Ketamine
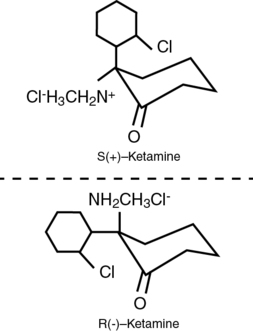
The ketamine molecule (2-(o-chlorophenyl)-2-methylamino-cyclohexamine) is chemically related to phencyclidine and contains a chiral center (C2 carbon of the cyclohexanone ring) with two optical isomers. The S-isomer is four times more potent, is associated with a faster recovery, and has a low incidence of psychomimetic effects, compared with the R-isomer. However, in North America, ketamine is sold primarily as a racemic mixture (Figure 74-1). It also contains benzethonium chloride as a preservative compound.
Mechanisms of action
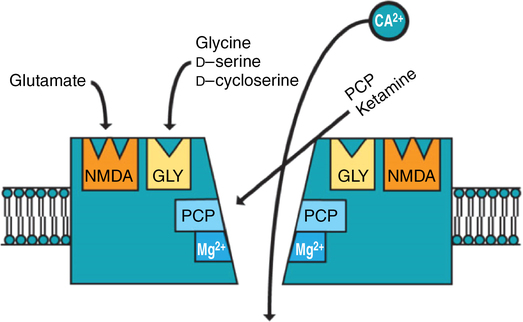
Ketamine is not a particularly selective drug, with multiple sites of action, including those in the central and peripheral nervous systems. The properties of ketamine are primarily mediated by noncompetitive antagonism at N-methyl-D-aspartate (NMDA) receptors, but the drug also has local anesthetic properties. The NMDA receptors consist of five subunits surrounding a central ion channel that is permeable to Ca2+, Na+, and K+ (Figure 74-2). Binding sites for Mg2+ and ketamine have been found inside this channel.