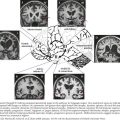
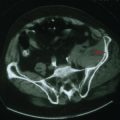
55 Ischemic Stroke
Etiology and Pathophysiology
Large Artery Occlusive Disease
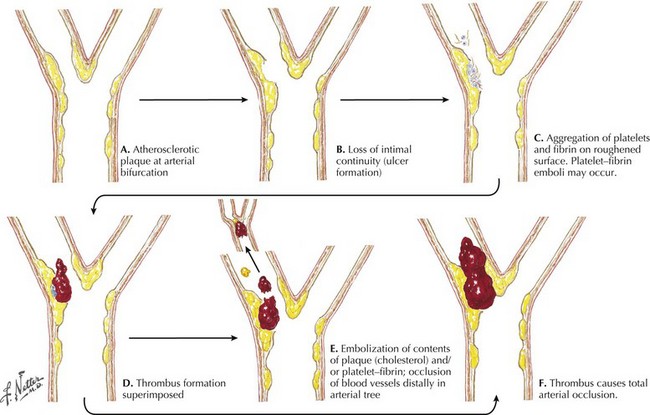
Atherosclerosis causes stenosis or occlusion of extracranial and intracranial arteries and is directly responsible for a significant percentage of cerebral ischemic events. Atheroma formation involves the progressive deposition of circulating lipids and ultimately fibrous tissue in the subintimal layer of the large and medium arteries, occurring most frequently at branching points (Fig. 55-1). Plaque formation is enhanced by blood-associated inflammatory factors as well as increased shear injury form uncontrolled blood pressure. Intraplaque hemorrhage, subintimal necrosis with ulcer formation, and calcium deposition can cause enlargement of the atherosclerotic plaque with consequent worsening of the degree of arterial narrowing.
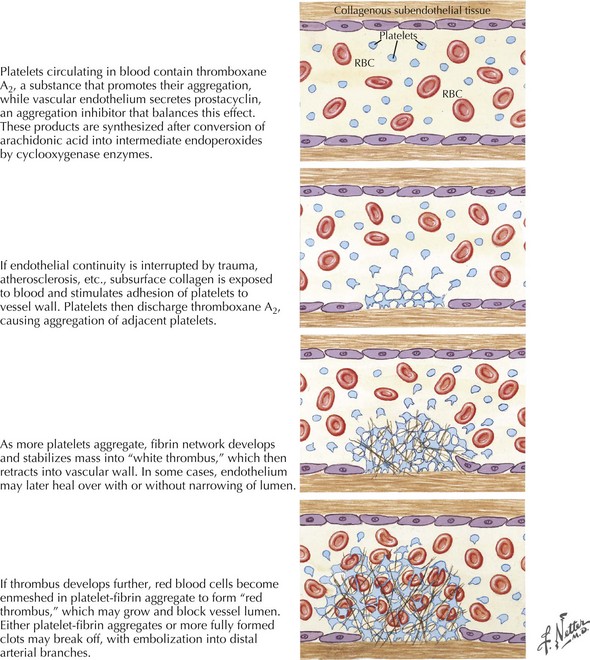
Disruption of the endothelial surface triggers thrombus formation within the arterial lumen through activation of nearby platelets by the subendothelial matrix. When platelets become activated they release thromboxane A2, causing further platelet aggregation. The development of a fibrin network stabilizes the platelet aggregate, forming a “white thrombus.” In areas of slowed or turbulent flow within or around the plaque the thrombus develops further, enmeshing red blood cells (RBCs) in the platelet–fibrin aggregate to form a “red thrombus” (Fig. 55-2). This remains poorly organized and friable for up to 2 weeks and presents a significant risk of propagation or embolization. Either the white or red thrombus, however, can dislodge and embolize to distal arterial branches. Large artery disease can cause ischemic strokes by either intra-arterial embolism as described above and, less commonly, hemodynamic ischemia or hypoperfusion through a significantly narrowed vessel.
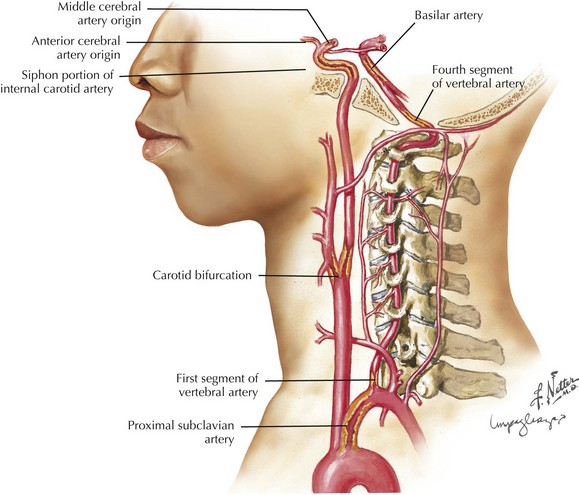
Frequent sites for carotid system or anterior circulation atherosclerosis are the origin of the internal carotid artery (ICA), the carotid siphon at the base of the brain (Fig. 55-3), and the main stem of the middle cerebral artery (MCA) and the anterior cerebral artery (ACA). The internal carotid artery at or around the bifurcation is usually affected in Caucasians whereas in Asian, Hispanic, and African-American populations, intracranial atherosclerosis may be more common than carotid disease. In the vertebrobasilar system, the origins of the vertebral arteries in the neck and the distal portion of the intracranial vertebral arteries are the most commonly affected areas. The basilar artery and origins of the posterior cerebral arteries (PCAs) are other sites.
Cardiac Embolism
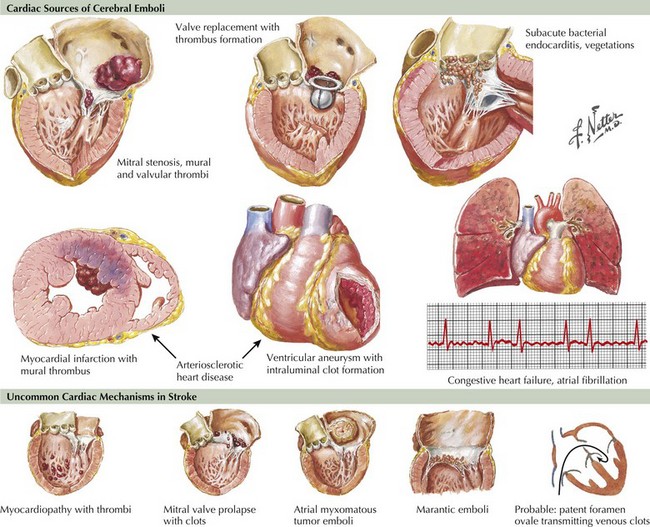
Several types of cardiac disease lead to cerebral embolism: cardiac arrhythmias, ischemic heart disease, valvular disease, dilated cardiomyopathies, atrial septal abnormalities, and intracardiac tumors (Fig. 55-4).
Small Vessel Disease (Lacunes)
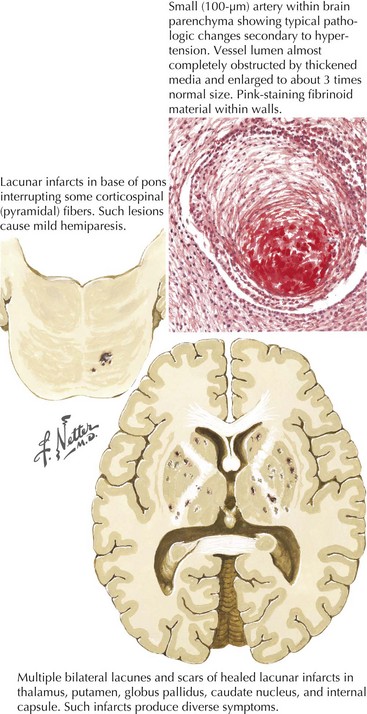
The capillary vessel and the end penetrating arteries that supply the basal ganglia, thalamus, internal capsule, and white matter tracts are not prone to atherosclerosis as in the large-caliber cerebral circulation but undergo a characteristic pathologic degeneration in response to endothelial damage. Fibrinoid degeneration with focal enlargement of the vessel wall, foam cell invasion of the lumen, and hemorrhagic rupture through the vessel wall characterize this process known as fibrinoid degeneration or lipohyalinosis. Occlusion of these arteries causes small (1–20 mm), discrete, and often irregular lesions called lacunes. As previously alluded to, lacunes do not involve the cortical ribbon and occur most often in the basal ganglia, thalamus, pons, internal capsule, and cerebral white matter and may cause discrete clinical syndromes but often go clinically unnoticed. Arterial hypertension and diabetes are the main risk factors (Fig. 55-5).
Arterial Dissection
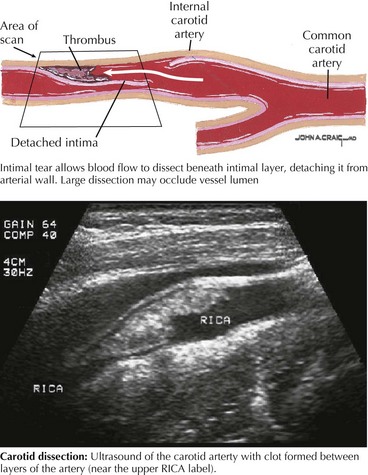
Dissection or tear within the extracranial ICA, particularly its pharyngeal and distal segments, or the extracranial vertebral artery, mainly in its first and third segments, are the two commonly affected vessels. Dissection occurring between the intima and media usually causes stenosis or occlusion of the affected artery, whereas dissection between the media and adventitia is associated with aneurysmal dilatation. Congenital abnormalities in the media or elastica of the arteries as seen in Marfan syndrome, fibromuscular dysplasia, and cystic medial necrosis can predispose patients to arterial dissection. Although often associated with acute trauma, arterial dissection may result from seemingly innocuous incidents, such as a fall while hiking or skiing; sports activities, particularly wrestling or diving into a wave; and paroxysms of coughing. The mechanism of stroke involves clot formation in the dissected wall of the vessel with distal propagation or embolization. Also, expansion of the clot in the dissected wall causes progressive narrowing of the lumen, leading to compromised blood flow and hypoperfusion or, ultimately, occlusion (Fig. 55-6).
Clinical Presentation
Large Artery Occlusive Disease
Carotid Artery Disease
Clinical Vignette
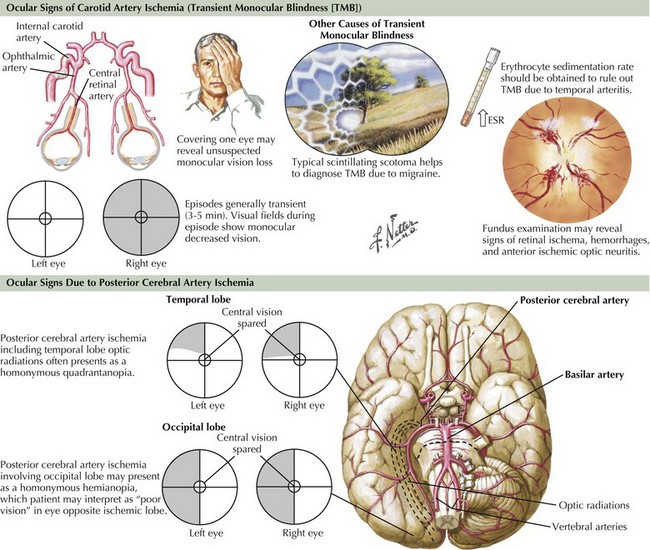
Another important clue to ICA disease is episodes of transient monocular blindness (TMB). TMB refers to the occurrence of temporary unilateral visual loss or obscuration that is classically described by careful observers as a horizontal or vertical “shade being drawn over one eye,” but most frequently as a “fog” or “blurring” in the eye, lasting 1–5 minutes. It often occurs spontaneously but at times is triggered by position changes. Positive phenomena such as sparkles, lights, or colors evolving over minutes are more typical of migrainous phenomena and help to differentiate such benign visual changes from the more serious TMB, a frequent harbinger of cerebral infarct within the carotid artery vasculature. Rarely, with critical ipsilateral internal carotid stenosis, gradual dimming or loss of vision when exposed to bright light, such as glare of snow on a sunlit background, can be reported and is due to limited vascular flow in the face of increased retinal metabolic demand. Besides carotid atherosclerosis, other etiologies of TMB include cardiac embolism and intrinsic ophthalmic artery disease due to processes such as atherosclerosis or arteritis (see Giant cell or Temporal arteritis in Chapter 11), as well as decreased retinal perfusion from glaucoma or increased intraocular pressure. It is not uncommon that homonymous field deficits are reported by patients as monocular visual loss off to the affected side, and careful questioning as to whether each eye was checked independently and whether the visual difficulty involved the perception of a quadrant or one half of the visual world is essential. For example, patients with left occipital infarctions or transient ischemia may report right-sided vision loss, but further questioning reveals that they were unable to read the right side of street signs or a license plate and while covering the “unaffected” left eye the seemingly abnormal right eye had retained vision within the distribution of the unaffected left homonymous field (Fig. 55-7).
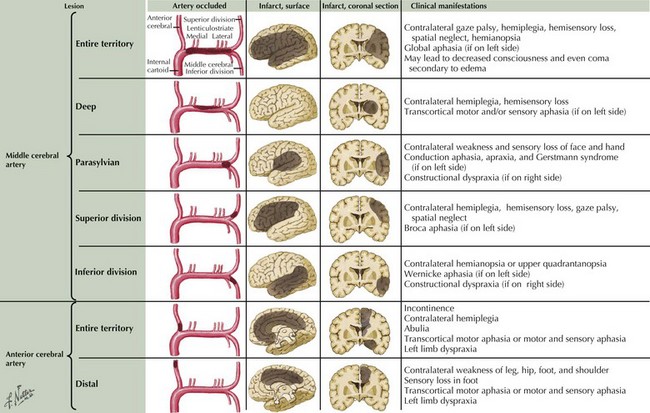
Neurologic findings vary by the location of the occlusion and presence of collateral circulation (Fig. 55-8). A large MCA stroke is usually seen in patients with MCA main stem occlusion without good collateral flow, whereas deep or parasylvian strokes are the most common presentation when enough collateral flow is present over the convexities.
Hemodynamic strokes usually involve the border zone territory between ACA and MCA (anterior border zone), MCA and PCA (posterior border zone), or between deep and superficial perforators (subcortical border zone) and cause typical clinical symptoms outlined in Table 55-1.
Table 55-1 Clinical Symptoms in Patients with Border Zone Strokes
| Stroke Location | Clinical Symptoms |
|---|---|
| Anterior border zone | Contralateral weakness (proximal > distal limbs and sparing face), transcortical motor aphasia (left-sided infarcts), mood disturbances (right-sided infarcts) |
| Posterior border zone | Homonymous hemianopsia, lower-quadrant-anopsia, transcortical sensory aphasia (left-sided infarcts), hemineglect, and anosognosia (right-sided infarcts) |
| Subcortical border zone | Brachiofacial hemiparesis with or without sensory loss, subcortical aphasia (left-sided infarcts) |
Vertebrobasilar Disease
Clinical Vignette
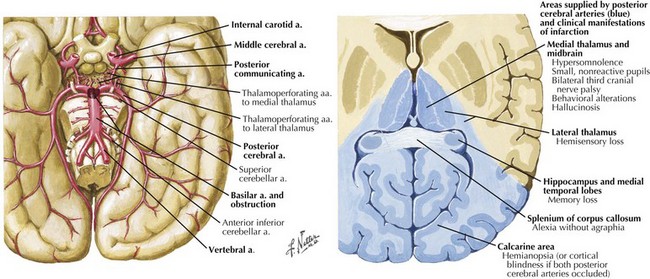
The vertebral arteries originate from the subclavian arteries in the neck. Stenosis or occlusion of the proximal subclavian arteries and the vertebral arteries at their origin rarely causes symptoms because of the concomitant development of adequate collateral circulation within the neck through the thyrocervical and costocervical trunks and other subclavian artery branches eventually flowing into the distal vertebral artery (see Fig. 55-3). More often, patients with subclavian and concomitant vertebral-origin stenosis have symptoms related only to upper extremity ischemia. They report pain, coolness, and weakness of the ipsilateral arm. Rarely does chronic atherosclerotic disease at the vertebral origins, even when bilateral, cause significant vertebrobasilar system flow reduction to cause symptoms. When stenosis or occlusion of the VA origin leads to TIAs or stroke, intra-arterial embolism is the commonly recognized mechanism. The embolus usually lodges in the distal vertebral artery, causing a PICA stroke, or passes through, leading to a “top of the basilar syndrome” (Table 55-2).
Table 55-2 Clinical Manifestations of Ischemia in the Vertebrobasilar System According to the Artery Involved*
| Involved Artery | Ischemic Manifestations |
|---|---|
| Vertebral or PICA penetrator arteries (Lateral medullary or Wallenberg syndrome) |
Ipsilateral limb ataxia and Horner syndrome, crossed sensory loss, vertigo, dysphagia, hoarseness |
| PICA | Vertigo, nausea, vomiting, gait ataxia |
| AICA | Gait and limb ataxia, dysfunction of ipsilateral CN-V, -VII, -VIII |
| SCA | Dysarthria and limb ataxia |
| _______________________________________________ | ______________________________________________________________________________ |
| PCA right | Contralateral visual field cut and sensory loss, visual neglect, prosopagnosia (inability to recognize faces) |
| Left | Contralateral visual field cut and sensory loss, alexia without agraphia, anomic or transcortical sensory aphasia, impaired memory and visual agnosia |
| Top of the basilar syndrome | Rostral brainstem–somnolence, vivid hallucinations, dreamlike behavior, and oculomotor dysfunction. Temporal + occipital regions—hemianopsia, fragments of Balint syndrome, agitated behavior, and amnestic dysfunction |
* AICA, anterior inferior cerebellar artery; PCA, posterior cerebral artery; PICA, posterior–inferior cerebellar artery; SCA, superior cerebellar artery
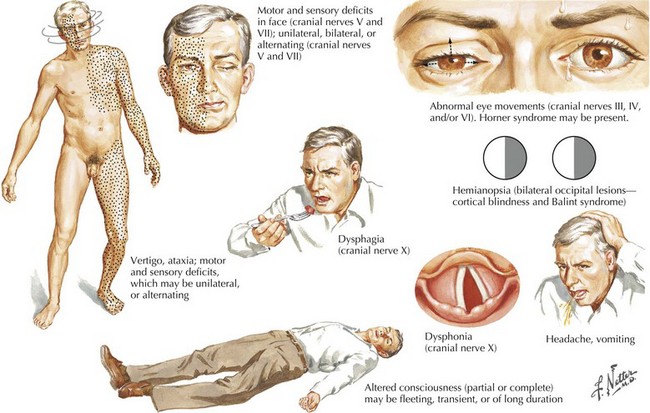
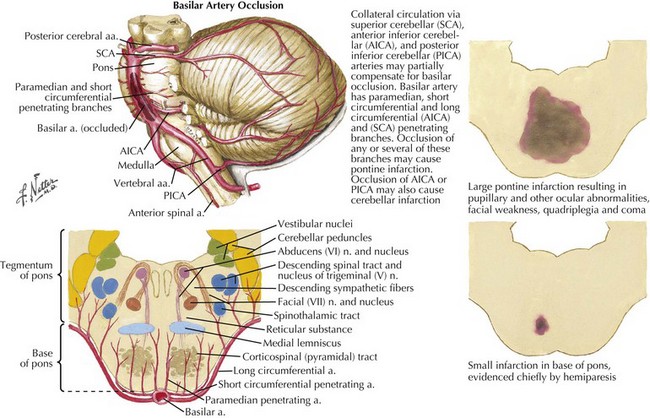
Atherosclerosis of the basilar artery most often affects its proximal and mid portions. Patients experience TIAs characterized by transient diplopia, dizziness, incoordination, and weakness affecting both sides at once or alternating between sides over minutes and even hours or days (Fig. 55-9). As in other occlusive large artery disease, some patients with severe basilar stenosis develop prominent headaches in the weeks before focal symptoms commence. The headache is thought to be from developing posterior circulation collateral flow. When stroke occurs, the most commonly affected area is the basis pons, with bilateral, often asymmetric, hemiparesis, pseudobulbar syndrome, abnormalities of eye movements (sixth nerve palsy, unilateral or bilateral internuclear ophthalmoplegia, ipsilateral conjugate gaze palsy, “one and one-half syndrome”), nystagmus, and if the reticular activating system is involved, coma (Fig. 55-10). Presence of coma or altered level of consciousness is dependent on collateral flow to the tegmentum from other vessels. If the pontine and midbrain tegmentum is spared, bilateral motor and sensory signs as well as varying degrees of ophthalmoplegia may be present without altered consciousness, such as in the “locked in” syndrome.
Embolus to the distal basilar artery leads to the classic top of the basilar syndrome (Fig. 55-11). Affected areas are the rostral brainstem (penetrator branches from distal basilar artery), the thalamus (penetrators of the proximal PCAs), and the medial temporal and occipital lobes. Clinical presentation includes bilateral homonymous hemianopsias or cortical blindness, confusion, vivid-formed visual hallucinations (peduncular hallucinations), and inability to form new memories. In contrast, emboli moving past the basilar tip cause only unilateral PCA occlusions, with isolated homonymous hemianopsias.
Lacunar Small Vessel Disease
Clinical Vignette
Lacunar strokes affecting the internal capsule, thalamus, striatum, or brainstem (see Fig. 55-5) can often be clinically distinguished from embolic disease by the tendency toward a more insidious onset, with deficits progressing or stuttering on over 2–4 days. Additionally, lacunar deficits have a relatively typical distribution; they affect the entire side of the body with motor and/or sensory symptoms without cortically based findings or visual changes. This is in contrast to middle cerebral artery cortical branch occlusions that tend to have a brachiofacial distribution often associated with other cognitive and/or visual signs.
Patients experiencing lacunar strokes can present with TIA in up to 15–20% of instances. TIAs are stereotypical, and tend to cluster over 2–5 days, at times occurring frequently over a 24-hour period and in a crescendo fashion. Signs and symptoms vary according to the location of the ischemia (Table 55-3).
Table 55-3 Most Frequent Lacunar Syndromes and Their Locations
| Clinical Syndrome | Location |
|---|---|
| Pure motor stroke: weakness equally involving face, arm, and leg | Internal capsule (posterior limb) or basis pontis |
| Pure sensory stroke: numbness or paresthesia equally involving face, arm, leg and usually trunk | Lateral thalamus (posteroventral nucleus) |
| Ataxic hemiparesis: weakness and incoordination in the arm and/or leg | Basis pontis or internal capsule |
| Dysarthria—clumsy hand: facial weakness, severe dysarthria and dysphagia, slight weakness, and clumsiness of the hand | Basis pontis |
| Rare sensorimotor stroke: combination of pure motor/pure sensory symptoms and findings | Thalamus internal capsule |
Diagnostic Approach
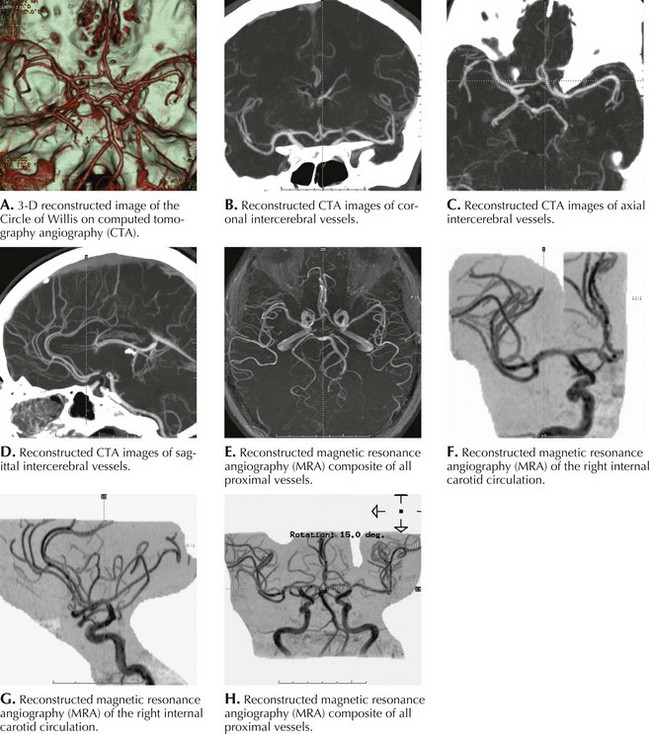
For every patient evaluated with ischemic stroke or TIA, the location of the lesion and mechanism should be investigated thoroughly to better predict potential complications and to most effectively direct treatment. CT and MRI brain scanning have greatly enhanced our ability to diagnose and follow neurologic disease as well as guide treatment. Noninvasive arterial imaging with CTA and MRA have largely replaced catheter angiography in the initial evaluation of cerebrovascular disease and show great promise in advancing acute stroke care (Fig. 55-12).
Anatomic Site
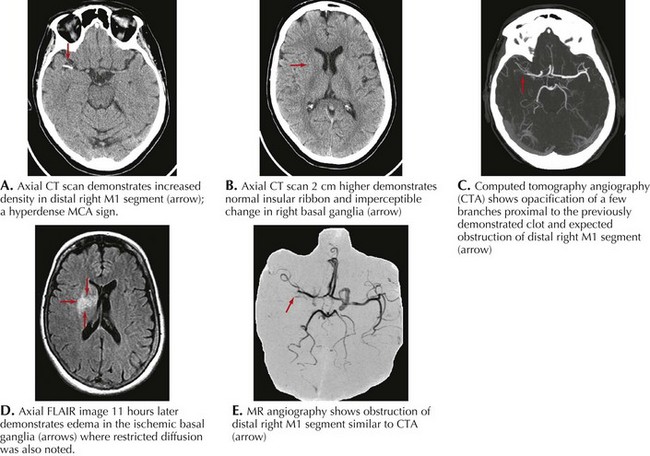
Brain CT examination, with its immediate availability in most hospitals and short scanning time, is usually the initial study performed in individuals presenting with an acute focal neurologic deficit. Its sensitivity to detect the presence of a primary cerebral hemorrhage or a hemorrhagic infarct is a crucial starting point in determining the future course of action such as the use of thrombolytics, the need for surgical intervention, and the degree of blood pressure control. The head CT is often normal in the first few hours of an ischemic stroke. However, in some cases, the presence of an acute arterial occlusion can be detected by the presence of a localized intraluminal hyperdense signal, often seen in patients with MCA occlusion (Fig. 55-13A), even when the brain parenchyma shows no evolving processes. Head CT may also show early infarct changes characterized by sulcal effacement or loss of gray–white matter differentiation (Fig. 55-13B). Such findings have important therapeutic implications. A CT angiogram can confirm the presence of a thrombus (Fig. 55-13C) and help guide further intervention concerning intravenous or intra-arterial tissue plasminogen activator (t-PA), anticoagulation, and management of blood pressure.
Diffusion-weighted MRI is the most sensitive and specific test for acute ischemia, and abnormalities have been demonstrated as early as 1 hour after symptom onset. Combined with concomitant perfusion scanning information, the ischemic penumbra (area of brain with compromised cerebral perfusion but without established infarction) can be defined, and decision concerning the risk and feasibility of reperfusion intervention can now be made more effectively and safely. Other MRI sequences, such as FLAIR and T2-weighted imaging, can show the area of stroke, often 6–12 hours after onset of symptoms (Fig. 55-13D and E).
Etiologic Mechanism
Complete assessment of cardiac function is essential and includes the electrical stability of the cardiac rhythm, myocardial contractility, valvular status, and whether a PFO is present. TEE provides more sensitivity and anatomic details and is preferred over the transthoracic approach for valvular lesions, intra-atrial abnormalities (PFO and ASD), and aortic arch disease. Ultrasound of the carotid arteries at their bifurcation in the neck and transcranial Doppler of the intracranial vessels can functionally assess cerebral flow and determine the presence of critically stenotic extracranial or circle of Willis arteries, respectively. Carotid ultrasound helps characterize the carotid plaque as “soft” consisting of cholesterol deposits and clot, which is more prone to ulceration and artery-to-artery embolization, or “hard” where the vessel wall has fibrosed and calcified over time, making it a less likely source of distal embolization. MRA or CTA of the head and neck is appropriate to assess patency of intracranial and extracranial arteries. The more recent addition of perfusion scanning helps define the effect of any stenotic lesion upon regional blood flow (Table 55-4).
| Imaging Method | Advantages | Disadvantages |
|---|---|---|
| MRI/MRA | DWI and PWI demonstrate the area of stroke and the area at risk (penumbra), respectively. | Prolonged test (30–60 min); patient must cooperate or sedation is required. Cannot be performed in patients with PCM. MRA can overestimate tight stenosis as an occluded vessel. |
| CTA/CTP | Images can be obtained rapidly (<5 min). | Patient must have normal renal function because CTA and CTP require high doses of contrast, 100 and 50 mL, respectively. |
| Ultrasonography of the neck | Easy to perform, can be done at the bedside | No detailed information about the vertebral arteries or the intracranial vessels. |
| TCD | Easy to perform, even at the bedside | Poor transtemporal windows limit the information about the intracranial vessels. |
* CTA, computed tomography angiography; CTP, computed tomography perfusion; DWI, diffusion-weighted imaging; PCM, pacemaker; PWI, perfusion-weighted imaging; TCD, transcranial Doppler
Renal failure or pacemaker devices limit the imaging studies that can be performed in patients with TIAs and strokes (Table 55-5). Gadolinium-based contrast agents have recently been linked to the development of nephrogenic systemic fibrosis and nephrogenic fibrosing dermopathy, often with serious and irreversible skin or organ pathology in patients with moderate to end-stage renal disease. The mechanism is unclear but thought to be due to stimulation of tissue fibrosis similar to that seen in scleroderma or eosinophilia-myalgia syndrome.
Table 55-5 Guidelines for Imaging in Ischemic Stroke/TIA Apropos Patient Renal Function and Pacemaker Presence*
| Normal Renal Function | Presence of PCM | Abnormal Renal Function |
|---|---|---|
| CT of the head, CT perfusion, and CTA of head and neck | CT of the head, CT perfusion, and CTA of head and neck | CT of the head, TCD and US |
| or | or | or |
| MRI of the head + DWI/PWI, MRA of the head and neck or |
CT of the head, TCD and US | MRI of the head MRA of head and neck without use of contrast |
| CT of the head, TCD and US |
* CTA, computed tomography angiography; DWI, diffusion-weighted imaging; PCM, pacemaker; PWI, perfusion-weighted imaging; TCD, transcranial Doppler; US, ultrasonography
Treatment
Treatment of the Acute Phase
A randomized double-blind trial of IV recombinant tissue plasminogen activator (rt-PA) in patients with ischemic stroke treated within the first 3 hours of symptom onset showed a 12% absolute (32% relative) increase in the number of patients with minimal or no disability at 3 months in the rt-PA group. The benefit was present for all the different stroke subtypes analyzed. Similar benefits of early treatment with rt-PA within a 3-hour window were also shown in a subpopulation analysis of patients in the ATLANTIS (Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke) trial. This treatment, since approved by the Food and Drug Administration (FDA), has become the standard of care for acute ischemic stroke, and all patients arriving to the hospital within the first 3 hours of symptom onset should be considered for IV rt-PA administration after appropriately screening for thrombolytic contraindications (Box 55-1).
Surgical Treatment
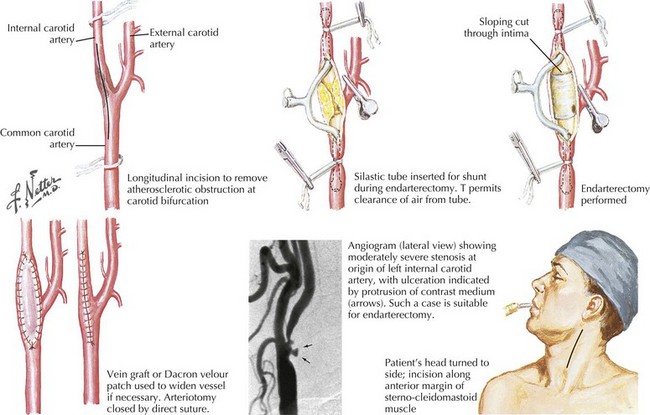
Carotid endarterectomy is one of the more common vascular procedures, with rates of perioperative mortality or stroke below 1% now achieved in many centers or practices (Fig. 55-14). A complication rate of less than 3–5% is thought to ensure overall patient benefit and most go home 1 or 2 days following surgery. Postoperative cranial neuropathies, cardiac complications, hyperperfusion syndrome with intracranial hemorrhages and rarely seizures can also occur but are rare.
Future Directions
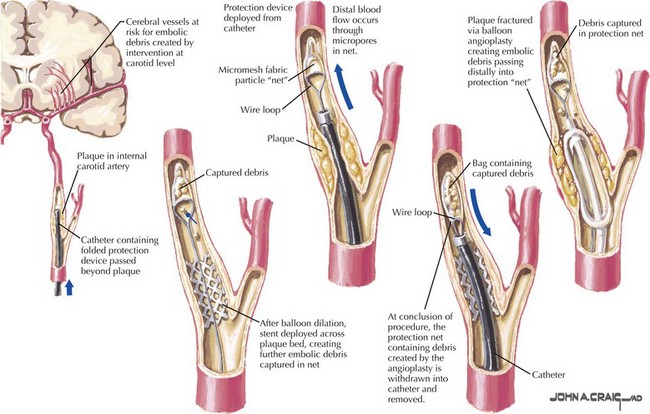
Endovascular techniques, such as angioplasty and stents (Fig. 55-15), will likely change the management approach for some patients with extracranial and intracranial disease. The recent Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) shows similar outcomes with carotid artery stenting (CAS) and CEA for the treatment of symptomatic and asymptomatic carotid stenosis (vascular event or death: 7.2% vs 6.8% at 2.5 years). However, 30-day stroke rates were significantly higher for stenting (4.1% vs 2.3%), whereas MI rates were higher for CEA (2.3% vs 1.1%). The choice of procedure therefore depends on a careful consideration of co-morbidities, individual risks, institutional experience, and patient preference.