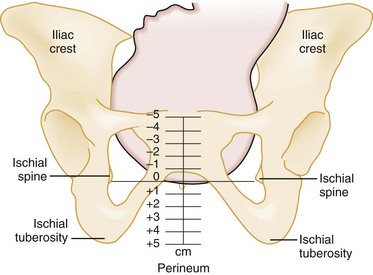
Station indicates the relationship of the presenting fetal part to the maternal ischial spines (Fig. 181-4).
Presentation specifies the anatomic part of the fetus leading through the birth canal.
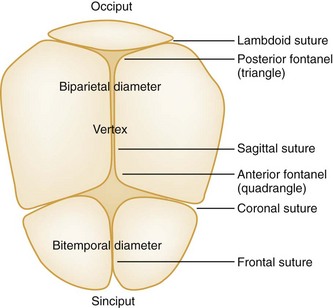
In 95% of all labors, the presenting part is the occiput, or vertex. On digital examination, a smooth surface with 360 degrees of firm bony contours and palpable suture lines is noted. Palpation of the suture lines and the fontanels where they join allows the examiner to determine the direction the fetus is facing. Three sutures radiate from the posterior fontanel, and four radiate from the anterior fontanel (Fig. 181-5). The lateral margins are examined carefully for fingers or facial parts that indicate compound or brow presentations.
When the clinician suspects rupture of membranes, a sterile speculum examination is performed. This may reveal pooling of amniotic fluid. Two tests to confirm the presence of amniotic fluid include a fernlike pattern when the fluid is allowed to dry on a microscope slide and the use of Nitrazine paper, which should turn blue, indicating an alkaline amniotic fluid (pH > 6). Although vaginal blood, cervical mucus, semen, and infection can interfere with results, sensitivities of both Nitrazine paper and ferning in detection of amniotic fluid are nearly 90%.16
Second Stage of Labor
The second stage of labor is characterized by a fully dilated cervix and accompanied by the urge to bear down and push with each uterine contraction. The fetal station is advanced to +3, with crowning of the presenting part as expulsion begins. Stage 2 uterine contractions may last 1 or 2 minutes and recur after a resting phase of less than 1 minute. The median duration of this stage is 50 minutes in nulliparous women and 20 minutes in multiparous women. More rapid progression through stage 2 should be anticipated for low-birth-weight premature infants. A prolonged second stage of labor is defined as more than 3 hours in nulliparous women if regional anesthesia is administered, more than 2 hours in nulliparous women without anesthesia or in multiparous women with regional anesthesia, and more than 1 hour in multiparous women without regional anesthesia.17 Prolonged second stage of labor is associated with an increase in maternal complications, including postpartum hemorrhage, infection, and severe vaginal lacerations.18
Antenatal Fetal Assessment: The assessment of a woman in the third trimester includes an assessment of fetal well-being. After 24 weeks’ gestation, the fetal condition affects clinical decision-making. During labor and delivery, the identification of fetal distress and appropriate intervention can reduce fetal morbidity and mortality.
Electronic Fetal Monitoring.: Intrapartum fetal assessment by electronic fetal monitoring is most useful during stage 1 of labor. Electronic fetal monitoring confirms true labor and may help diagnose fetal distress. Tracings of fetal heart rate and uterine activity provide information that in combination with clinical data can presage fetal distress due to hypoxia and provide a window for intervention.
Fetal heart rate tracings have several components that can be assessed: baseline heart rate, variability, accelerations, decelerations, and diagnostic patterns. Baseline heart rate, by definition, is the average fetal heart rate during a 10-minute period (in the absence of a uterine contraction) and is the most important aspect of fetal heart rate monitoring. Fetal bradycardia is defined as a baseline rate of less than 110 beats per minute; fetal tachycardia is defined as a baseline rate of more than 160 beats per minute.19
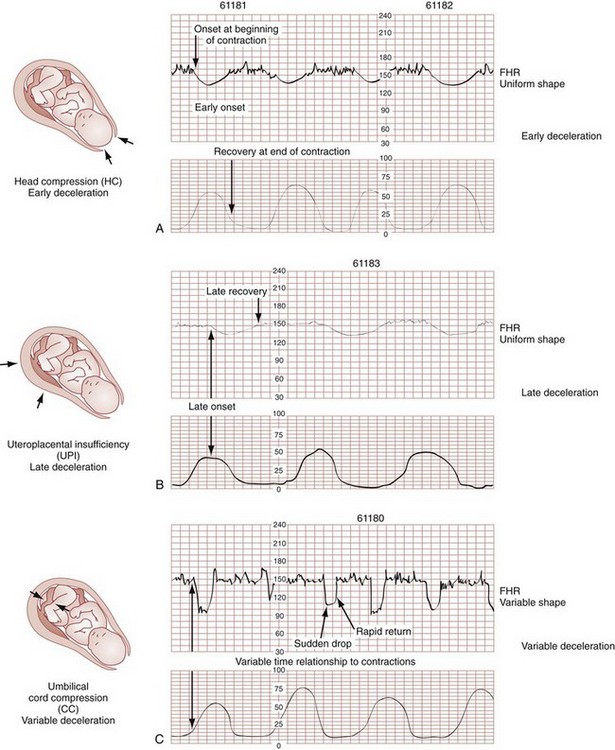
Decelerations in fetal heart rate are more complicated, and their interpretation should be integrated with the clinical situation. There are three types of deceleration: variable, early, and late (Fig. 181-6). These terms refer to the timing of the deceleration relative to the uterine contraction.
Late decelerations are more serious and most often indicate uteroplacental insufficiency. The tracing contours are generally smooth, with the heart rate nadir occurring well after maximal uterine contraction (typically 30 seconds or more afterward).19 The lag, slope, and magnitude of late decelerations correlate with increasing fetal hypoxia. Late decelerations are particularly ominous in association with poor variability, nonreactivity, and baseline bradycardia. When these findings are present, immediate obstetric consultation for delivery to prevent further hypoxia is indicated. The need for newborn resuscitation should be anticipated and preparation for critical care established for these deliveries. Overall, 30% of infants with late decelerations have good outcomes. The remaining 70% have suboptimal outcomes related to either the underlying pathologic condition or hypoxia.
Ultrasonography.: Ultrasonographic techniques have wide application in obstetric care. In the third trimester or during labor, ultrasonography can provide crucial information pertaining to impending delivery. When a technician and radiologist are available, the gestational age, biophysical profile, and amniotic fluid index as well as a survey of fetal and placental anatomy may be discerned (Table 181-1). The parameters of immediate interest in the ED are fetal viability (specifically in utero gestation and fetal heart rate), lie, and presentation. Ultrasonography may also reveal multiple gestations, allowing preparation and early communication with other specialists (from obstetrics, neonatology, and anesthesia). In 1991 the American College of Obstetricians and Gynecologists (ACOG) published recommendations regarding the indications for ultrasonography in the third trimester (Box 181-2). A 2- to 5-MHz transducer is appropriate for all bedside transabdominal sonographic assessments. Transvaginal ultrasonography is relatively contraindicated in the peripartum period, particularly in the cases of PROM and placenta previa.20
Table 181-1
Biophysical Profile: 30 Minutes of Ultrasonographic Observation
| ELEMENT ASSESSED | NORMAL SCORE = 2 | ABNORMAL SCORE = 0 |
| Fetal heart rate reactivity | 2 accelerations >15 beats/minute for >15 seconds | <2 accelerations |
| Amniotic fluid index | 1 pocket >1 cm in orthogonal planes | No large pockets |
| Fetal muscle tone | 1 episode of active flexion-extension with full return to flexed posture | <1 episode or slow, incomplete actions |
| Body movements | 3 discrete moves | ≤2 |
| Breathing motions | 1 episode of fetal breathing of at least 60 seconds in duration during 30 minutes of observation | No breathing activity or The absence of 1 episode of fetal breathing of at least 60 seconds in duration during 30 minutes of observation |
Delivery: As stage 2 of labor progresses, preparation for delivery should be under way. A radiant warmer should be available and heated. Neonatal resuscitation adjuncts, such as a towel, scissors, umbilical clamps, bulb suction, airway management equipment (oxygen, bag-mask device with appropriate-sized masks, and endotracheal intubation and suctioning equipment for meconium), and equipment to achieve vascular access, should be available. Most deliveries require only basic equipment to cut and clamp the umbilical cord, to suction the mouth and nose, and to dry and stimulate the infant. A nurse should be at the bedside to coach and to provide reassurance to the mother.
Controlled, coordinated expulsion with coaching to sustain each push aids with crowning and delivery of the head. When the fetus is crowning, care is exercised to have the delivery occur in a slow, controlled manner. Precipitous delivery is more likely to cause maternal injuries, such as perineal, rectal, urethral, labial, vaginal, and uterine lacerations, and fetal injuries.21
One described technique to potentially decrease the rate of maternal injury is the modified Ritgen maneuver.22 In the modified Ritgen maneuver, a towel-draped, gloved hand is used to stretch the perineum and gently exert pressure on the chin of the fetus. The second hand puts pressure on the occiput superiorly, guiding the head into slight extension. When the head is at the perineum, this slight extension of the head promotes delivery by positioning the head so that its smallest diameter passes through the pelvic outlet and perineum.
Episiotomy.: Previously, the need for episiotomy in normal deliveries was an area of controversy. The original potential benefit of episiotomy included the substitution of a straight surgical incision for a ragged, uncontrolled traumatic laceration. Theoretically, the surgical approach decreased the incidence of severe rectovaginal tears. This rationale led to widespread routine episiotomy, with a medial incision used in the United States and a mediolateral incision used in the Commonwealth countries.
Recent literature has shown that both types of incisions increase maternal morbidity, and they are no longer recommended for uncomplicated deliveries. Women receiving episiotomies have been shown to have a higher incidence of perineal trauma and maternal blood loss during delivery and more pelvic pain, sexual dysfunction, and urinary incontinence in the postpartum period.23
Episiotomy should be performed only for specific indications, such as shoulder dystocia or breech delivery. When the decision is made to use an episiotomy, the procedure should be done before excessive stretching of the perineal muscles occurs but near the time of delivery to avoid excessive bleeding. Common practice is to cut the episiotomy when the head is visible during a contraction and the introitus opens to a diameter of 3 or 4 cm. Most authors currently recommend a mediolateral incision to avoid perineal tears and rectal involvement; this is particularly true for a complicated delivery in which lacerations extending the surgical incision are likely (Fig. 181-7).
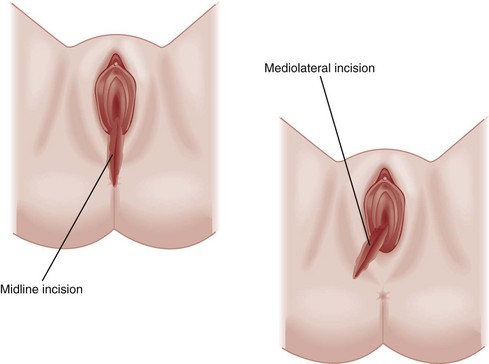
Figure 181-7 A mediolateral episiotomy incision is preferred to a strictly midline incision. (Redrawn from www.aurorahealthcare.org/healthgate/images/exh44028a_ma.jpg.)
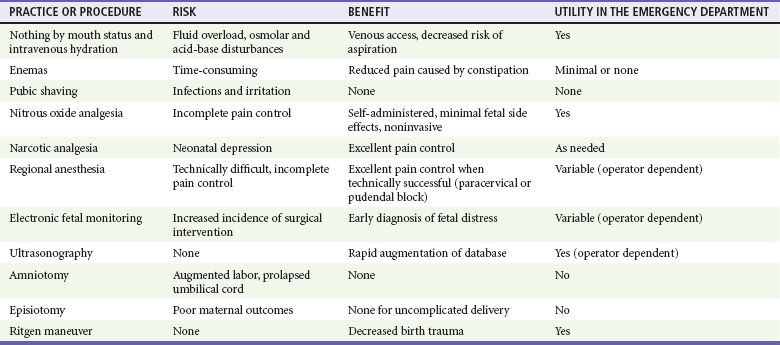
Table 181-2 summarizes some of the adjuncts used in normal labor and delivery, with recommendations for their use in the high-risk setting of an ED delivery.
Third Stage of Labor
These signs usually occur within 5 to 10 minutes of the delivery of the infant but may extend to 30 minutes. The risk of postpartum hemorrhage increases beyond 18 minutes and is in fact up to six times more likely after 30 minutes.25 Although the placenta may be delivered expectantly, active management reduces the length of the third stage of labor and thereby decreases the risk of postpartum hemorrhage. Active management includes the administration of uterotonics, gentle traction of the clamped umbilical cord with mild pressure applied above the symphysis pubis, and uterine massage after delivery.26 Be aware that any attempt to deliver the placenta before it separates is contraindicated.
Normally a three-vessel structure, the umbilical cord is filled with a connective tissue known as Wharton’s jelly and is approximately 50 to 60 cm long and 12 mm in diameter. Normal architecture places the two umbilical arteries on either side of the single umbilical vein. A two-vessel cord (one umbilical artery) occurs in 1 of 500 deliveries, is more common in African Americans, and is a result of aplasia or atrophy. Approximately 30% of two-vessel cord infants have congenital defects. An association also exists between fetal structural anomalies and placental vascular occlusion or thrombosis.27
The placenta should also be examined for abnormalities. Clots adherent to the uterine aspect may indicate placental abruption. Accessory lobes (succenturiate placenta if completely separate) and abnormal cord insertion are common abnormalities. The umbilical cord and placenta routinely should be held for pathologic review.28 The discovery of an incomplete placenta or membranes should alert the emergency physician to the possibility of postpartum complications and should be documented.
Specific Disorders
Third-Trimester Complications Associated with Delivery
Premature Labor
Perspective.: Premature or preterm labor and fetal immaturity are the leading causes of neonatal mortality. Preterm labor is defined as uterine contractions with cervical changes before 37 weeks of gestation. Women with premature labor are a heterogeneous group. Many underlying conditions result in preterm labor, which accounts for 9.6% of all pregnancies but 70% of all perinatal mortality.29 Factors linked to this problem include substance abuse, history of preterm delivery, multiple gestations, placental anomalies, infections, and lifestyle or psychosocial stressors30 (Box 181-3). The unexpected nature of premature labor often results in an ED visit. When delivery is not imminent, the patient can be moved to the obstetrics unit for further care.
Clinical Features.: The diagnosis of preterm labor requires the identification of uterine activity and cervical changes before 37 weeks of gestation. Early maternal signs and symptoms include an increase or change in vaginal discharge, pain resulting from uterine contractions (sometimes perceived as low back pain), pelvic pressure, vaginal bleeding (usually bloody show), and fluid leak.
Diagnostic Strategies.: If uterine contractions and cervical changes are present and the estimated fetal weight on ultrasonography is less than 2500 g, the diagnosis of premature labor is likely. The differentiation of false labor (Braxton Hicks contractions) from true labor is best done by electrical monitoring. Ultrasonography may aid in the diagnosis because fetal breathing movements make the diagnosis of false labor unlikely. The initial evaluation of a woman with possible preterm labor includes urinalysis, complete blood count, and pelvic ultrasonography. If delivery is not imminent, these studies can be performed in the ED or obstetrics area, whichever would provide the best monitoring. When possible, these patients should be transferred to a perinatal center with an associated intensive care unit.
Management.: A viable fetus and healthy mother are indications for medical management directed toward the prolongation of gestation. Preterm labor should not be postponed with medical management in the cases of fetal compromise, major congenital anomalies, intrauterine infection, placental abruption, eclampsia, significant cervical dilation, or, most important, PROM.31
The treatment of preterm labor involves multiple modalities. Tocolytics and fetal maturation therapy combined with bed rest and hydration are used in the hope of prolonging pregnancy (Box 181-4). These patients optimally should be transferred to an appropriate center before delivery whenever possible because medical management fails in more than 25% of preterm patients in whom it is attempted.32
Tocolysis: The two classically used tocolytics are magnesium sulfate and beta-mimetic drugs. Other medications that have been shown to be effective include prostaglandin synthetase inhibitors (nonsteroidal anti-inflammatory drugs [NSAIDs]) and calcium channel blockers.33 When they are indicated and in coordination with an obstetric consultant, tocolytics initiated in the ED may arrest premature labor, preventing imminent delivery in 75 to 80% of patients for 48 to 72 hours.29
Magnesium Sulfate: Magnesium sulfate competitively inhibits calcium uptake into smooth muscle and allows relaxation. The efficacy of magnesium sulfate as a tocolytic agent has not been clearly established.34 Women treated with magnesium require monitoring. Magnesium produces respiratory and neurologic depression at elevated levels, exacerbated by renal insufficiency. Pulmonary edema and cardiac dysrhythmias have also been reported.29 These effects can be reversed rapidly by the administration of calcium-containing solutions (i.e., 1 g of 10% calcium gluconate solution) (Table 181-3).
Table 181-3
| DRUG | EFFECT ON LABOR |
| Barbiturates | Can stop labor in anesthetic doses |
| Alcohol | Decreases oxytocin release, smooth muscle relaxant |
| Cocaine | Increased prematurity, placental infarction |
| Caffeine or aminophylline | Increased duration of labor |
| Narcotics | Increased latent phase, slow dilation (minimal effect once in active labor) |
| Atropine, scopolamine | Lower uterine segment relaxation, decreased frequency of contractions |
| Halothane | Strong inhibition of labor |
| IV nitroglycerin | Profound uterine relaxation |
Beta-Mimetics: Beta-mimetics (ritodrine and terbutaline) cause smooth muscle relaxation by activating enzymes that bind calcium to the sarcoplasmic reticulum. This effect is mediated by beta2 receptors that increase cyclic adenosine monophosphate concentrations in the myometrium. The beta-mimetic is titrated to effect because the dosage needed to eliminate uterine activity varies. In one meta-analysis, beta-mimetics and magnesium sulfate had similar efficacy in elimination of contractions.33
Despite evidence that beta-mimetics are effective tocolytics, their side effect profile is somewhat prohibitive for their selection over other options. Maternal pulmonary edema, myocardial ischemia, and cardiac dysrhythmia are the main adverse effects of high-dose beta-mimetics. Pulmonary edema is more likely to occur in mothers with preexisting cardiac disease, multiple gestation, and maternal infection. Pulmonary edema is caused by high-output failure and tends to occur when there is sustained maternal tachycardia of more than 120 beats/minute. Beta-mimetics should be gradually titrated according to uterine activity and maternal heart rate.30
Other beta-mimetic side effects include gluconeogenesis and glycogenolysis, which can be a problem for diabetic mothers. Surveillance of the urine for glucose and ketones is recommended. Fetal heart stimulation can result in both fetal dysrhythmia and cardiac hypertrophy. Also, some studies have shown an association between beta-mimetics and an increased incidence of fetal intraventricular hemorrhage.35
NSAIDs: The prostaglandin synthetase inhibitors, specifically indomethacin and sulindac, have been shown to be as effective as magnesium and the beta-mimetics in multiple trials. However, in the fetus, pulmonary hypertension, ductus arteriosus constriction, renal insufficiency, necrotizing enterocolitis, and intraventricular hemorrhage have been reported with NSAID use. Potential maternal side effects include a prolonged bleeding time and renal insufficiency.33,36
Calcium Channel Blockers: Calcium channel blockers have also been used as tocolytics with success, reducing muscle contractility by their effect on transmembrane calcium influx. Nifedipine or nicardipine may be given.37 Equal in efficacy to ritodrine and magnesium sulfate, calcium channel blockers have been shown to have relatively good maternal and fetal side effect profiles.30
Aggressive titratable tocolytics are best for the initial 24 to 48 hours of preterm labor. After uterine contractions have been stopped, the patient can usually be maintained with oral agents, although the benefits of maintenance tocolysis, studied to date primarily with beta-mimetics and magnesium, have yet to be shown.33 It is important to review the contraindications to tocolytics before initiation of these therapies (Box 181-5). Any patient receiving tocolytics should be externally monitored (electrically) for signs of fetal distress.
Premature Rupture of Membranes
Clinical Features.: PROM, also known as amniorrhexis, is defined as rupture of the amniotic and chorionic membranes before the onset of labor. It affects 3% of all gestations.38 During pregnancy, the chorionic and amniotic membranes protect the fetus from infection and provide an environment that allows fetal growth and movement. The amniotic fluid is constantly exchanged by fetal swallowing and urination and umbilical cord transfer. The fetal airway also contains a secreted fluid that allows fetal breathing movements, promoting fetal respiratory development. This fluid is produced at 5 mL/kg/hr at term gestation and is resorbed rapidly by the pulmonary lymphatics, blood vessels, and upper airway at birth.
The word premature in PROM refers to rupture before labor, not to fetal prematurity. In 8% of PROM cases, the fetus is at or near term, and PROM may result in normal labor. When PROM occurs before 37 weeks, it is called preterm PROM and is associated with significant fetal morbidity and mortality. PROM is the inciting event in one third of all preterm deliveries.39
Diagnostic Strategies.: The diagnosis of PROM usually can be established by history and physical examination. The patient usually describes a spontaneous gush of watery fluid followed by a mild persistent seepage. In most cases, the patient suggests the diagnosis and usually is correct. Urinary incontinence or excess vaginal or cervical secretions are occasionally confused with PROM.
Examination of women with potential PROM is performed under sterile conditions to prevent ascending infection. Direct digital examination of the cervix is avoided. The incidence of infection has been shown to be proportional to the number of examinations. The identification of amniotic fluid was previously discussed. Table 181-4 summarizes the bedside testing modalities available to confirm the diagnosis of PROM. Visualization of the cervix for prolapsed cord or abnormal fetal presentation (prolapse of a small part) is performed during the evaluation for effacement and dilation. Culture specimens for group B streptococci, Chlamydia trachomatis, and Neisseria gonorrhoeae should be obtained.39
Table 181-4
Bedside Testing for Premature Rupture of Membranes
| METHOD | RESULT |
| Nitrazine | Amniotic fluid (pH >6.5) will turn nitrazine paper blue; normal vaginal secretions (pH <5.5) will leave nitrazine paper yellow |
| Ferning | Amniotic fluid crystallizes |
| Smear combustion | Amniotic fluid, when flamed, turns white and crystallizes |
| Vaginal secretions caramelize and turn brown |
Management.: When the diagnosis of PROM is established, management depends on several factors: the gestational age and fetal maturity, the presence of active labor, the presence or absence of infection, the presence of placental abruption, and the degree of fetal well-being or distress.39 Fetal heart rate monitoring, obstetric consultation, and admission are indicated.
In the immature fetus (24-31 weeks of gestation), administration of corticosteroids can accelerate pulmonary maturation and, some studies suggest, also decrease rates of intraventricular hemorrhage and necrotizing enterocolitis.40 The benefit of this strategy has been shown with preterm PROM; however, this therapy is less well documented for PROM. In PROM, treatment with steroids seems to decrease the incidence and severity of hyaline membrane disease but may increase the risk of maternal infectious complications. Rupture of the membranes also stimulates fetal lung maturation, making it more difficult to establish a treatment benefit in PROM compared with preterm PROM. Therefore, patients with PROM between 31 and 33 weeks’ gestation are usually managed expectantly (unless fetal pulmonary maturity can be confirmed); those at or beyond 34 weeks of gestation are generally delivered.40 When gestational age is less than 26 weeks, the latent interval to delivery is often 1 week. Tocolytics seem an obvious choice, but their use is controversial. When tocolytics are used, the goal is to temporize, allowing time for both corticosteroid and antibiotic therapy to take effect. These treatment decisions should be coordinated with the receiving obstetrician.
All patients with PROM should be assessed for intra-amniotic infection. Infectious complications should be diagnosed and treated before the mother has overt clinical signs of infection. Preterm PROM is usually treated with intravenous ampicillin (or amoxicillin) and erythromycin.40 Of note, amoxicillin–clavulanic acid has been found to increase the risk of necrotizing enterocolitis in several studies and is therefore not recommended.39,40 Treatment of term PROM is indicated when the patient is group B streptococcus positive or has not been tested. The signs and symptoms of chorioamnionitis are late manifestations of advanced infection and are discussed next.
Chorioamnionitis
Chorioamnionitis occurs when vaginal or cervical bacteria ascend into the uterus, instigating an inflammation of the chorion and amnion layers of the amniotic sac.41 It occurs in 1 to 10% of all pregnancies, and risk factors include prolonged labor, PROM, excessive vaginal examinations, and recent amniocentesis. Box 181-6 summarizes the findings and evaluation of chorioamnionitis. Chorioamnionitis may result in prolonged first- and second-stage labor and decreased responsiveness to oxytocin. Early, aggressive treatment, even before evidence of infection occurs, decreases neonatal morbidity and delays delivery, allowing fetal maturation.42,43
Vertical Transmission of Human Immunodeficiency Virus
Emergency deliveries may involve women who are known to be HIV positive in addition to women who are infected but have never been tested. The latter group generally includes pregnant women with little or no prenatal care who are at risk for precipitous delivery. In 2000, between 280 and 370 infants were born in the United States with HIV infection. Of these, 29% were born to mothers undiagnosed with HIV infection before delivery.44 Transmission may occur in the antepartum, intrapartum, or postpartum (breast-feeding) period. Because intrapartum transmission accounts for up to 75% of vertically transmitted HIV infections, antiretroviral therapy on presentation, even while labor progresses, can decrease vertical HIV transmission.45 Potential mechanisms of transmission include microtransfusion during contractions, absorption of virus through the mucous membranes, and even invasion through the epithelium. Risk factors for transmission include high viral loads, prolonged rupture of membranes, maternal drug use, vaginal delivery, and breast-feeding (see Table 181-4).46,47
In November 2002, the Food and Drug Administration approved the OraQuick Rapid HIV-1 Antibody Test (OraSure Technologies, Bethlehem, Pa).48 Point-of-care testing for HIV with a median turnaround time of 45 minutes realistically allows the clinician to initiate intrapartum and neonatal antiretroviral therapy when the test result is positive. Serologic confirmation is recommended, but emergent interventions can proceed on the basis of the bedside result.49 Since 1994, it has been known that immediate treatment during labor can significantly decrease vertical transmission to the newborn.50
A positive HIV test result in some cases may allow a change in the method of delivery. Cesarean section decreases the rate of HIV transmission compared with vaginal delivery methods. In a 1999 meta-analysis from the United States and Europe, vertical HIV transmission was decreased by cesarean section with an odds ratio of 0.43 (95% confidence interval, 0.33-0.56). The protective effect of surgical delivery over other delivery methods persisted even when the data were stratified for receipt of antiretroviral therapy.51 This finding was tempered by an increase in maternal morbidity and mortality in HIV-positive women undergoing cesarean section because of an increased incidence of endometritis, maternal sepsis, pneumonia, and transfusion.52 Taking into account the risks and benefits, current ACOG and Department of Health and Human Services panel guidelines recommend elective cesarean section for all HIV-infected women with viral loads of more than 1000 copies/mL at 38 weeks of gestation.53
Ideally, decisions about the mode of delivery and the need for antiretroviral therapy can be made before the rupture of membranes. The risk of transmission increases after that time and continues to increase as the fetus traverses the birth canal.53 When antiretroviral therapy and elective cesarean section are used, the likelihood of vertical transmission is reduced by 87% compared with that for women receiving neither intervention.51
This relatively new diagnostic and therapeutic burden placed on the emergency physician faced with an imminent delivery is challenging. Data indicate that antiretroviral treatment and the mode of delivery make labor a true emergency for an HIV-positive patient. In addition, pregnant women with advanced HIV disease have a higher incidence of premature births, postpartum endometritis, and perinatal mortality.47 Currently, the main obstacle to this type of care is the availability of point-of-care HIV testing. Since the late 1990s, the results supporting this approach have become increasingly robust and seem to justify the efforts.54,55
Complicated Delivery
Principles of Disease
Dystocia and Malpresentation.: Dystocia, or abnormal labor progression, accounts for one third of all cesarean sections and half of primary cesarean sections. Because rapid surgical resolution is unavailable to the emergency physician, intrapartum management skills are important.
In order of increasing incidence, brow, face, shoulder, and breech presentations are the most common malpresentations (Table 181-5). True fetopelvic disproportion is much less common. Cesarean section is indicated when labor arrest or cord prolapse coexists with these presentations.56
Table 181-5
Relative Incidence of Malpresentations
| MALPRESENTATION | INCIDENCE |
| Breech presentation | 1/25 live births |
| Shoulder dystocia | 1/300 live births |
| Face presentation | 1/550 live births |
| Brow presentation | 1/1400 live births |
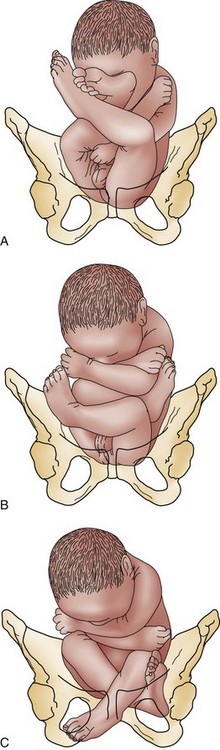
Perspective.: Breech is the most common malpresentation, occurring in just less than 4% of all deliveries.57 Three types of breech presentation exist: frank, incomplete, and complete (Fig. 181-8; Box 181-7). The main mechanical problem with breech presentations is that the buttocks and legs do not provide a sufficient wedge, hindering cervical accommodation of the relatively larger head. In addition, because the presenting part does not completely occlude the cervical opening, umbilical cord prolapse may occur.
Correlated with this abnormal presentation are several factors: prematurity, multiparity, fetal abnormalities, prior breech presentation, polyhydramnios, and uterine abnormalities.57
Overall, one third of breech fetal deaths are believed to be preventable. Asphyxia is often due to umbilical cord prolapse or entrapment of the head. Fetal head and neck trauma can occur if inappropriate delivery techniques are used. Scheduled cesarean section for these patients reduces the potential for ED presentation. However, emergency physicians should be prepared for vaginal delivery of breech presentations in the event of premature or unforeseen labor in the absence of immediate surgical services.58
Diagnostic Strategies.: Before labor, Leopold’s maneuvers facilitate the diagnosis of breech presentation. In the case of breech presentation, Leopold’s first maneuver identifies a firm, round mass (the head) in the fundus of the uterus. The third maneuver reveals the softer breech at the pelvic inlet. For the emergency physician, active labor restricts the use of Leopold’s maneuvers; thus vaginal examination is required.
If time allows, ultrasound examination is indicated to obtain information on the type of breech presentation, gestational age and fetal weight, and position of the fetal arms and neck.59 If the fetus has a hyperextended neck, vaginal delivery is associated with a 70% incidence of spinal cord injuries. If possible, labor should be delayed to allow cesarean section.60 Likewise, if the arms are over the head, they increase the dystocia when the head enters the birth canal. If ultrasound examination reveals anencephaly or massive hydrocephaly, vaginal breech delivery should be allowed to continue because cesarean section is undesirable.
Management.: Premature infants in the breech position often deliver spontaneously without difficulty. As the infant comes to term, dystocia becomes increasingly common. With commitment to a vaginal delivery, knowledge of breech dystocia mechanics may allow atraumatic delivery. The key goals are to maximize the size of the passage and to minimize the dystocia of the after-coming head. Box 181-8 summarizes the actions associated with successful vaginal breech delivery.
The Mauriceau maneuver is the use of the fetal oral aperture to flex the fetal neck and draw in the chin. Because fetal neck extension is associated with cord injuries and worsening dystocia, this maneuver is useful to ensure a successful vaginal delivery. This maneuver should be attempted only once the fetal elbows and chin have entered the pelvic inlet to avoid inducing the Moro reflex (in which fetal head flexion results in the arms being suddenly extended).59 While the Mauriceau maneuver is used, the fetal pelvis should be supported to avoid abdominal injuries. Generous episiotomy may even be necessary to facilitate the Mauriceau maneuver in a full-term infant. If the after-coming head cannot be delivered quickly, the chances of good fetal outcome are poor. For term infants, labor arrest, asphyxia, or brachial plexus injuries are potential complications of vaginal breech deliveries.
Perspective.: Shoulder dystocia is the second most common malpresentation, occurring in 0.6 to 1.4% of all vaginal vertex deliveries.61 In contrast to a breech presentation, which may be diagnosed in the antepartum period, shoulder dystocia develops in the intrapartum period. Maternal and fetal factors are associated with shoulder dystocia. Maternal factors include diabetes, obesity, and precipitous or protracted labor; fetal factors include macrosomia, postmaturity, and erythroblastosis fetalis. The combination of prenatal data, estimated fetal weight, and fetal biometry cannot reliably identify most deliveries complicated by shoulder dystocia. The fact that shoulder dystocia responds well to a variety of intrapartum maneuvers means that delivery skill is an important determinant of fetal outcome.
The consequences of shoulder dystocia can be devastating. As with breech presentation, infant complications are more common and severe than maternal complications. Traumatic brachial plexus injuries, clavicular fractures, and hypoxic brain injury are all well-documented complications.61 Maternal complications are related to traumatic delivery and include vaginal, perineal, and anal sphincter tears as well as urinary incontinence.62
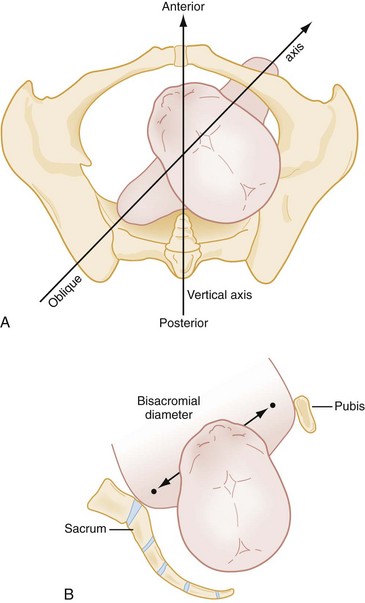
Diagnostic Strategies: Shoulder dystocia is diagnosed clinically by the inability to deliver either shoulder. The fetal head may appear to retract toward the maternal perineum. This finding is known as the “turtle sign.” Traction on the head extends and abducts the shoulders, increasing the bisacromial diameter and worsening the dystocia. Figure 181-9 shows the normal and abnormal relationship of the shoulders to the birth canal and illustrates why the bisacromial diameter is an important element of fetal biometry.
Normally, the shoulders negotiate the maternal pelvis in sequential fashion, anterior shoulder first. With shoulder dystocia, both shoulders attempt to clear the maternal pelvis simultaneously. In addition to the turtle sign, examination often reveals that the fetal shoulders are on a vertical axis (rather than oblique). These findings in combination with an arrested delivery confirm the diagnosis of shoulder dystocia.63
Management.: When shoulder dystocia becomes evident, knowledge of intrapartum delivery maneuvers can be lifesaving. Successful vaginal delivery is most likely when a directed sequential approach to each maneuver is used. Rapid resolution of shoulder dystocia is important to avoid fetal asphyxia and resultant central nervous system injury. Obstetric and neonatology assistance may improve the outcome, and aggressive attempts to obtain help in these areas are warranted.
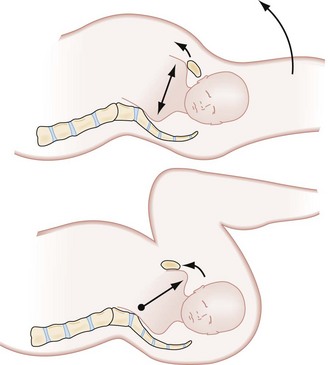
The most important first step is to use McRoberts’ maneuver (Fig. 181-10). Maternal leg flexion to a knee-chest position may disengage the anterior shoulder, allowing rapid vaginal delivery to follow. This maneuver “walks” the pubic symphysis over the anterior shoulder and flattens the sacrum, helping the fetus pass through the birth canal one shoulder at a time. This method, although requiring very little effort, is successful in 40% of shoulder dystocia cases when it is used alone.64
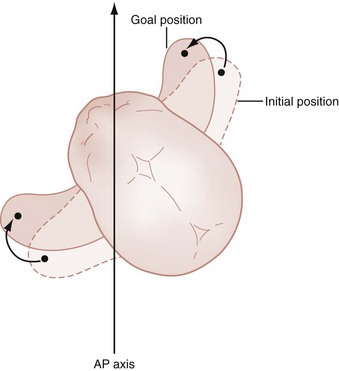
Use of these intrapartum maneuvers resolves most cases of shoulder dystocia. However, if delivery is still impossible, more drastic interventions are warranted. It may be possible to decrease the bisacromial diameter by pushing the most accessible shoulder toward the fetal chest, termed Rubin’s maneuver (Fig. 181-11). Often, both shoulders assume the same attitude, decreasing the bisacromial diameter and allowing delivery. Attempts to manipulate the shoulders for Rubin’s maneuver may be transabdominal, through the introitus (anterior shoulder), or through the episiotomy (posterior shoulder).
If the shoulders remain undeliverable, the next step is to use Wood’s corkscrew maneuver. In this process, the impacted shoulders are released through rotation of the fetus 180 degrees. Fetal rotation is achieved by pushing the most accessible shoulder in toward the chest.65 The fetal axilla can be snared with a digit, or a hand can be slid in along the fetal spine to sweep the hips and generate rotation. Wood’s corkscrew maneuver is difficult to perform but should be attempted before reaching for an arm. A slightly oblique anteroposterior position for the shoulders provides the largest passage through the pelvic outlet.
At this juncture, if the fetus remains trapped and several attempts have failed to yield delivery, consideration of delivery of an arm is appropriate. A hand is introduced along the posterior aspect of the posterior shoulder. This would be a tight fit, requiring tactile identification of fetal anatomy. The posterior arm is swept across the chest, bringing the fetal hand up to the chin. Attempts to splint the humerus may prevent fractures and brachial plexus injuries. The fetal hand is grasped and pulled out of the birth canal across the face, delivering the posterior shoulder. The mnemonic HELPER (Box 181-9) has been proposed to help keep these steps organized and to facilitate a sequential approach. These steps successfully deliver almost all cases of shoulder dystocia.
Face, Brow, and Compound Presentations.: Face and brow presentations yield a larger engaging aspect of the fetal head and predispose to labor arrest. Although these abnormal presentations can be diagnosed with ultrasonography or Leopold’s maneuvers, most are discovered during labor by vaginal examination. Approximately half are discovered during the second stage of labor.
The engaging diameter of the head in vertex position is approximately 0.8 cm less than a face presentation and 1.5 cm less than a brow presentation. Face presentations are described with the chin as a reference point (e.g., mentum anterior). Face presentation is managed expectantly. The obstetric adage “if a face presentation is progressing, leave it alone” arises from the fact that mentum anterior presentations usually deliver vaginally and mentum transverse presentations frequently rotate to become mentum anterior. Brow presentations, occurring when the fetal head is partially flexed, also spontaneously convert to vertex or face presentations in more than 50% of cases.57
Perspective.: Although twin gestations accounted for less than 1% of all deliveries historically, there has been a recent increase in the frequency of twin and triplet or higher births (65 and 500%, respectively) because of the increasing use of infertility treatments.66 In 2003 twin deliveries accounted for 31.5 per 1000 live births. Because multiple gestation deliveries have a higher incidence of preterm labor and low birth weights, both maternal and fetal complication rates are correspondingly increased.67
Diagnostic Strategies: Most women with multiple gestations have the situation identified well before the third trimester. In patients who have had little or no prenatal care, bedside ultrasonography allows rapid diagnosis and early preparation. The stages of labor for twins and other multiple gestations are similar to the stages for a singleton. Nulliparous women experience longer labors than multiparous women, with an overall labor duration that is similar to that of singleton pregnancies. Of importance to the emergency physician is a relatively short latent phase of labor, with rapid progression to the active phase. The active phase is usually longer, however. The prolongation of the active phase is due to overdistention of the uterus plus malpresentation and may allow time for obstetric assistance to arrive.
Vertex twin A and vertex twin B occur in approximately 40% of deliveries. In the remaining 60%, one or both of the twins has a malpresentation, usually twin B.68 Delivery problems with twin B cause most of the preventable perinatal mortality of twin gestation.69
Management.: The presentation of the twins is an important determinant for the safety of vaginal delivery. Twins who are vertex-vertex, the most common presentation, can be delivered vaginally barring any other obstetric complication. If twin B is nonvertex, some obstetricians recommend cesarean section to prevent delivery-related complications for twin B. External cephalic version may also be attempted to convert twin B to a cephalic, longitudinal lie and then proceed to vaginal delivery. Breech extraction is a third, more difficult, option. The general consensus is that if twin A is nonvertex, cesarean section is the preferred route. In such cases, efforts should be made to delay delivery until an operative approach can be used. Proceeding vaginally can result in interlocking of twins, associated with a high mortality.70
Umbilical Cord–Related Emergencies
Umbilical Cord Prolapse
Clinical Features.: Umbilical cord prolapse occurs when the umbilical cord precedes the fetal presenting part or the presenting part does not fill the birth canal completely. Most instances of cord prolapse are unexpected and develop during the second stage of labor.
Cord prolapse has a variable rate of association with different fetal presentations. Compound, shoulder, and breech presentations yield gaps and a relatively poor dilating wedge. Table 181-6 summarizes the rates of umbilical cord prolapse with various fetal presentations. Overall, malpresentations account for 50% of cord prolapse cases; therefore, prolapse may be the first indication of a malpresentation.71 The reported incidence of cord prolapse ranges from 0.1 to 0.6% of all deliveries, and associated perinatal mortality is estimated to be just below 10%.72,73
Table 181-6
Conditions Associated with Umbilical Cord Prolapse
| PRESENTATION | INCIDENCE (%) |
| Vertex | 0.14 |
| Breech | 2.5-3.0 |
| Frank breech | 0.4 |
| Complete breech | 5 |
| Incomplete breech | 10 |
| Shoulder | 5-10 |
| Compound | 10-20 |
| Face or brow | Rare |
Diagnostic Strategies.: Umbilical cord prolapse may be overt or occult, requiring a pelvic examination to reveal the umbilical cord lying beside the presenting part. The diagnosis also may be made with Doppler ultrasonography. In most cases, the diagnosis is obvious, and the cord is encountered at the perineum or introitus.
Management.: Whenever a prolapsed cord occurs with a viable infant, cesarean section is the delivery method of choice. If surgical delivery is available, maneuvers to preserve umbilical circulation should be instituted immediately. The mother should be placed in the knee-chest position with the bed in the Trendelenburg position as the presenting part is manually elevated off the umbilical cord. It is crucial that the mother be instructed to refrain from pushing to avoid further compression of the cord. Placement of a Foley catheter and instillation of 500 to 750 mL of saline into the bladder may help lift the fetus off the cord, particularly in the first stage of labor.73
Preparation for an emergency (“crash”) cesarean section should be under way. The time from prolapse to surgical intervention is an important factor in fetal outcome. Perinatal mortality rates are significantly higher for out-of-hospital cases (approximately 40%) versus those within a monitored setting (approximately 3%), and outcomes correlate with time from diagnosis to delivery.73 This finding has implications for the availability of obstetric and surgical backup for the ED.
If surgical delivery cannot be done in a timely fashion, funic reduction (manual replacement of the cord into the uterus) and rapid vaginal delivery may be the only options available. The same maneuvers to decrease cord compression are used, and the umbilical cord is pushed gently, in a retrograde fashion, above the presenting part. Manipulation and cord trauma is kept to a minimum because the resultant vasospasm can cause fetal hypoxia. After funic reduction, the development of umbilical cord body coils or nuchal loops is common and should be anticipated.74
Cord Entanglement
Long umbilical cords are associated with true knots as well as with entanglements and prolapse. Because the fetal limbs are short and flexed in most presentations, they are rarely involved. Loops around the neck and body do occur, however. Umbilical cord loops can be single or multiple, and there are reports of six nuchal loops. Risk factors for excessive cord length include increasing parity and fetal weight. Although generally benign, they may result in fetal complications, such as nonreassuring fetal status (fetal distress) and respiratory distress.75
Maternal Complications of Labor and Delivery
Postpartum Hemorrhage
Clinical Features.: Postpartum hemorrhage is the most common complication of labor and delivery. Defined as hemorrhage of more than 500 mL after vaginal delivery, it affects 5 to 10% of all deliveries and accounts for up to 25% of obstetric deaths.76 Postpartum hemorrhage is divided into primary and secondary categories. The primary category includes blood loss that occurs within the first 24 hours; the secondary category is hemorrhage 24 hours to 6 weeks after delivery. The clinical picture is as expected with any type of hemorrhage, although because of maternal adaptations during pregnancy, the patient may not show signs of shock until more than 1500 mL of volume is lost.77
Differential Considerations.: The differential diagnosis of primary postpartum hemorrhage includes uterine atony, genital tract trauma, retained placental tissue, and coagulopathies, or the four t‘s: tone, trauma, tissue, and thrombin.
Uterine Atony: The most common cause of serious immediate postpartum hemorrhage is laxity of the uterus after delivery. It accounts for 75 to 90% of postpartum hemorrhage cases.78 Postpartum bleeding from the placental implantation site normally is limited by contraction of the myometrium, constricting the spiral arteries. If the uterus does not contract, ongoing hemorrhage will occur. Predisposing factors include overdistention of the uterus (multiple gestations, fetal macrosomia, and polyhydramnios), prolonged labor, chorioamnionitis, use of tocolytics, and general anesthesia with halogenated compounds. Despite the myriad causes, uterine atony is a diagnosis of exclusion. Physical examination to rule out obstetric trauma and retained products of conception ought to be done before the diagnosis is reached. On examination, the uterus is palpable as a soft, boggy mass.
Maternal Birth Trauma: Maternal birth trauma is the second most common cause of postpartum hemorrhage, accounting for 20% of the cases. Uncontrolled delivery, macrosomia, episiotomy, nulliparity, maternal coagulopathy, operative delivery, prolonged second stage of labor, preeclampsia, and malpresentation may result in maternal birth-related trauma.79 Although genitourinary structures are most commonly involved, any part of the birth canal–associated anatomy may be injured, with resultant postpartum hemorrhage. Tears and lacerations may involve the perineum, rectum, cervix, vagina, vulva, and urethra. Blood vessels beneath the vulvar or vaginal epithelium can also be injured without frank hemorrhage, resulting in the formation of large, contained hematomas. These hematomas may go unrecognized for hours, gradually enlarging and possibly resulting in hemorrhagic shock. This type of hemorrhage should be suspected whenever there is evidence of ongoing blood loss and no identifiable obstetric site of bleeding (a firm uterus and negative examination findings for lacerations). Delayed postpartum hemorrhage at these sites can also occur and is often a diagnostic challenge. Physical examination may reveal uterine displacement (lateral or cephalad), and confirmation by radiologic means may be used in stable patients. Management, decided in conjunction with specialists, may be expectant, involve vascular embolization, or require surgical intervention, depending on the severity of clinical presentation.79
Retained Products of Conception: Approximately 10% of postpartum hemorrhage cases are due to retained placental tissue. Normally, the plane of cleavage between the zona basalis and the zona spongiosa results in clean separation of the placenta from the uterus. When this occurs, the placental tissue delivers as a single unit, without evidence of fragmentation. On occasion, accessory placental tissue exists as succenturiate placenta, but this also should cleave normally and deliver spontaneously.
Any placental defect or evidence of accessory placental tissue may signify a retained cotyledon (part of the embryo). Retained fragments prevent myometrial constriction and result in hemorrhage. Inappropriate traction on the placenta during stage 3 of labor can result in tears with retained products of conception, which may cause immediate and delayed postpartum hemorrhage. Ultrasonography may be used in the diagnosis of retained placenta; an empty or fluid-filled uterus provides a high negative predictive value, and an expanded endometrium or solid echogenic mass within the uterus provides evidence of retention.80,81
Placenta accreta, placenta increta, and placenta percreta describe various degrees of abnormal placental attachment to the uterus. Placental villi may invade the myometrium at the site of implantation, firmly rooting the placenta and obliterating the normal cleavage plane. Thus abnormal attachment results in retained products of conception and postpartum hemorrhage. When the placenta adheres to the myometrium without the intervening decidua basalis, it is termed placenta accreta. In placenta increta, the villi extend into the myometrium. In placenta percreta, the placenta penetrates the full thickness of the myometrium.82
The current incidence of placenta accreta is approximately 3 per 1000 deliveries, a relative increase from past decades. Associated risk factors include multiparity, prior cesarean sections, placenta previa, previous curettage, and uterine anomalies.83
Coagulopathies: A speedy search for the refractory cause of hemorrhage is warranted because coagulopathies may complicate obstetric hemorrhage. Disseminated intravascular coagulation (DIC) can occur as a consequence of placental abruption, eclampsia, amniotic fluid embolism, postpartum infections, and dilution of clotting factors caused by aggressive volume resuscitation. Also, retained products of conception and dead fetal tissue contain excess thromboplastin, which can initiate DIC. All women with severe postpartum hemorrhage should be evaluated for DIC. As with DIC from nonobstetric causes, clinical signs of bleeding are associated with hypofibrinogenemia, thrombocytopenia, and elevated levels of fibrin split products and D-dimer.84
Appropriate management entails hemodynamic support as well as correction of coagulopathies. In fact, investigations have reported the successful use of recombinant factor VIIa for severe cases of postpartum hemorrhage.85
Uterine Exploration and Removal of the Placenta: In the face of ongoing hemorrhage and retained products of conception, attempts to remove the placenta manually are indicated. The procedure entails risk of infection, perforation, and increased hemorrhage but may be the most expeditious way to control bleeding. Before beginning, the patient is placed on a monitor, good vascular access is established, and blood products are available. Also, a Foley catheter may be placed to reduce bladder distention and to monitor urinary output.86 The umbilical cord is traced through the cervical os to the placenta, allowing the identification of a placental margin. The placental membranes are digitally perforated, and the placenta is gradually divided from the myometrium. The palmar surface of the hand is directed toward the placenta, with care taken to avoid uterine perforation. After removal of the placenta, the uterus is explored for retained cotyledons. Removal of any more fragments still present requires curettage of the uterine cavity by an obstetrician. Placenta accreta, placenta percreta, and placenta increta may be diagnosed in this way because these are not digitally dissectible.
Once it is emptied, the uterus is stimulated to contract. Uterine massage, oxytocin, and prostaglandins may be used. Prophylactic antibiotic administration at the time of manual placenta extraction is somewhat debated in the literature. If it is used, a single dose of metronidazole and either ampicillin or cefazolin may be given.86
Uterine Packing: Uterine packing to decrease postpartum hemorrhage was widely used previously but now is uncommon. For the emergency physician, this technique may be used to create tamponade, preventing further blood loss. The procedure has limited morbidity and is straightforward. The physician introduces 15 to 20 yards of 4-inch gauze with a ring forceps and packs it into the uterus by a layering technique. A special “uterine packer” is available to help direct gauze high into the uterus but is not necessary. Another option is to place a Foley catheter or Sengstaken-Blakemore tube into the uterus and to instill the balloon with saline.86
Opponents of packing point out that an atonic uterus may accommodate a large volume of packing and blood without effective tamponade. Packing may also increase the risk of postpartum infection even when prophylactic antibiotics are given. As with all uterine manipulation and instrumentation, some risk of perforation also exists. Because pelvic embolization and hysterectomy sometimes are not immediately available to the emergency physician, the importance of uterine packing as a temporizing measure is increased.87
Pelvic Vessel Embolization: Pelvic bleeding post partum can be difficult to control. Hysterectomy as a solution results in infertility and brings with it all the complications of general anesthesia and major surgery. Radiographic embolization of the bleeding vessels by an interventional radiologist is another option. The procedure does not require an anesthesiologist, operating room, or obstetrician and may be more readily available on an emergent basis. Reported success rates of embolization in control of postpartum hemorrhage range from 95 to 100%.86
A catheter is placed in the aorta and fluoroscopically guided to the bleeding sites that are imaged by radiopaque dye. The vessels are embolized with absorbable gelatin sponges placed through the catheter. Common sites of bleeding include the uterine artery, pudendal artery, and hypogastric artery. Because only the smallest involved branches are embolized and recanalization usually occurs, future reproductive capability is generally preserved.88
Uterotonic Agents: Although they are commonly applied on delivery of the placenta, uterotonic agents also have special application in the case of postpartum hemorrhage. Uterotonics, such as oxytocin, ergot alkaloids, and prostaglandins, control bleeding by inducing myometrial contraction. Oxytocin is considered to be first-line treatment, given either intramuscularly or intravenously. Ergot alkaloids, such as methylergonovine and ergotamine, may induce hypertension and are therefore contraindicated in patients with preeclampsia or other comorbid conditions. Finally, prostaglandins may also be used (e.g., misoprostol), although they have shown no clear advantage over oxytocin or ergot alkaloids in published reports.86
Hysterectomy: Most postpartum hemorrhages are controllable with uterotonics and massage or uterine exploration for products of conception. Rarely, hemorrhage continues despite the interventions outlined. Life-threatening obstetric bleeding may require emergency hysterectomy. The desire to preserve the patient’s reproductive capabilities should not be given priority if her life is in jeopardy.89
Uterine Inversion
Perspective.: Uterine inversion is an uncommon but serious complication of delivery that occurs during stage 4 of labor. The resultant postpartum hemorrhage can be severe and life-threatening, accounting for a maternal mortality rate of up to 15%. Uterine inversion is relatively rare, complicating 1 in 2000 deliveries.90 It is classified by duration as well as by degree of inversion. Risk factors include excessive fundal pressure during delivery, forceful traction on the umbilical cord (especially in conjunction with a fundal placenta), placenta accreta, maternal congenital abnormalities of the uterus, use of magnesium sulfate in the antepartum period, and primiparity.90,91
Clinical Features.: Clinically, the patient notes the sudden onset of severe abdominal pain. Abdominal examination reveals tenderness and an absence of the uterine corpus, which is potentially visualized at the cervical os or bulging from the introitus. Profuse bleeding leading to hemodynamic instability can also occur. Ultrasound examination may assist in the diagnosis. Once uterine inversion is identified, the appropriate mobilization of resources should begin simultaneously with efforts to re-establish the correct anatomic position of the uterus.
Management.: As with other causes of postpartum hemorrhage, initial management involves aggressive fluid resuscitation.
If initial attempts fail and a cervical ring develops, pharmacologic attempts to relax the uterus are indicated. Sedation and tocolytics can be used to facilitate uterine replacement. Terbutaline and magnesium sulfate have been used successfully to relax cervical rings. When the uterus has been repositioned, the muscle relaxants should be halted, and oxytocin and prostaglandin therapy should be initiated. Firm manual pressure through the introitus should be maintained until uterine contraction begins, the cervical ring contracts, and the uterus can no longer invert. If all these measures fail and surgical backup becomes available, halogenated anesthetics may be used to induce relaxation of the cervical rings with or without an attempt at surgical repair.90 Once the uterine inversion is resolved, an assessment must be made to screen for uterine perforation, adherent placenta, and vaginal lacerations.79
Uterine Rupture
Perspective.: Criticism of the high rate of cesarean delivery in the United States has led to an advocacy of vaginal birth after cesarean (VBAC). Prior cesarean section is no longer an automatic indication for repeated cesarean delivery. The high success rate and relative safety of VBAC are countered partly by the risk of uterine rupture. Dehiscence of a surgical scar occurs in 0.6% of VBAC deliveries.92 As more women have VBAC, emergency physicians can expect to encounter uterine rupture, which is associated with high morbidity and mortality rates.
Clinical Features.: Uterine rupture is an unpredictable event occurring late in pregnancy or as stage 1 of labor transitions to the active phase. It is defined as a full-thickness uterine wall perforation. The severity of rupture ranges from simple scar dehiscence to complete fetal extrusion. It may be spontaneous, but it is most often linked with previous uterine surgery.93 Other risk factors for uterine rupture include congenital uterine abnormalities, trauma, and medications used for labor induction and abortions.79 As the degree of fetal expulsion through the rupture increases, the fetal mortality rate increases as well. Minimal fetal extrusion results in a perinatal mortality rate of less than 1%, whereas complete extrusion results in a 10 to 20% mortality rate. Maternal death is rare, but significant hemorrhage is common, complicating one third of cases. Maternal genitourinary injury may also occur in association with uterine rupture.
Diagnostic Strategies and Difficulties.: The diagnosis of uterine rupture may be difficult because pain is not always present. In fact, clinical presentation of uterine rupture ranges from nonreassuring fetal heart rate patterns to frank maternal hemorrhagic shock.79 Intrapartum vaginal bleeding may signal the problem, but its absence by no means precludes rupture. Prolonged fetal heart rate deceleration, indicating fetal distress, is the most reliable sign of fetal extrusion.80 Emergency ultrasonography may reveal a protruding amniotic sac, hemoperitoneum, or the site of myometrial rupture; however, good sensitivity data are lacking.93
Management.: If uterine rupture is suspected, delivery should be hastened to limit fetal hypoxia. Emergency cesarean section is the best method to speed delivery and to repair the injury. The ACOG guidelines for uterine rupture identify a 30-minute window of opportunity that maximizes fetal outcome.94 At surgery, the maternal condition dictates whether uterine repair or hysterectomy is indicated. In the absence of opportunity for emergency laparotomy, appropriate interventions remain speculative. Uterotonic agents may exacerbate the rupture and are contraindicated.
Amniotic Fluid Embolism
Amniotic fluid embolism is a rare and catastrophic complication of labor and delivery. The incidence rate is 6.0 and 14.8 per 100,000 in primigravid and multiparous deliveries, respectively.95 Although the mechanism is not well understood, it is thought to involve the spread of amniotic fluid through the maternal vasculature, activating either a complement or anaphylactic cascade. Cesarean delivery, forceps- or vacuum-assisted delivery, uterine rupture, eclampsia, placenta previa, and placental abruption have been found to have a significant association with amniotic fluid embolism. Patients will demonstrate moderate to severe pulmonary hypertension, right ventricular failure, and, in severe cases, left ventricular failure. The diagnosis is clinically evident during labor, during delivery, or within 48 hours of delivery. It is characterized by the sudden onset of dyspnea, hypoxia, altered mental status, seizure, hemorrhage (from coagulopathy), fetal compromise, or cardiovascular collapse. DIC occurs in approximately 50%, and both maternal and fetal mortality rates are high. Treatment is generally supportive and may include assisted ventilation, central hemodynamic monitoring, vasopressors or inotropes, and blood products.96–99
Postpartum Endometritis
Perspective.: Puerperal infections affect 5% of all vaginal deliveries and 10% of all cesarean sections. Operative delivery, prolonged rupture of membranes, lack of prenatal care, prolonged stage 2 labor, use of intrauterine monitoring, and frequent vaginal examinations have been linked to these ascending gynecologic infections.100 It is estimated that sepsis results in up to 15% of maternal deaths worldwide.101 Causative organisms for these infections include gram-positive cocci and gram-negative coliforms. Less commonly, Chlamydia and Mycoplasma species have been implicated.
Clinical Features.: Endometritis is the most common puerperal infection, usually developing on the second or third day post partum. Typically, the lochia has a foul odor and the white blood cell count is elevated. Fever and abdominal pain indicate greater severity of infection, often warranting inpatient care and intravenous antibiotics. A coexistent surgical wound infection is often present. A search for retained products of conception is indicated, particularly if bleeding is ongoing.
Management.: Treatment is empirical and directed at gram-positive, gram-negative, and anaerobic organisms. A combination of clindamycin and an aminoglycoside is recommended. Another commonly used antibiotic regimen is a second- or third-generation cephalosporin plus metronidazole.102 Most patients with postpartum endometritis require admission.
Peripartum Cardiomyopathy
Perspective.: For unclear reasons, the peripartum period is associated with the relatively sudden onset of cardiomyopathy in healthy women without evidence of prior cardiac disease. Estimates indicate that peripartum cardiomyopathy (PPCM) occurs in 1 of 4000 pregnancies; reported risk factors include advanced maternal age, preeclampsia, gestational hypertension, multiparity, and African American race.103 Proposed causes include viral, immunologic, hormonal, toxic, and genetic factors, but no specific cause is found in most cases. Reported mortality rates for PPCM range from 0 to 19%.104
Clinical Features.: Symptom onset varies, as does the severity of the cardiomyopathy. Onset is usually days to weeks after delivery, and symptoms range from mild fatigue to acute pulmonary edema. PPCM is often unrecognized in its milder form, leading to the consensus that the condition may be more prevalent than reported. Dyspnea on exertion, orthopnea, and fatigue may be easily misinterpreted as normal in a mildly anemic woman who is breast-feeding a new infant at home. The clinician should not dismiss these symptoms because severe congestive heart failure, thromboembolism, and dysrhythmias may ensue.
Management.: Treatment with diuretics, vasodilators, and oxygen relieves the symptoms in many cases. Angiotensin-converting enzyme inhibitors are contraindicated if PPCM occurs during the last month of pregnancy (owing to teratogenicity) but should be considered a mainstay of treatment post partum. Amlodipine (a dihydropyridine calcium channel blocker) may also have a role in the treatment of PPCM.105,106
Cardiac function returns to normal in half of patients with PPCM during the following 6 months. Others have residual left ventricular dysfunction, although the overall survival rate at 5 years is 94%.107 The presence of cardiomyopathy after one pregnancy does not predict recurrence during subsequent pregnancies.108 Most obstetricians recommend against future pregnancies, however, especially if some residual cardiac function impairment exists. If such a pregnancy cannot be avoided, it should be considered high risk and observed closely.
Postpartum Depression
Perspective.: Postpartum depression is likely to be underdiagnosed, but it is estimated that it affects 10 to 15% of mothers. Although in many cases it is self-limited, the condition has been recognized as having important consequences for the mother, infant, and family. Risk factors for postpartum depression include previously diagnosed depression and neuroticism, inadequate spousal support, adverse socioeconomic factors, recent life stressors, and emergency delivery.109
Clinical Features.: Postpartum depression patients present with symptoms not unlike those of other major depressive disorders. These symptoms include depressed mood, anhedonia, loss of appetite, insomnia, fatigue, decreased concentration, feelings of guilt and worthlessness, and suicidal ideation.110 Most women with postpartum depression do not have vegetative signs or symptoms. Symptoms peak at 10 to 12 weeks post partum, although some cases are diagnosed up to 1 year post partum.111
Management.: Early identification and referral are the key components of therapy. Dismissal of postpartum fatigue as normal, without consideration of the diagnosis of postpartum depression, can be disastrous. Not only does this condition contribute to marital discord, maternal risk for suicide, and even infanticide, but studies have shown that children of depressed mothers have an increased incidence of delayed cognitive, psychological, neurologic, and motor development.112 Therefore, sensitivity to the possibility of postpartum depression is crucial to successful treatment.
References
1. Pons, PT. Prehospital considerations in the pregnant patient. Emerg Med Clin North Am. 1994;12:1.
2. Moscovitz, HC, et al. Care and outcome of out-of-hospital deliveries. Acad Emerg Med. 2000;7:757.
3. MacDorman, MF, Munson, ML, Kirmeyer, S. Fetal and perinatal mortality, United States, 2004. Natl Vital Stat Rep. 2007;56:1.
4. Brunette, DD. Prehospital and emergency department delivery: A review of eight years experience. Ann Emerg Med. 1989;18:1116.
5. Elixhauser, A, et al. Health care for children and youth in the United States: 2001 annual report on access, utilization, quality, and expenditures. Ambul Pediatr. 2002;2:419.
6. Chasnoff, JJ, et al. Cocaine use in pregnancy. N Engl J Med. 1985;313:666.
7. Verenson, A, et al. Perinatal morbidity associated with violence experienced by pregnant women. Am J Obstet Gynecol. 1994;170:1760.
8. Abbott, J, et al. Domestic violence against women: Incidence and prevalence in an emergency department population. JAMA. 1995;273:1763.
9. Katz, VL, Hansen, AR. Complications in the emergency transport of pregnant women. South Med J. 1990;83:7.
10. Ayres, RJJr. Legal considerations in prehospital care. Emerg Med Clin North Am. 1993;11:853.
11. Strobos, J. Tightening the screw: Statutory and legal supervision of interhospital patient transfers. Ann Emerg Med. 1991;20:302.
12. Lee, NG. Update on EMTALA. Am J Nurs. 2000;100:57.
13. Albers, L. The evidence for physiologic management of the active phase of the first stage of labor. J Midwifery Womens Health. 2007;52:207.
14. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. No. 62: Intrapartum fetal heart rate monitoring. Obstet Gynecol. 2005;105(Pt 1):1161.
15. Trott, A. Diagnostic modalities in gynecologic and obstetric emergencies. Emerg Med Clin North Am. 1987;5:405.
16. Medina, TM, Hill, DA. Preterm premature rupture of membranes: Diagnosis and management. Am Fam Physician. 2006;73:659.
17. American College of Obstetrics and Gynecology Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445.
18. Altman, MR, Lydon-Rochelle, MT. Prolonged second stage of labor and risk of maternal and perinatal outcomes: A systematic review. Birth. 2006;33:315.
19. Stout, MJ, Cahill, AG. Electronic fetal monitoring: Past, present, and future. Clin Perinatol. 2011;38:127.
20. Vintzileos, AM, et al. Use of ultrasound in the labor and delivery. J Matern Fetal Neonatal Med. 2010;23:269.
21. Shiono, P, et al. Midline episiotomies: More harm than good? Obstet Gynecol. 1990;75:765.
22. Jonsson, ER, et al. Modified Ritgen’s maneuver for anal sphincter injury at delivery: A randomized control trial. Obstet Gynecol. 2008;112:212.
23. Webb, DA, Culhane, J. Hospital variation in episiotomy use and the risk of perineal trauma during childbirth. Birth. 2002;29:132.
24. Norwitz, ER, Robinson, JN, Repke, JT. Labor and delivery. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics: Normal and Problem Pregnancies. New York: Churchill Livingstone; 2003:353–390.
25. Magann, EF, et al. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105:290.
26. Bair, ME, Williams, J. Management of the third stage of labor. J Midwifery Womens Health. 2007;52:412.
27. Macpherson, T. Fact and fancy: What can we really tell from the placenta? Arch Pathol Lab Med. 1991;115:672.
28. Salatia, CM, Vintzilcos, AM. Why all placentas should be examined by a pathologist in 1990. Am J Obstet Gynecol. 1990;163:1282.
29. Katz, VL, Farmer, RM. Controversies in tocolytic therapy. Clin Obstet Gynecol. 1999;42:801.
30. Goldenberg, RL. The management of preterm labor. Obstet Gynecol. 2002;100:1020.
31. ACOG Committee on Practice Bulletins; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologist. Number 43, May 2003. Management of preterm labor. Obstet Gynecol. 2003;101(Pt 1):1039.
32. Canadian Preterm Labor Investigators Group. Treatment of preterm labor with the β-adrenergic agonist ritodrine. N Engl J Med. 1992;327:308.
33. Rodts-Palenik, S, Morrison, JC. Tocolysis: An update for the practitioner. Obstet Gynecol Surv. 2002;57:S9.
34. Crowther, CA, et al. Magnesium sulfate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev. (4):2002.
35. Pryde, PG, et al. Risk-benefit effects of tocolytic therapy. Expert Opin Drug Saf. 2004;3:639.
36. Sawdy, RJ, Lye, S, Fisk, NM, Bennett, PR. A double-blind randomized study of fetal side effects during and after the short-term maternal administration of indomethacin, sulindac, and nimesulide for the treatment of preterm labor. Am J Obstet Gynecol. 2003;188:1046.
37. Conde-Agudelo, A, et al. Nifedipine in the management of preterm labor: A systemic review and metaanalysis. Am J Obstet Gynecol. 2011;204:134.e1.
38. Wenstrom, KD, et al. Premature rupture of membranes. Obstet Gynecol Clin North Am. 1992;19:241.
39. ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 80: Premature rupture of membranes. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2007;109:1007.
40. Yudin, MH, et al. Antibiotic therapy in preterm premature rupture of the membranes. J Obstet Gynaecol Can. 2009;31:863.
41. Curran, CA. Perianesthesia care following obstetric emergencies at risk for multisystem organ dysfunction. J Perianesth Nurs. 2005;20:185.
42. Gorgas, DL. Infections related to pregnancy. Emerg Med Clin North Am. 2008;26:345.
43. Kenyon, S, Boulvain, M, Neilson, J. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. (2):2003.
44. Office of Inspector General, Reducing Obstetrician Barriers to Offering HIV Testing. Washington, DC:Department of Health and Human Services; 2002.
45. Kotler, DP. Human immunodeficiency virus and pregnancy. Gastroenterol Clin North Am. 2003;32:437.
46. McGowan, JP, Shah, SS. Prevention of perinatal HIV transmission during pregnancy. J Antimicrob Chemother. 2000;46:657.
47. Minkoff, H. Human immunodeficiency virus infection in pregnancy. Am Coll Obstet Gynecol. 2003;101:797.
48. Centers for Disease Control and Prevention. Approval of a new rapid test for HIV antibody. MMWR Morb Mortal Wkly Rep. 2002;51:1051.
49. Centers for Disease Control and Prevention. Rapid point-of-care testing for HIV-1 during labor and delivery—Chicago, Illinois, 2002. MMWR Morb Mortal Wkly Rep. 2003;52:866.
50. Mofenson, LM, Centers for Disease Control and PreventionU.S. Public Health Service Task Force. U.S. Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1–infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Recomm Rep. 2002;51:1.
51. International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of HIV-1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977.
52. Louis, J, Landon, MB, Gersnoviez, RJ. Perioperative morbidity and mortality among human immunodeficiency virus infected women undergoing cesarean delivery. Obstet Gynecol. 2007;110:385.
53. Legardy-Williams, JK, et al. Prevention of mother-to-child transmission of HIV-1: The role of caesarean delivery. Clin Perinatol. 2010;37:777.
54. Moodley, D, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum transmission of HIV-1. J Infect Dis. 2003;187:725.
55. Simonds, RJ, et al. Impact of zidovudine use on risk and risk factors for perinatal transmission of HIV. AIDS. 1998;12:301.
56. Stallard, TC, Burns, B. Emergency delivery and perimortem C-section. Emerg Med Clin North Am. 2003;21:679.
57. Stitely, ML, Gherman, RB. Labor with abnormal presentation and position. Obstet Gynecol Clin North Am. 2005;32:165.
58. Mukhopadhyay, S, Arulkumaran, S. Breech delivery. Best Pract Res Clin Obstet Gynaecol. 2002;16:31.
59. Kotaska, A, et al. Vaginal delivery of breech presentation. J Obstet Gynaecol Can. 2009;31:557.
60. Seffah, JD, Armah, JO. Antenatal ultrasonography for breech delivery. Int J Gynaecol Obstet. 2000;68:7.
61. Jevitt, CM. Shoulder dystocia: Etiology, common risk factors, and management. J Midwifery Womens Health. 2005;50:485.
62. Mazouni, C, et al. Maternal morbidity associated with obstetrical maneuvers in shoulder dystocia. Eur J Obstet Gynecol Reprod Biol. 2006;129:15.
63. Stallard, TC, Burns, B. Emergency delivery and perimortem C-section. Emerg Med Clin North Am. 2003;21:679.
64. Gurewitsch, ED. Optimizing shoulder dystocia management to prevent birth injury. Clin Obstet Gynecol. 2007;50:592.
65. Benrubi, GI. Handbook of Obstetric and Gynecologic Emergencies. Philadelphia: Lippincott Williams & Wilkins; 2005.
66. American College of Obstetrics and Gynecologists; Society for Maternal-Fetal Medicine; ACOG Joint Editorial Committee. ACOG Practice Bulletin #56: Multiple gestation: complicated twin, triplet, and high-order multifetal pregnancy. Obstet Gynecol. 2004;104:869.
67. Carroll, MA, Yeomans, ER. Vaginal delivery of twins. Clin Obstet Gynecol. 2006;49:154.
68. Apfelbaum, JD, Colwell, CB, Roe, EJ. Precipitous breech delivery of twins. Prehosp Emerg Care. 2000;4:78.
69. Adams, DM, Chervenak, FA. Intrapartum management of twin gestation. Clin Obstet Gynecol. 1990;33:52.
70. Carroll, MA, Yeomans, ER. Vaginal delivery of twins. Clin Obstet Gynecol. 2006;49:154.
71. Barnett, WM. Umbilical cord prolapse: A true obstetrical emergency. J Emerg Med. 1989;7:149.
72. Kinugasa, M, et al. Antepartum detection of cord presentation by transvaginal ultrasonography for term breech presentation: Potential prediction and prevention of cord prolapse. J Obstet Gynaecol. 2007;33:612.
73. Lin, MG. Umbilical cord prolapse. Obstet Gynecol Surv. 2006;61:269.
74. Barrett, JM. Funic reduction for the management of umbilical cord prolapse. Am J Obstet Gynecol. 1991;165:654.
75. Baergen, RN, et al. Morbidity, mortality, and placental pathology in excessively long umbilical cords: Retrospective study. Pediatr Dev Pathol. 2001;4:144.
76. Higgins, S. Obstetric haemorrhage. Emerg Med. 2003;15:227.
77. Curran, C. Intrapartum emergencies. J Obstet Gynecol Nurs. 2003;32:802.
78. Dildy, GA. Postpartum hemorrhage: New management options. Clin Obstet Gynecol. 2002;45:330.
79. Mirza, FG, Gaddipati, S. Obstetric emergencies. Semin Perinatol. 2009;33:97.
80. Lazebnik, N, Lazebnik, RS. The role of ultrasound in pregnancy-related emergencies. Radiol Clin North Am. 2004;42:315.
81. Shen, O, Rabinowitz, R, Eisenberg, VH, Samuelhoff, A. Transabdominal sonography before uterine exploration as a predictor of retained placental fragments. J Ultrasound Med. 2003;22:561.
82. Zahn, CM, Yoemans, ER. Postpartum hemorrhage, placenta accreta, uterine inversion, and puerperal hematomas. Clin Obstet Gynecol. 1990;33:422.
83. Belfort, MA, Publications Committee, Society for Maternal-Fetal Medicine. Placenta accreta. Am J Obstet Gynecol. 2010;203:430.
84. Gerbasi, FR, et al. Increased intravascular coagulation associated with pregnancy. Obstet Gynecol. 1990;75:385.
85. Hamaekers, AE, et al. Successful use of recombinant factor VIIa for treatment of severe postpartum hemorrhage. Am J Crit Care. 2006;15:399.
86. Rajan, PV, Wing, DA. Postpartum hemorrhage: Evidence-based medical interventions for prevention and treatment. Clin Obstet Gynecol. 2010;53:165.
87. Goldrath, MH. Uterine tamponade for the control of acute uterine bleeding. Am J Obstet Gynecol. 1983;147:869.
88. Vegas, G, Illescas, T, Munoz, M, Perez-Pinar, A. Selective pelvic arterial embolization in the management of obstetric hemorrhage. Eur J Obstet Gynecol Reprod Biol. 2006;127:68.
89. Stanco, LM, et al. Emergency peripartum hysterectomy and associated risk factors. Am J Obstet Gynecol. 1993;168:879.
90. Hostetler, DR, Bosworth, MF. Uterine inversion: A life-threatening obstetric emergency. J Am Board Fam Pract. 2000;13:120.
91. Lago, JD. Presentation of acute uterine inversion in the emergency department. Am J Emerg Med. 1991;9:239.
92. Chauhan, SP, et al. Maternal and perinatal complications with uterine rupture in 142,075 patients who attempted vaginal birth after cesarean delivery: A review of the literature. Am J Obstet Gynecol. 2003;189:408.
93. Kaakaji, Y, Nghiem, HV, Nodell, C, Winter, TC. Sonography of obstetrics and gynecologic emergencies. AJR Am J Roentgenol. 2000;174:641.
94. ACOG practice bulletin. Vaginal birth after previous cesarean delivery. Number 5, July 1999. Int J Gynaecol Obstet. 1999;66:197.
95. Kramer, MS, et al. Amniotic-fluid embolism and medical induction of labor: A retrospective, population-based cohort study. Lancet. 2006;368:1444.
96. Davies, S. Amniotic fluid embolism and isolated DIC. Can J Anaesth. 2000;47:481.
97. Green, BT, Umana, E. Amniotic fluid embolism. South Med J. 2000;93:721.
98. Stafford, I, Sheffield, J. Amniotic fluid embolism. Obstet Gynecol Clin North Am. 2007;34:545.
99. Conde-Agudelo, A, Romero, R. Amniotic fluid embolism: An evidence-based review. Am J Obstet Gynecol. 2009;201:445.
100. Faro, S. Postpartum endometritis. Clin Perinatol. 2005;32:803.
101. Van Dillen J, et al. Maternal sepsis: Epidemiology, etiology and outcome. Curr Opin Infect Dis. 2010;23:249.
102. Maharaj, D. Puerperal pyrexia: A review. Part II. Obstet Gynecol Surv. 2007;62:400.
103. Ramaraj, R, Sorrell, V. Peripartum cardiomyopathy: Causes, diagnosis, and treatment. Cleve Clin J Med. 2009;76:289.
104. Elkayam, U. Clinical characteristics of peripartum cardiomyopathy in the United States: Diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58:659.
105. Pearson, GD, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and the Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183.
106. Packer, M, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. N Engl J Med. 1996;335:1107.
107. Felker, GM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077.
108. Witlin, AG, et al. Peripartum cardiomyopathy: An ominous diagnosis. Am J Obstet Gynecol. 1997;176:182.
109. Koo, V, Lynch, J, Cooper, S. Risk of postnatal depression after emergency delivery. J Obstet Gynecol. 2003;29:246.
110. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 2000.
111. Steinberg, SI, Bellavance, F. Characteristics and treatment of women with antenatal and postpartum depression. Int J Psychiatry Med. 1999;29:209.
112. Gjerdingen, DK, Yawn, BP. Postpartum depression screening: Importance, methods, barriers, and recommendations for practice. J Am Board Fam Med. 2007;20:280.