Chapter 7 Knee Ultrasound
![]() Additional videos for this topic are available online at www.expertconsult.com.
Additional videos for this topic are available online at www.expertconsult.com.
Knee Anatomy
The knee joint is a synovial joint that consists of hyaline cartilage articulations between the femur, the tibia, and the patella (Fig. 7-1). The fibrocartilage menisci are C-shaped structures between the femur and the tibia. A prominent joint recess, the suprapatellar recess, extends superiorly from the knee joint between the patella and the femur and communicates with the medial and lateral joint recesses, which extend over the medial and lateral aspects of the femoral condyles beneath the patellar retinaculum.1 In the sagittal plane, the quadriceps fat pad is located anteriorly between the suprapatellar recess and quadriceps tendon, and the prefemoral fat pad is located between the suprapatellar recess and the femur. The infrapatellar fat pad of Hoffa is an intracapsular but extrasynovial fat pad between the anterior knee joint and the patellar tendon. Various bursae exist around the anterior knee joint, including the prepatellar bursa anterior to the patella, the superficial infrapatellar bursa anterior to the distal patellar tendon, and the deep infrapatellar bursa between the patellar tendon and proximal tibia. Additional bursae are present around the medial knee, including the pes anserinus bursa deep to the pes anserinus tendons and the semimembranosus-tibial collateral ligament bursa, which has an inverted U shape located at the joint line between the medial collateral ligament and the semimembranosus tendon.2,3 These latter two bursae do not communicate with the knee joint. A more common bursa is the semimembranosus-medial gastrocnemius bursa, which, when distended, is called a Baker cyst. This bursa communicates to the knee joint in 50% of adults who are older than 50 years and becomes a common recess for joint fluid and intra-articular bodies.4
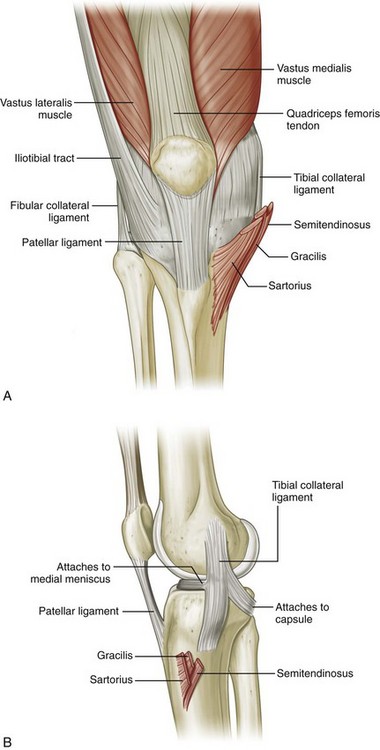
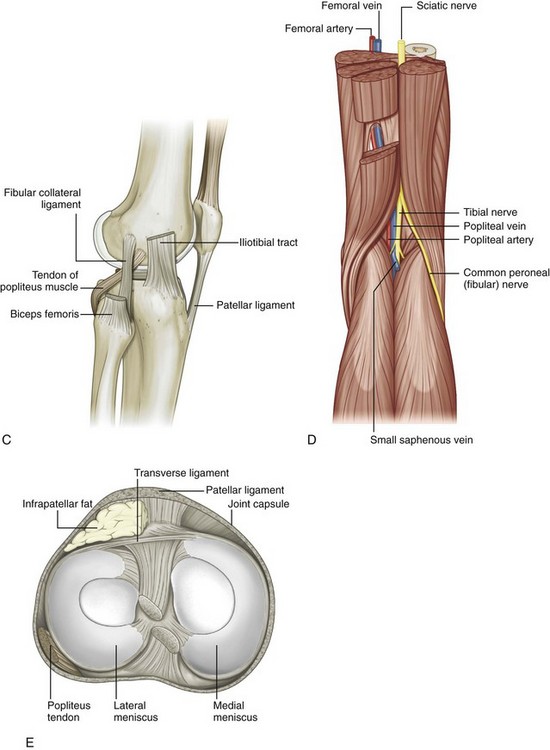
FIGURE 7-1 Knee anatomy.
(From Drake R, Vogl W, Mitchell A: Gray’s anatomy for students. Philadelphia, 2005, Churchill Livingstone.)
The knee joint is stabilized by a number of ligaments. Medially, the medial collateral ligament extends from the medial femoral condyle to the tibia in the coronal plane. Thin, deep layers of the medial collateral ligament (meniscofemoral and meniscotibial ligaments) extend from the meniscus to the femur and tibia, whereas a thicker, more superficial layer (tibial collateral ligament) extends from the femur to insert distally on the tibia deep to the pes anserinus.5 Superficial to the medial collateral ligament is found the deep crural fascia.5 The lateral or fibular collateral ligament originates from the lateral femur and extends over the popliteus tendon to insert on the lateral aspect of the fibula with the biceps femoris tendon.6 Other supporting structures of the posterolateral knee include the popliteofibular ligament and the arcuate ligament. The popliteofibular ligament extends from the popliteus tendon to the styloid process of the proximal fibula, whereas the arcuate ligament extends from the femur and joint capsule to the fibula tip as well. When a fabella is present, another posterolateral structure is the fabellofibular ligament. The anterior and posterior cruciate ligaments within the intercondylar notch extend from the femur to the proximal tibia as intracapsular but extrasynovial structures.
With regard to tendons around the knee, anteriorly the quadriceps femoris tendon inserts on the superior patellar pole, although superficial fibers extend over the patella (termed the prepatellar quadriceps continuation) to insert on the tibial tuberosity as part of the patellar tendon.7 The medial and lateral patellar retinaculum extends from each side of the patella to the femur; the medial aspect is reinforced by the medial patellofemoral ligament, which extends from the medial patella to the adductor tubercle region of the medial femoral condyle.8 The distal aspect of the vastus tibial, often termed the vastus medialis obliquus, blends with the medial patellar retinaculum to insert onto the medial patella.8–10 Medially and anteriorly, the sartorius, gracilis, and semitendinosus tendons insert on the tibia near the tibial collateral ligament as the pes anserinus (a helpful mnemonic is “Say Grace before Tea” where S, Sartorius; G, Gracilis; and T, semiTendinosis). Posterior and proximal to the pes anserinus, the semimembranosus primarily inserts on the tibia just beyond the tibia articular surface, although the distal anatomy is quite complex.11 Posteriorly, the medial and lateral heads of the gastrocnemius originate from the posterior aspect of the femoral condyles. Laterally, the biceps femoris tendon and lateral collateral ligament attach to the lateral margin of the fibular head.12 The direct arm of the long head of the biceps femoris tendon inserts on the lateral aspect of the fibula with the lateral collateral ligament, whereas the anterior arm of the long head biceps femoris inserts more anterior on the fibula. The short head of the biceps femoris also has two insertions: the direct arm insertion on the proximal fibula medial to the long head and the anterior arm insertion on the proximal tibia.6 The popliteus tendon originates at the lateral aspect of the femur, lies within a groove or sulcus of the femur, and courses obliquely with its muscle belly located between the posterior aspect of the tibia and the tibial artery and vein. Anterolaterally, the iliotibial tract or band inserts on the Gerdy tubercle of the proximal tibia.
Ultrasound Examination Technique
Table 7-1 is a checklist for a knee ultrasound examination. Examples of diagnostic knee ultrasound reports are available online at www.expertconsult.com (see eBox 7-1 and 7-2).
| Structures/Pathologic Features | Location of Interest |
|---|---|
| Anterior |
eBox 7-1 Sample Diagnostic Knee Ultrasound Report
Normal
Examination: Ultrasound of the Right Knee
Findings: The extensor mechanism, including the quadriceps tendon, patella, and patellar tendon, is normal without bursal abnormalities. No significant joint effusion or synovial hypertrophy. The medial collateral and lateral collateral ligaments are normal. Unremarkable iliotibial tract, biceps femoris, popliteus tendon, and common peroneal nerve. No Baker cyst. Limited evaluation of the menisci is unremarkable.
Impression: Unremarkable ultrasound examination of the right knee.
eBox 7-2 Sample Diagnostic Knee Ultrasound Report
Abnormal
Examination: Ultrasound of the Right Knee
History: Pain, evaluate for cyst
Findings: The extensor mechanism, including the quadriceps tendon, patella, and patellar tendon, is normal. There is a moderate-sized joint effusion and no synovial hypertrophy or intra-articular body. The medial and lateral collateral ligaments are normal, as is the iliotibial tract, biceps femoris, popliteus tendon, and common peroneal nerve. There is medial compartment joint space narrowing and osteophyte formation with mild extrusion of the body of the medial meniscus, which is abnormally hypoechoic. No parameniscal cyst. There is a Baker cyst measuring 2 × 2 × 6 cm. Abnormal hypoechogenicity is noted at the inferior margin of the Baker cyst. There is also a hypoechoic cleft involving the posterior horn of the medial meniscus, which extends to the articular surface.
Anterior Evaluation
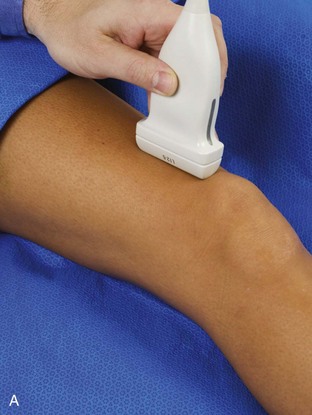
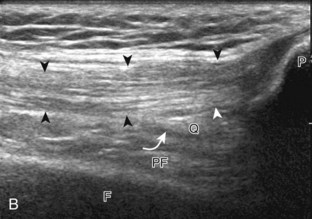
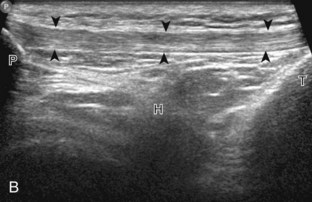
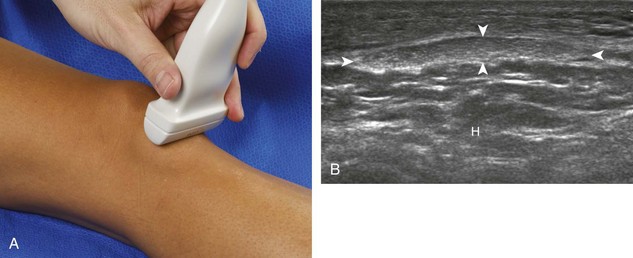
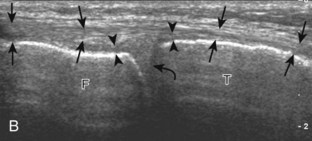
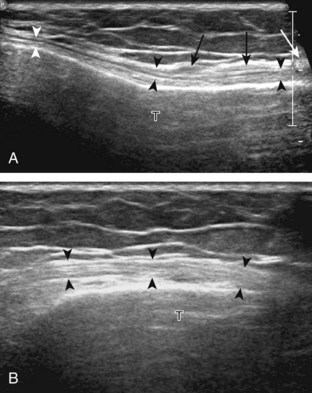
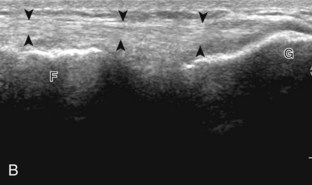
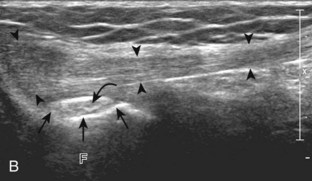
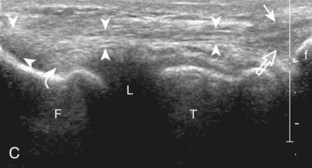
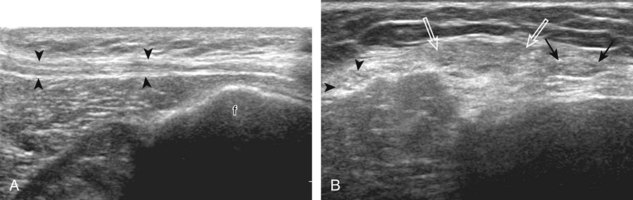
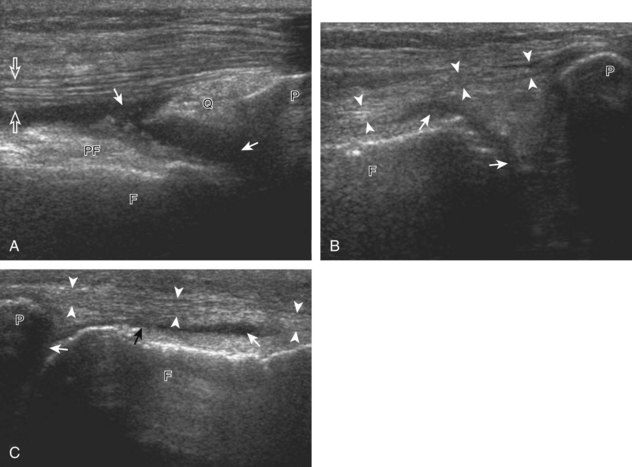
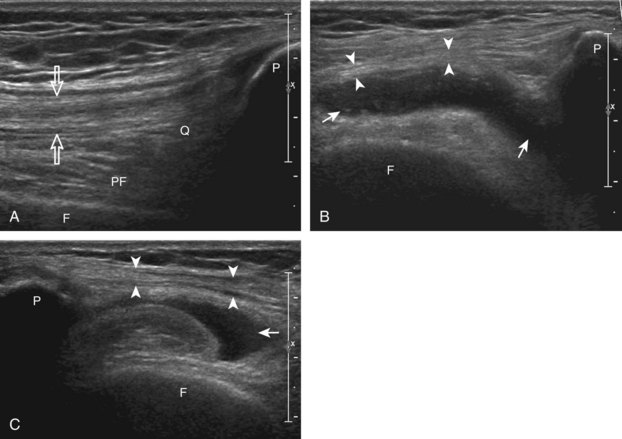
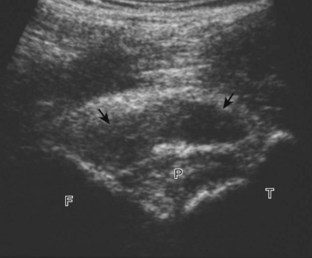
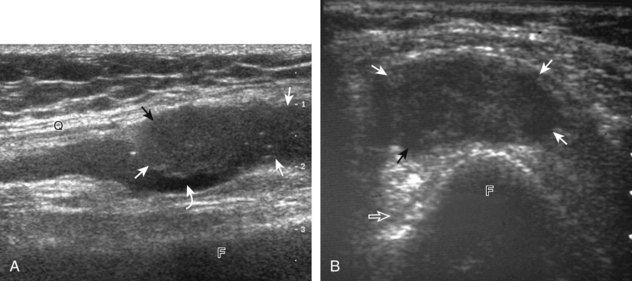
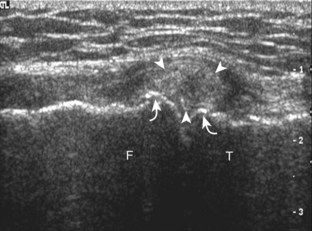
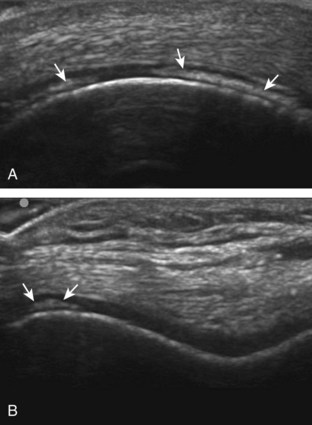
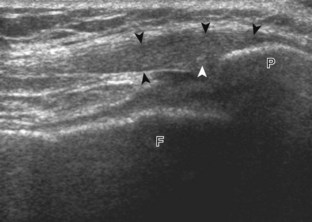
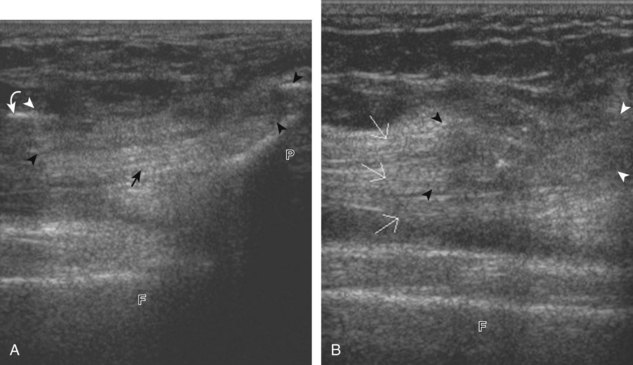
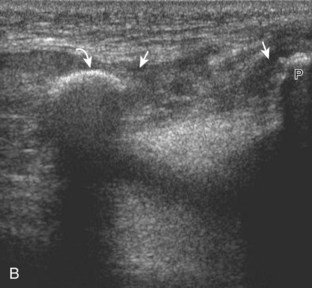
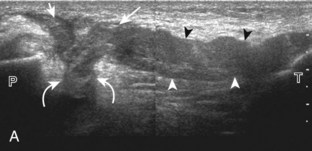
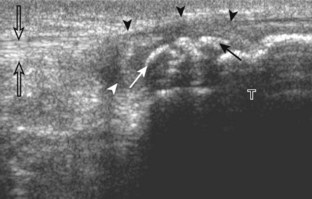
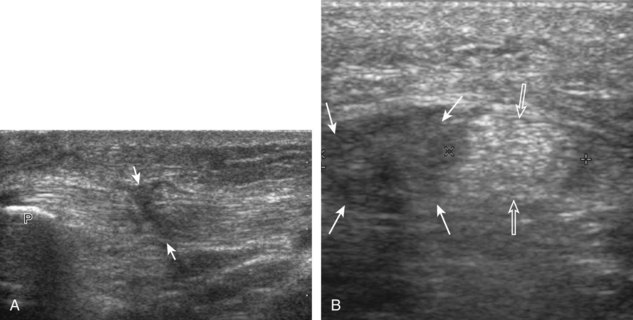
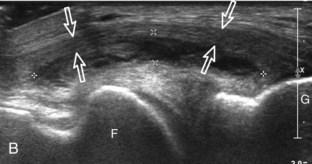
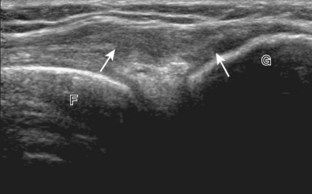
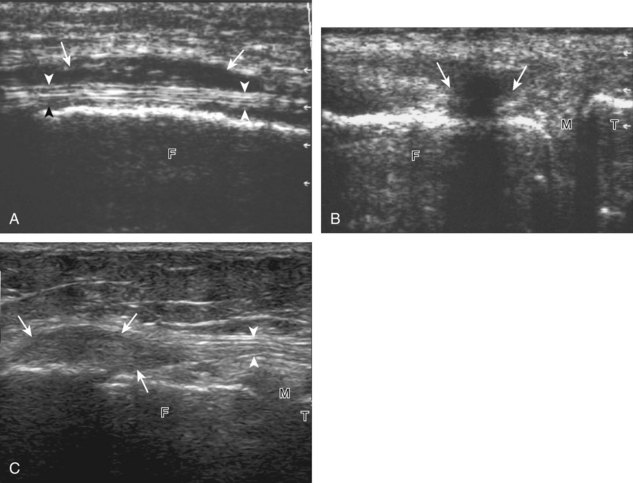
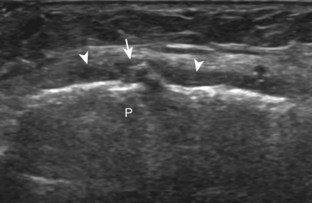
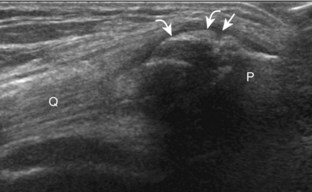
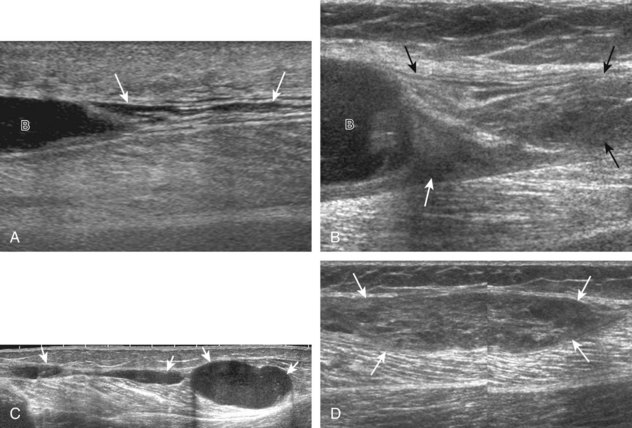
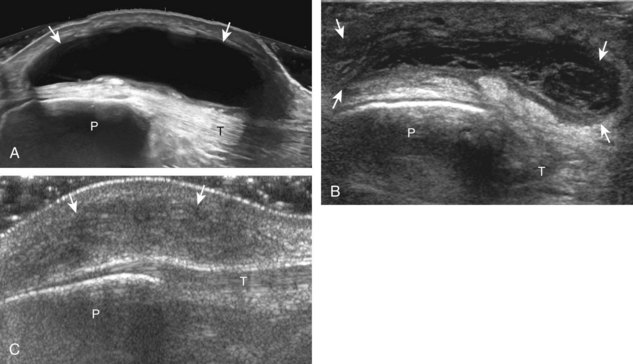
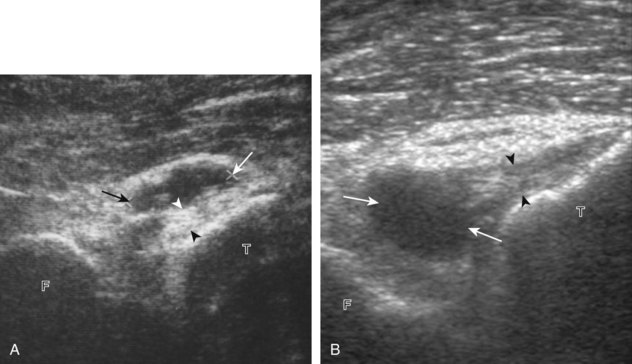
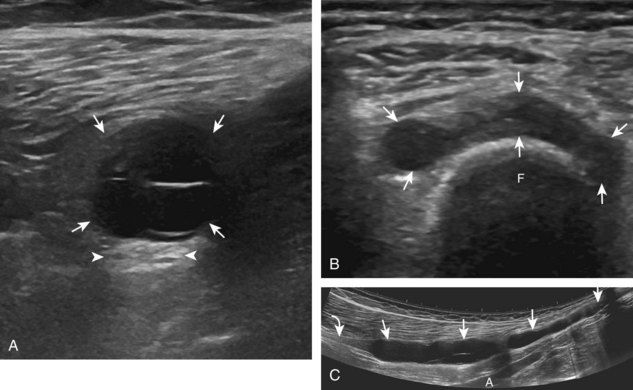
The primary structures evaluated from the anterior approach are the quadriceps tendon, the patella, the patellar tendon, the patellar retinaculum, the suprapatellar recess, the medial and lateral recesses, and the bursa around the anterior knee. Examination is begun in the sagittal plane proximal to the patella (Fig. 7-2A). This plane demonstrates the normal hyperechoic and fibrillar appearance of the quadriceps tendon (see Fig. 7-2B). Slight flexion of the knee with a pad or roll behind the knee is often helpful because this position straightens and tenses the extensor mechanism to reduce tendon anisotropy. Often, the trilaminar appearance of the quadriceps tendon can be appreciated, with the rectus femoris as the anterior layer, the combined vastus medialis and intermedius as the middle layer, and the vastus intermedius as the deepest layer (see Quadriceps Femoris Injury). The quadriceps tendon is also evaluated in short axis (Fig. 7-3A and B). Returning to the quadriceps tendon in long axis, the suprapatellar recess is identified deep to the quadriceps tendon and evaluated for anechoic or hypoechoic joint fluid, which would separate the quadriceps fat pad (located superficial) from the prefemoral fat pad (located deep) (see Fig. 7-2). Slight knee flexion also shifts fluid from other parts of the knee joint into the suprapatellar recess. The transducer is then moved inferiorly below the patella in the sagittal plane to visualize the hyperechoic, fibrillar, and uniform patellar tendon (Fig. 7-4A and B). The Hoffa infrapatellar fat pad appears minimally hyperechoic or isoechoic to muscle deep to the patellar tendon. The transducer should also be floated on a layer of gel over the proximal patellar tendon and patella to evaluate for patellar fracture, as well as prepatellar bursal fluid, because the latter may be easily redistributed out of view with the slightest transducer pressure. The region around the distal patellar tendon is also evaluated for superficial and deep infrapatellar bursal fluid; minimal fluid in the latter is considered physiologic (see Other Bursae). Although long axis is most important in evaluation of extensor mechanism abnormalities, imaging should also be completed in short axis to ensure a thorough evaluation, especially with the patellar tendon, where a focal abnormality may not be located in midline (Fig. 7-5A and B).
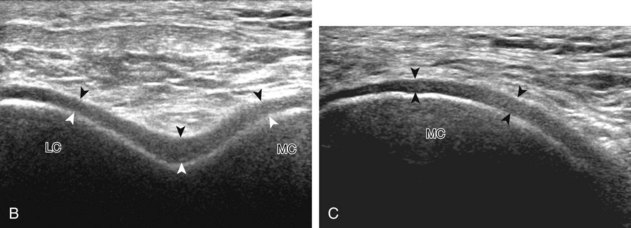
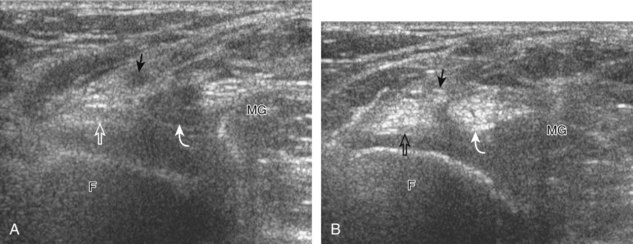
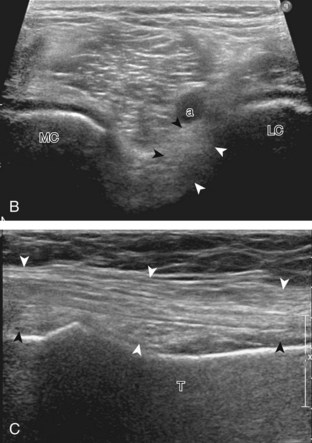
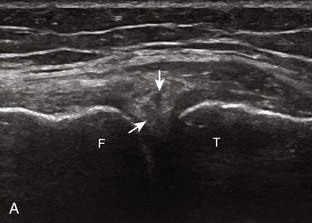
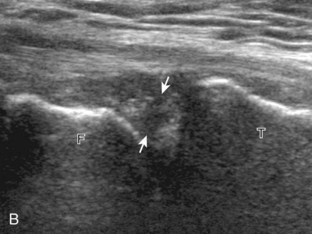
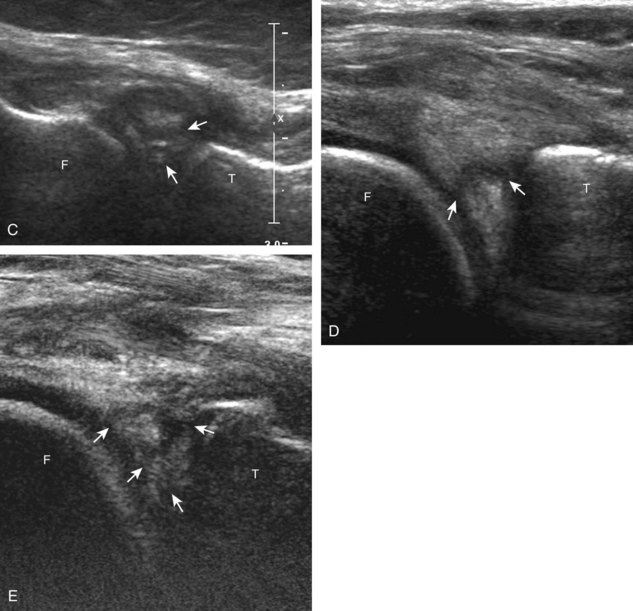
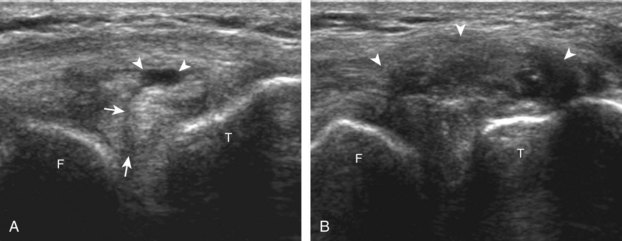
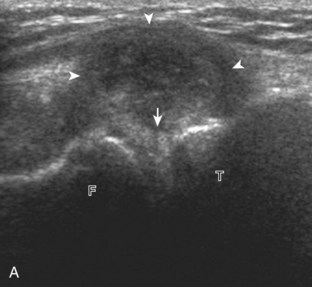
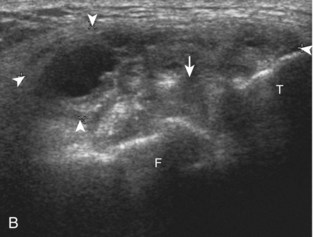
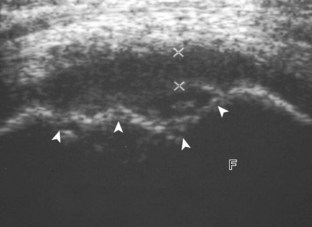
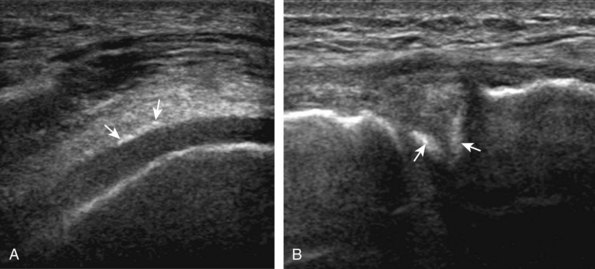
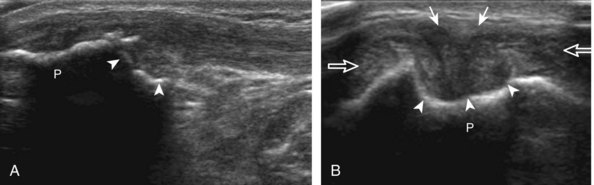
The transducer is then moved to both the medial and lateral margins of the patella in the transverse plane to visualize the thin hyperechoic patellar retinaculum as well as distention of the medial and lateral recesses that are continuous with the suprapatellar recess, which is more apparent when the knee is completely extended (Fig. 7-6A and B). One must be careful not to displace joint fluid from view with transducer pressure (see Joint Effusion and Synovial Hypertrophy). The patellar retinaculum may demonstrate three defined layers.8 Within the medial patellar retinaculum, the medial patellofemoral ligament may be identified as hyperechoic with a compact fibrillar echotexture, which extends from the adductor tubercle of the femur to the patella. Finally, with the knee in flexion, the hypoechoic hyaline cartilage that covers the trochlea of the anterior femur can be visualized in the transverse plane superior to the patella (Fig. 7-7A and B), and the hypoechoic hyaline cartilage covering the anterior and central aspects of the femoral condyles can be seen in the parasagittal plane (see Fig. 7-7C).13
Medial Evaluation
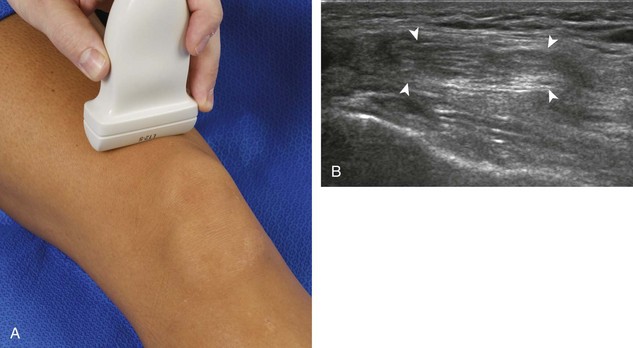
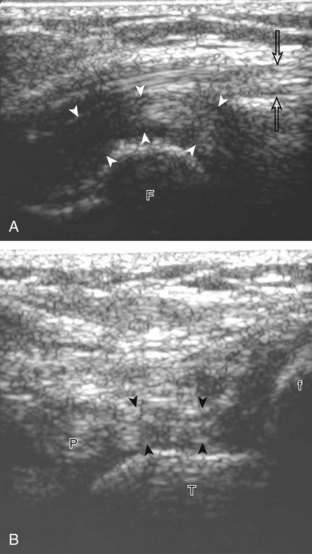
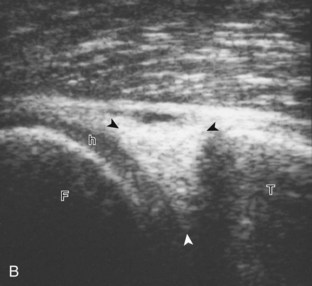
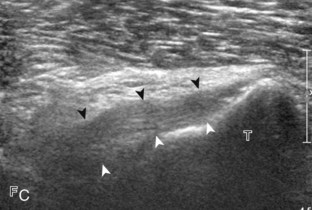
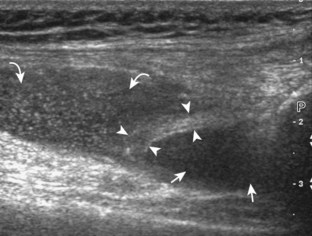
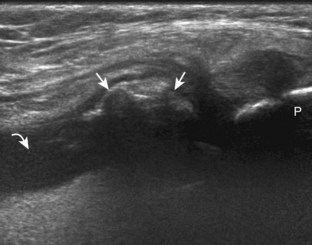
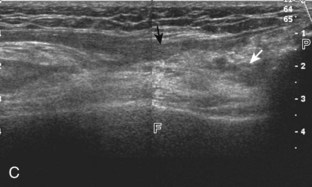
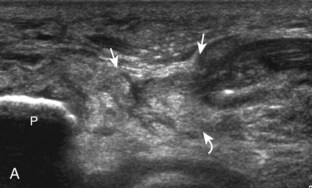
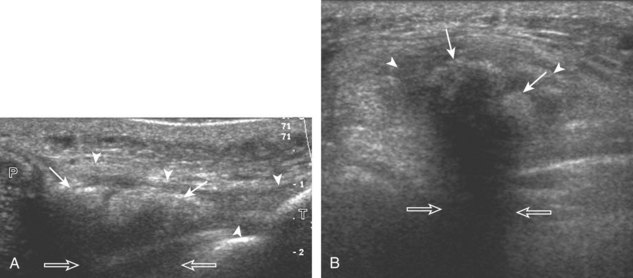
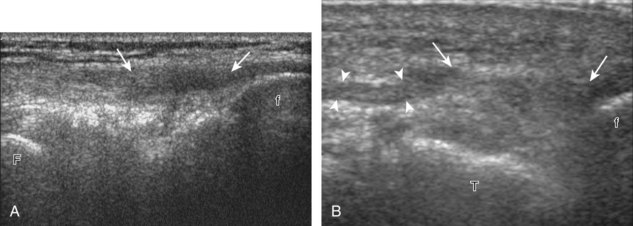
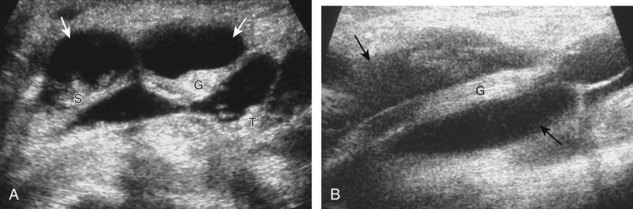
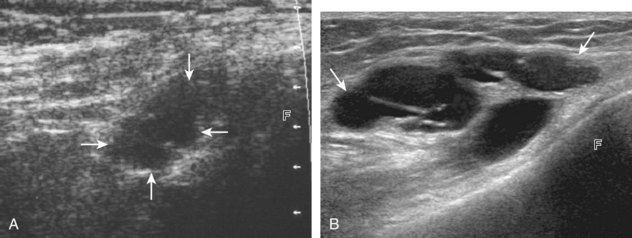
For medial knee evaluation, the patient remains supine and rotates the hip externally to gain access to the medial structures. The structures of interest include the medial collateral ligament (composed of several layers), the body and anterior horn of the medial meniscus, and the pes anserinus.5 To begin, the transducer is placed in the coronal plane along the medial joint line, which is identified by the bone contours of the femoral condyle and the proximal tibia (Fig. 7-8A).14 The thick hyperechoic and fibrillar superficial layer of the medial collateral ligament (or tibial collateral ligament) is easily identified in long axis (see Fig. 7-8B); it extends proximally from the medial femoral condyle and extends distally and slightly anterior to the proximal tibial metaphysis. With rotation of the transducer short axis to the tibial collateral ligament, the anteroposterior extent of this structure can be appreciated (Fig. 7-9A and B). By toggling the transducer along the long axis of the tibial collateral ligament, the borders of the ligament can be better appreciated because the ligament fibers become hypoechoic as a result of anisotropy and the adjacent soft tissues remain hyperechoic (see Fig. 7-9C). Returning to the coronal plane or long axis to the tibial collateral ligament, the thinner hyperechoic deep layers of the medial collateral ligament, also called the meniscofemoral and meniscotibial ligaments, are identified from the meniscus to the femur and tibia, respectively (see Fig. 7-8B). The fibrocartilage meniscus is identified as a triangular hyperechoic structure between the femur and the tibia. The transducer is then moved anteriorly from the coronal plane to the oblique-sagittal plane to visualize the anterior horn of the medial meniscus.
Returning back to the coronal plane long axis to the tibial collateral ligament, the transducer is moved distally beyond the joint line along the tibial collateral ligament and slightly anterior to its attachment on the tibia, about 4 to 5 cm beyond the joint line (Fig. 7-10A). Here, the pes anserinus can be seen as three hyperechoic tendons superficial to the tibial collateral ligament that converge onto the tibia. Toggling the transducer is often helpful because this will cause the tendons of the pes anserinus superficial to the tibial collateral ligament to appear hypoechoic from anisotropy and be more conspicuous. By turning the transducer to the oblique-axial plane along the long axis of each pes anserinus tendon, the individual sartorius, gracilis, and semitendinosus tendons can be seen; they extend to their tibial attachment as the pes anserinus (see Fig. 7-10B). The more proximal aspects of the pes anserinus tendons can also be visualized when the posterior knee is evaluated. One potential pitfall in evaluation of the posterior aspect of the medial meniscus body is misinterpretation of the adjacent semimembranosus tendon anisotropy as a meniscal cyst. Identification of a hypoechoic round structure just distal to the meniscus with an associated groove in the tibial cortex represents anisotropy of the semimembranosus tendon at its tibial insertion (Fig. 7-11). The normal semimembranosus tendon may be confirmed with the transducer repositioned long axis and perpendicular to the tendon to demonstrate the normal hyperechoic and fibrillar echotexture.
Lateral Evaluation
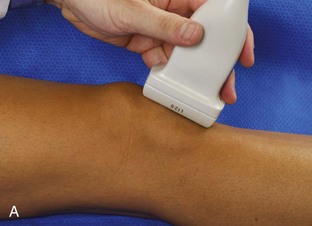
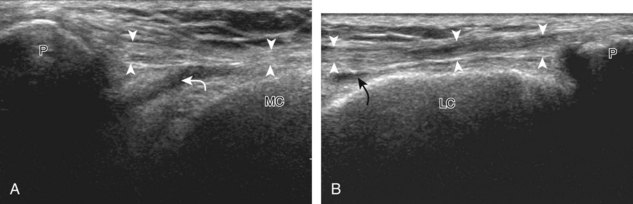
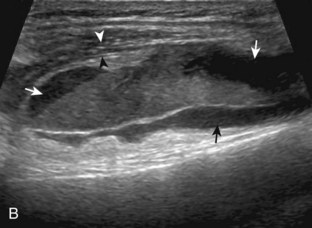
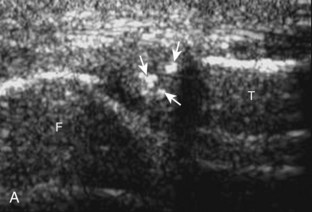
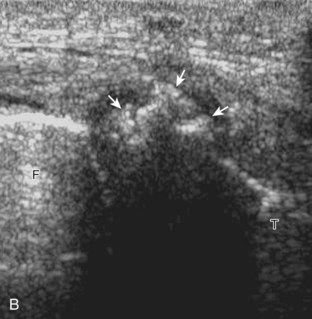
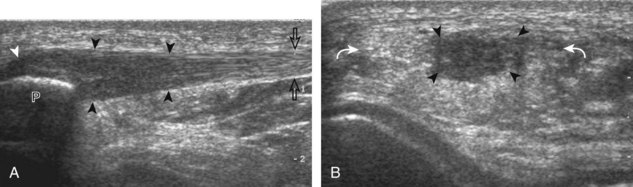
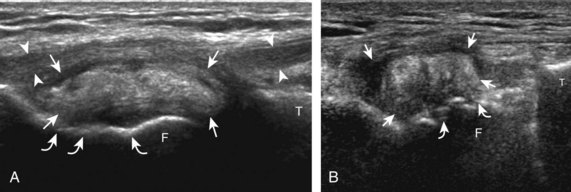
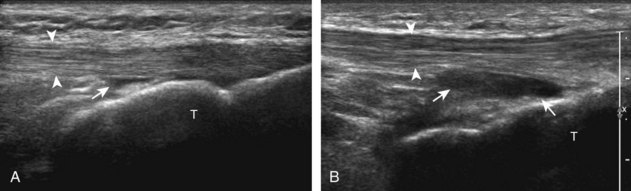
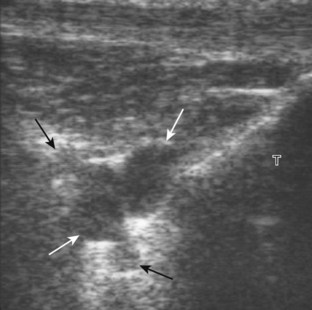
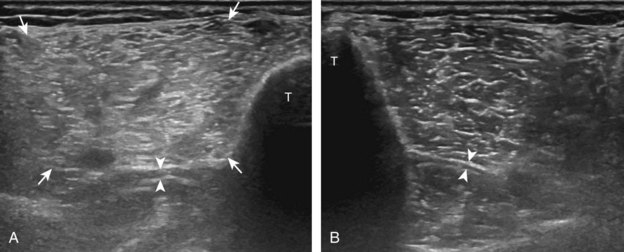
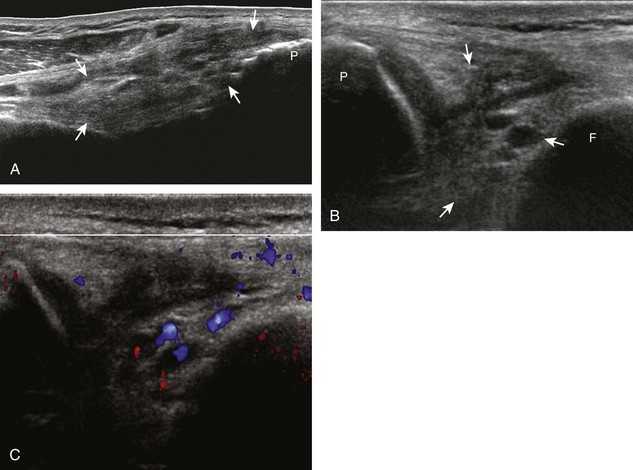
For evaluation of the lateral knee structures, the leg is internally rotated, or the patient rolls partly onto the contralateral side. Structures of interest laterally include the iliotibial tract, the lateral (or fibular) collateral ligament, the biceps femoris tendon, the supporting structures of the posterolateral corner of the knee, and the common peroneal nerve. To begin, the transducer may be initially placed over the anterior knee long axis to the patellar tendon. The transducer is then moved laterally (Fig. 7-12A). As one leaves the patellar tendon, the next fibrillar structure identified is the iliotibial tract or band, which inserts on the Gerdy tubercle of the proximal tibia (see Fig. 7-12B). It is important to evaluate the tissues between the iliotibial tract and the distal femur more proximally for disorders related to iliotibial band friction syndrome. Next, the transducer is moved laterally to the coronal plane over the lateral femoral condyle. At this location, an important bony landmark is identified: the groove or sulcus for the popliteus tendon.14 After this groove is identified, the proximal aspect of the transducer is fixed to the femur while the distal aspect is rotated posteriorly toward the fibular head (Fig. 7-13A). In this position, the hyperechoic and fibrillar echotexture of the lateral collateral ligament is seen, which extends from the lateral femoral condyle to the lateral aspect of the fibular head (see Fig. 7-13B and C). The proximal aspect of the lateral collateral ligament extends over the popliteus tendon located within the femoral groove. The distal insertion on the fibula may appear thickened and heterogeneous owing to the bifurcating distal biceps femoris tendon seen both superficial and deep to the lateral collateral ligament (see Fig. 7-13C).15 One must be aware that slight valgus angulation of the knee joint may cause a wavy appearance to the lateral collateral ligament and possible anisotropy. This can be minimized with the patient positioned so that the opposite knee is flexed under the knee being examined; this position places the knee in slight varus angulation.
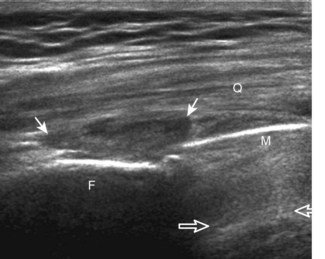
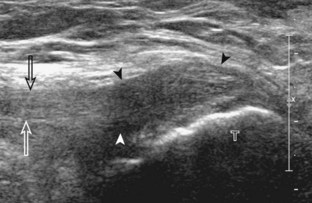
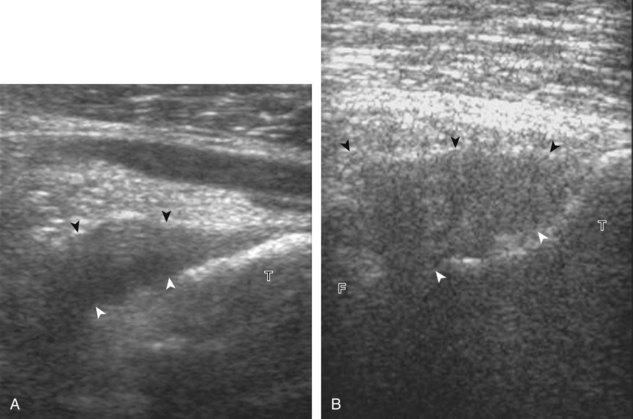
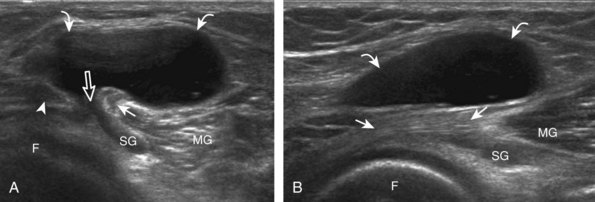
After the transducer is moved along the lateral collateral ligament to its fibular attachment, the distal aspect of the transducer is fixed to the fibular head while the proximal aspect is rotated posteriorly to the coronal plane (Fig. 7-14A) to bring the biceps femoris tendon into view; this tendon is differentiated from ligament by the less compact fibrillar echotexture and the associated hypoechoic muscle more proximally (see Fig. 7-14B). Both the lateral collateral ligament and the biceps femoris tendon insert onto the lateral aspect of the proximal fibula. The distal biceps femoris may appear heterogeneous as fibers bifurcate both superficial and deep to the lateral collateral ligament at the fibula, which should not be mistaken for tendinosis (see Fig. 7-13C).15 As the transducer is then moved posteriorly from the biceps femoris in the coronal plane, the relatively hypoechoic appearance of the common peroneal nerve can be seen in long axis (Fig. 7-15A), although the more proximal aspect is best evaluated from a posterior approach with the patient prone in short axis. Evaluation of the posterolateral aspect of the knee proximal to the fibula demonstrates the relative locations of the lateral collateral ligament, the biceps femoris, and the common peroneal nerve (see Fig. 7-15B).
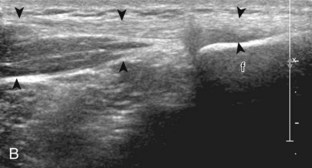
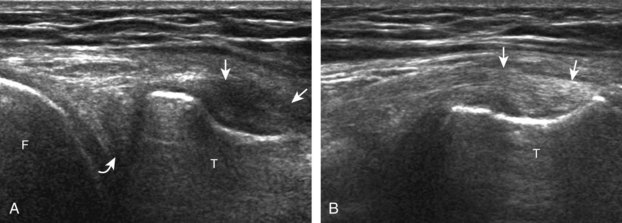
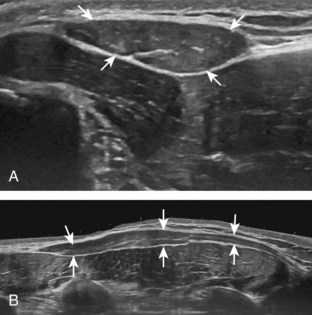
FIGURE 7-14 Biceps femoris.
A, Coronal imaging shows (B) the biceps femoris (arrowheads). f, fibula.
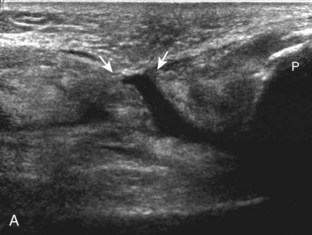
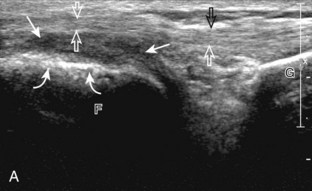
Returning to the popliteus groove in the lateral femoral condyle in the coronal plane, the popliteus tendon may be followed as it curves posteriorly around the joint. The adjacent hyperechoic fibrocartilage body and anterior horn of the lateral meniscus may also be evaluated. Because of the curved course of the popliteus tendon, this tendon is assessed in segments to avoid misinterpretation of hypoechoic anisotropy as tendon abnormality (Fig. 7-16A). The popliteus muscle is best evaluated from a posterior approach, in which the muscle belly is located between the tibia and the tibial vessels (see Posterior Evaluation). Finally, a hyperechoic extension from the popliteus tendon at the joint line may be seen, which attaches to the fibular styloid, called the popliteofibular ligament (see Fig. 7-16B).16 Other supporting structures of the posterolateral corner, such as the arcuate ligament and the possible fabellofibular ligament, are difficult to identify.
Posterior Evaluation
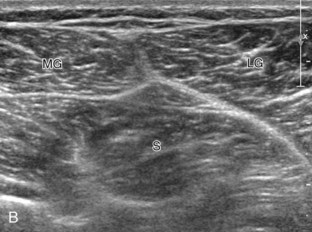
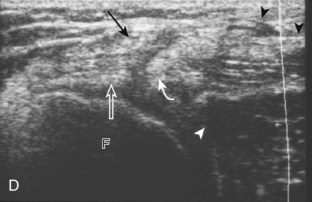
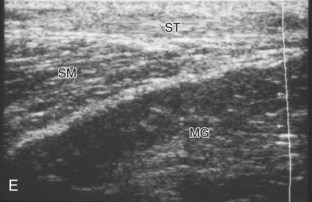
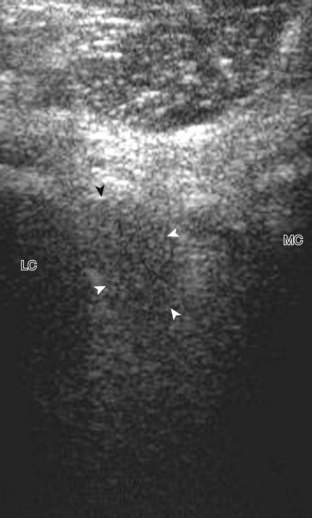
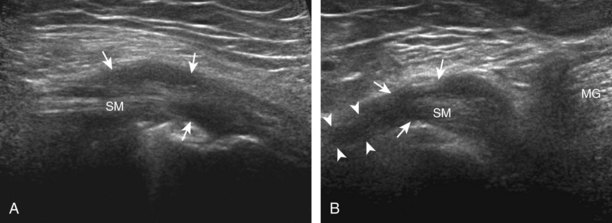
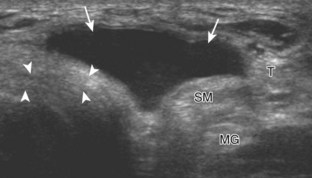
To evaluate the posterior structures of the knee, the patient is turned prone. The structures and pathology of interest include a Baker cyst, the posterior horns of the menisci, the cruciate ligaments, and the neurovascular structures of the posterior knee. Examination begins with evaluation for a Baker cyst. One technique is to initially place the transducer in the transverse plane over the mid-calf (Fig. 7-17A).4 At this location, three distinct muscles are identified: the soleus anteriorly and the medial and lateral heads of the gastrocnemius muscle superficially (see Fig. 7-17B). The transducer is then moved superiorly along the medial aspect of the medial head of the gastrocnemius muscle (see Fig. 7-17C). As the transducer approaches the knee joint, the distinct hyperechoic semimembranosus tendon is identified just medial to the medial head of the gastrocnemius tendon and muscle over the medial femoral condyle (see Fig. 7-17D). This is the location where distention of a semimembranosus-medial gastrocnemius bursa or Baker cyst is seen. The smaller round and hyperechoic tendon of the semitendinosus is also seen in short axis directly superficial to the semimembranosus tendon. The course of the medial head of the gastrocnemius tendon is not parallel to that of the semimembranosus tendon; therefore, it may be difficult to have both tendons appear hyperechoic in the same plane. One pitfall is incorrect interpretation of the semimembranosus tendon or the medial head of gastrocnemius tendon anisotropy as a small Baker cyst (Fig. 7-18A and B).4 Toggling the transducer while imaging the tendons in short axis can create anisotropy (helping to identify the tendons) and eliminate anisotropy (avoiding the pitfall interpreting anisotropy as a Baker cyst) (Video 7-1)![]() . If a Baker cyst is identified, the transducer is then turned in the sagittal plane to evaluate the extent of the Baker cyst and to assess for rupture (see Fig. 7-17E). The semitendinosus can also be imaged from this point distally to its insertion at the pes anserinus.
. If a Baker cyst is identified, the transducer is then turned in the sagittal plane to evaluate the extent of the Baker cyst and to assess for rupture (see Fig. 7-17E). The semitendinosus can also be imaged from this point distally to its insertion at the pes anserinus.
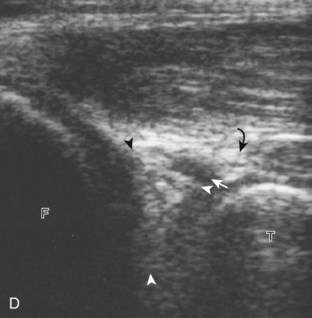
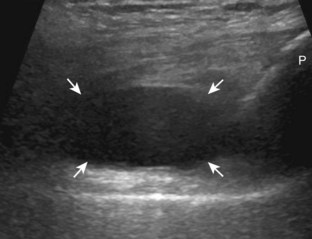
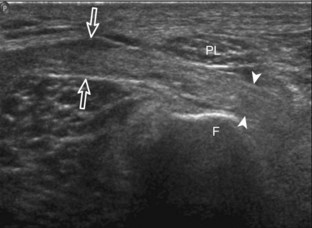
The transducer is then moved over the medial aspect of the posterior knee in the sagittal plane (Fig. 7-19A). At this location, the posterior horn of the medial meniscus is evaluated; this structure normally appears hyperechoic and triangular (see Fig. 7-19B). It may be important to use a lower-frequency transducer (5 or 7 MHz) to assess the posterior horns of the menisci and cruciate ligaments adequately. Toward the medial aspect of the medial meniscus posterior horn, the semimembranosus can be seen as it inserts on the posteromedial tibial cortex, just beyond the meniscus at a prominent concavity or sulcus in the bone. With anisotropy, the normal semimembranosus tendon may appear hypoechoic and may potentially simulate a meniscal cyst (see Fig. 7-11). The transducer is then moved toward the midline in the sagittal plane, and the posterior cruciate ligament is seen with its attachment to the posterior tibia, identified by characteristic bone contours (see Fig. 7-19C). The normal posterior cruciate ligament may appear artifactually hypoechoic as a result of anisotropy, but its thickness should be uniform and less than 1 cm.17 Anisotropy of the posterior cruciate ligament may be reduced with the heel-toe maneuver or the use of beam steering (available on some ultrasound machines). The transducer is then moved laterally to assess the posterior horn of the lateral meniscus, although accurate identification of pathology is difficult in this location because the popliteus tendon and sheath cross at the peripheral aspect of the lateral meniscus (see Fig. 7-19D).
The transducer is then turned to the transverse plane and is positioned over the intercondylar notch (Fig. 7-20A). Normally, this space should be hyperechoic, which contains the anterior cruciate ligament along the lateral aspect and the adjacent hyperechoic fat (see Fig. 7-20B).18 Identification of the anterior cruciate ligament may be improved by toggling the transducer because the normal ligament becomes hypoechoic relative to the adjacent hyperechoic fat as a result of anisotropy. Finally, the popliteal artery and vein are evaluated in short axis and long axis. The muscle belly of the popliteus is located between these vessels and the tibia (Fig. 7-20C). The tibial nerve can be followed proximally to its junction with the common peroneal nerve at the sciatic nerve, which is evaluated with the posterior thigh. Although the sciatic nerve demonstrates a honeycomb appearance from hypoechoic nerve fascicles and surrounding hyperechoic connective tissue, the smaller peripheral nerve branches may consist of only a few hypoechoic fascicles.
Joint Abnormalities
Joint Effusion and Synovial Hypertrophy
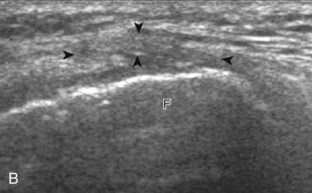
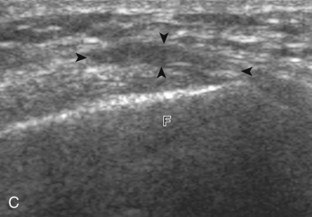
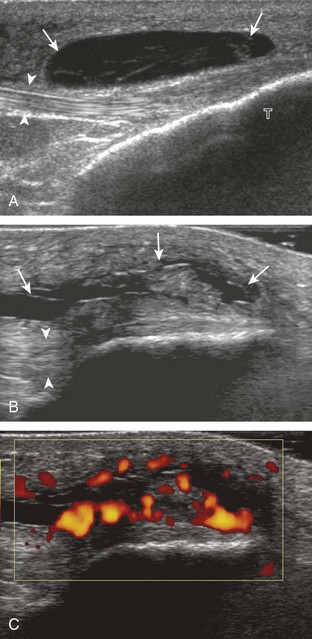
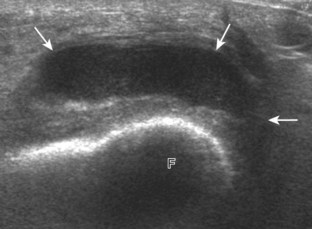
Increased joint fluid in the knee is characterized by anechoic or hypoechoic distention of the suprapatellar recess. With slight knee flexion, joint recess distention preferentially occurs deep to the quadriceps tendon (Fig. 7-21), where fluid extends superiorly from between the patella and the femur. In the setting of a small joint effusion, often joint fluid is only identified superolateral to the patella with the knee in flexion, so this area should always be assessed. In knee extension, joint distention may be seen only medial or more likely lateral to the patella in the transverse plane (Fig. 7-22).19 When imaging these areas, it is important not to apply too much pressure with the transducer because this can collapse the joint recess and displace the joint fluid out of view (Video 7-2)![]() . Joint fluid may also collect in the popliteus tendon sheath or in a Baker cyst when there is a communication with the posterior knee joint. A superior patellar plica, which typically is located in the transverse plane through the suprapatellar recess superior to the patella, may uncommonly completely separate the suprapatellar recess into two compartments (Fig. 7-23). In the setting of an intra-articular fracture, several layers of varying echogenicity within the joint may be visible as a lipohemarthrosis (Fig. 7-24).20
. Joint fluid may also collect in the popliteus tendon sheath or in a Baker cyst when there is a communication with the posterior knee joint. A superior patellar plica, which typically is located in the transverse plane through the suprapatellar recess superior to the patella, may uncommonly completely separate the suprapatellar recess into two compartments (Fig. 7-23). In the setting of an intra-articular fracture, several layers of varying echogenicity within the joint may be visible as a lipohemarthrosis (Fig. 7-24).20
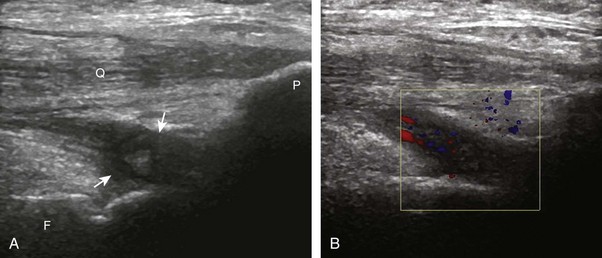
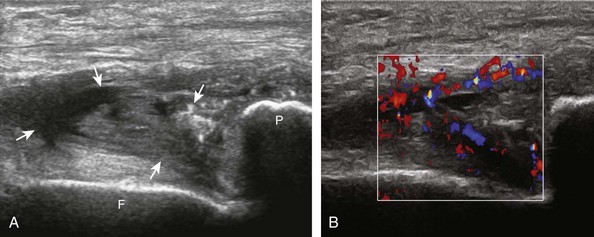
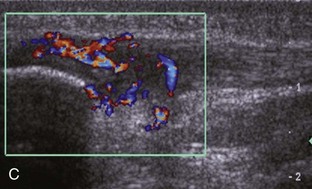
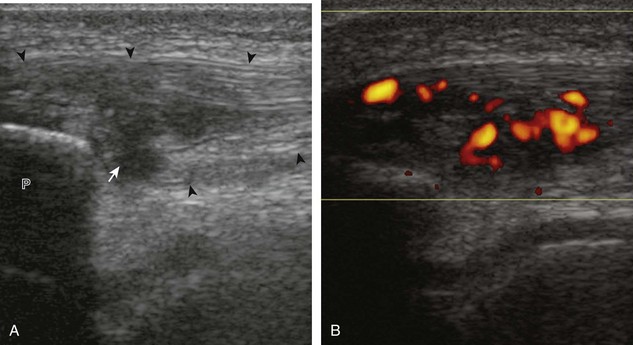
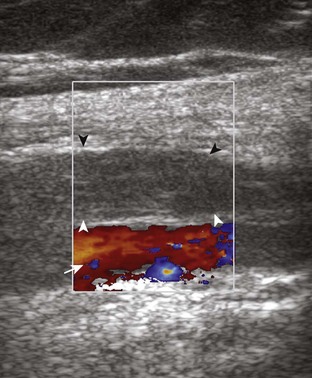
The causes of joint effusion are many; however, ultrasound including color or power Doppler imaging cannot distinguish between aseptic and septic effusion (Figs. 7-25 and 7-26). If joint recess distention is not anechoic but rather hypoechoic, isoechoic, or hyperechoic to muscle, then considerations include complex fluid versus synovial hypertrophy. Compressibility of the joint recess, redistribution of recess contents or swirling of the contents with compression or joint movement, and lack of internal flow on color or power Doppler imaging all suggest complex fluid rather than synovial hypertrophy.21 The differential diagnosis for complex fluid includes inflammation (including infection) (see Fig. 7-26) and hemorrhage (Fig. 7-27; see Fig. 7-24). If there is concern for infection, percutaneous aspiration should be considered. Inflammatory synovial hypertrophy may be associated with cortical erosions, characterized by cortical irregularity and discontinuity, often associated with increased blood flow on color or power Doppler imaging. Although synovial hypertrophy may also result from inflammation, such as rheumatoid arthritis and crystal deposition (Fig. 7-28), synovial proliferative disorders such as pigmented villonodular synovitis22 (Fig. 7-29), lipoma arborescens,23 and synovial osteochondromatosis24 are other considerations, with possible hyperechoic foci seen in the last condition. The differential diagnosis for mixed hyperechoic and hypoechoic tissue associated with the suprapatellar recess with compressible vascular channels is synovial hemangioma (see Vascular Abnormalities).25 Localized nodular synovitis may also occur in the knee joint recesses, and it typically appears hypoechoic and noncompressible with possible increased through-transmission (Fig. 7-30).26 Dynamic imaging may demonstrate snapping of synovial hypertrophy. In the setting of a total knee arthroplasty, abnormal synovial hypertrophy may cause snapping, termed patellar clunk syndrome (Fig. 7-31) (Video 7-3)![]() .27 Within joint fluid, hyperechoic and shadowing intra-articular bodies may be identified, commonly in a Baker cyst (see Baker Cyst) or suprapatellar recess (Fig. 7-32). When an intra-articular body is identified, the hyaline articular cartilage should be evaluated for a donor site (Fig. 7-33).
.27 Within joint fluid, hyperechoic and shadowing intra-articular bodies may be identified, commonly in a Baker cyst (see Baker Cyst) or suprapatellar recess (Fig. 7-32). When an intra-articular body is identified, the hyaline articular cartilage should be evaluated for a donor site (Fig. 7-33).
Cartilage Abnormalities
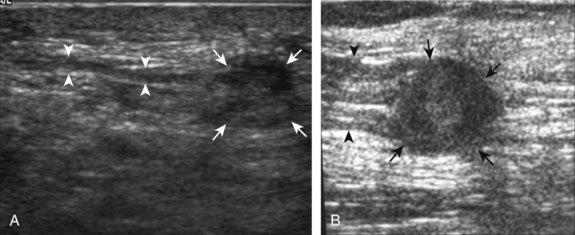
One common cause of joint effusion is a cartilage abnormality. Meniscal degeneration may appear as heterogeneous or internal hypoechogenicity, whereas meniscal tear appears as a well-defined anechoic or hypoechoic cleft that extends to the articular surface, or possibly meniscal irregularity and truncation (Fig. 7-34). Sensitivity and specificity for diagnosis of meniscal tears using ultrasound have been described as 85% and 86%, respectively.28 Because ultrasound is limited with respect to evaluation of the knee menisci as a result of incomplete or poor visualization, magnetic resonance imaging (MRI) remains the imaging method of choice for evaluation of the menisci.29 However, evaluation of the menisci can be accomplished in minutes, and pathologic features are often seen. The posterior horn of the medial meniscus is the most common site for tears, so evaluation should be at least considered here. Meniscal tears often are associated with pain with transducer pressure directly over the abnormality.
Parameniscal cysts can be diagnosed at ultrasound with a reported accuracy of 88%.30 They are typically associated with an adjacent meniscal tear, although parameniscal cysts adjacent to the anterior horn of the lateral meniscus are less likely to have an associated meniscal tear.31 Parameniscal cysts are usually multilocular and characteristically are located at the joint line at the base of the meniscus (Fig. 7-35).30 Whereas some parameniscal cysts are anechoic with increased through-transmission, others are complex cysts and are hypoechoic (Fig. 7-36).32 Small meniscal cysts may be located within the meniscus; larger parameniscal cysts can be quite extensive and typically are associated with a meniscal tear. A medial parameniscal cyst may extend some distance from the meniscal tear, so the possible diagnosis of parameniscal cyst should be considered with any multilocular cyst around the knee, and possible meniscal extension should always be sought. It is important not to misinterpret the normal semimembranosus tendon insertion on the adjacent tibia as a parameniscal cyst; this tendon often appears oval and hypoechoic in evaluation of the posterior horn medial meniscus as a result of anisotropy given the oblique course of the tendon (see Fig. 7-11).
In addition to meniscal tear and degeneration, other meniscal pathology includes meniscal extrusion in the setting of osteoarthritis (Fig. 7-37).33 Abnormal displacement of the meniscus relative to the tibia is often appreciated in the coronal plane deep to the tibial collateral ligament, where associated edema of the tibial collateral ligament is possible.34 In contrast to medial meniscal extrusion, extrusion of the anterior horn and body of the lateral meniscus may be a variation of normal.35 Abnormal symptomatic meniscal displacement may occur with knee flexion and extension and be visualized dynamically (Video 7-4)![]() .36 One hallmark of osteoarthritis is the osteophyte, which can be reliably assessed at the knee with ultrasound.37
.36 One hallmark of osteoarthritis is the osteophyte, which can be reliably assessed at the knee with ultrasound.37
Other cartilage abnormalities may involve the hyaline articular cartilage, such as cartilage thinning or defect (Fig. 7-38; see Fig. 7-33B).37–39 In the setting of osteoarthritis, femoral articular cartilage thickness is best assessed in knee flexion in the parasagittal plane and correlates with MRI to a better degree than imaging of the trochlear cartilage in the transverse plane.13 If an intra-articular body is identified, the hyaline cartilage should be evaluated for a defect as a potential donor site.40 Another cartilage abnormality relates to deposition of calcification, which can involve both the fibrocartilage meniscus (Fig. 7-39) and the hyaline articular cartilage (Fig. 7-40). Calcification deposition within cartilage is seen as hyperechoic foci and occurs in pseudogout (calcium pyrophosphate dihydrate crystal deposition disease), among other conditions.41,42 Unlike pseudogout, the deposition of monosodium urate crystals with gout is on the surface of the cartilage (Fig. 7-41), which can be seen on the meniscus (Video 7-5)![]() and hyaline cartilage; the latter, termed the double contour sign, disappears when serum urate levels are below 6 mg/dL.42–44
and hyaline cartilage; the latter, termed the double contour sign, disappears when serum urate levels are below 6 mg/dL.42–44
Tendon and Muscle Abnormalities
Quadriceps Femoris Injury
Ultrasound is well suited to evaluate the extnsor mechanism of the knee because the tendons are superficial and relatively large.45 With regard to the quadriceps tendon, tendinosis will appear hypoechoic and swollen, with continuous tendon fibers visible and possible calcification and increased flow on color or power Doppler imaging, which often correlates with patient symptoms (Fig. 7-42).46 The term tendinosis is used rather than tendinitis because this condition primarily represents a degenerative process without acute inflammatory cells. Partial-thickness tears appear as superimposed, well-defined hypoechoic or anechoic clefts or incomplete disruption of tendon fibers.47 Partial-thickness tears of the quadriceps tendon may involve only one or two of the three layers while sparing other layers of the quadriceps tendon (Fig. 7-43). Full-thickness tears are characterized by complete tendon disruption, tendon retraction, joint fluid that tracks from the suprapatellar recess through the tendon defect, and secondary wavy appearance of the patellar tendon resulting from a low-lying patella (Fig. 7-44).48 A retracted tendon stump may show posterior refraction shadowing, or it may be associated with a hyperechoic bone avulsion fragment. A displaced avulsion fracture fragment from the superior patellar pole may be seen in both partial-thickness and full-thickness quadriceps tendon tears.47 Dynamic imaging during evaluation of the quadriceps tendon is often helpful in the diagnosis of a full-thickness tear because lack of tendon movement or translation across the abnormal segment or movement of the tendon stump or avulsion fragment away from the patella indicates complete tear (Video 7-6)![]() . Dynamic imaging can be accomplished by squeezing the patient’s thigh, slightly flexing the knee, or manually pressing inferiorly on the patella. This method is helpful with a subacute tear, when hemorrhage may fill the torn tendon gap with echogenic material, and also with a chronic tear, in which a complete tear may demonstrate partial healing.
. Dynamic imaging can be accomplished by squeezing the patient’s thigh, slightly flexing the knee, or manually pressing inferiorly on the patella. This method is helpful with a subacute tear, when hemorrhage may fill the torn tendon gap with echogenic material, and also with a chronic tear, in which a complete tear may demonstrate partial healing.
Patellar Tendon Injury
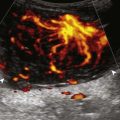
Tendinosis and partial-thickness tears may also involve the proximal patellar tendon, also termed jumper’s knee (Figs. 7-45 and 7-46). At ultrasound, tendinosis appears as hypoechoic swelling with continuous tendon fibers.49 The presence of more clearly defined hypoechoic or anechoic clefts suggests a superimposed interstitial tear. Marked hyperemia from neovascularity may be seen with color and power Doppler imaging, which is associated with a higher level of pain.50 In the setting of a penetrating injury or laceration, it is important to assess the abnormal tendon in both long and short axis because spared and intact tendon fibers exclude a full-thickness tear (Fig. 7-47, online). With a full-thickness patellar tendon tear, there is complete tendon fiber discontinuity (Fig. 7-48). Similar to quadriceps tendon tears, tendon retraction, refraction shadowing at the torn tendon stump, and a wavy patellar tendon may be present; however, the patella may be high-riding in contrast to quadriceps tear, in which the tendon may be positioned low. Dynamic imaging may also be used to confirm tendon discontinuity and to identify tendon retraction in the setting of a full-thickness tendon tear by minimally flexing the knee or by manually pressing superiorly on the patella. Hypoechoic swelling of the distal patellar tendon, swelling of the nonossified cartilage, and possible fragmentation of the tibial tuberosity are consistent with Osgood-Schlatter disease, a painful condition that affects the distal patellar tendon insertion from repetitive trauma in an adolescent (Fig. 7-49).51 Similar changes may involve the proximal patellar tendon at the inferior pole of the patella, termed Sinding-Larsen-Johansson disease.51 A central defect or persistent hypoechoic area in the central patellar tendon may be seen when this segment of the tendon is used for anterior cruciate ligament reconstruction (Fig. 7-50).52 After total knee arthroplasty, the patellar tendon may be swollen as an expected finding.53
Other Knee Tendon Injuries
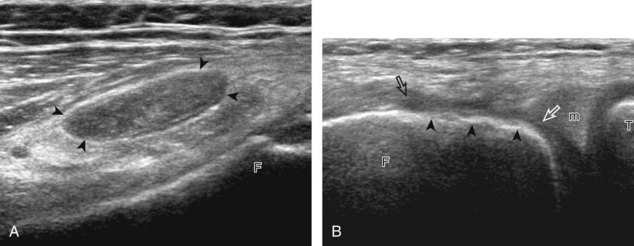
Other tendon abnormalities around the knee are less common. However, tendinosis may involve the semimembranosus (Fig. 7-51) or biceps femoris. Normal bifurcation of the distal biceps femoris tendon around the lateral collateral ligament should not be confused with tendinosis (see Fig. 7-13C).15 One other disorder that deserves mention is iliotibial friction band syndrome.54,55 In this condition, chronic and repetitive contact between the iliotibial tract and the lateral femoral condyle may produce hypoechoic edema, inflammation, and possibly adventitious bursa formation deep to the iliotibial tract, with a possible thickened iliotibial tract (Fig. 7-52). Acute trauma may also cause injury to the iliotibial tract (Fig. 7-53).
Gout
In the setting of gout, the patellar tendon (Fig. 7-54) (Video 7-7)![]() and popliteus tendon (Fig. 7-55) (Video 7-8)
and popliteus tendon (Fig. 7-55) (Video 7-8)![]() are prone to involvement; therefore, these sites should be included when evaluating for inflammatory arthritis. Even with asymptomatic hyperuricemia, involvement of the distal patellar tendon is not uncommon.56 At ultrasound, a gouty tophus appears hyperechoic and amorphous, often with an anechoic or hypoechoic halo.57,58 A tophus is often more difficult to delineate in a tendon given that both are hyperechoic; however, real-time imaging shows lack of fibrillar echotexture within the tophus. Shadowing may also be seen deep to the tophus, which is more often due to refraction rather than true shadowing from calcification of the tophus (see Fig. 7-54B). Hyperemia may also be present, as may adjacent cortical erosion (see Fig. 7-55B) and soft tissue extension of the tophus.
are prone to involvement; therefore, these sites should be included when evaluating for inflammatory arthritis. Even with asymptomatic hyperuricemia, involvement of the distal patellar tendon is not uncommon.56 At ultrasound, a gouty tophus appears hyperechoic and amorphous, often with an anechoic or hypoechoic halo.57,58 A tophus is often more difficult to delineate in a tendon given that both are hyperechoic; however, real-time imaging shows lack of fibrillar echotexture within the tophus. Shadowing may also be seen deep to the tophus, which is more often due to refraction rather than true shadowing from calcification of the tophus (see Fig. 7-54B). Hyperemia may also be present, as may adjacent cortical erosion (see Fig. 7-55B) and soft tissue extension of the tophus.
Ligament and Bone Abnormalities
Medial Collateral Ligament
Sonographic evaluation of the ligaments around the knee is most effective for the superficially located ligaments, such as the medial collateral and lateral collateral ligaments. Transducer position long axis to a ligament is the most important plane, although any abnormality is also assessed short axis to the ligament as well. With regard to the tibial collateral ligament, a grade 1 sprain is characterized by adjacent hypoechoic or anechoic fluid but an intact ligament (Fig. 7-56A). Edema around the tibial collateral ligament may not be traumatic because it may be secondary to meniscal extrusion and osteoarthritis (see Fig. 7-37).34 With a grade 2 injury or partial-thickness tear, the normally hyperechoic and compact fibrillar echotexture is replaced by abnormal hypoechogenicity and possible adjacent hypoechoic or anechoic fluid. With a grade 3 injury or full-thickness tear, there is complete disruption of the ligamentous fibers with heterogeneous hemorrhage and fluid (see Fig. 7-56B). Overall, a tibial collateral ligament injury is suggested when greater than 6 mm thick at the femoral attachment or greater than 3.6 mm thick at the tibial attachment.59 Dynamic imaging may also be used to assess the integrity of the medial collateral ligament because medial joint space widening with valgus stress less than 5 mm represents a grade 1 injury, 5 to 10 mm represents a grade 2 injury, and greater than 10 mm indicates a grade 3 injury.59 A segment of the tibial collateral ligament that is thickened with intact fibers yet no symptoms is compatible with a remote injury (see Fig. 7-56C) or prior total knee arthroplasty.53 Because the tibial collateral ligament is a relatively flat structure, it is important to assess the entire anterior to posterior extent in short axis. It is not uncommon to have complete fiber discontinuity involving the anterior fibers, but with intact fibers posteriorly. A bursa may also be found between the superficial and deep layers of the medial collateral ligament.60
Lateral Collateral Ligament
The lateral collateral ligament is an important structure that is a part of the posterolateral ligamentous complex.16 Injuries may create a swollen, hypoechoic appearance or complete discontinuity (Fig. 7-57).61 Distal ligamentous avulsions may be associated with a hyperechoic fibular fracture fragment. In this situation, the ligament is structurally intact but functionally completely torn. Other supporting structures of the posterolateral corner include the popliteofibular ligament. Because this ligament may be difficult to visualize, it is often helpful to use other signs of posterolateral corner injury, such as lateral collateral ligament tear and abnormal widening of the lateral joint space with varus stress, where lateral joint space of more than 10.5 mm can predict those who will require posterolateral corner repair or reconstruction.61
Cruciate Ligaments
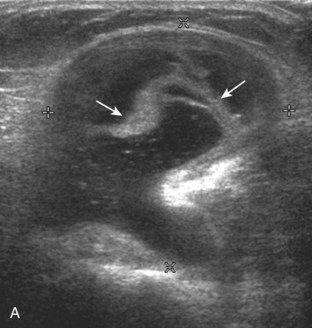
With regard to the anterior cruciate and posterior cruciate ligaments, each can be partially visualized at sonography, but MRI is considered the imaging test of choice in their evaluation. At sonography, the posterior cruciate ligament is considered abnormal if it is hypoechoic or anechoic and swollen more than 1 cm thick (Fig. 7-58).17 A torn posterior cruciate ligament may be focally disrupted or diffusely enlarged.62 Hyperechoic bone avulsions from the posterior aspect of the tibia are also possible.63 An anterior cruciate ligament tear is diagnosed at ultrasound when the normally hyperechoic ligament in the lateral aspect of the intercondylar notch, when imaged transversely, is abnormally hypoechoic or anechoic (Fig. 7-59).18,64 Dynamic stress views have also been used in conjunction with ultrasound to identify abnormal anterior tibial translation as an indirect sign of anterior cruciate ligament tear.65 Although evaluation for anterior and posterior cruciate ligament tears is limited with ultrasound, ganglion cysts associated with the cruciate ligaments (discussed later) may extend posteriorly and can be visible at sonography.66
Osseous Injury
With regard to ultrasound of bone, the osseous surfaces have a characteristic contour that is important for orientation, especially when evaluating ligaments. The normal bone cortex is smooth and continuous. Any focal step-off deformity, especially if point tenderness with transducer pressure, should raise concern for fracture (Fig. 7-60).67,68 With regard to the patella, it is important not to misinterpret a bipartite or tripartite patella, which is a normal variation, as a fracture.69 Unlike a fracture, this normal variation is isolated to the upper outer quadrant of the patella, has more irregular osseous margins, and is often asymptomatic; correlation with radiography is also important (Fig. 7-61).
Bursae and Cysts
Baker Cyst
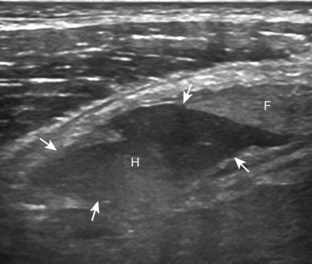
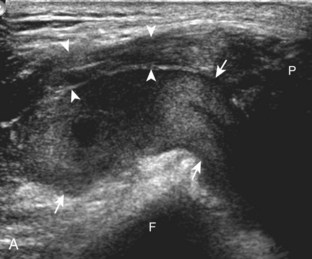
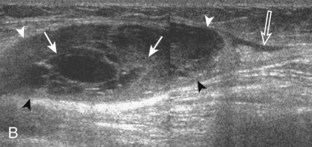
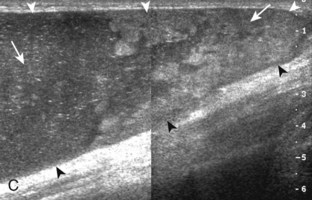
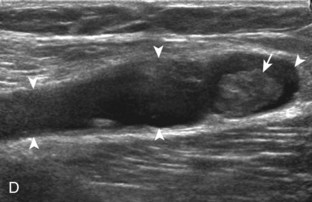
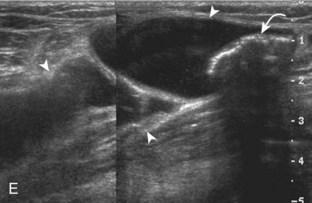
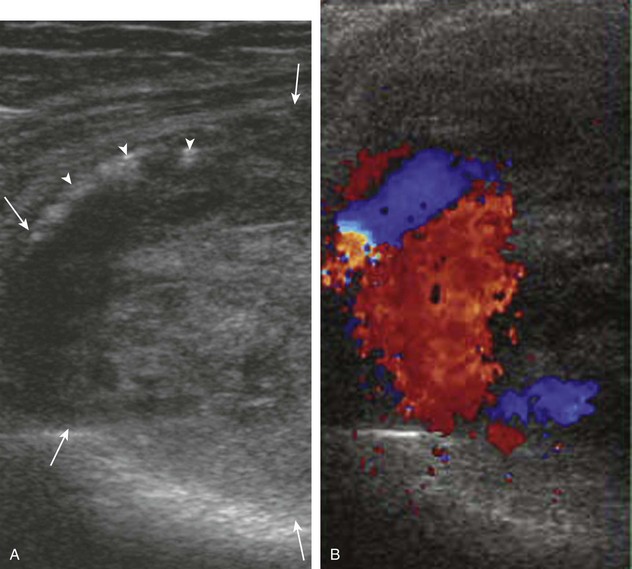
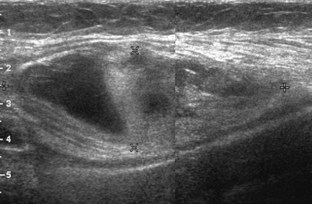
Besides parameniscal cysts described in the preceding section, other cystic abnormalities are often seen around the knee. One of the most common is distention of the semimembranosus-medial gastrocnemius bursa, which results in Baker (or popliteal) cyst.4 Although distention of this bursa may occur from local irritation or inflammation, more commonly it becomes distended with joint fluid through communication with the knee joint. Present in 50% of adults who are older than 50 years, this communication is acquired by a combination of degenerative weakening of the intervening capsule and increased intra-articular pressure and joint fluid from internal derangement.4 In the pediatric population, Baker cyst may be associated with underlying arthritis or joint hypermobility.70 Accurate diagnosis of Baker cyst relies on identification of the characteristic channel or neck between the semimembranosus and the medial head of the gastrocnemius tendon, which connects the bursa to the knee joint via the subgastrocnemius bursa. The result is a C-shaped fluid collection, concave lateral, which wraps around the medial head of the gastrocnemius tendon and muscle (Fig. 7-62).
A Baker cyst may be distended with anechoic or hypoechoic fluid. The presence of isoechoic or hyperechoic material within a Baker cyst may represent complex fluid, hemorrhage, or synovial hypertrophy (inflammatory or the result of proliferative synovial conditions such as pigmented villonodular synovitis) (Fig. 7-63). Hyperechoic and shadowing intra-articular bodies are also commonly present within a Baker cyst (see Fig. 7-63E). It is important to evaluate the inferior margin of the Baker cyst in the sagittal plane, which is normally well defined and smooth. The presence of hypoechoic fluid beyond the confines of the Baker cyst suggests rupture, which can produce diffuse edema or reactive cellulitis that typically is located superficial to the medial head of the gastrocnemius muscle and may extend to the ankle (Fig. 7-64). A more chronically ruptured Baker cyst may result in a heterogeneous mass-like area in the calf, usually superficial to the medial head of the gastrocnemius muscle (see Fig. 7-64C and D). In this situation, it is important to differentiate a ruptured Baker cyst from a soft tissue neoplasm; identification of the Baker cyst communication to the knee joint between the medial head of the gastrocnemius and semimembranosus tendons is critical in this differentiation. Extension of a Baker cyst deep to the calf musculature is uncommon, and extension within the muscle is rare, so such findings should raise concern for another etiology, such as sarcoma. Ultrasound-guided aspiration and steroid injection of Baker cyst may be considered, although re-accumulation of fluid from the knee joint is possible (see Chapter 9).
Other Bursae
Several other bursae around the knee in addition to Baker cysts may become distended. Medially and anteriorly, the pes anserinus bursa is located deep to the pes anserinus adjacent to the medial tibia, but it may be extensive (Fig. 7-65).2 Symptoms referable to the pes anserinus rarely correspond to a tendon or bursal abnormality but rather are associated with knee osteoarthritis.71 Posterior and superior to the pes anserinus and at the joint line, the semimembranosus-tibial collateral ligament bursa takes the form of an inverted U shape as it wraps around the semimembranosus tendon (Figs. 7-66 and 7-67).3 The prepatellar bursa is located anterior to the patella (Fig. 7-68).72 The superficial infrapatellar bursa (Fig. 7-69) and deep infrapatellar bursa (Fig. 7-70) are located around the distal patellar tendon, the latter of which normally contains minimal fluid (see Fig. 7-70A).73 A bursa may be distended with anechoic or hypoechoic fluid, although complex fluid, hemorrhage, or synovial hypertrophy may range from hypoechoic to hyperechoic, with possible increased flow on color or power Doppler imaging. Causes of bursal distention include trauma; inflammation, such as infection, rheumatoid arthritis, and gout; and other synovial proliferative disorders. The presence of pain with transducer pressure and hyperemia on color or power Doppler imaging suggest true inflammation or bursitis rather than mechanical or reactive bursal fluid. Knowledge of these common bursae allows one to distinguish an abnormal bursa from a nonspecific fluid collection or abscess. At points of abnormal mechanical friction or contact, an adventitious bursa may form, such as after knee amputation (Fig. 7-71).74 Unlike a Baker cyst, the previously described bursae do not normally communicate to the knee joint; therefore, ultrasound-guided percutaneous aspiration and injection may be warranted.
Ganglion Cysts
Ganglion cysts have a propensity to be located at several areas around the knee.75 The exact cause of ganglion cysts is not known, but synovial herniation, tissue degeneration, and tissue response to trauma are several possibilities.75 One common site is around the cruciate ligaments, where a ganglion cyst may extend into the soft tissue and bone (Fig. 7-72).66 Ganglion cysts may also occur posteriorly at the gastrocnemius tendon origins (Fig. 7-73) and anteriorly in the Hoffa infrapatellar fat pad (Fig. 7-74). Most ganglion cysts demonstrate lobular margins and internal septations with a multilocular appearance. Ganglion cysts may be anechoic, with increased through-transmission, or hypoechoic, without increased through-transmission when small. Percutaneous aspiration reveals thick and clear gelatinous fluid. The differential diagnosis of a soft tissue multilocular cyst is a parameniscal cyst, with the latter located around the joint line, which extends from the meniscus. If a large cyst is identified at ultrasound that is not multilocular (such as a ganglion cyst) and not in the expected location of a bursa, then a hypoechoic solid neoplasm must be considered. Intraneural ganglion cysts of the peroneal nerve are discussed later.
Peripheral Nerve Abnormalities
Ultrasound evaluation of the knee includes imaging of peripheral nerves. The common peroneal nerve near the fibula is predisposed to pathology, which includes direct injury or entrapment between the peroneus longus muscle and fibula (Fig. 7-75) (Video 7-9)![]() . The findings of peripheral nerve entrapment include hypoechoic swelling of the involved nerve at and just proximal to the site of entrapment, with transition to normal-appearing nerve distally. Transducer pressure over the abnormal nerve often elicits symptoms.
. The findings of peripheral nerve entrapment include hypoechoic swelling of the involved nerve at and just proximal to the site of entrapment, with transition to normal-appearing nerve distally. Transducer pressure over the abnormal nerve often elicits symptoms.
An intraneural ganglion cyst characteristically involves the peroneal nerve (Fig. 7-76). More common in patients with a high body mass index, joint fluid from the proximal tibiofibular joint may extend to the peroneal nerve via the articular branch to create the peroneal nerve ganglion cyst.76 Such cysts originate near the fibular neck but can extend proximally to the level of the sciatic nerve and beyond, both proximal in the sciatic nerve and distal in the tibial nerve.77 At ultrasound, intraneural peroneal nerve ganglion cysts are hypoechoic and often multilobular and track along the course of the involved nerves.76 As with any peripheral nerve disorder, it is important to evaluate the distal musculature for signs of denervation (increased echogenicity) and possible atrophy (Fig. 7-77). Intraneural ganglion cysts have been described in 18% of patients with isolated peroneal mononeuropathy.78
One additional application for peripheral nerve evaluation is in the setting of a knee amputation patient who presents with symptoms of a neuroma. After nerve transection, a neuroma is an expected finding as the nerve attempts to regenerate.79 Ultrasound can locate each neuroma and importantly determine which neuroma is responsible for symptoms through transducer palpation. Neuromas will appear hypoechoic in continuity with the involved peripheral nerve (Fig. 7-78).80 Deeper neuromas may be difficult to identify in the presence of surrounding muscle atrophy, which attenuates the ultrasound beam. Peripheral nerve sheath tumors are discussed in Chapter 2.
Vascular Abnormalities
Evaluation of the posterior knee should always include assessment of the popliteal vasculature. The differential diagnosis for a cyst in the popliteal region includes aneurysm and pseudoaneurysm. Ultrasound can show the characteristic to-and-fro appearance of blood flow from the adjacent vessel into a pseudoaneurysm with color and power Doppler imaging (Fig. 7-79).81 A soft tissue hematoma may also manifest as a soft tissue mass (Fig. 7-80). The popliteal vein also should be assessed for thrombosis, which causes the popliteal vein to be noncompressible without flow (Fig. 7-81) (Video 7-10)![]() .82 The differential diagnosis for a cyst adjacent to the popliteal artery also includes adventitial cystic disease of the popliteal artery.83 Although hemangiomas and vascular malformations are discussed in Chapter 2, synovial hemangioma deserves mention here in that it most commonly involves the suprapatellar recess of the knee, appearing as mixed hyperechoic and hypoechoic tissue with compressible vascular channels (Fig. 7-82).25
.82 The differential diagnosis for a cyst adjacent to the popliteal artery also includes adventitial cystic disease of the popliteal artery.83 Although hemangiomas and vascular malformations are discussed in Chapter 2, synovial hemangioma deserves mention here in that it most commonly involves the suprapatellar recess of the knee, appearing as mixed hyperechoic and hypoechoic tissue with compressible vascular channels (Fig. 7-82).25
1 Fenn S, Datir A, Saifuddin A. Synovial recesses of the knee: MR imaging review of anatomical and pathological features. Skeletal Radiol. 2009;38:317–328.
2 Forbes JR, Helms CA, Janzen DL. Acute pes anserine bursitis: MR imaging. Radiology. 1995;194:525–527.
3 Rothstein CP, Laorr A, Helms CA, et al. Semimembranosus-tibial collateral ligament bursitis: MR imaging findings. AJR Am J Roentgenol. 1996;166:875–877.
4 Ward EE, Jacobson JA, Fessell DP, et al. Sonographic detection of Baker’s cysts: comparison with MR imaging. AJR Am J Roentgenol. 2001;176:373–380.
5 De Maeseneer M, Van Roy F, Lenchik L, et al. Three layers of the medial capsular and supporting structures of the knee: MR imaging-anatomic correlation. Radiographics. 2000;20:S83–S89.
6 Munshi M, Pretterklieber ML, Kwak S, et al. MR imaging, MR arthrography, and specimen correlation of the posterolateral corner of the knee: an anatomic study. AJR Am J Roentgenol. 2003;180:1095–1101.
7 Wangwinyuvirat M, Dirim B, Pastore D, et al. Prepatellar quadriceps continuation: MRI of cadavers with gross anatomic and histologic correlation. AJR Am J Roentgenol. 2009;192:W111–116.
8 Phornphutkul C, Sekiya JK, Wojtys EM, et al. Sonographic imaging of the patellofemoral medial joint stabilizing structures: findings in human cadavers. Orthopedics. 2007;30:472–478.
9 Hubbard JK, Sampson HW, Elledge JR. Prevalence and morphology of the vastus medialis oblique muscle in human cadavers. Anat Rec. 1997;249:135–142.
10 Jan MH, Lin DH, Lin JJ, et al. Differences in sonographic characteristics of the vastus medialis obliquus between patients with patellofemoral pain syndrome and healthy adults. Am J Sports Med. 2009;37:1743–1749.
11 Beltran J, Matityahu A, Hwang K, et al. The distal semimembranosus complex: normal MR anatomy, variants, biomechanics and pathology. Skeletal Radiol. 2003;32:435–445.
12 Huang GS, Yu JS, Munshi M, et al. Avulsion fracture of the head of the fibula (the “arcuate” sign): MR imaging findings predictive of injuries to the posterolateral ligaments and posterior cruciate ligament. AJR Am J Roentgenol. 2003;180:381–387.
13 Yoon CH, Kim HS, Ju JH, et al. Validity of the sonographic longitudinal sagittal image for assessment of the cartilage thickness in the knee osteoarthritis. Clin Rheumatol. 2008;27:1507–1516.
14 De Maeseneer M, Vanderdood K, Marcelis S, et al. Sonography of the medial and lateral tendons and ligaments of the knee: the use of bony landmarks as an easy method for identification. AJR Am J Roentgenol. 2002;178:1437–1444.
15 Smith J, Sayeed YA, Finnoff JT, et al. The bifurcating distal biceps femoris tendon: potential pitfall in musculoskeletal sonography. J Ultrasound Med. 2011;30:1162–1166.
16 Sekiya JK, Jacobson JA, Wojtys EM. Sonographic imaging of the posterolateral structures of the knee: findings in human cadavers. Arthroscopy. 2002;18:872–881.
17 Cho KH, Lee DC, Chhem RK, et al. Normal and acutely torn posterior cruciate ligament of the knee at US evaluation: preliminary experience. Radiology. 2001;219:375–380.
18 Ptasznik R, Feller J, Bartlett J, et al. The value of sonography in the diagnosis of traumatic rupture of the anterior cruciate ligament of the knee. AJR Am J Roentgenol. 1995;164:1461–1463.
19 Hong BY, Lim SH, Cho YR, et al. Detection of knee effusion by ultrasonography. Am J Phys Med Rehabil. 2010;89:715–721.
20 Bianchi S, Zwass A, Abdelwahab IF, et al. Sonographic evaluation of lipohemarthrosis: clinical and in vitro study. J Ultrasound Med. 1995;14:279–282.
21 Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487.
22 Lin J, Jacobson JA, Jamadar DA, et al. Pigmented villonodular synovitis and related lesions: the spectrum of imaging findings. AJR Am J Roentgenol. 1999;172:191–197.
23 Learch TJ, Braaton M. Lipoma arborescence: high-resolution ultrasonographic findings. J Ultrasound Med. 2000;19:385–389.
24 Roberts D, Miller TT, Erlanger SM. Sonographic appearance of primary synovial chondromatosis of the knee. J Ultrasound Med. 2004;23:707–709.
25 Greenspan A, Azouz EM, Matthews J, 2nd., et al. Synovial hemangioma: imaging features in eight histologically proven cases, review of the literature, and differential diagnosis. Skeletal Radiol. 1995;24:583–590.
26 Huang GS, Lee CH, Chan WP, et al. Localized nodular synovitis of the knee: MR imaging appearance and clinical correlates in 21 patients. AJR Am J Roentgenol. 2003;181:539–543.
27 Koh YG, Kim SJ, Chun YM, et al. Arthroscopic treatment of patellofemoral soft tissue impingement after posterior stabilized total knee arthroplasty. Knee. 2008;15:36–39.
28 Wareluk P, Szopinski KT. Value of modern sonography in the assessment of meniscal lesions. Eur J Radiol. 2011. Oct 5 [Epub ahead of print]
29 Azzoni R, Cabitza P. Is there a role for sonography in the diagnosis of tears of the knee menisci? J Clin Ultrasound. 2002;30:472–476.
30 Rutten MJ, Collins JM, van Kampen A, et al. Meniscal cysts: detection with high-resolution sonography. AJR Am J Roentgenol. 1998;171:491–496.
31 De Smet AA, Graf BK, del Rio AM. Association of parameniscal cysts with underlying meniscal tears as identified on MRI and arthroscopy. AJR Am J Roentgenol. 2011;196:W180–186.
32 Seymour R, Lloyd DC. Sonographic appearances of meniscal cysts. J Clin Ultrasound. 1998;26:15–20.
33 Naredo E, Cabero F, Palop MJ, et al. Ultrasonographic findings in knee osteoarthritis: a comparative study with clinical and radiographic assessment. Osteoarthritis Cartilage. 2005;13:568–574.
34 Blankenbaker DG, De Smet AA, Fine JP. Is intra-articular pathology associated with MCL edema on MR imaging of the non-traumatic knee? Skeletal Radiol. 2005;34:462–467.
35 Miller TT, Staron RB, Feldman F, et al. Meniscal position on routine MR imaging of the knee. Skeletal Radiol. 1997;26:424–427.
36 Chan SK, Robb CA, Singh T, et al. Medial dislocation of the medial meniscus. J Bone Joint Surg Br. 2010;92:155–157.
37 Abraham AM, Goff I, Pearce MS, et al. Reliability and validity of ultrasound imaging of features of knee osteoarthritis in the community. BMC Musculoskelet Disord. 2011;12:70.
38 Kazam JK, Nazarian LN, Miller TT, et al. Sonographic evaluation of femoral trochlear cartilage in patients with knee pain. J Ultrasound Med. 2011;30:797–802.
39 Sureda D, Quiroga S, Arnal C, et al. Juvenile rheumatoid arthritis of the knee: evaluation with US. Radiology. 1994;190:403–406.
40 Felus J, Kowalczyk B, Lejman T. Sonographic evaluation of the injuries after traumatic patellar dislocation in adolescents. J Pediatr Orthop. 2008;28:397–402.
41 Ciapetti A, Filippucci E, Gutierrez M, et al. Calcium pyrophosphate dihydrate crystal deposition disease: sonographic findings. Clin Rheumatol. 2009;28:271–276.
42 Filippucci E, Riveros MG, Georgescu D, et al. Hyaline cartilage involvement in patients with gout and calcium pyrophosphate deposition disease: an ultrasound study. Osteoarthritis Cartilage. 2009;17:178–181.
43 Thiele RG, Schlesinger N. Diagnosis of gout by ultrasound. Rheumatology (Oxford). 2007;46:1116–1121.
44 Thiele RG, Schlesinger N. Ultrasonography shows disappearance of monosodium urate crystal deposition on hyaline cartilage after sustained normouricemia is achieved. Rheumatol Int. 2010;30:495–503.
45 Friedman L, Finlay K, Popovich T, et al. Sonographic findings in patients with anterior knee pain. J Clin Ultrasound. 2003;31:85–97.
46 Pfirrmann CW, Jost B, Pirkl C, et al. Quadriceps tendinosis and patellar tendinosis in professional beach volleyball players: sonographic findings in correlation with clinical symptoms. Eur Radiol. 2008;18:1703–1709.
47 La S, Fessell DP, Femino JE, et al. Sonography of partial-thickness quadriceps tendon tears with surgical correlation. J Ultrasound Med. 2003;22:1323–1329. quiz 30–31
48 Bianchi S, Zwass A, Abdelwahab IF, et al. Diagnosis of tears of the quadriceps tendon of the knee: value of sonography. AJR Am J Roentgenol. 1994;162:1137–1140.
49 Khan KM, Bonar F, Desmond PM, et al. Patellar tendinosis (jumper’s knee): findings at histopathologic examination, US, and MR imaging. Victorian Institute of Sport Tendon Study Group. Radiology. 1996;200:821–827.
50 Hoksrud A, Ohberg L, Alfredson H, et al. Color Doppler ultrasound findings in patellar tendinopathy (jumper’s knee). Am J Sports Med. 2008;36:1813–1820.
51 De Flaviis L, Nessi R, Scaglione P, et al. Ultrasonic diagnosis of Osgood-Schlatter and Sinding-Larsen-Johansson diseases of the knee. Skeletal Radiol. 1989;18:193–197.
52 Jarvela T, Paakkala T, Kannus P, et al. Ultrasonographic and power Doppler evaluation of the patellar tendon ten years after harvesting its central third for reconstruction of the anterior cruciate ligament: comparison of patients without or with anterior knee pain. Am J Sports Med. 2004;32:39–46.
53 Lee J, Robinson G, Finlay K, et al. Evaluation of the quadriceps tendon, patellar tendon, and collateral ligaments after total knee arthroplasty: appearances in the early postoperative period. Can Assoc Radiol J. 2006;57:291–298.
54 Bonaldi VM, Chhem RK, Drolet R, et al. Iliotibial band friction syndrome: sonographic findings. J Ultrasound Med. 1998;17:257–260.
55 Muhle C, Ahn JM, Yeh L, et al. Iliotibial band friction syndrome: MR imaging findings in 16 patients and MR arthrographic study of six cadaveric knees. Radiology. 1999;212:103–110.
56 Puig JG, de Miguel E, Castillo MC, et al. Asymptomatic hyperuricemia: impact of ultrasonography. Nucleosides Nucleotides Nucleic Acids. 2008;27:592–595.
57 Thiele RG. Role of ultrasound and other advanced imaging in the diagnosis and management of gout. Curr Rheumatol Rep. 2011;13:146–153.
58 de Avila Fernandes E, Kubota ES, Sandim GB, et al. Ultrasound features of tophi in chronic tophaceous gout. Skeletal Radiol. 2011;40:309–315.
59 Lee JI, Song IS, Jung YB, et al. Medial collateral ligament injuries of the knee: ultrasonographic findings. J Ultrasound Med. 1996;15:621–625.
60 Jose J, Schallert E, Lesniak B. Sonographically guided therapeutic injection for primary medial (tibial) collateral bursitis. J Ultrasound Med. 2011;30:257–261.
61 Sekiya JK, Swaringen JC, Wojtys EM, et al. Diagnostic ultrasound evaluation of posterolateral corner knee injuries. Arthroscopy. 2010;26:494–499.
62 Miller TT. Sonography of injury of the posterior cruciate ligament of the knee. Skeletal Radiol. 2002;31:149–154.
63 Hsu CC, Tsai WC, Chen CP, et al. Ultrasonographic examination of the normal and injured posterior cruciate ligament. J Clin Ultrasound. 2005;33:277–282.
64 Skovgaard Larsen LP, Rasmussen OS. Diagnosis of acute rupture of the anterior cruciate ligament of the knee by sonography. Eur J Ultrasound. 2000;12:163–167.
65 Palm HG, Bergenthal G, Ehry P, et al. Functional ultrasonography in the diagnosis of acute anterior cruciate ligament injuries: a field study. Knee. 2009;16:441–446.
66 DeFriend DE, Schranz PJ, Silver DA. Ultrasound-guided aspiration of posterior cruciate ligament ganglion cysts. Skeletal Radiol. 2001;30:411–414.
67 Boutry N, Dupont S, Glaude E, et al. Segond fracture revealed by ultrasonography. J Ultrasound Med. 2005;24:1431–1435.
68 Pearce T, Cobby M. Radiographically occult fracture of the tibial epiphysis: sonographic findings with CT correlation. J Clin Ultrasound. 2011;39:425–426.
69 Blankstein A, Cohen I, Salai M, et al. Ultrasonography: an imaging modality enabling the diagnosis of bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2001;9:221–224.
70 Neubauer H, Morbach H, Schwarz T, et al. Popliteal cysts in paediatric patients: clinical characteristics and imaging features on ultrasound and MRI. Arthritis. 2011;2011:751593.
71 Uson J, Aguado P, Bernad M, et al. Pes anserinus tendino-bursitis: what are we talking about? Scand J Rheumatol. 2000;29:184–186.
72 Aguiar RO, Viegas FC, Fernandez RY, et al. The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography. AJR Am J Roentgenol. 2007;188:W355–358.
73 Viegas FC, Aguiar RO, Gasparetto E, et al. Deep and superficial infrapatellar bursae: cadaveric investigation of regional anatomy using magnetic resonance after ultrasound-guided bursography. Skeletal Radiol. 2007;36:41–46.
74 Ahmed A, Bayol MG, Ha SB. Adventitious bursae in below knee amputees: case reports and a review of the literature. Am J Phys Med Rehabil. 1994;73:124–129.
75 Bui-Mansfield LT, Youngberg RA. Intraarticular ganglia of the knee: prevalence, presentation, etiology, and management. AJR Am J Roentgenol. 1997;168:123–127.
76 Young NP, Sorenson EJ, Spinner RJ, et al. Clinical and electrodiagnostic correlates of peroneal intraneural ganglia. Neurology. 2009;72:447–452.
77 Spinner RJ, Desy NM, Amrami KK. Sequential tibial and peroneal intraneural ganglia arising from the superior tibiofibular joint. Skeletal Radiol. 2008;37:79–84.
78 Visser LH. High-resolution sonography of the common peroneal nerve: detection of intraneural ganglia. Neurology. 2006;67:1473–1475.
79 Thomas AJ, Bull MJ, Howard AC, et al. Peri operative ultrasound guided needle localisation of amputation stump neuroma. Injury. 1999;30:689–691.
80 Tagliafico A, Altafini L, Garello I, et al. Traumatic neuropathies: spectrum of imaging findings and postoperative assessment. Semin Musculoskelet Radiol. 2010;14:512–522.
81 Kapoor BS, Haddad HL, Saddekni S, et al. Diagnosis and management of pseudoaneurysms: an update. Curr Probl Diagn Radiol. 2009;38:170–188.
82 Hamper UM, DeJong MR, Scoutt LM. Ultrasound evaluation of the lower extremity veins. Radiol Clin North Am. 2007;45:525–547. ix
83 Franca M, Pinto J, Machado R, et al. Case 157: bilateral adventitial cystic disease of the popliteal artery. Radiology. 2010;255:655–660.