Chapter 95 Kyphotic Cervical Deformity
Kyphotic cervical spine deformity can be caused by advanced degenerative disease, systemic arthritides, trauma, neoplastic disease, and postsurgical (iatrogenic) causes.1 The most common cause is postsurgical.2
The postsurgical development of cervical kyphosis can follow both ventral and dorsal approaches. Following ventral approaches, kyphosis may develop secondary to pseudarthrosis or failure to restore anatomic cervical lordosis during surgery. Ventral discectomy without graft placement is associated with a 33% rate of kyphosis.3 Following dorsal surgery, kyphosis may develop and progress secondary to disruption of the dorsal elements (interspinous ligaments, laminae, and facet joints). The incidence of kyphosis after laminectomy ranges from 14% to 47%.4,5
Cervical kyphosis is biomechanically unfavorable for the cervical musculature. The kyphosis will tend to progress as axial loads of the head produce a bending moment through the moment arm of the cervical spine.6 This will often produce mechanical pain that improves when the patient is supine.7 Often, excessive degeneration of the cervical discs occurs that contributes to cervical pain. As the kyphosis progresses, the patient’s field of view, swallow, and respiration may be affected. The patient may develop low back pain and accelerated degeneration by hyperlordosing the lumbar spine to accommodate for the cervical deformity.
Clinical Presentation
Patients often present with mechanical neck pain. This pain is typically worse in the upright position and with exertion and improved with rest and recumbancy. Patients can also present with radiculopathy or myelopathy from compression on neural elements. As the kyphosis worsens, there is increased stress on the ventral spinal cord from tethering of the cord by the dentate ligaments. This may adversely affect the spinal cord vasculature and lead to worsened myelopathic symptoms.8,9 Patients with severe deformity may present with swallowing dysfunction and in some cases nutritional deficiency, compromise of horizontal gaze, and compromised breathing.10,11
Clinical Evaluation
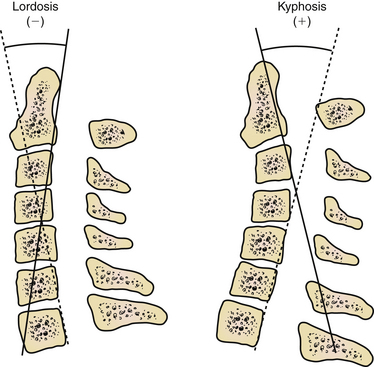
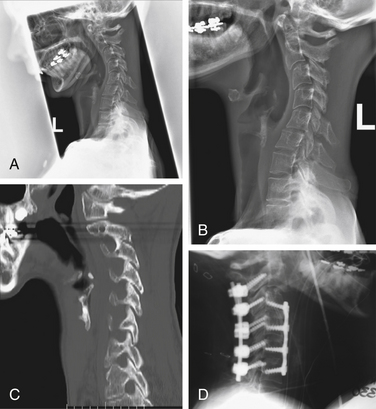
The deformity should be evaluated radiographically as well. Evaluate the cervical deformity using static upright and dynamic (flexion-extension) radiographs. The thoracic spine should be evaluated if deformity is suspected at the cervicothoracic junction. By using radiographs, the sagittal angle of the deformity may be measured (Fig. 95-1), and other abnormalities may be identified, such as subluxation and pseudarthrosis. Pseudarthrosis and existing fusion can be better clarified by using thin-cut CT scan. The ability of the alignment of the cervical spine to be reduced can also be assessed with extension or supine radiographs. Standing long-cassette radiographs can be useful to assess overall sagittal balance. In designing the treatment strategy, thoracic hyperkyphosis, lumbar hyperlordosis, and ankylosed joints must be taken into account.
Deformity Correction Strategies
The cervical spine may be exposed to coronal plane, rotational, axial (subsidence), or, most commonly, sagittal plane deformation stresses. It is important to establish goals of treatment prior to surgery. Decompression of the neural elements should be the first priority. Most commonly, the surgical approach will address the compression directly, but occasionally, this is dealt with indirectly by deformity correction. Ferch et al.12 demonstrated an association of improved myelopathy with restored lordosis in cervical kyphotic deformity patients. Fusion at abnormal levels may also prevent damage to the cord from micromotion.
Sagittal plane deformity may be addressed ventrally,13–18 dorsally,19–21 or both.18,19,22–27 If there is imaging evidence of ventral compression, then a ventral procedure should be considered. If the deformity is fixed ventrally without dorsally fused joints, then a ventral decompression with fusion can be used to correct the kyphosis. If the dynamic radiographs demonstrate normal movement and a normal lordosis can be achieved in extension, then a dorsal surgical reconstruction can adequately address the pathology. If the deformity is fixed owing to dorsally fused joints (ankylosing), then a dorsal osteotomy can be used to correct the kyphosis.10,11,28 Finally, a flexible deformity can be corrected posturally or with traction and then be fused dorsally.
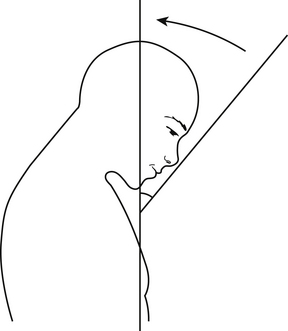
The goal for restoration of sagittal alignment is unclear. A definition of normal lordosis does not exist. However, Gore et al. measured lordotic angles in the cervical spine of patients with osteoarthritis using the perpendicular of the C2 and C7 vertebral bodies. Lordosis of 16 to 22 degrees was observed in men and 25 degrees in women.29 However, it is likely that if a patient is not restored to neutral or a lordotic posture, the deformity will continue to progress over time. Another useful measure, especially in rigid cervical kyphosis (e.g., ankylosing spondylosis), is the chin-brow vertical angle, which will help to determine whether the patient will be able to see the horizon (Fig. 95-2). Finally, overall sagittal and coronal balance is important. Ideally, the head should be balanced over the sacrum.
Ventral Strategies
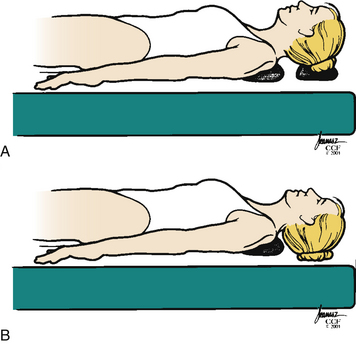
The ventral strategy uses both posture and biomechanical principles to correct the cervical deformity. Initially positioning the head on a towel or foam doughnut with the neck in a neutral or only slightly extended position allows adequate exposure for the approach but without compromising the spinal cord. After the decompression is complete, the towel or doughnut can be removed, allowing further extension of the cervical spine (Fig. 95-3).
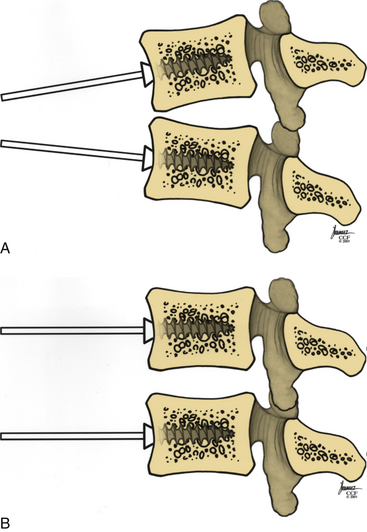
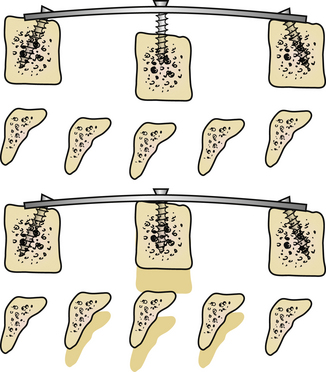
Distraction posts should be placed into the vertebral bodies in a convergent manner (Fig. 95-4A). This will allow distraction on the posts to provide further lordosis (Fig. 95-4B).
Long-segment corpectomies, greater than two vertebral bodies, have been used to correct ventral deformity. However, the authors believe that long-segment corpectomies should be avoided. Biomechanical studies show that three-segment corpectomies allow more movement than two-level corpectomies do.30 Long-segment corpectomies are more likely to fail at the terminal end because of the amount of force at the terminal screw-bone interface. Vaccarro et al. found that the early failure rate of two-segment corpectomies was 9% compared with 50% for three-segment corpectomies.31 In straight or kyphotic spines, corpectomies do not maintain as much sagittal angle correction as they do in lordotic spines.32
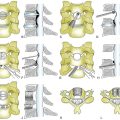
An alternative method for ventral correction is multilevel discectomy and fusion and/or a combined short-segment corpectomy. Leaving an intermediate vertebral body allows adequate decompression and provides additional intermediate fixation points for security of fixation and deformity correction (Fig. 95-5). Wei-bing et al.33 showed that two-level corpectomies had a higher rate of plate and graft migration requiring reoperation than did a combined construct of one-level anterior cervical discectomy and fusion (ACDF) plus one-level corpectomy. Oh et al.34 did not show a difference in clinical outcome between single-level corpectomy versus two-level ACDF.
The degree of correction required must be considered preoperatively to help plan the number of levels to be involved for deformity correction. Although this is often simply estimated, some recent studies may be helpful. Cabraja et al.35 found that a ventral or two-level corpectomy achieved a segmental correction of 6.2 degrees and an overall cervical lordosis correction of 8.8 degrees. However, using ventral correction only, they found that there was an approximately 2-degree loss of correction over an average 33-month follow-up period. Ferch et al.12 demonstrated that a ventral only approach can improve overall lordosis an average of 11 degrees. Eighty-five percent of the patients in their series were restored to neutral or lordotic alignments.
Dorsal Strategies
It is uncommon to use a dorsal strategy alone to correct cervical deformity because it is difficult to reduce a kyphotic deformity and achieve adequate lordosis from a dorsal approach alone. But if the deformity is flexible and no ventral decompression is required, then it should be possible to correct the deformity from a dorsal approach alone. If there is evidence of ventral compression, then a combined approach should be considered.
There are many instrumentation choices. The most common method is lateral mass fixation at the levels of C3-6. The C7 lateral masses are often not large enough to hold a screw, and screw placement often makes it difficult to align rods across the cervicothoracic junction. Lateral mass fixation might not be an optimal stabilizing anchor in some patients.20,23,36
It is also possible to use cervical pedicle screws for the dorsal correction of cervical deformity. Abumi et al.19 have demonstrated their usefulness in deformity correction, achieving a correction from 28.4 to 5.1 degrees of kyphosis with all patients achieving solid fusion. It should be emphasized that lordosis was not achieved with the dorsal procedure alone. Kotani et al.37 have demonstrated that these screw systems are biomechanically equivalent to combined ventral plate and dorsal wiring.
Combined Strategies
In general, combined ventral and dorsal approaches allow improved deformity correction because they allow ventral lengthening with dorsal shortening. In fixed (or inflexible) deformity with ankylosed dorsal elements, a combined ventral and dorsal approach may be required. Also, if the deformity involves the cervicothoracic junction, a combined approach should be considered. The addition of a dorsal construct aids in preventing deformity progression at the cervicothoracic junction by using a long moment arm strategy.38
Ankylosing spondylosis often requires a 540-degree procedure. A dorsal osteotomy is performed first. Some authors have proposed performing a C7-T1 osteotomy under local anesthesia in the seated position.39 After the osteotomy is performed, the patient can be placed in the supine position, and a ventral corpectomy or multilevel ACDF can be performed to allow decompression and ventral release for the correction of the deformity. Ventral instrumentation can then be applied. Careful attention should be paid to the patient’s chin-brow vertical angle, which can help to determine whether the patient will be able to look to the horizon and in front of himself or herself when walking. Owing to the large moment arm above and below the level of the fusion, dorsal instrumentation should also be used to prevent later deformity. The stages of the operation often need to be tailored depending on the basis of the anatomy of the deformity.
If the deformity is to be corrected by using a 540-degree approach, a ventral decompression and release should be performed without graft or instrumentation. The patient may then be positioned prone to expose the dorsal cervical spine. At this point, an assistant may adjust the head holder to achieve the appropriate alignment that should be determined with the aid of fluoroscopy and direct visualization. The dorsal instrumentation may then be placed and contoured to secure deformity correction. Rib or iliac crest bone graft can then be harvested to provide autogenous bone graft. The patient will then again be placed supine, and the ventral strut graft can be placed and secured with ventral instrumentation. Utilizing this combined approach, Abumi et al.19 were able to improve preoperative kyphosis of 30.8 degrees to 0.5 degrees at final follow-up.
Extension Osteotomy
Some cervical kyphotic deformities, for example, ankylosing spondylosis, may produce an extreme fixed flexion deformity at the cervicothoracic junction. This deformity can be treated with an extension osteotomy at the cervicothoracic junction.28,39,40 The procedure as initially described was performed under local anesthesia with the patient awake and sitting.11,28,39 However, this was primarily due to early limitations in intubating patients with severe kyphotic deformity. Subsequently, the procedure has been described under general anesthesia in the prone position with intraoperative nerve monitoring.11 Positioning the patient remains a significant challenge, owing to the degree of deformity. Positioning often requires additional padding and table adjustment to gain access for a dorsal approach. The head must be placed in a halo frame and attached to an adjustable head holder.
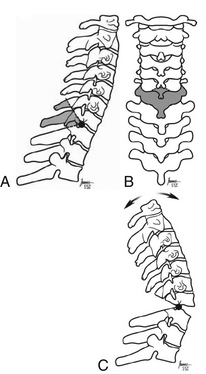
After positioning the cervicothoracic junction is exposed. A wide laminectomy is performed at C7 with partial laminectomy at C6 and T1. The spinous process of C6 is removed. The ankylosed C7 and T1 facet joints are then removed to expose the C8 nerve roots beyond the foramina. Then the C6 and T1 pedicles are removed to avoid impingement (Fig. 95-6). One surgeon then adjusts the head while the other surgeon watches for evidence of impingement or subluxation. Electrophysiologic monitoring continues as the deformity is corrected. If a change is noted, leads should be checked, and blood pressure should be optimized. If the signals do not correct, the dura should be evaluated for compression. If none of these correct the signals, the deformity can be returned to its original alignment. A wake-up test can also be considered.
As the kyphosis is corrected, a fracture will eventually develop ventrally. This is the most difficult portion of the operation to control. Various instrumentation and techniques have been used to try to control this portion of the reduction, including articulated halo jackets,28 prebent loops and wires,41 temporary malleable rods,42 and hinged rods.43 Tokala et al.44 described a more ventrally based wedge osteotomy to try to allow a more controlled closure.
Other surgeons have described using a ventral release prior to the dorsal osteotomy to help control the correction.45–47 However, this can be a difficult approach in patients with severe chin-on-chest deformities.
Instrumentation Techniques
Ventral cervical plates, either constrained or nonconstrained, can be used for multilevel fusions and will allow stabilization for fusion and also achieve lordosis.48 Dynamic ventral fixation devices allow sagittal correction along with controlled deformation in the axial plane.6 The controlled subsidence encourages bone healing via Wolff’s law and also off-loads stresses at the screw-bone interface. Nunley et al.49 found that there was improved clinical outcome with dynamic plates rather than fixed plates.
There are many options for dorsal instrumentation. Interspinous wiring may be used, but lateral mass fixation has been shown to provide greater rigidity than wire fixation alone.36 Lateral mass and cervical pedicle screws have similar biomechanical stability and have been shown to be superior in resisting axial rotation compared to cervical laminar hooks.50 There have not been studies looking specifically at fusion rates using lateral mass screws. Cervical pedicle screws are more difficult and dangerous to place but may increase load sharing across the disc space compared to lateral mass screws.51
Interbody and corpectomy grafts are often used for deformity correction. The choice of grafts includes autologous iliac crest, which has a fusion rate of 98% to 100%,52 but there is a risk of persistent donor site morbidity.53 The allograft fusion rate using fibula is 86.6% when combined with instrumentation.54 The use of titanium cage with autologous graft has been reported to have a fusion rate of 97.8%.55 Other options include cages made of polyetheretherketone or carbon fiber filled with morselized autologous or allograft bone. Corpectomy grafts are more prone to technical failures, particularly at the distal end of long constructs. Sasso et al.56 demonstrated increased failure rates with long (more than two-body) corpectomy constructs.
Bone morphogenic protein-2 can be used to promote fusion. However, its use in the ventral neck is associated with significant soft tissue swelling in 23% to 37% of patients.53 It is used primarily to promote dorsal arthrodesis.
Intraoperative Neurologic Monitoring
There are multiple methods to monitor intraoperative neurologic function. The Stagnara wake-up test has been described as the gold standard.11,41 The wake-up test definitively determines the patient’s neurologic function; however, it does take expert anesthesia to administer, and because it takes time to perform, the critical time window for reversal of deficit may be missed.
Care must be taken in interpreting neurologic monitoring. The use of SSEPs alone should be avoided, as there can be significant postoperative neurologic deficits despite normal intraoperative recordings.57,58 MEPs can be used also and have a high sensitivity and specificity, but there are inconsistent data regarding correlation of MEP changes to neurologic outcome.40,59 A 20% decrease in amplitude of the MEPs is considered a significant neurologic change. In the event that this occurs, technical problems should be assessed, and hemodynamic parameters should be optimized. If the amplitude remains decreased, the surgical maneuver preceding the change should be reversed.60
Outcomes
Clinical
There have been no randomized clinical trials evaluating the clinical outcome of cervical deformity correction. In studies in which horizontal gaze was restored, patients were satisfied with their outcomes.44,61 Cabraja et al.35 found a statistically significant improvement in visual analogue scale and modified Japanese Orthopaedic Association Scale for patients receiving ventral or dorsal surgery for deformity correction. The study did not find a difference between the ventral and dorsal groups. Using a combined surgical approach, Nottmeier et al. described preoperative symptom improvement of 97.5%.62
Radiographic
By using a ventral only approach, overall cervical lordosis was improved in the ventral group by 9 to 32 degrees, and segmental lordosis by 6.2 degrees.62 However, the 2 degrees of ventral correction was lost over an average of 33-month follow-up.35
By using a dorsal only approach, approximately 6.5 to 54 degrees of improvement in overall lordosis can be achieved.35,63 The chin-brow vertical angle can be corrected an average of 35 to 52 degrees.63
Nottmeier et al.62 reviewed their combined approaches and found that they had an average correction of 22 degrees. They showed a fusion rate of 97.5%.
Complications
Complications of the ventral approach include vocal cord palsy, dysphagia, tracheal or esophageal injury, vertebral artery injury, graft failure or displacement, hardware failure, and fracture and wound complications. Overall operative and perioperative complications of 22% to 33% have been reported.52,54
The dorsal wedge osteotomy for the correction of flexion deformity has a potential high incidence of morbidity.11 Complications include infection and respiratory and cardiovascular problems. There is a potential for vertebral artery injury during the osteotomy or neurologic injury during deformity correction. The resultant neurologic injury may range from minor nerve root irritation to spinal cord compression and quadriplegia. After deformity correction, there is risk of subluxation of C7 on T1, with resultant bony nonunion.
Circumferential fusion has a complication rate of about 32% to 33%.22,26 These complications can include neurologic deficits, wound infection, plate dislodgement, pseudarthrosis, dysphonia, requirement of tracheostomy and PEG tube, and death. Many of these are early transient complications, with about 5% persisting long term.26
Conclusion
Deformity of the cervical spine has multiple causes, the most common being prior surgery. Patients may present with symptoms related to the deformity itself (e.g., swallowing dysfunction) or with neurologic deficit. If deformity is causing symptoms, it should be corrected. Surgical goals should be decompression of neural elements, restoration of lordotic alignment, and prevention of further deformity. Controversy remains regarding the optimal approach to achieve these goals, and some situations may require combined approaches (Fig. 95-7). The ventral approach has been found to be most beneficial in restoring sagittal alignment. However, the dorsal approach has been shown to be beneficial in maintaining alignment. There is more likelihood of having perioperative complications in this type of surgery, but many of these complications are transient. The majority of patients have both relief of their mechanical symptoms and improvement of their neurologic function.
Cabraja M., Abbushi A., Koeppen D., et al. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15-E19.
Etame A.B., Wang A.C., Than K.D., et al. Outcomes after surgery for cervical spine deformity: review of the literature. Neurosurg Focus. 2010;28(3):E14-E21.
Hoh D.J., Khoueir P., Wang M.Y. Management of cervical deformity in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E9.
Kaptain G.J., Simmons N., Replogic R.E., et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg Spine. 2000;93:199-204.
Koller H., Hempfing A., Ferraris L., et al. 4- and 5-level anterior fusions of the cervical spine: review of literature and clinical results. Eur Spine J. 2007;16(12):2055-2071.
Mummaneni P.V., Dhall S.S., Rodts G.E., Haid R.W. Circumferential fusion for cervical kyphotic deformity. J Neurosurg Spine. 2008;9(6):515-521.
1. Johnston F.G., Crockard H.A. One stage internal fixation and anterior fusion in complex cervical spinal disorders. J Neurosurg. 1995;82:234-238.
2. Albert T.J., Vacarro A. Postlaminectomy kyphosis. Spine (Phila Pa 1976). 1998;23:2738-2745.
3. Laing R.J., Ng I., Seeley H.M., et al. Prospective study of clinical and radiological outcome after anterior cervical discectomy. Br J Neurosurg. 2001;15:319-323.
4. Kaptain G.J., Simmons N., Replogic R.E., et al. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg Spine. 2000;93:199-204.
5. Ryken T.C., Heary R.F., Matz P.G., et al. Cervical laminectomy for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11(2):142-149.
6. Benzel E.C. Biomechanics of spine stabilization. Rolling Meadows, IL: American Association of Neurological Surgeons; 2001.
7. Katsuura A., Hukuda S., Imanaka T., et al. Anterior cervical plate used in degenerative disease can maintain cervical lordosis. J Spine Disord. 1996;9:470-476.
8. Breig A., El-Nadi A.F. Biomechanics of the cervical spinal cord: relief of contact pressure on and overstretching of the spinal cord. Acta Radiol Diagn. 1964;4:602-624.
9. Masini M., Maranhao V. Experimental determination of the effect of progressive sharp-angle spinal deformity on the spinal cord. Eur Spine J. 1997;6:89-92.
10. Belanger T.A., Milam R.A.I., Roh J.S., Bohlman H.H. Cervicothoracic extension osteotomy for chin-on-chest deformity in ankylosing spondylitis. J Bone Joint Surg [Am]. 2005;87:1732-1738.
11. McMaster M.J. Osteotomy of the cervical spine in ankylosing spondylitis. J Bone Joint Surg [Br]. 1997;79:197-203.
12. Ferch R.D., Shad A., Cadoux-Hudson T.A., Teddy P.J. Anterior correction of cervical kyphotic deformity: effects on myelopathy, neck pain, and sagittal alignment. J Neurosurg. 2004;100(1 Suppl Spine):13-19.
13. Buttler J.C., Whitecloud T.S.III. Postlaminectomy kyphosis: causes and surgical management. Clin Orthop North Am. 1992;23:505-511.
14. Cattrell H.S., Clark G.L.Jr. Cervical instability following multiple laminectomies in children. J Bone Joint Surg [Am]. 1967;49:713-720.
15. Steinmetz M.P., Kager C.D., Benzel E.C. Ventral correction of postsurgical cervical kyphosis. J Neurosurg. 2003;98:1-7.
16. Stewart T.J., Steinmetz M.P., Benzel E.C. Techniques for the ventral correction of postsurgical cervical kyphotic deformity. Neurosurgery. 2005;56(Suppl 1):191-195.
17. Herman J.M., Sonntag V.K. Cervical corpectomy and plate fixation for post-laminectomy kyphosis. J Neurosurg. 1994;82:234-238.
18. Zdeblick T.A., Bohlman H.H. Cervical kyphosis and myelopathy: treatment by anterior corpectomy and strut-grafting. J Bone Joint Surg [Am]. 1989;71:170-182.
19. Abumi K., Shono Y., Taneichi H. Correction of cervical kyphosis using pedicle screw fixation systems. Spine (Phila Pa 1976). 1999;24:2389-2396.
20. Anderson P.A., Henley M.B., Grady M.S., et al. Posterior cervical arthrodesis with AO reconstruction plates and bone graft. Spine (Phila Pa 1976). 1991;16:S72-S79.
21. Callahan R.A., Johnson R.M., Margolis R.N. Cervical facet fusion for control of instability following laminectomy. J Bone Joint Surg [Am]. 1977;59:991-1002.
22. Mummaneni P.V., Dhall S.S., Rodts G.E., Haid R.W. Circumferential fusion for cervical kyphotic deformity. J Neurosurg Spine. 2008;9(6):515-521.
23. Heller J.G., Silcox D.H.I., Sutterlin C.E.I. Complications of posterior cervical plating. Spine (Phila Pa 1976). 1995;20:2442-2448.
24. McAfee P.C., Bohlman H.H., Ducker T.B. One stage anterior cervical decompression and posterior stabilization. A study of one hundred patients with a minimum of two years of follow-up. J Bone Joint Surg [Am]. 1995;77:1791-1800.
25. O’Shaughnessy B.A., Liu J.C., Hsieh P.C., et al. Surgical treatment of fixed cervical kyphosis with myelopathy. Spine (Phila Pa 1976). 2008;33:771-778.
26. Schultz K.D., McLaughlin M.R., Haid R.W., et al. Single-stage anterior-posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg Spine. 2000;93(2):214-221.
27. Steinmetz M.P., Stewart T.J., Kager C.D., et al. Cervical deformity correction. Neurosurgery. 2007;60:S0-S97.
28. Urist M.R. Osteotomy of the cervical spine: report of a case of ankylosing spondylitis. J Bone Joint Surg [Am]. 1958;40:833-843.
29. Gore D.R., Sepic S.B., Gardner G.M. Roentgenographic findings of the cervical spine in asymptomatic people. Spine (Phila Pa 1976). 1986;11:521-524.
30. Porter R.W., Crawford N.R., Chamberlain R.H., et al. Biomechanical analysis of multilevel cervical corpectomy and plate constructs. J Neurosurg Spine. 2003;99(1):98-103.
31. Vaccaro A.R., Fatalyn S.P., Scuderi G.J., et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11:415.
32. Thakar S., Vedantam A., Rashekhar V. Correlation between change in graft height and change in segmental angle following central corpectomy for cervical spondylotic myelopathy. J Neurosurg Spine. 2008;9:158-166.
33. Wei-bing X., Wun-Jer S., Gang L., et al. Reconstructive techniques study after anterior decompression of multilevel cervical spondylotic myelopathy. J Spinal Disord Tech. 2009;22(7):511-515.
34. Oh M.C., Zhang H.Y., Park J.Y., Kim K.S. Two-level anterior cervical discectomy versus one-level corpectomy in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2009;34(7):692-696.
35. Cabraja M., Abbushi A., Koeppen D., et al. Comparison between anterior and posterior decompression with instrumentation for cervical spondylotic myelopathy: sagittal alignment and clinical outcome. Neurosurg Focus. 2010;28(3):E15-E19.
36. Fehlings M.G., Cooper P.R., Errico T.J. Posterior plates in the management of cervical instability: long-term results in 44 patients. J Neurosurg. 1994;81:341-349.
37. Kotani Y., Cunningham B.W., Abumi K., McAfee P.C. Biomechanical analysis of cervical stabilization systems: an assessment of transpedicular screw fixation in the cervical spine. Spine (Phila Pa 1976). 1994;19:2529-2539.
38. Lapsiwala S., Benzel E.C. Surgical management of cervical myelopathy dealing with the cervical thoracic junction. Spine J. 2006;6(Suppl 6):S268-S273.
39. Simmons E.H. The surgical correction of flexion deformity of the cervical spine in ankylosing spondylitis. Clin Orthop Relat Res. 1972;86:132-143.
40. Hoh D.J., Khoueir P., Wang M.Y. Management of cervical deformity in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E9.
41. Shimizu K., Matsushita M., Fujibayashi S., et al. Correction of kyphotic deformity of the cervical spine in ankylosing spondylitis using general anesthesia and internal fixation. J Spinal Disord. 1996;9:540-543.
42. Mehdian S.M., Jaffray D., Eisenstein S. Correction of severe cervical kyphosis in ankylosing spondylitis by traction. Spine (Phila Pa 1976). 1992;17:237-240.
43. Khoueir P., Hoh D.J., Wang M.Y. Use of hinged rods for controlled osteoclastic correction of a fixed cervical kyphotic deformity in ankylosing spondylitis. J Neurosurg Spine. 2008;8(6):579-583.
44. Tokala D.P., Lam K.S., Freeman B.J., et al. C7 decancellisation closing wedge osteotomy for the correction of fixed cervico-thoracic kyphosis. Eur Spine J. 2007;16:1471-1478.
45. Bhojraj S.Y., Dasgupta D., Dewoolkar L.V. One-stage “front” and “back” correction for rigid cervical kyphosis. A safer technique of correction for a rare case of adult-onset Still’s disease. Spine (Phila Pa 1976). 1993;18:1904-1908.
46. Mummaneni P.V., Mummaneni V.P., Haid R.W., et al. Cervical osteotomy for the correction of chin-on-chest deformity in ankylosing spondylitis. Technical note. Neurosurg Focus. 2003;14(1):E9.
47. Bouchard J.A., Feibel R.J. Gradual multiplanar cervical osteotomy to correct kyphotic ankylosing spondylitic deformities. Can J Surg. 2005;14:215-218.
48. Koller H., Hempfing A., Ferraris L., et al. 4- and 5-level anterior fusions of the cervical spine: review of literature and clinical results. Eur Spine J. 2007;16(12):2055-2071.
49. Nunley P.D., Jawahar A., Kerr E.Jr., et al. Choice of plate may affect outcomes for single versus multilevel ACDF: results of a prospective randomized single-blind trial. Spine J. 2009;9(2):121-127.
50. Espinoza-Larios A., Ames C.P., Chamberlain R.H., et al. Biomechanical comparison of two-level cervical locking posterior screw/rod and hook/rod techniques. Spine J. 2007;7:194-204.
51. Dunlap B.J., Karaikovic E.E., Park H-S., et al. Load sharing properties of cervical pedicle screw-rod constructs versus lateral mass screw-rod constructs. Eur Spine J. 2010;19:803-808.
52. Eleraky M.A., Lllanos C., Sonntag V.K.H. Cervical corpectomy: report of 185 cases and review of the literature. J Neurosurg Spine. 1999;90(1):35-41.
53. Ryken T.C., Heary R.F., Matz P.G., et al. Techniques of cervical interbody grafting. J Neurosurg Spine. 2009;11(2):203-220.
54. Mayr M.T., Subach B.R., Comey C.H., et al. Cervical spinal stenosis: outcome after anterior corpectomy, allograft reconstruction and instrumentation. J Neurosurg Spine. 2002;96:10-16.
55. Zeena D., Morgan H., Coimbra C. Titanium cage reconstruction after cervical corpectomy. J Neurosurg Spine. 2003;99(1):3-7.
56. Sasso R.C., Ruggiero R.A.J., Reilly T.M., Hall P.V. Early reconstruction failures after multilevel corpectomy. Spine (Phila Pa 1976). 2003;23:2455-2461.
57. Ben-David B., Haller G., Taylor P. Anterior spinal fusion complicated by paraplegia: a case report of a false-negative somatosensory-evoked potential. Spine (Phila Pa 1976). 1987;12:536-539.
58. Lesser R.P., Raudzens P., Luders H., et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986;19:22-25.
59. Resnick D.K., Anderson P.A., Kaiser M.G., et al. Electrophysiological monitoring during surgery for cervical degenerative myelopathy and radiculopathy. J Neurosurg Spine. 2009;11:245-252.
60. Langeloo D.D., Journee H.L., Pavlov P.W., de Kleuver M. Cervical osteotomy in ankylosing spondylitis: evaluation of new developments. Eur Spine J. 2006;15:493-500.
61. Simmons E.D.J., DiStefano R.J., Zheng Y., Simmons E.H. Thirty-six years experience of cervical extension osteotomy in ankylosing spondylitis: techniques and outcomes. Spine (Phila Pa 1976). 2006;31:3006-3012.
62. Nottmeier E.W., Deen H.G., Patel N., Birch B. Cervical kyphotic deformity correction using 360-degree reconstruction. J Spinal Disord Tech. 2009;22(6):385-391.
63. Etame A.B., Wang A.C., Than K.D., et al. Outcomes after surgery for cervical spine deformity: review of the literature. Neurosurg Focus. 2010;28(3):E14-E21.