Kinesiology of Mastication and Ventilation
PART 1: MASTICATION
OSTEOLOGY AND TEETH
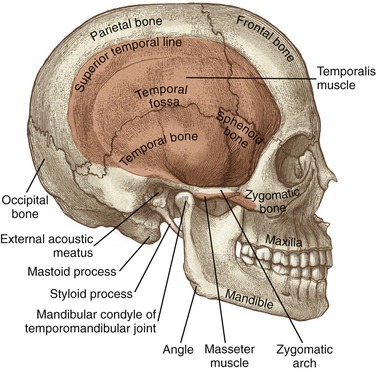
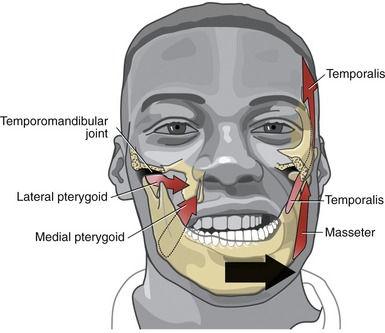
Figure 11-1 highlights some of the surface anatomy associated with the TMJ. The mandibular condyle fits within the mandibular fossa of the temporal bone. The condyle can be palpated just anterior to the external auditory meatus (i.e., the opening into the ear). The cranial attachment of the temporalis muscle fills a broad, slightly concave region of the skull known as the temporal fossa. The temporal, parietal, frontal, sphenoid, and zygomatic bones all contribute to the temporal fossa.
Individual Bones
MANDIBLE: The mandible is the largest of the facial bones (see Figure 11-1). It is a very mobile bone, suspended from the cranium by the muscles, ligaments, and capsule of the TMJ. Muscles of mastication attach either directly or indirectly to the mandible. Muscle contraction positions the teeth embedded within the mandible firmly against the teeth embedded within the fixed maxillae.
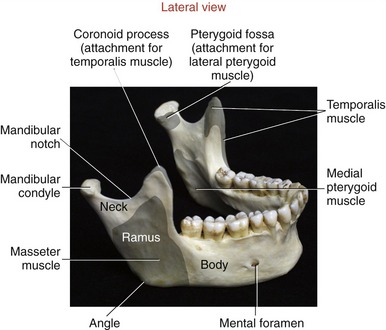
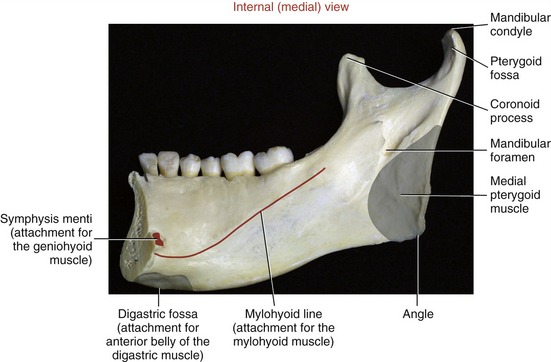
The two main parts of the mandible are the body and the two rami (Figure 11-2). The body, the horizontal portion of the bone, accepts the lower 16 adult teeth (Figure 11-3). The rami of the mandible project vertically from the posterior aspect of the body (see Figure 11-2). Each ramus has an external and internal surface and four borders. The posterior and inferior borders of the ramus join at the readily palpable angle of the mandible. The masseter and medial pterygoid muscles—two powerful muscles of mastication—share similar attachments in the region of the angle of the mandible.
At the superior end of the ramus are the coronoid process, mandibular condyle, and mandibular notch. The coronoid process is a triangular projection of thin bone that extends upward from the anterior border of the ramus. This process is the primary inferior attachment of the temporalis muscle. The mandibular condyle extends upward from the posterior border of the ramus. The condyle forms the convex bony component of the TMJ. Extending between the coronoid process and mandibular condyle is the mandibular notch. The mandibular neck is a slightly constricted region located immediately below the condyle. The lateral pterygoid muscle attaches to the anterior-medial surface of the mandibular neck, within a depression called the pterygoid fossa (Figures 11-2 and 11-4).
MAXILLA: The right and left maxillae fuse to form a single maxilla, or upper jaw. The maxilla is fixed within the skull through rigid articulations to adjacent bones (see Figure 11-1). The maxillae extend superiorly, forming the floor of the nasal cavity and the orbit of the eyes. The lower horizontal portions of the maxillae accept the upper teeth.
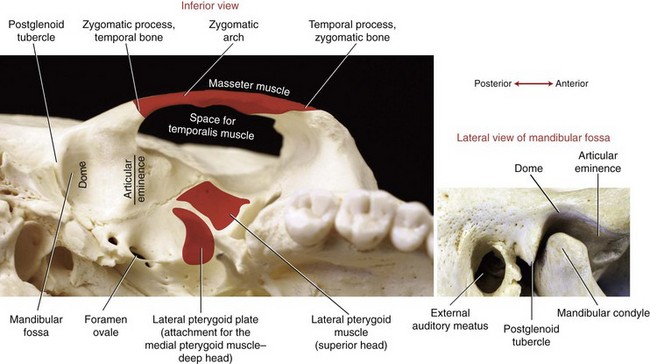
TEMPORAL BONE: Two temporal bones exist—one on each side of the cranium. The mandibular fossa forms the bony concavity of the TMJ, highlighted in a side view in the lower part of Figure 11-5. The highest point of the fossa is the dome, often very thin and membranous (see main illustration in Figure 11-5). The fossa is bound anteriorly by the articular eminence and posteriorly by the postglenoid tubercle and the tympanic part of the temporal bone. On full opening of the mouth, the condyles of the mandible slide anteriorly and inferiorly across the pair of sloped articular eminences.
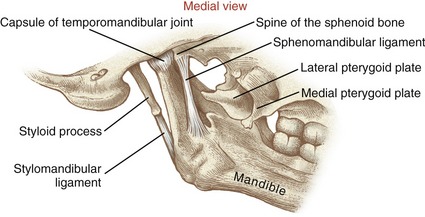
The styloid process is a long slender extension of bone that protrudes from the inferior aspect of the temporal bone (see Figure 11-1). The pointed process serves as an attachment for the stylomandibular ligament (to be discussed further) and three small muscles (styloglossus, stylohyoid, and stylopharyngeus). The zygomatic process of the temporal bone forms the posterior half of the zygomatic arch (see main illustration in Figure 11-5).
ZYGOMATIC BONE: The right and left zygomatic bones constitute the major part of the cheeks and the lateral orbits of the eyes (see Figure 11-1). The temporal process of a zygomatic bone contributes the anterior half of the zygomatic arch (see Figure 11-5). A large part of the masseter muscle attaches to the zygomatic bone and the adjacent zygomatic arch.
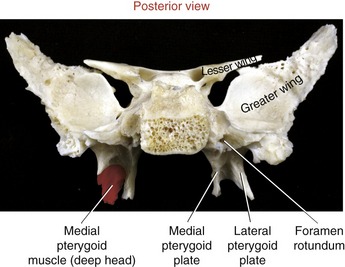
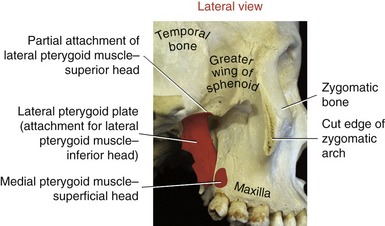
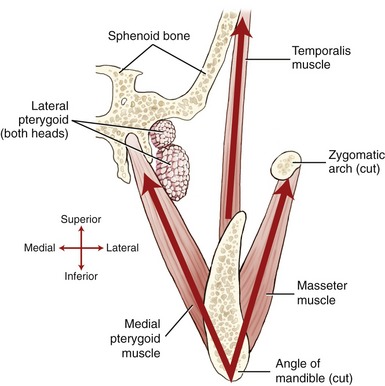
SPHENOID BONE: Although the sphenoid bone does not contribute to the structure of the TMJ, it does provide proximal attachments for the medial and lateral pterygoid muscles. When articulated within the cranium, the sphenoid bone lies transversely across the base of the skull. The relevant osteologic features of the sphenoid bone are its greater wing, medial pterygoid plate, and lateral pterygoid plate (Figure 11-6). When a section of the zygomatic arch is removed, the lateral surfaces of the greater wing and lateral pterygoid plate are revealed (Figure 11-7).
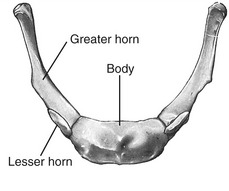
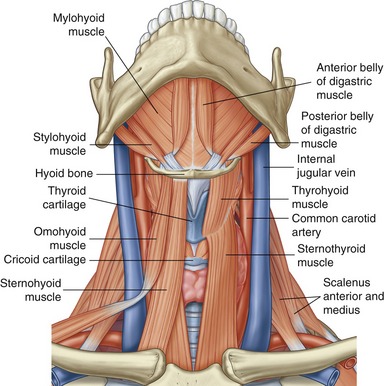
HYOID BONE: The hyoid is a U-shaped bone that can be palpated at the base of the throat, just anterior to the body of the third cervical vertebra (Figure 11-8). The body of the hyoid is convex anteriorly. The bilateral greater horns form its slightly curved sides. The hyoid is suspended primarily by a bilateral pair of stylohyoid ligaments. Several muscles involved with moving of the tongue, swallowing, and speaking attach to the hyoid bone (see Figure 11-21).
Teeth
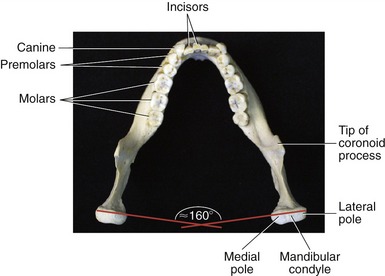
The maxillae and mandible each contain 16 permanent teeth (see Figure 11-3 for names of lower teeth). The structure of each tooth reflects its function in mastication (Table 11-1).
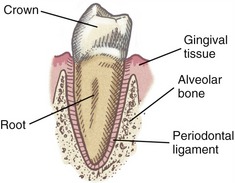
Each tooth has two basic parts: crown and root (Figure 11-9). Normally the crown is covered with enamel and is located above the gingiva (gum). The root of each tooth is embedded in alveolar bone. The periodontal ligaments help attach the roots of the teeth within their sockets.
ARTHROLOGY OF THE TEMPOROMANDIBULAR JOINT
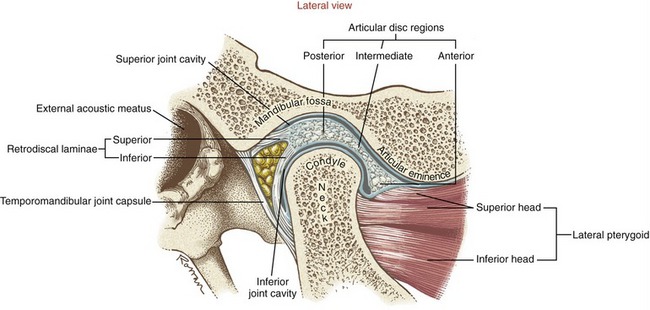
The temporomandibular joint (TMJ) is a loosely fitting articulation formed between the mandibular condyle and the mandibular fossa of the temporal bone (see Figures 11-1 and 11-5). It is a synovial joint that permits a wide range of rotation as well as translation. An articular disc cushions the potentially large and repetitive forces inherent to mastication. The disc separates the joint into two synovial joint cavities (Figure 11-10). The inferior joint cavity is between the inferior aspect of the disc and the mandibular condyle. The larger superior joint cavity is between the superior surface of the disc and the segment of bone formed by the mandibular fossa and the articular eminence.
Although the right and left TMJs function together, each retains its ability to function relatively independently. Mastication is typically performed asymmetrically, with one side of the mandible exerting a greater biting force than the other. The dominant side is often referred to as the “working” side, whereas the nondominant side is referred to as the “balancing” side.24 Different demands are placed on the muscles and joints of the working and balancing sides.
Osseous Structure
MANDIBULAR CONDYLE: The mandibular condyle is flattened from front to back, with its medial-lateral length twice as long as its anterior-posterior length (see Figure 11-3). The condyle is generally convex, possessing short projections known as medial and lateral poles. The medial pole is more prominent than the lateral. While the mouth is opening and closing, the outside edge of the lateral pole can be palpated as a point under the skin just anterior to the external auditory meatus.
The articular surface of the mandibular condyle is lined with a thin but dense layer of fibrocartilage. This tissue absorbs forces associated with mastication better than hyaline cartilage, and it has a superior reparative process.65 Both of these functions are important, given the extraordinary demands placed on the TMJ.
MANDIBULAR FOSSA: The mandibular fossa of the temporal bone is divided into two surfaces: articular and nonarticular. The articular surface of the fossa is formed by the articular eminence, occupying the sloped anterior wall of the fossa (see Figures 11-5 and 11-10). This thick and smooth load-bearing surface is lined with a thick layer of fibrocartilage. Full opening of the mouth requires that each condyle slide forward across the articular eminence. The slope of the articular eminence is, on average, 55 degrees from the horizontal plane.29 The magnitude of the slope partially determines the kinematic path of the condyle during opening and closing of the mouth.
The nonarticular surface of the mandibular fossa consists of a very thin layer of bone and fibrocartilage that occupies much of the superior (dome) and posterior walls of the fossa (see Figure 11-5). This thin region is not an adequate load-bearing surface. A large upward force applied to the chin can fracture this region of the fossa, possibly even sending bone fragments into the cranium.
Articular Disc
The disc is divided into three regions: posterior, intermediate, and anterior (see Figure 11-10). The shape of each region allows the disc to accommodate to the varying contours of the condyle and the fossa. The posterior region of the disc is convex superiorly and concave inferiorly. The concavity accepts most of the condyle, much like a ball-and-socket joint. The extreme posterior region attaches to the loosely organized retrodiscal laminae, containing collagen and elastin fibers. Connections made by the laminae anchor the disc posteriorly to bone. A meshwork of fat, blood vessels, and sensory nerves fills the space between the superior and inferior laminae.
The thickness of the disc varies between its anterior and posterior regions. The thinnest intermediate region is only 1 mm thick.36 The anterior and posterior regions, however, are about two to three times thicker. The disc is constricted at its intermediate region.55 The constriction, flanked by the adjacent thicker anterior and posterior regions, forms a dimple on the disc’s inferior surface. In maximal intercuspation, the dimpled intermediate region of the disc should fit between the anterior-superior edge of the condyle and the articular eminence of the fossa. The disc position protects the condyle as it slides forward across the articular eminence during the later phase of opening the mouth widely.
Capsular and Ligamentous Structures
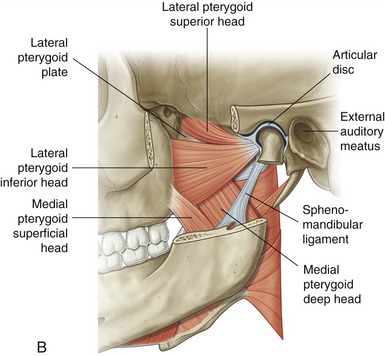
FIBROUS CAPSULE: The TMJ and disc are surrounded by a loose fibrous capsule. The internal surfaces of the capsule are lined with a synovial membrane. Superiorly the capsule attaches to the rim of the mandibular fossa, as far anterior as the articular eminence. Inferiorly the capsule attaches to the periphery of the articular disc and to the superior neck of the mandible. Anteriorly the capsule and part of the anterior edge of disc attach to the tendon of the superior head of the lateral pterygoid muscle (see Figure 11-10).
LATERAL LIGAMENT: The primary ligament reinforcing the TMJ is the lateral (temporomandibular) ligament (Figure 11-11, A). The lateral ligament has been described as a combination of horizontal and oblique fibers (see Figure 11-11, B).71 The more superficial oblique fibers course in an anterior-superior direction, from the posterior neck of the mandible to the lateral margins of the articular eminence and zygomatic arch. The deeper horizontal fibers share similar temporal attachments. They course horizontally and posteriorly to attach into the lateral pole of the mandibular condyle.
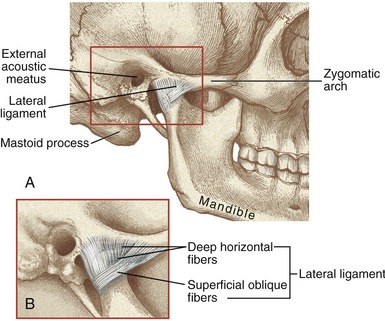
FIGURE 11-11. A, The lateral ligament of the temporomandibular joint. B, The lateral ligament’s main fibers: oblique and horizontal.
The primary function of the lateral ligament is to stabilize the lateral side of the capsule. Tears or excessive elongation of the lateral ligament may cause the disc to migrate medially by an unopposed pull of the superior head of the lateral pterygoid muscle. As described in the discussion of arthrokinematics, the oblique fibers of the lateral ligament have a special function in guiding the movement of the condyle during opening of the mouth.55
Osteokinematics
The osteokinematics of the mandible are most often described as protrusion and retrusion, lateral excursion, and depression and elevation (Figures 11-13 to 11-15). All of these movements occur to varying degrees during mastication. For a more detailed analysis of mandibular movements, the reader is encouraged to consult the classic work by Posselt,61 thoroughly summarized by Okeson.55
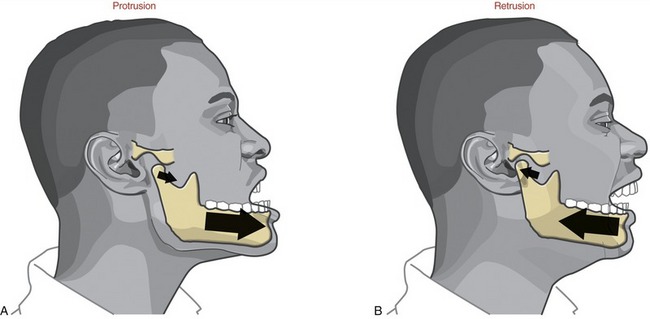
FIGURE 11-13. Protrusion (A) and retrusion (B) of the mandible.
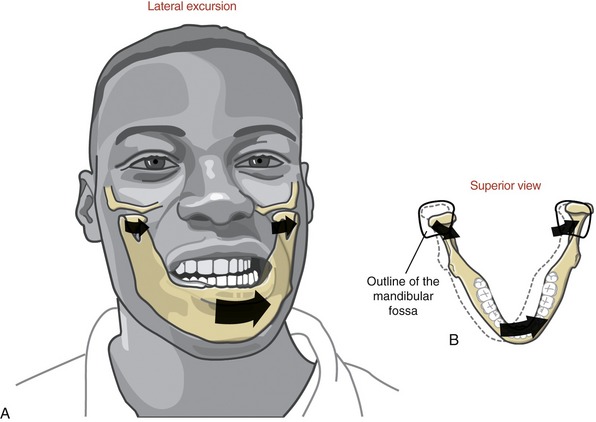
FIGURE 11-14. Lateral excursion of the mandible (A) shown combined with horizontal plane rotation (B).
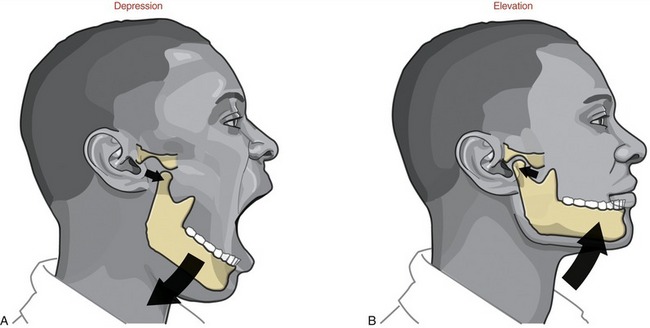
FIGURE 11-15. Depression (A) and elevation (B) of the mandible.
PROTRUSION AND RETRUSION: Protrusion of the mandible occurs as it translates anteriorly without significant rotation (see Figure 11-13, A). Protrusion is an important component of the mouth’s opening maximally. Retrusion of the mandible occurs in the reverse direction (see Figure 11-13, B). Retrusion provides an important component of closing the widely opened and protruded mouth.
LATERAL EXCURSION: Lateral excursion of the mandible occurs primarily as a side-to-side translation (see Figure 11-14, A). The direction (right or left) of active lateral excursion can be described as either contralateral or ipsilateral to the side of the primary muscle action. In the adult, an average of 11 mm (about 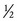 inch) of maximal unilateral excursion is considered normal.74 Lateral excursion of the mandible is usually combined with other relatively slight translations and rotations. Normally the specific path of movement is guided by the shape of the mandibular fossa and position of the articular disc.
inch) of maximal unilateral excursion is considered normal.74 Lateral excursion of the mandible is usually combined with other relatively slight translations and rotations. Normally the specific path of movement is guided by the shape of the mandibular fossa and position of the articular disc.
DEPRESSION AND ELEVATION: Depression of the mandible causes the mouth to open, a fundamental component of chewing (see Figure 11-15, A). Maximal opening of the mouth typically occurs during actions such as yawning and singing. In the adult the mouth can be opened an average of 50 mm as measured between the incisal edges of the upper and lower front teeth.2,35,74 The interincisal opening is typically large enough to fit three adult “knuckles” (proximal interphalangeal joints). Typical mastication, however, requires an average maximal opening of 18 mm—about 36% of maximum (sufficient to accept one adult knuckle). Being unable to fit two knuckles between the edges of the upper and lower incisors is usually considered abnormal in the average sized adult. Elevation of the mandible closes the mouth—an action used to grind food during mastication (see Figure 11-15, B).
Arthrokinematics
PROTRUSION AND RETRUSION: During protrusion and retrusion the mandibular condyle and disc translate anteriorly and posteriorly, respectively, relative to the fossa (see Figure 11-13). The condyle and disc follow the downward slope of the articular eminence. The mandible slides slightly downward during protrusion and upward during retrusion. The path of movement varies depending on the degree of opening of the mouth.
LATERAL EXCURSION: Lateral excursion involves primarily a side-to-side translation of the condyle and disc within the fossa. Slight multiplanar rotations are typically combined with lateral excursion.55 Figure 11-14, B, shows an example of lateral excursion combined with slight horizontal plane rotation. The left condyle forms a pivot point within the fossa as the right condyle rotates slightly anteriorly and medially.
DEPRESSION AND ELEVATION: Opening and closing of the mouth occur by depression and elevation of the mandible, respectively. During these movements, each TMJ experiences a combination of rotation and translation among the mandibular condyle, articular disc, and fossa. No other joint in the body experiences such a large proportion of translation and rotation. Because rotation and translation occur simultaneously, the axis of rotation is constantly moving. In the ideal case the movements within both TMJs result in a maximal range of mouth opening with a minimal stress placed on the articular surfaces.
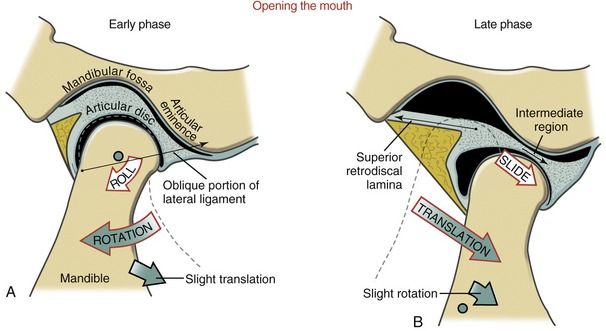
The arthrokinematics of opening the mouth are depicted for an early and a late phase in Figure 11-16. The early phase, constituting the first 35% to 50% of the range of motion, involves primarily rotation of the mandible relative to the cranium.66,88 As depicted in Figure 11-16, A, the condyle rolls posteriorly within the concave inferior surface of the disc. (The direction of the roll is described relative to the rotation of a point on the ramus of the mandible.) The rolling motion swings the body of the mandible inferiorly and posteriorly. The axis of rotation is not fixed but migrates within the vicinity of the condyles.24,60
The rolling motion of the condyle stretches the oblique portion of the lateral ligament. The increased tension in the ligament helps to initiate the late phase of the mouth’s opening.57,71
The late phase of opening the mouth consists of the final 50% to 65% of the total range of motion. This phase is marked by a gradual transition from primary rotation to primary translation. The transition can be readily appreciated by palpating the condyle of the mandible during the full opening of the mouth. During the translation the condyle and disc slide together in a forward and inferior direction against the slope of the articular eminence (see Figure 11-16, B). At the end of opening, the axis of rotation shifts inferiorly. The exact point of the axis is difficult to define because it depends on the person’s unique rotation-to-translation ratio. At the later phase of opening, the axis is usually below the neck of the mandible.24
MUSCLE AND JOINT INTERACTION
Innervation of the Muscles and Joints
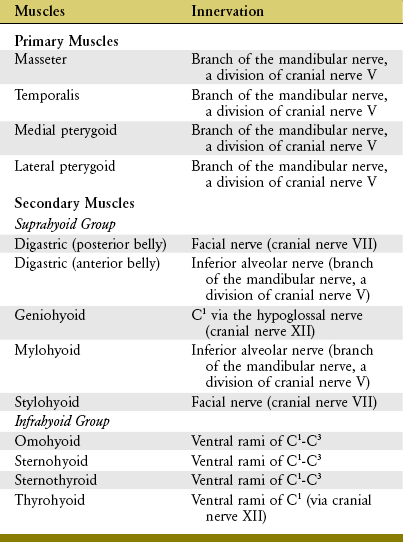
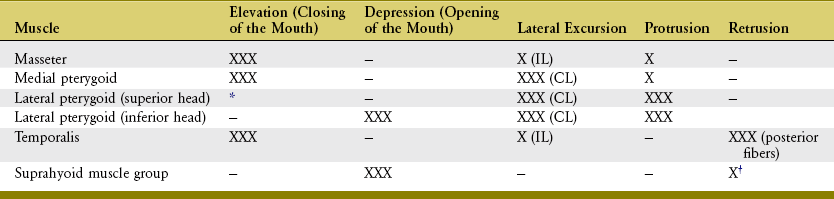
The muscles of mastication and their innervation are listed in Table 11-2. Based primarily on size, the muscles of mastication are divided into two groups: primary and secondary. The primary muscles are the masseter, temporalis, medial pterygoid, and lateral pterygoid. The secondary muscles are much smaller. The primary muscles of mastication are innervated by the mandibular nerve, a division of the trigeminal nerve (cranial nerve V). This nerve exits the skull via the foramen ovale, which is just medial and slightly anterior to the mandibular fossa (see Figure 11-5).
The central part of the disc within the TMJ lacks sensory innervation. The periphery of the disc, capsule, lateral ligament, and retrodiscal tissues, however, possess pain fibers and mechanoreceptors.75,87 In addition, mechanoreceptors and sensory nerves from oral mucosa, periodontal ligaments, and muscles provide the nervous system with a rich source of proprioception. This sensory information helps protect the soft oral tissues, such as the tongue and cheeks, from trauma caused by the teeth during chewing or speaking. Furthermore, the sensation helps coordinate the neuromuscular reflexes that synchronize the functional interaction among the muscles of the TMJ and in the craniocervical region. The sensory innervation from the TMJ is carried through two branches of the mandibular nerve: auriculotemporal and masseteric.75
Muscular Anatomy and Function
PRIMARY MUSCLES OF MASTICATION: The primary muscles of mastication are the masseter, temporalis, medial pterygoid, and lateral pterygoid. Refer to Appendix III, Part C for a summary of muscle attachments.
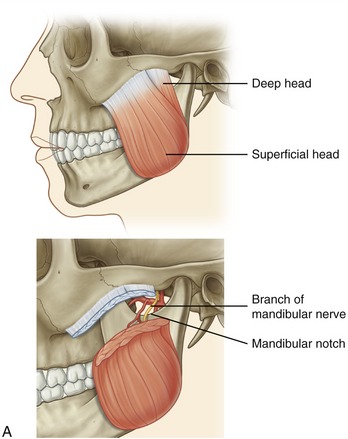
Masseter: The masseter is a thick, strong muscle, easily palpable just above the angle of the mandible (Figure 11-17, A). The muscle, as a whole, originates from the zygomatic arch and zygomatic bone (see Figures 11-1 and 11-5) and inserts inferiorly on the external surface of the ramus of the mandible (see Figure 11-2).
The masseter has superficial and deep heads (see Figure 11-17, A). The fibers of the larger, more superficial head travel inferiorly and posteriorly, attaching inferiorly near the angle of the mandible. The fibers from the smaller deep head attach inferiorly to the upper region of the ramus of the mandible, close to the base of the coronoid process.
The actions of both heads of the masseter are essentially the same. Bilateral contraction elevates the mandible to bring the teeth into contact during mastication.55,75 The line of force of the muscle is nearly perpendicular to the biting surface of the molars. The primary function of the masseter, therefore, is to develop large forces between the molars for effective grinding and crushing of food. Bilateral action of the masseters also protrudes the mandible slightly. Unilateral contraction of the masseter, however, causes slight ipsilateral excursion of the mandible. Such an action may occur during a lateral grinding motion while chewing (Figure 11-18). The multiple actions of the masseter are necessary for effective mastication.
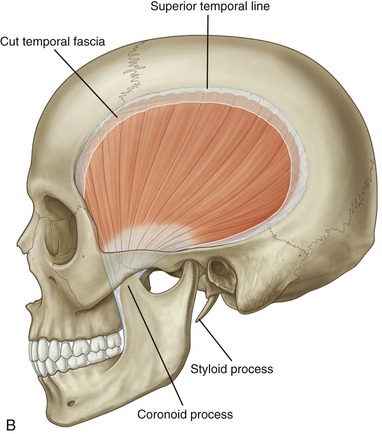
Temporalis: The temporalis is a flat, fan-shaped muscle that fills much of the concavity of the temporal fossa of the skull (see Figure 11-17, B). From its cranial attachment, the muscle forms a broad tendon that narrows distally as it passes through a space formed between the zygomatic arch and the lateral side of the skull (see Figure 11-5). The muscle attaches distally to the coronoid process and to the anterior edge and medial surface of the ramus of the mandible (see Figure 11-2). Bilateral contractions of the temporalis muscles elevate the mandible. The more oblique posterior fibers elevate and retrude the mandible.55
Similar to the masseter, the temporalis courses slightly medially as its approaches its distal attachment. Unilateral contraction of the temporalis, therefore, as when chewing in a side-to-side manner, causes slight ipsilateral excursion of the mandible (see Figure 11-18).
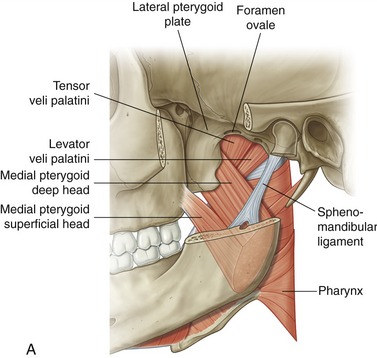
Medial Pterygoid: The medial pterygoid muscle arises from two heads (Figure 11-19, A). The much larger deep head attaches on the medial surface of the lateral pterygoid plate of the sphenoid bone (see Figures 11-5 and 11-6). The smaller superficial head attaches to a region of the posterior side of the maxilla, just above the third molar (see Figure 11-7).75 Both heads course nearly parallel with the masseter muscle and attach on the internal surface of the ramus, near the angle of the mandible (see Figures 11-2 and 11-4).
The actions of the two heads of the medial pterygoid are essentially identical. Acting bilaterally, the medial pterygoid elevates and, to a limited extent, protrudes the mandible. Because of the oblique line of force of the muscle relative to the frontal plane, a unilateral contraction of the medial pterygoid produces a very effective contralateral excursion of the mandible (see Figure 11-18).
Lateral Pterygoid: The lateral pterygoid muscle is generally described as a bipennate muscle with two distinct heads (see Figure 11-19, B).20,52,55,75 The superior head arises from the greater wing of the sphenoid bone (see Figures 11-5 and 11-7). The considerably larger inferior head arises from the lateral surface of the lateral pterygoid plate and adjoining region of the maxilla (see Figure 11-7).
As a whole, the lateral pterygoid muscle traverses nearly horizontally to insert into (1) the neck of the mandible and the pterygoid fossa, (2) the articular disc, and (3) the capsule of the TMJ (review Figure 11-10).* Although the subject continues to be debated,9 many sources state that about 65% of the fibers of the superior head attach into the pterygoid fossa (see Figure 11-2), whereas the remaining fibers attach into the medial wall of the capsule and part of the medial side of the articular disc. The inferior head attaches within the pterygoid fossa and adjacent neck of the mandible.
The precise action and role of the two heads of the lateral pterygoid muscle during mastication is controversial and not completely understood.52,53,82 The lack of understanding partially reflects the muscle’s deep location and subsequent technical challenge to electromyographic study.20 Most authors, however, agree that unilateral contraction of both heads of the lateral pterygoid produces contralateral excursion of the mandible (see Figure 11-18). Unilateral muscle contraction also rotates the ipsilateral condyle anterior-medially within the horizontal plane. Usually a given right or left lateral pterygoid muscle contracts synergistically with other muscles during mastication. For example, as depicted in Figure 11-18, a biting motion that involves left lateral excursion is controlled by the right lateral and medial pterygoid muscles and by the left masseter and temporalis.
Bilateral contraction of both heads of the lateral pterygoid muscle produces a strong protrusion of the mandible.42 As fully described in the discussion of muscular control of opening and closing of the mouth, the two heads of the lateral pterygoid muscles are active at different phases of opening and closing of the mouth. (For this and other morphologic considerations, some authors have argued that the two heads of the lateral pterygoid are actually separate muscles.20) Most sources suggest that the inferior head is the primary depressor of the mandible, especially during resisted opening of the mouth.44,52,55,59 The superior head, in contrast, helps control the tension within the disc and its position during resisted closure of the jaw.44,52 This action is especially important during resisted, unilateral closure of the jaw, such as when biting down on a hard piece of candy.
SECONDARY MUSCLES OF MASTICATION: The suprahyoid and infrahyoid muscles are considered secondary muscles of mastication (Figure 11-21). These muscles are listed in Table 11-2. Forces produced by these muscles are transferred either directly or indirectly to the mandible. The suprahyoid muscles attach between the base of the cranium, hyoid bone, and mandible; the infrahyoid muscles attach superiorly to the hyoid and inferiorly to the thyroid cartilage, sternum, and scapula. The mandibular attachments of three of the suprahyoid muscles—anterior belly of the digastric, geniohyoid, and mylohyoid—are shown in Figure 11-4. Appendix III, Part C includes the attachments of the suprahyoid and infrahyoid muscles.
With the hyoid bone stabilized by sufficient activation of the infrahyoid muscles, the suprahyoid muscles can assist with depression of the mandible and thereby opening of the mouth.8 The suprahyoid and infrahyoid muscles are also involved in speech, tongue movement, and swallowing and in controlling of boluses of food before swallowing.
SUMMARY OF INDIVIDUAL MUSCLE ACTION: Table 11-3 provides a summary of the individual actions of the muscles of mastication.
MUSCULAR CONTROL OF OPENING AND CLOSING OF THE MOUTH:
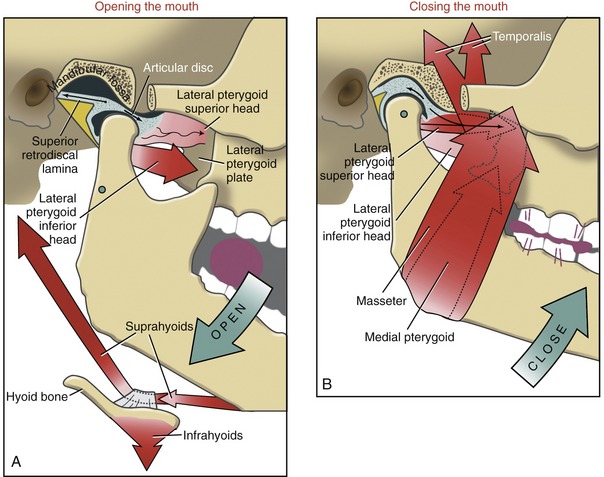
Opening the Mouth: Opening the mouth is performed primarily through contraction of the inferior head of the lateral pterygoid and the suprahyoid group of muscles. This action is depicted in Figure 11-22, A as the mouth opens in preparation to bite on a grape. The inferior head of the lateral pterygoid is primarily responsible for the forward translation (protrusion) of the mandibular condyle. This muscle is also involved in a force-couple with the contracting suprahyoid muscles. The force-couple rotates the mandible around its axis of rotation, shown as a green opened circle below the neck of the mandible. Although mandibular rotation is minimal during the late phase of opening the mouth, it does facilitate the extremes of this action. Gravity also assists with opening the mouth.
Closing the Mouth: Closing the mouth against resistance is performed primarily by contraction of the masseter, medial pterygoid, and temporalis muscles (see Figure 11-22, B). These muscles all have a very favorable moment arm (leverage) for this action. The more oblique posterior fibers of the temporalis muscle also retrude the mandible. This action translates the mandible in a posterior-superior direction, helping to reseat the condyle within the fossa.
Although the muscular action is not completely understood or agreed on, the superior head of the lateral pterygoid is likely active eccentrically during closing of the mouth. The activation tends to be greatest on the “working” side of the mandible (i.e., that side most involved with chewing).55 Eccentric activation exerts a forward tension on the disc and neck of the mandible (see Figure 11-22, B). The tension helps to stabilize and optimally position the disc between the condyle and articular eminence. The muscle activation also helps balance the strong retrusion force generated by the posterior fibers of the temporalis.
TEMPOROMANDIBULAR DISORDERS
The term temporomandibular disorders (TMDs) is broad and often vague and refers to a number of clinical problems that involve the masticatory system.81 TMDs are typically associated with impairments involving the muscles, the joint, or both.63,78 In addition to pain during movement, the signs and symptoms of TMDs include joint sounds (“popping”), reduced molar bite forces, reduced range in opening of the mouth, headaches, joint locking, and referred pain to the face and scalp.* Many factors are associated with the causes of TMD, including stress or other emotional disturbances, daily oral parafunctional habits (e.g., grinding of teeth, repetitive biting of the lip or tongue), asymmetric muscle activity, sleep bruxism, chronic forward head posturing, or sensitization of the central nervous system. Although most cases are self-limiting, a small percentage may progress to osteoarthritis, which can lead to significant degenerative changes within the joint, remodeling of bone, and a marked loss of function.41,68
No single mechanical or physiologic explanation can account for the myriad symptoms associated with TMD.34,67 The pathomechanics involved with a particular disorder may stem from increased joint stress from abnormal anatomy or dentition; internal derangement of the disc; or trauma, such as a fall, blow to the face, or cervical whiplash injury. Other predisposing factors may include chronic overloading of the joint and rheumatic disease.25,41 Often, however, the exact cause of TMDs is unknown.
The treatment for TMDs is mixed and depends primarily on the nature of the underlying problem. The multiple symptoms associated with TMDs often require collaborative treatments from a team of clinicians, which may include dentists, physicians, physical therapists, and psychologists.11,27,39,55,56 The more common conservative treatments for TMDs are listed in the box.
Discussing the relative clinical effectiveness of the different conservative treatments for TMDs is not in the scope of this chapter. Briefly, however, it is worth noting that a few clinical studies have reported that therapeutic exercise, manual therapy, splint therapy, and patient education can reduce pain and improve range of jaw motion in persons with TMDs.10,27,41,48 Not all studies concur with these findings, however.48,49,76 The conflicting results as to the effectiveness of treatment for TMDs result from, in part, the design of the research studies. Many of the studies have not optimally controlled for confounding variables, such as dissimilar treatment interventions or use of subjects with widely varying severities of pathology.
Surgical intervention is relatively rare for persons with TMDs and usually is performed only when the pain is so great or motion so limited that the quality of life is significantly reduced. In addition to arthrocentesis, surgery may involve arthroscopy to inspect the joint and remove adhesions, condylotomy to realign the condyle relative to the disc, arthrotomy (open joint procedures such as disc repositioning and discectomy), and TMJ replacement.21 Surgery is usually ineffective if performed without other more conservative interventions.
PART 2: VENTILATION
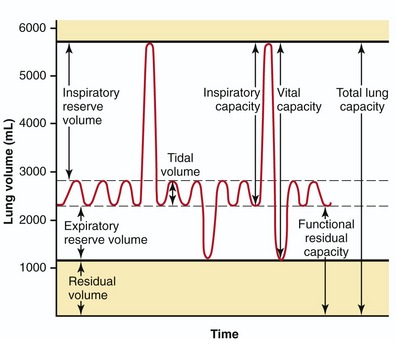
Figure 11-23 shows the lung volumes and capacities in the normal adult. As depicted, the total lung capacity is about 5.5 to 6 L of air. Vital capacity, normally about 4500 mL, is the maximum volume of air that can be exhaled after a maximal inhalation. Tidal volume is the volume of air moved in and out of the lungs during each ventilation cycle. At rest, tidal volume is about 0.5 L, approximately 10% of vital capacity.
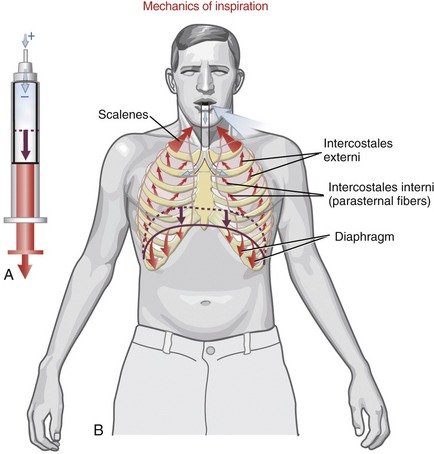
Ventilation is driven by a combination of active and passive forces that alter the volume within the expandable thorax. The change in intrathoracic volume causes a change in air pressure as described by Boyle’s law. This law states that, given a fixed temperature and mass, the volume and pressure of a gas, such as air, are inversely proportional. Increasing the volume within the chamber of a piston, for example, lowers the pressure of the contained air. Because air flows spontaneously from high to low pressure, the relatively high air pressure outside the piston forces air into an opening at the top of the piston. In other words, the negative pressure created within the piston sucks air into its chamber (Figure 11-24, A). This analogy between the thorax and the piston can be very helpful in understanding the mechanics of ventilation. As will be described, much of the physics of human ventilation is based on the inverse relationship between volume and pressure of a gas.
During inspiration the intrathoracic volume is increased by contraction of the muscles that attach to the ribs and sternum (see Figure 11-24, B). As the thorax expands, the pressure within the interpleural space, which is already negative, is further reduced, creating a suction that expands the lungs. The resulting expansion of the lungs reduces alveolar pressure below atmospheric pressure, ultimately drawing air from the atmosphere to the lungs.
ARTHROLOGY
The thorax, or rib cage, is a closed system that functions as a mechanical bellows for ventilation. The internal aspect of the thorax is sealed from the outside by several structures (Box 11-1). Although this chapter focuses on the thorax as a mechanical bellows, the thorax also protects cardiopulmonary organs and large vessels; serves as a structural base for the cervical spine; and provides a site for attachment of muscles that either directly or indirectly act on the head, neck, and extremities.
Articulations within the Thorax
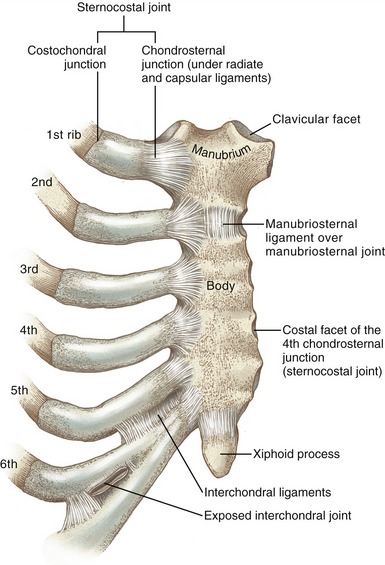
MANUBRIOSTERNAL JOINT: The manubrium fuses with the body of the sternum at the manubriosternal joint (Figure 11-25). This fibrocartilaginous articulation is classified as a synarthrosis, similar to the structure of the pubic symphysis. A partial disc fills the cavity of the manubriosternal joint, completely ossifying late in life. Before ossification, the joint may contribute modestly to expansion of the thorax.
STERNOCOSTAL JOINTS: Bilaterally the anterior cartilaginous ends of the first seven ribs articulate with the lateral sides of the sternum. In a broad sense, these articulations are referred to as sternocostal joints (see Figure 11-25). Because of the intervening cartilage between the bones of the ribs and the sternum, however, each sternocostal joint is structurally divided into costochondral and chondrosternal junctions.
The chondrosternal junctions are formed between the medial ends of the cartilage of the ribs and the small concave costal facets on the sternum. The first chondrosternal junction is a synarthrosis, providing a relatively stiff connection with the sternum.75 The second through the seventh joints, however, are synovial in nature, permitting slight gliding motions. Fibrocartilaginous discs are sometimes present, especially in the lower joints, where cavities are frequently absent. Each synovial joint is surrounded by a capsule that is strengthened by radiate ligaments.
INTERCHONDRAL JOINTS: The opposed borders of the cartilages of ribs 5 through 10 form small, synovial-lined interchondral joints, strengthened by interchondral ligaments (see Figure 11-25). Ribs 11 and 12 do not attach anteriorly to the sternum.
COSTOCORPOREAL AND COSTOTRANSVERSE JOINTS: The posterior end of the ribs attaches to the vertebral column via the costocorporeal (costovertebral) and costotransverse joints. The costocorporeal joints connect the heads of each of the 12 ribs to the corresponding sides of the bodies of the thoracic vertebrae. The costotransverse joints connect the articular tubercles of ribs 1 to 10 to the transverse processes of the corresponding thoracic vertebrae. Ribs 11 and 12 usually lack costotransverse joints. The anatomy and ligamentous structures of these joints are described and illustrated in Chapter 9 (see Figure 9-51).
THORACIC INTERVERTEBRAL JOINTS: Movement within the thoracic vertebral column occurs primarily at the interbody and apophyseal joints within the region. It is likely that forced ventilation is associated with modest movement at these joints, although this topic has not been thoroughly investigated. The structure and function of these joints is described in Chapter 9.
Changes in Intrathoracic Volume during Ventilation
VERTICAL CHANGES: During inspiration the vertical diameter of the thorax is increased primarily by contraction and subsequent lowering of the dome of the diaphragm muscle (see Figure 11-24, B). During quiet expiration the diaphragm relaxes, allowing the dome to recoil upward to its resting position.
ANTERIOR-POSTERIOR AND MEDIAL-LATERAL CHANGES: Elevation and depression of the ribs and sternum produce changes in the anterior-posterior and medial-lateral diameters of the thorax. To varying degrees, all articulations within the thorax contribute to these changes in diameter.
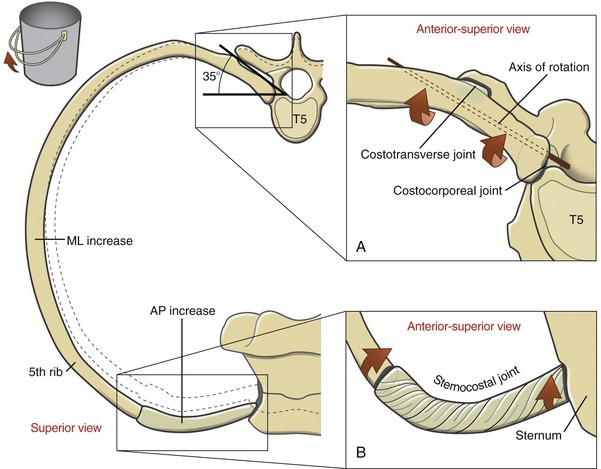
During inspiration the shaft of the ribs elevates in a path generally perpendicular to the axis of rotation that courses between the costotransverse and costocorporeal joints (Figure 11-26). The downward sloped shaft of the ribs rotates upward and outward, increasing the intrathoracic volume in both anterior-posterior and medial-lateral diameters. A slight rotation of the posterior joints produces a relatively large displacement of the shaft of the ribs. This mechanism is somewhat similar to the rotation of a bucket handle. During forced inspiration the movement of the ribs is combined with slight extension throughout the thoracic spine.
The specific path of movement of a given rib depends partially on its unique shape and on the spatial orientation of the axis of rotation that runs through the costotransverse and costocorporeal joints. In the upper six ribs the axis is displaced horizontally approximately 25 to 35 degrees from the frontal plane; in the lower six ribs the axis is displaced horizontally approximately 35 to 45 degrees from the frontal plane. (The anatomic specimen used to illustrate Figure 11-26, A shows an approximate 35-degree horizontal displacement from the frontal plane.) This slight difference in angulations causes the upper ribs to elevate slightly more in the anterior direction, thereby facilitating the forward and upward movement of the sternum.
The elevating ribs and sternum create slight bending and twisting movements within the pliable cartilages associated with the joints of the thorax. As depicted in Figure 11-26, B, torsion created in the twisted cartilage within a sternocostal joint stores a component of the energy used to elevate the ribs. The energy is partially recaptured during expiration, as the rib cage recoils to its relatively constricted state.
MUSCULAR ACTIONS DURING VENTILATION
A great deal is still to be learned about the specific functions of the muscles of ventilation. Some methods used to study this topic are listed in the box.*
In addition, clinical observations of the effects of muscle paralysis after spinal cord injury have helped tremendously in the understanding of the normal function of the ventilatory muscles.32,51,77
As will be described, any muscle that attaches to the thorax can potentially assist with the mechanics of ventilation. More specifically, a muscle that increases intrathoracic volume is a muscle of inspiration; a muscle that decreases intrathoracic volume is a muscle of expiration. The detailed anatomy and innervation of the muscles of ventilation are found throughout Appendix III, Part C, in particular in the section on muscles related primarily to ventilation.
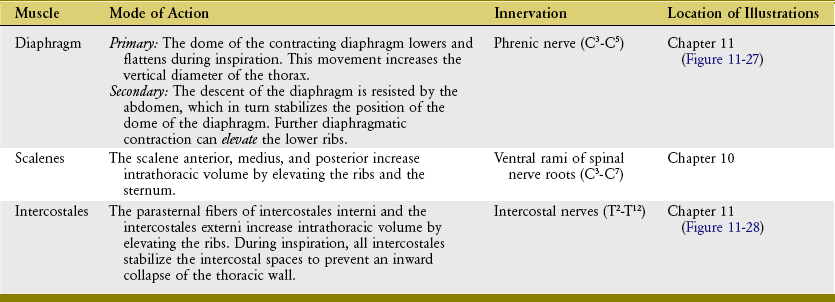
Muscles of Quiet Inspiration
The muscles of quiet inspiration are the diaphragm, scalenes, and intercostales (review Figure 11-24). These muscles are considered primary because they are typically active during all work intensities. Active contraction of the diaphragm muscle is dedicated totally toward the mechanics of inspiration. The intercostales and the scalene muscles, however, also stabilize and rotate parts of the axial skeleton. The mode of action and innervation of the primary muscles of inspiration are summarized in Table 11-4.
DIAPHRAGM MUSCLE: The diaphragm is a dome-shaped, thin, musculotendinous sheet of tissue that separates the thoracic cavity from the abdominal cavity. Its convex upper surface is the floor of the thoracic cavity, and its concave lower surface is the roof of the abdominal cavity.
Because of the position of the liver within the abdomen, the right side of the resting diaphragm lies slightly higher than the left. During quiet inspiration, the dome of the diaphragm drops about 1.5 cm. During forced inspiration the diaphragm flattens and may drop as far as 6 to 10 cm.75 At maximum inspiration the right side descends to the level of the body of T11; the left side descends to the level of the body of T12.
The diaphragm is the most important muscle of inspiration, performing 60% to 80% of the work of the ventilatory process.1,62 The muscle’s predominant role in inspiration is largely a result of its ability to increase intrathoracic volume in all three diameters: vertical, medial-lateral, and anterior-posterior. A given level of muscle contraction therefore yields a relatively large drop in intrathoracic pressure.
The diaphragm is the first muscle to be activated by the nervous system during an inspiratory effort.70 With the lower ribs stabilized, the initial contraction of the diaphragm causes a lowering and flattening of its dome (Figure 11-27). This lowering piston action substantially increases the vertical diameter of the thorax. This action is the primary method by which the diaphragm increases intrathoracic volume. An additional increase in volume requires resistance from within the abdomen. The descent of the diaphragm into the abdominal cavity is resisted by an increase in intra-abdominal pressure; by compressed abdominal contents; and by passive tension in stretched abdominal muscles, such as the transversus abdominis. At some point this abdominal resistance stabilizes the position of the dome of the diaphragm, allowing its continued contraction to elevate the lower six ribs. The elevation can be visualized by reversing the direction of the arrowheads in Figure 11-27. As described earlier, elevation of the ribs expands the thorax in the anterior-posterior and medial-lateral diameters.
SCALENE MUSCLES: The scalenus anterior, medius, and posterior muscles attach between the cervical spine and the upper two ribs (see Chapter 10). If the cervical spine is assumed to be well stabilized, bilateral contraction of the muscles increases intrathoracic volume by elevating the upper ribs and attached sternum. The scalene muscles are active, along with the diaphragm, during every inspiration cycle.13,38,70
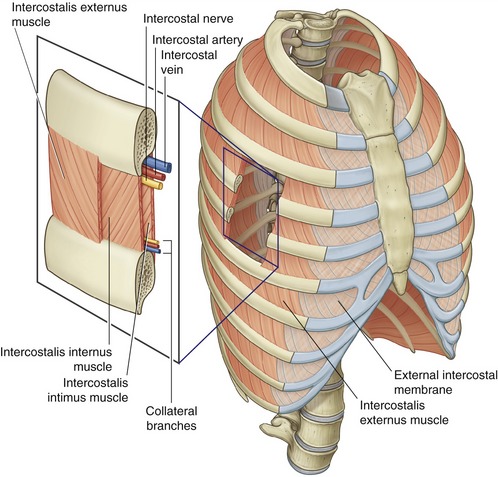
Anatomy: The intercostales are a thin, three-layer set of muscles that occupy the intercostal spaces. Each set of intercostal muscles within a given intercostal space is innervated by an adjacent intercostal nerve (Figure 11-28).
The intercostales externi are most superficial, analogous in depth and fiber direction to the obliquus abdominis externus of the trunk (see Chapter 10). There are 11 per side, and each intercostalis externus arises from the lower border of a rib and inserts on the upper border of the rib below (see Figure 11-28, inset). Fibers travel obliquely between ribs in an inferior and medial direction. The intercostales externi are most developed laterally. Anteriorly, within the region of the sternocostal joints, the intercostales externi are replaced by a thin external intercostal membrane.
The intercostales interni are deep to the externi and are analogous in depth and fiber direction to the obliquus abdominis internus of the trunk. There are also 11 per side, with each muscle occupying one intercostal space, in a manner similar to that in the intercostales externi. A major difference, however, is that the fibers of the intercostales interni travel perpendicular to the fibers of the intercostales externi (see Figure 11-28, inset). The intercostales interni are most developed anteriorly within the region of the sternocostal joints; posteriorly, the muscles terminate as the internal intercostal membrane.
Primarily because of differences in function, recent research literature typically refers to the intercostales interni as two different sets of muscle fibers: the parasternal intercostals, occupying the region of the sternocostal joints, and the interosseous intercostals, occupying the more lateral and posterior-lateral intercostal spaces.15 This terminology will be used in subsequent discussions.
Finally, the intercostales intimi are the deepest and least developed of the intercostales. Often referred to as the “innermost intercostals,” these muscles run parallel and deep to the intercostales interni (see Figure 11-28, inset). Fibers of the intercostales intimi located near the angle of the ribs (often designated as subcostales muscles) may cross one or two intercostal spaces. The intercostales intimi are most developed in the lower thorax. The actions of these deep and relatively inaccessible muscles have not been extensively studied. It is tempting to speculate, however, that they have actions similar to those of the adjacent intercostales interni.75
Function of the Intercostales Externi and Interni Muscles: The intercostales externi and interni muscles are often informally referred to as the “external and internal intercostals,” respectively. The specific actions of the intercostal muscles during ventilation are not completely understood and are controversial.15 The disagreement on this subject is traced back to the teachings of Galen (circa 130-200 ad), Leonardo da Vinci (1452-1519), and Vesalius (1514-1564).15 In more recent times the conventional teaching on this topic is that the external intercostals muscles drive inspiration, and the internal intercostals drive forced expiration.75 To a large extent these functions are based on the contrasting lines of force (fiber direction) of the muscles relative to the axis of rotation through the posterior end of the ribs. In theory, isolated contraction of an external intercostal muscle has greater leverage to elevate the lower rib than to depress the upper. Conversely, an isolated contraction of an internal intercostal muscle has greater leverage to depress the upper rib than to elevate the lower.15
Although the proposed relatively simple reciprocal actions of the external and internal intercostals have generally been supported through EMG and other methods of research, the overall muscular kinesiology is far more complicated.* De Troyer and colleagues provide a compelling argument that the action of any given intercostal muscle is influenced not only by its fiber direction and line of pull but also, perhaps more important, by factors associated with the specific region where the muscle resides.15 These regional-specific factors include the local muscles’ force- and torque-generating capability (based on cross-sectional areas and moment arm lengths, respectively), curvature of the ribs, stabilizing influence of other muscles, and most important, differing intensities of neural drive.14,15,28
• The external intercostal muscles are primary muscles of inspiration.14,70 The effectiveness of this action is greatest in the dorsal and upper (cranial) regions of the thorax and diminishes in a ventral-to-caudal direction.14,86
• The parasternal fibers of the internal intercostal muscles are primary muscles of inspiration.70 The effectiveness of this action, however, diminishes in a cranial-to-caudal direction.16,86
• The interosseous fibers of the internal intercostal muscles are primary muscles of forced expiration.5 The effectiveness of this action persists throughout the thorax.
In addition to functioning as muscles of inspiration or expiration, the lateral set of intercostal muscles (both external and internal) show considerable activation during axial rotation of the trunk. In a similar manner as the “oblique abdominals” (see Chapter 10), the external intercostals are most active during contralateral trunk rotation, and the more internal intercostals are most active during ipsilateral trunk rotation.64 The relative contribution of these muscles to the overall biomechanics of axial rotation of the trunk is uncertain.
In addition to expanding the intrathoracic volume during inspiration, contraction of the external and parasternal intercostal muscles also adds a degree of rigidity to the rib cage.4,14,28 Although often overlooked, this stabilizing function is a very important component of ventilation.6 With the assistance of the scalene muscles, the splinting action on the ribs prevents the thoracic wall from being partially sucked inward by the reduced intrathoracic pressure caused by contraction of the diaphragm.
As the intercostal muscles contract to stiffen the thoracic cage during inspiration, muscles located in the pharyngeal region also contract slightly to stiffen and dilate the upper airway. One of the main upper airway dilator muscles is the genioglossus, a dominant extrinsic muscle of the tongue.75 The neural control of this muscle during breathing has been extensively studied, primarily because of its possible role in obstructive sleep apnea.4,69
Muscles of Forced Inspiration
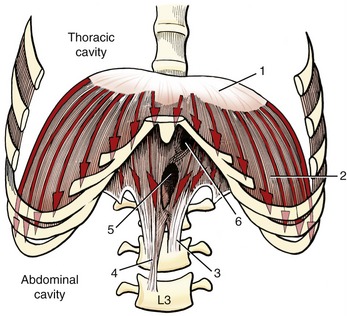
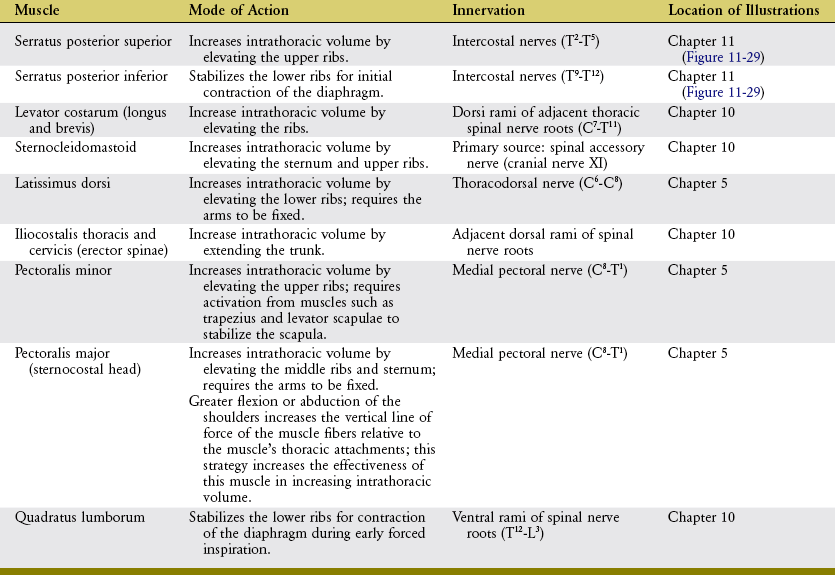
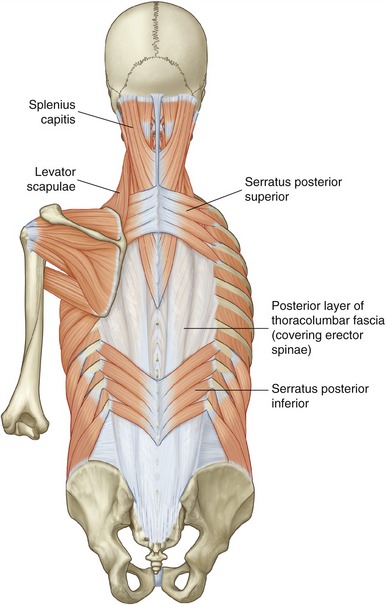
Forced inspiration requires additional muscles to assist the primary muscles of inspiration. As a group, the additional muscles are referred to as muscles of forced inspiration, or accessory muscles of inspiration. Table 11-5 lists a sample of several muscles of forced inspiration, including their mode of action. Each muscle has a line of action that can directly or indirectly increase intrathoracic volume. Most muscles listed in Table 11-5 are illustrated elsewhere in this textbook. The serratus posterior superior and serratus posterior inferior are illustrated in Figure 11-29.
Muscles of Forced Expiration
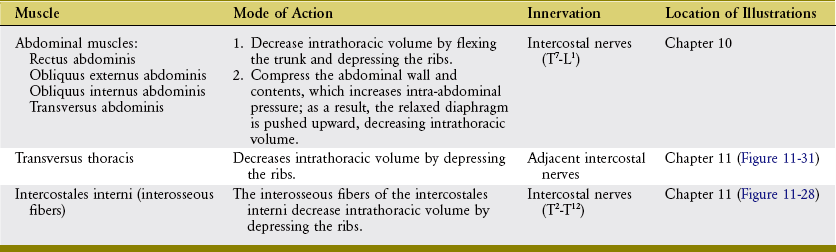
During forced expiration, active muscle contraction is required to rapidly reduce intrathoracic volume. Muscles of forced expiration include the four abdominal muscles, the transversus thoracis, and the interosseous fibers of the intercostales interni (Figure 11-30). The mode of action of the muscles of forced expiration is summarized in Table 11-6.
ABDOMINAL MUSCLES: The “abdominal” muscles include the rectus abdominis, obliquus externus abdominis, obliquus internus abdominis, and transversus abdominis (see Chapter 10). Contraction of these muscles has a direct and indirect effect on forced expiration. By acting directly, contraction of the abdominal muscles flexes the thorax and depresses the ribs and sternum. These actions rapidly and forcefully reduce intrathoracic volume, such as when coughing, sneezing, or vigorously exhaling to the limits of the expiratory reserve volume. When acting indirectly, contraction of the abdominal muscles—especially the transversus abdominis—increases intra-abdominal pressure and compresses the abdominal viscera. The increased pressure can forcefully push the relaxed diaphragm upward, well into the thoracic cavity (see Figure 11-30). In this manner, active contraction of the abdominal muscles takes advantage of the parachute-shaped diaphragm to help expel air from the thorax. As described in Chapter 10, the increased intra-abdominal pressure is also used during activities involving the Valsalva maneuver, including defecation, childbirth, and lifting of loads or stabilization of the lumbar spine.
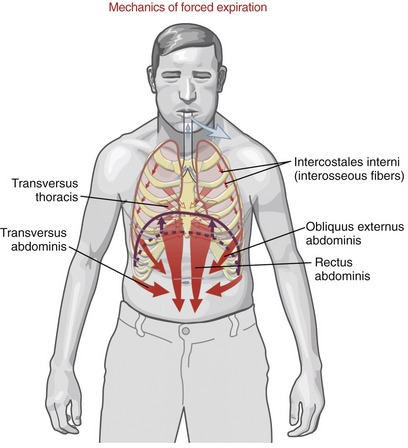
TRANSVERSUS THORACIS AND INTERCOSTALES INTERNI: The transversus thoracis (also referred to as the triangularis sterni) is a muscle of forced expiration.16,17 The muscle is located on the internal side of the thorax, running horizontally and obliquely superiorly between the lower third of the sternum and the sternocostal joints of the adjacent four or five ribs (Figure 11-31). The muscle’s neural activation is synchronized with the abdominal muscles and interosseous fibers of the internal intercostals during forced expiration.15,17
REFERENCES
1. Aliverti, A, Cala, SJ, Duranti, R, et al. Human respiratory muscle actions and control during exercise. J Appl Physiol. 1997;83:1256–1269.
2. Baltali, E, Zhao, KD, Koff, MF, et al. A method for quantifying condylar motion in patients with osteoarthritis using an electromagnetic tracking device and computed tomography imaging. J Oral Maxillofac Surg. 2008;66:848–857.
3. Buescher, JJ. Temporomandibular joint disorders. Am Fam Physician. 2007;76:1477–1482.
4. Butler, JE. Drive to the human respiratory muscles. Respir Physiol Neurobiol. 2007;159:115–126.
5. Butler, JE, Gandevia, SC. The output from human inspiratory motoneurone pools. J Physiol. 2008;586:1257–1264.
6. Butler, JE, McKenzie, DK, Gandevia, SC. Discharge frequencies of single motor units in human diaphragm and parasternal muscles in lying and standing. J Appl Physiol. 2001;90:147–154.
7. Cala, SJ, Kenyon, CM, Lee, A, et al. Respiratory ultrasonography of human parasternal intercostal muscle in vivo. Ultrasound Med Biol. 1998;24:313–326.
8. Castro, HA, Resende, LA, Bérzin, F, König, B. Electromyographic analysis of superior belly of the omohyoid muscle and anterior belly of the digastric muscle in mandibular movements. Electromyogr Clin Neurophysiol. 1998;38:443–447.
9. Christo, JE, Bennett, S, Wilkinson, TM, Townsend, GC. Discal attachments of the human temporomandibular joint. Aust Dent J. 2005;50:152–160.
10. Cleland, J, Palmer, J. Effectiveness of manual physical therapy, therapeutic exercise, and patient education on bilateral disc displacement without reduction- of the temporomandibular joint: a single-case design. J Orthop Sports Phys Ther. 2004;34:535–548.
11. Cooper, BC, Kleinberg, I. Establishment of a temporomandibular physiological state with neuromuscular orthosis treatment affects reduction of TMD symptoms in 313 patients. Cranio. 2008;26:104–117.
12. De Troyer, A. Relationship between neural drive and mechanical effect in the respiratory system. Adv Exp Med Biol. 2002;508:507–514.
13. De Troyer, A, Estenne, M. Functional anatomy of the respiratory muscles. Clin Chest Med. 1988;9:175–193.
14. De Troyer, A, Gorman, RB, Gandevia, SC. Distribution of inspiratory drive to the external intercostal muscles in humans. J Physiol. 2003;546:943–954.
15. De Troyer, A, Kirkwood, PA, Wilson, TA. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717–756.
16. De Troyer, A, Legrand, A, Gevenois, PA, Wilson, TA. Mechanical advantage of the human parasternal intercostal and triangularis sterni muscles. J Physiol. 1998;513:915–925.
17. De Troyer, A, Ninane, V, Gilmartin, JJ, et al. Triangularis sterni muscle use in supine humans. J Appl Physiol. 1987;62:919–925.
18. De Troyer, A, Peche, R, Yernault, JC, Estenne, M. Neck muscle activity in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150:41–47.
19. Decramer, M. Hyperinflation and respiratory muscle interaction. Eur Respir J. 1997;10:934–941.
20. Desmons, S, Graux, F, Atassi, M, et al. The lateral pterygoid muscle, a heterogeneous unit implicated in temporomandibular disorder: a literature review. Cranio. 2007;25:283–291.
21. Dolwick, MF. Temporomandibular joint surgery for internal derangement. Dent Clin North Am. 2007;51:195–208.
22. Estenne, M, DeTroyer, A. Relationship between respiratory muscle electromyogram and rib cage motion in tetraplegia. Am Rev Respir Dis. 1985;132:53–59.
23. Estenne, M, Yernault, JC, De, TA. Rib cage and diaphragm-abdomen compliance in humans: effects of age and posture. J Appl Physiol. 1985;59:1842–1848.
24. Ferrario, VF, Sforza, C, Miani, A, Jr., et al. Open-close movements in the human temporomandibular joint: does a pure rotation around the intercondylar hinge axis exist? J Oral Rehabil. 1996;23:401–408.
25. Fink, M, Tschernitschek, H, Stiesch-Scholz, M. Asymptomatic cervical spine dysfunction (CSD) in patients with internal derangement of the temporomandibular joint. Cranio. 2002;20:192–197.
26. Finucane, KE, Panizza, JA, Singh, B. Efficiency of the normal human diaphragm with hyperinflation. J Appl Physiol. 2005;99:1402–1411.
27. Furto, ES, Cleland, JA, Whitman, JM, Olson, KA. Manual physical therapy interventions and exercise for patients with temporomandibular disorders. Cranio. 2006;24:283–291.
28. Gandevia, SC, Hudson, AL, Gorman, RB, et al. Spatial distribution of inspiratory drive to the parasternal intercostal muscles in humans. J Physiol. 2006;573:263–275.
29. Gokalp, H, Turkkahraman, H, Bzeizi, N. Correlation between eminence steepness and condyle disc movements in temporomandibular joints with internal derangements on magnetic resonance imaging. Eur J Orthod. 2001;23:579–584.
30. Goldman, JM, Rose, LS, Williams, SJ, et al. Effect of abdominal binders on breathing in tetraplegic patients. Thorax. 1986;41:940–945.
31. Goldstein, DF, Kraus, SL, Williams, WB, et al. Influence of cervical posture on mandibular movement. J Prosthet Dent. 1984;52:421–426.
32. Gollee, H, Hunt, KJ, Allan, DB, et al. A control system for automatic electrical stimulation of abdominal muscles to assist respiratory function in tetraplegia. Med Eng Phys. 2007;29:799–807.
33. Graff-Radford, SB. Temporomandibular disorders and other causes of facial pain. Curr Pain Headache Rep. 2007;11:75–81.
34. Guarda-Nardini, L, Manfredini, D, Ferronato, G. Temporomandibular joint total replacement prosthesis: current knowledge and considerations for the future. Int J Oral Maxillofac Surg. 2008;37:103–110.
35. Hansdottir, R, Bakke, M. Joint tenderness, jaw opening, chewing velocity, and bite force in patients with temporomandibular joint pain and matched healthy control subjects. J Orofac Pain. 2004;18:108–113.
36. Hansson, T, Oberg, T, Carlsson, GE, Kopp, S. Thickness of the soft tissue layers and the articular disk in the temporomandibular joint. Acta Odontol Scand. 1977;35:77–83.
37. Hawkes, EZ, Nowicky, AV, McConnell, AK. Diaphragm and intercostal surface EMG and muscle performance after acute inspiratory muscle loading. Respir Physiol Neurobiol. 2007;155:213–219.
38. Hudson, AL, Gandevia, SC, Butler, JE. The effect of lung volume on the co-ordinated recruitment of scalene and sternomastoid muscles in humans. J Physiol. 2007;584:261–270.
39. Iglarsh, ZA, Snyder-Mackler, L. Temporomandibular joint and the cervical spine. In: Richardson JV, Iglarsh ZA, eds. Clinical orthopaedic physical therapy. Philadelphia: Saunders, 1994.
40. Ioi, H, Matsumoto, R, Nishioka, M, et al. Relationship of TMJ osteoarthritis/osteoarthrosis to head posture and dentofacial morphology. Orthod Craniofac Res. 2008;11:8–16.
41. Ismail, F, Demling, A, Hessling, K, et al. Short-term efficacy of physical therapy compared to splint therapy in treatment of arthrogenous TMD. J Oral Rehabil. 2007;34:807–813.
42. Lafreniere, CM, Lamontagne, M, el-Sawy, R. The role of the lateral pterygoid muscles in TMJ disorders during static conditions. Cranio. 1997;15:38–52.
43. Le Bars, P, Duron, B. Are the external and internal intercostal muscles synergist or antagonist in the cat? Neurosci Lett. 1984;51:383–386.
44. Mahan, PE, Wilkinson, TM, Gibbs, CH, et al. Superior and inferior bellies of the lateral pterygoid muscle EMG activity at basic jaw positions. J Prosthet Dent. 1983;50:710–718.
45. Marchand, E, Decramer, M. Respiratory muscle function and drive in chronic obstructive pulmonary disease. Clin Chest Med. 2000;21:679–692.
46. McKenzie, DK, Gorman, RB, Tolman, J, et al. Estimation of diaphragm length in patients with severe chronic obstructive pulmonary disease. Respir Physiol. 2000;123:225–234.
47. McLean, L. The effect of postural correction on muscle activation amplitudes recorded from the cervicobrachial region. J Electromyogr Kinesiol. 2005;15:527–535.
48. McNeely, ML, Armijo, OS, Magee, DJ. A systematic review of the effectiveness of physical therapy interventions for temporomandibular disorders. Phys Ther. 2006;86:710–725.
49. Minakuchi, H, Kuboki, T, Matsuka, Y, et al. Randomized controlled evaluation of non-surgical treatments for temporomandibular joint anterior disk displacement without reduction. J Dent Res. 2001;80:924–928.
50. Mizuno, M. Human respiratory muscles: fibre morphology and capillary supply. Eur Respir J. 1991;4:587–601.
51. Morgan, MD, Gourlay, AR, Silver, JR, et al. Contribution of the rib cage to breathing in tetraplegia. Thorax. 1985;40:613–617.
52. Murray, GM, Bhutada, M, Peck, CC, et al. The human lateral pterygoid muscle. Arch Oral Biol. 2007;52:377–380.
53. Murray, GM, Phanachet, I, Uchida, S, Whittle, T. The role of the human lateral pterygoid muscle in the control of horizontal jaw movements. J Orofac Pain. 2001;15:279–292.
54. Nicolakis, P, Erdogmus, B, Kopf, A, et al. Exercise therapy for craniomandibular disorders. Arch Phys Med Rehabil. 2000;81:1137–1142.
55. Okeson, JP. Management of temporomandibular disorders and occlusion, ed 6. St. Louis: Mosby; 2005.
56. Orlando, B, Manfredini, D, Salvetti, G, Bosco, M. Evaluation of the effectiveness of biobehavioral therapy in the treatment of temporomandibular disorders: a literature review. Behav Med. 2007;33:101–118.
57. Osborn, JW. The temporomandibular ligament and the articular eminence as constraints during jaw opening. J Oral Rehabil. 1989;16:323–333.
58. Perez del, PA, Doblare, M. Finite element analysis of the temporomandibular joint during lateral excursions of the mandible. J Biomech. 2006;39:2153–2163.
59. Phanachet, I, Whittle, T, Wanigaratne, K, Murray, GM. Functional properties of single motor units in the inferior head of human lateral pterygoid muscle: task firing rates. J Neurophysiol. 2002;88:751–760.
60. Piehslinger, E, Celar, AG, Celar, RM, Slavicek, R. Computerized axiography: principles and methods. Cranio. 1991;9:344–355.
61. Posselt, U. Movement areas of the mandible. J Prosthet Dent. 1957;7:375–385.
62. Ratnovsky, A, Elad, D. Anatomical model of the human trunk for analysis of respiratory muscles mechanics. Respir Physiol Neurobiol. 2005;148:245–262.
63. Ries, LG, Alves, MC, Berzin, F. Asymmetric activation of temporalis, masseter, and sternocleidomastoid muscles in temporomandibular disorder patients. Cranio. 2008;26:59–64.
64. Rimmer, KP, Ford, GT, Whitelaw, WA. Interaction between postural and respiratory control of human intercostal muscles. J Appl Physiol. 1995;79:1556–1561.
65. Robinson, PD. Articular cartilage of the temporomandibular joint: can it regenerate? Ann R Coll Surg Engl. 1993;75:231–236.
66. Rocabado, M. Arthrokinematics of the temporomandibular joint. Dent Clin North Am. 1983;27:573–594.
67. Rollman, GB, Gillespie, JM. The role of psychosocial factors in temporomandibular disorders. Curr Rev Pain. 2000;4:71–81.
68. Rutkiewicz, T, Könönen, M, Suominen-Taipale, L, et al. Occurrence of clinical signs of temporomandibular disorders in adult Finns. J Orofac Pain. 2006;20:208–217.
69. Saboisky, JP, Butler, JE, Fogel, RB, et al. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–2221.
70. Saboisky, JP, Gorman, RB, De Troyer, A, et al. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol. 2007;102:772–780.
71. Sato, H, Strom, D, Carlsson, GE. Controversies on anatomy and function of the ligaments associated with the temporomandibular joint: a literature survey. J Orofac Pain. 1995;9:308–316.
72. Schiffman, EL, Fricton, JR, Haley, DP, Shapiro, BL. The prevalence and treatment needs of subjects with temporomandibular disorders. J Am Dent Assoc. 1990;120:295–303.
73. Singh, B, Panizza, JA, Finucane, KE. Breath-by-breath measurement of the volume displaced by diaphragm motion. J Appl Physiol. 2003;94:1084–1091.
74. Sinn, DP, de Assis, EA, Throckmorton, GS. Mandibular excursions and maximum bite forces in patients with temporomandibular joint disorders. J Oral Maxillofac Surg. 1996;54:671–679.
75. Standring, S. Gray’s anatomy: the anatomical basis of clinical practice, ed 40. St Louis: Elsevier; 2009.
76. Stiesch-Scholz, M, Fink, M, Tschernitschek, H, Rossbach, A. Medical and physical therapy of temporomandibular joint disk displacement without reduction [see comment]. Cranio. 2002;20:85–90.
77. Strakowski, JA, Pease, WS, Johnson, EW. Phrenic nerve stimulation in the evaluation of ventilator-dependent individuals with C4- and C5-level spinal cord injury. Am J Phys Med Rehabil. 2007;86:153–157.
78. Suvinen, TI, Kemppainen, P. Review of clinical EMG studies related to muscle and occlusal factors in healthy and TMD subjects. J Oral Rehabil. 2007;34:631–644.
79. Takahashi, S, Suzuki, N, Asazuma, T, et al. Factors of thoracic cage deformity that affect pulmonary function in adolescent idiopathic thoracic scoliosis. Spine. 2007;32:106–112.
80. Takazakura, R, Takahashi, M, Nitta, N, Murata, K. Diaphragmatic motion in the sitting and supine positions: healthy subject study using a vertically open magnetic resonance system. J Magn Res Imaging. 2004;19:605–609.
81. Tanaka, E, Detamore, MS, Mercuri, LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307.
82. Tanaka, E, Hirose, M, Inubushi, T, et al. Effect of hyperactivity of the lateral pterygoid muscle on the temporomandibular joint disk. J Biomech Eng. 2007;129:890–897.
83. Taskaya-Yilmaz, N, Ceylan, G, Incesu, L, Muglali, M. A possible etiology of the internal derangement of the temporomandibular joint based on the MRI observations of the lateral pterygoid muscle. Surg Radiol Anat. 2005;27:19–24.
84. Urmey, W, Loring, S, Mead, J, et al. Upper and lower rib cage deformation during breathing in quadriplegics. J Appl Physiol. 1986;60:618–622.
85. Vassilakopoulos, T, Zakynthinos, S, Roussos, C. Respiratory muscles and weaning failure. Eur Respir J. 1996;9:2383–2400.
86. Wilson, TA, Legrand, A, Gevenois, PA, De Troyer, A. Respiratory effects of the external and internal intercostal muscles in humans. J Physiol. 2001;530:319–330.
87. Wink, CS, St, OM, Zimny, ML. Neural elements in the human temporomandibular articular disc. J Oral Maxillofac Surg. 1992;50:334–337.
88. Yustin, DC, Rieger, MR, McGuckin, RS, Connelly, ME. Determination of the existence of hinge movements of the temporomandibular joint during normal opening by Cine-MRI and computer digital addition. J Prosthodont. 1993;2:190–195.
89. Zaugg, M, Lucchinetti, E. Respiratory function in the elderly. Anesthesiol Clin North Am. 2000;18:47–58.
90. Zeleznik, J. Normative aging of the respiratory system. Clin Geriatr Med. 2003;19:1–18.
STUDY QUESTIONS
1. Explain the mechanism by which the intermediate region of the articular disc within the temporomandibular joint (TMJ) protects the joint throughout the late phase of opening the mouth.
2. Compare the distal attachments of the medial and lateral pterygoid muscles. Which attachments form a “functional sling” with the masseter muscle?
3. Explain how, in theory, an overly depressed scapulothoracic joint could predispose internal derangement of the articular disc of the TMJ.
4. Compare the differences in structure and functional demands normally placed on articular and nonarticular parts of the mandibular fossa.
5. Describe the functional role of the oblique fibers of the lateral ligament of the TMJ in opening the mouth.
6. Explain the function of the temporalis muscles in closing the mouth.
7. Describe the synergistic relationship between the masseter and contralateral medial pterygoid muscle during the production of shearing (grinding) force between the molars.
8. Using Figure 11-22 as a guide, describe the specific function of the lateral pterygoid muscle during opening and closing of the mouth.
9. Describe how the inferior head of the lateral pterygoid muscle and the suprahyoid muscles act synergistically during a rapid opening of the mouth.
10. List the bones that make up temporal fossa of the cranium.
PART 2: VENTILATION
11. Describe the function of the diaphragm muscle during inspiration, and explain why it is considered the most important muscle of ventilation.
12. Explain how the sternocostal head of the pectoralis major could function as an effective muscle of forced inspiration.
13. How could a chronically lowered (flattened) diaphragm muscle negatively affect the mechanics of ventilation?
14. List the articulations that most likely affect the anterior-posterior and medial-lateral dimensions of the thorax during ventilation.
15. What structures seal the inferior and superior poles of the thoracic cavity?
16. Explain how the normal “tone” within the “abdominal” muscles contributes to the mechanics of inspiration.
17. How does paralysis of the intercostal muscles in a person with quadriplegia contribute to the pathomechanics of “paradoxic breathing”?
18. Describe the changes in intrathoracic and intra-abdominal pressure during forced expiration.
19. List factors explaining why quiet expiration is considered a “passive” process.
20. List the muscles most likely rendered fully paralyzed after a complete spinal cord lesion at the bony level of T4.
 Answers to the study questions can be found on the Evolve website.
Answers to the study questions can be found on the Evolve website.