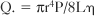Chapter 7 Ischemic Heart Disease
ISCHEMIC HEART DISEASE
Chest Radiograph
Heart Size
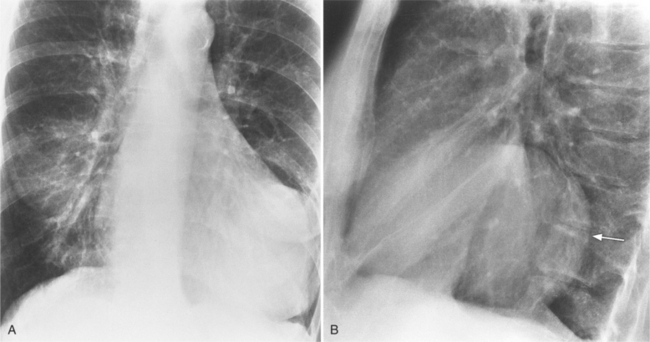
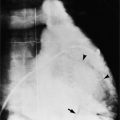
Initially, most patients undergoing myocardial infarction have a normal heart size. The assessment of an enlarged heart on a supine portable chest film is rather inaccurate and usually not necessary for clinical management. Severe cardiomegaly generally indicates long-standing coronary artery disease with previous infarction or with a major complication (Fig. 7-1).
Aneurysms and Ruptures
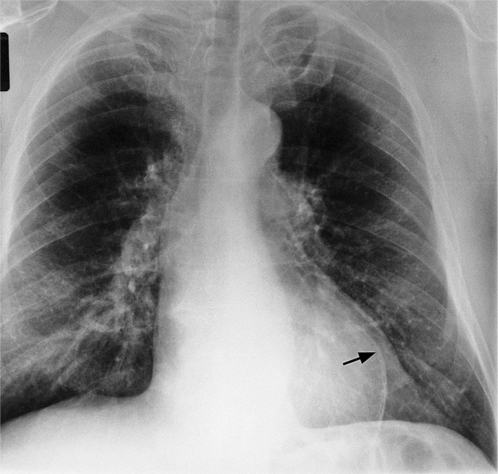
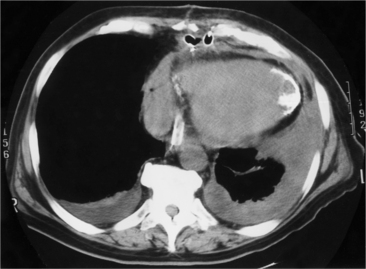
In addition to chronic left ventricular failure, left ventricular enlargement may also be the result of a true or false aneurysm, chronic mitral regurgitation, or rarely cardiac rupture. Heart enlargement is not a feature of acute mitral regurgitation or rupture of the interventricular septum because the left ventricle needs several hours to several days to dilate enough to be visible on the chest film. The most frequent site of a true left ventricular aneurysm is in the anterolateral and apical wall. Although left ventricular aneurysms may involve any wall segment, aneurysms in the posterolateral wall are frequently false aneurysms. A false left ventricular aneurysm exists when the left ventricle ruptures into a site of previous pericardial adhesions so that the rupture is contained by the pericardium. An increase in size of the left ventricular aneurysm on serial studies is suggestive of a false aneurysm and warrants urgent, definitive evaluation. Calcification of the anterolateral and apical region of the left ventricle usually takes several years after the myocardial infarction that produced the scarring (Fig. 7-2).
Coronary Angiography
Uses and Analysis
Patients with Angina Class II to IV or Unstable Angina
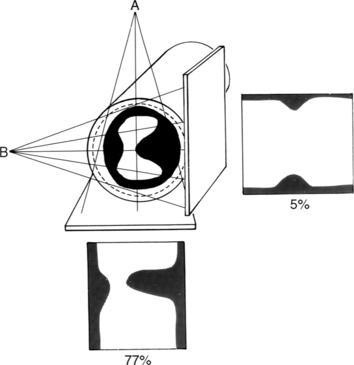
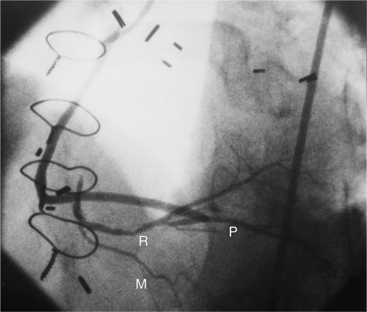
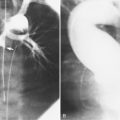
Many patients with moderate or severe stable angina or unstable angina do not respond adequately to medical therapy. PCI or CABG surgery may even improve their left ventricular systolic function. Coronary angiography is used mainly to identify atherosclerotic coronary stenosis, and less often, congenital anomalies and manifestations of other diseases. Each coronary artery is opacified so that its origin, course, and termination are visualized at least in two orthogonal projections. The orthogonal projections are necessary because atherosclerotic stenoses tend to be eccentric; a minor plaque in one projection may appear as a major stenosis in the opposite oblique view (Fig. 7-3).
Progression of Atherosclerosis
Coronary atherosclerosis begins as lipid deposition in the arterial wall, which appears grossly as a raised, fatty streak. As the lesion progresses, a fibrous cap develops over the endothelial lipid deposit. Disruption of an atherosclerotic plaque results in fissuring and intraluminal thrombosis (Fig. 7-4). The thrombus may lead to intermittent vessel occlusion and unstable angina. Large ulcers at the site of the plaque can cause formation of a fixed thrombus and a chronic occlusion resulting in acute myocardial infarction. Severe stenoses tend to progress to total occlusion about three times more frequently than less severe lesions.
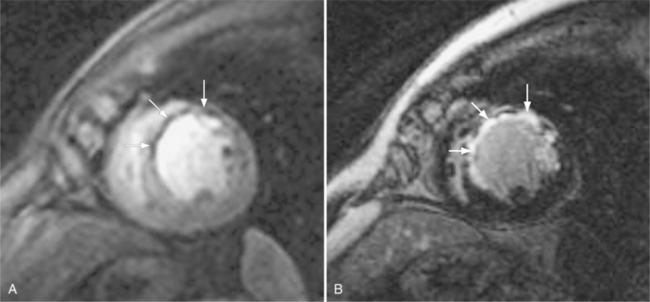
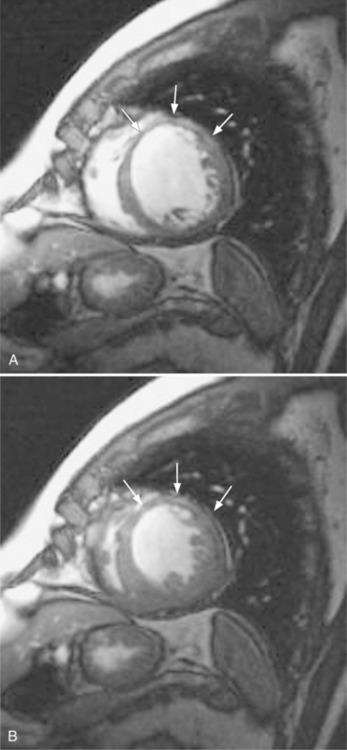
These two conditions of reversible left ventricular dysfunction are important to recognize because vigorous treatment of the thrombus or spasm in myocardial stunning and relief of the obstruction in myocardial hibernation may reverse the impaired ventricular performance and potentially salvage the jeopardized myocardium. Stress echocardiography is the modality of choice to assess left ventricular wall motion abnormalities. Stress cardiac magnetic resonance imaging (CMRI) has demonstrated better sensitivity and specificity, but is less available. Furthermore, CMRI is capable of providing useful information about left ventricular viability (Fig. 7-5).
Extent and Location of Stenoses
Common Lesion Sites
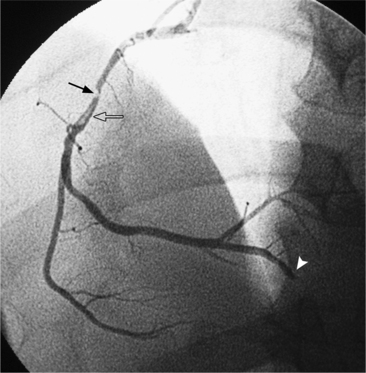
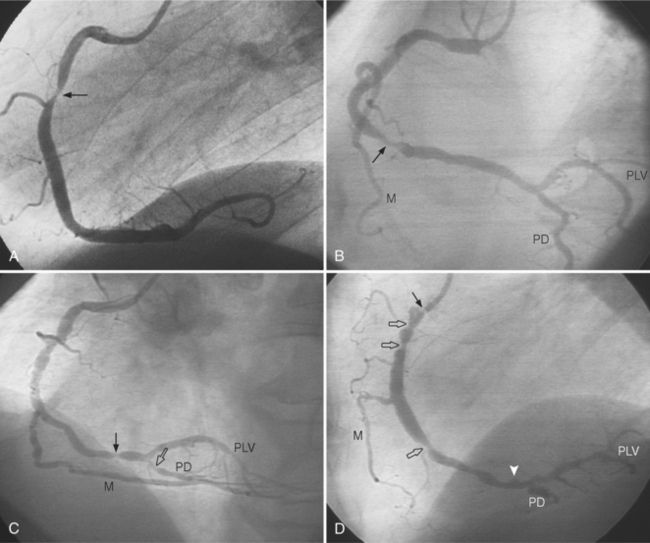
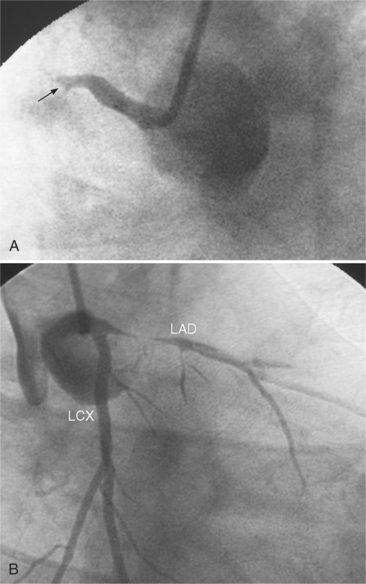
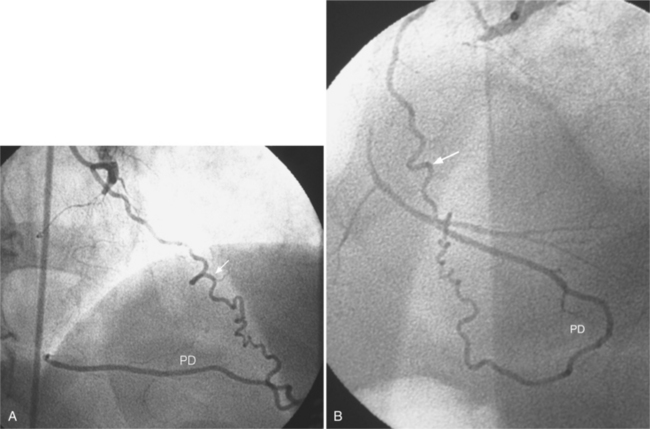
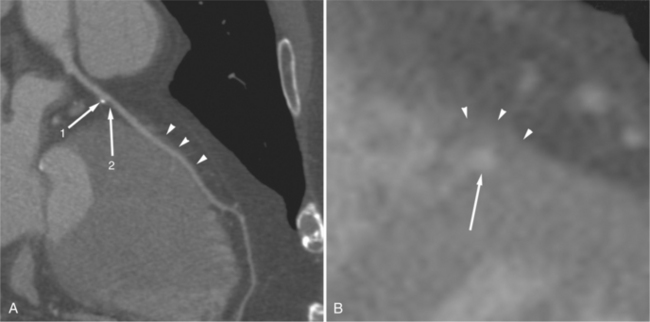
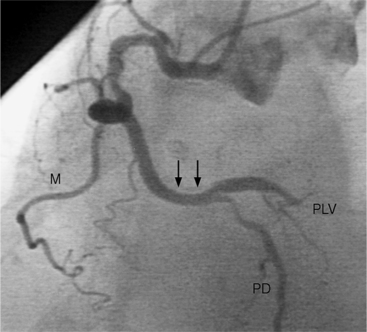
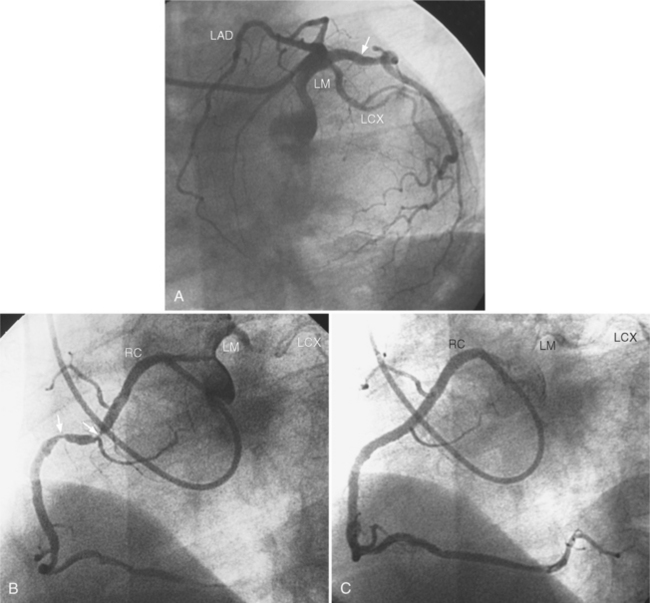
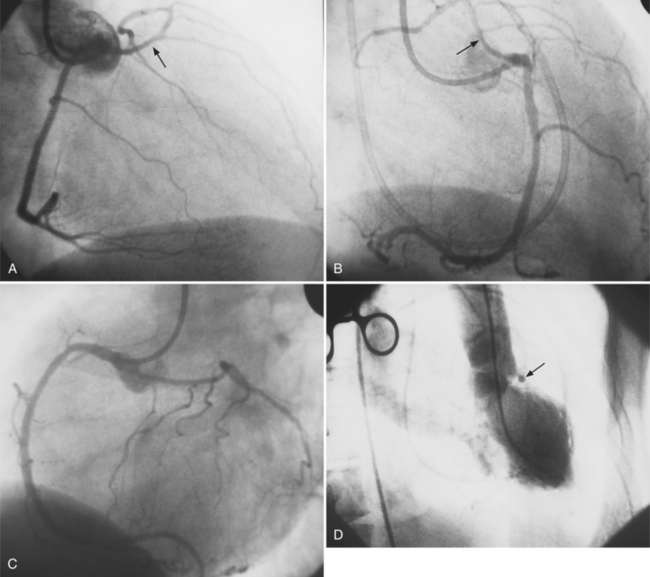
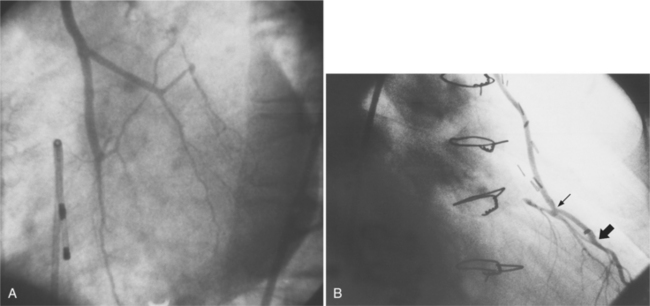
In the right coronary artery, most severe stenoses develop in its proximal half, although there are occasional severe plaques at the bifurcation of the posterior descending and posterior left ventricular arteries (Fig. 7-6). The right ventricular marginal branch frequently has a severe stenosis at its origin.
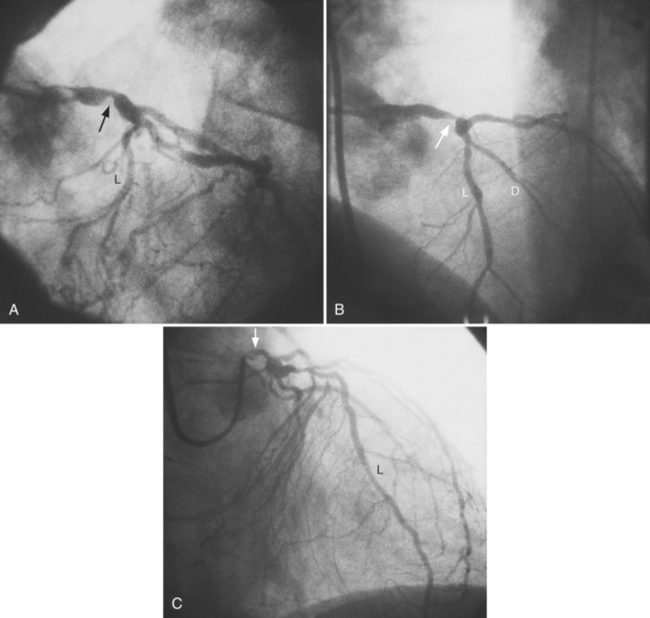
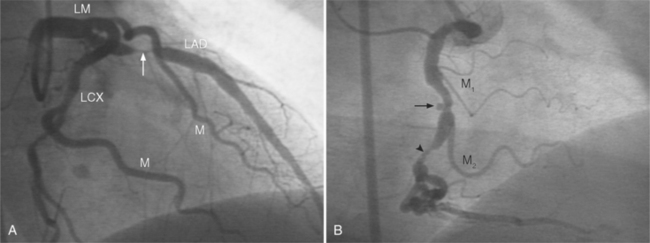
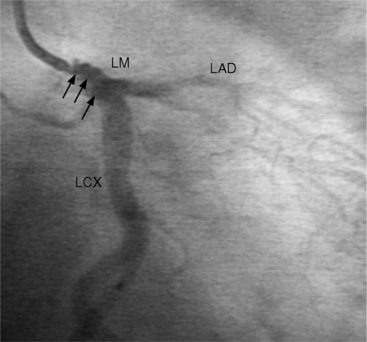
The left main coronary artery should not taper. It is usually narrowed either at its ostium or at the bifurcation of the left anterior descending and circumflex arteries. Occasionally, the entire main arterial segment may be uniformly narrowed but usually one of its ends is more severely involved. Detection of plaques in this segment is particularly important because severe lesions are associated with an increased mortality during cardiac catheterization (Fig. 7-7).
Left Main Equivalent Disease
An example of a left main equivalent lesion would be a severe left anterior descending artery stenosis when an occluded right coronary artery is supplied by collaterals from the left anterior descending artery. Here one lesion controls the blood supply to the bulk of the heart. A similar example would be a stenosis in a long left anterior descending artery that extends completely around the apex in place of the usual posterior descending artery. Here also a significant percentage of myocardium is affected by a single stenosis.
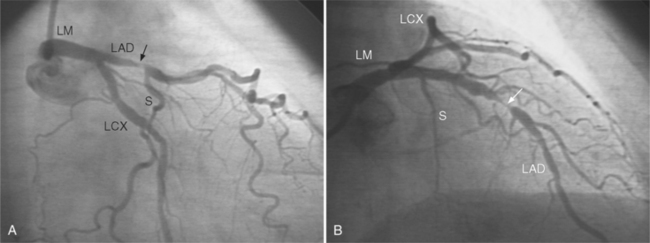
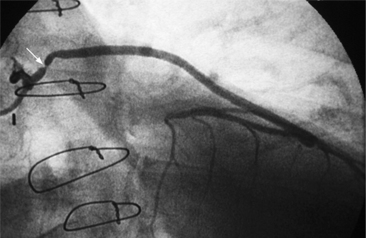
In the left anterior descending artery, stenoses before or after the first large septal branch may have different clinical implications. Patients with chronic stable angina who have a severe stenosis before the first septal branch have a statistically higher mortality when compared with patients who have a stenosis distal to this branch (Fig. 7-8). The first septal branch can supply nearly half of the interventricular septum and a contractile portion of the ventricle and is closely related to the conduction system. A stenosis before the first septal branch also frequently involves a large diagonal branch that supplies a portion of the lateral wall. This correlation does not hold in unstable angina pectoris, where there is no association between severe plaques before and after the first septal branch.
Morphology of Coronary Atherosclerosis
Angiographic Appearance
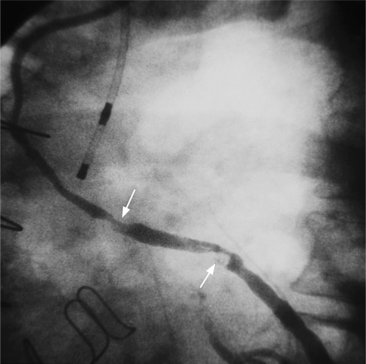
Atherosclerotic plaques tend to have eccentric and sharp edges that help distinguish them from spasm, which is usually smooth and fusiform. Thrombus within the artery may be distinguished from plaques if contrast medium flows on both sides of the lucency (Fig. 7-9). Occlusive thrombi may be impossible to distinguish from a fibrosed artery, but sharp, slanting edges are more typical of thrombus.
Grading System
Plaque morphology can be analyzed by location, shape, and severity (Box 7-1). Each lesion is resolved into length, calcification, involvement of major branches, presence of adjacent thrombus, tortuosity of the involved segment, and eccentricity of the plaque edges.
Relationship to Coronary Syndrome
Both clinical and angiographic studies have confirmed that angiographic morphology is correlated with unstable coronary syndromes. Simple plaques with a smooth fibrous covering, smooth borders, and an hourglass configuration are associated with stable angina. Complex lesions with plaque rupture, intraplaque hemorrhage, and irregular borders in eccentric stenoses are associated with unstable angina and myocardial infarction (Fig. 7-10).
Myocardial Infarctions
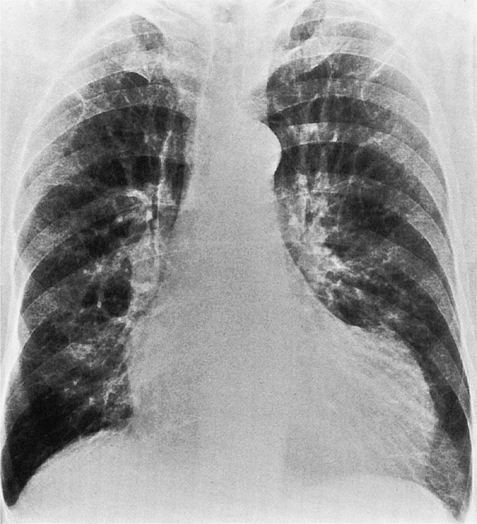
Multiple myocardial infarctions result from extensive coronary disease and occur with greater frequency in persons with diabetes. You are more likely to find a decreased ejection fraction in patients with diabetes mellitus but patients with diabetes and those without do not differ significantly in number and extent of severe stenosis. Type II hyperlipoproteinemia is associated with extensive coronary calcifications and severe involvement of the distal distribution of the coronary arteries (Fig. 7-11). These patients may have an unusual edge in the left sinus of Valsalva at the ostium of the left coronary artery, which can make catheterization difficult. Patients with type IV hyperlipoproteinemia have a distribution of stenoses similar to that in patients with normal lipid findings.
Other Causes of Stenosis
There are many causes of coronary stenosis other than atherosclerosis. Frequently, the cause can only be determined by clinical correlation with a systemic disease (Box 7-2). Even then, in the adult age range, it is often impossible to exclude coexisting atherosclerosis. The clinical constellation of chest pain, a positive exercise test, and a normal coronary arteriogram is referred to as syndrome X. The cause of the syndrome is unknown, is not related to large vessel spasm, and may be related to abnormalities in precapillary vessels that are too small to be seen with coronary angiography. In contrast, myocardial infarction can occur with a normal coronary arteriogram. This event is rare and has been caused by thrombosis with recanalization, coronary spasm, cocaine abuse, viral myocarditis, chest trauma, and carbon monoxide intoxication.
Interpretation of Arterial Stenoses on Angiography
Determining Severity
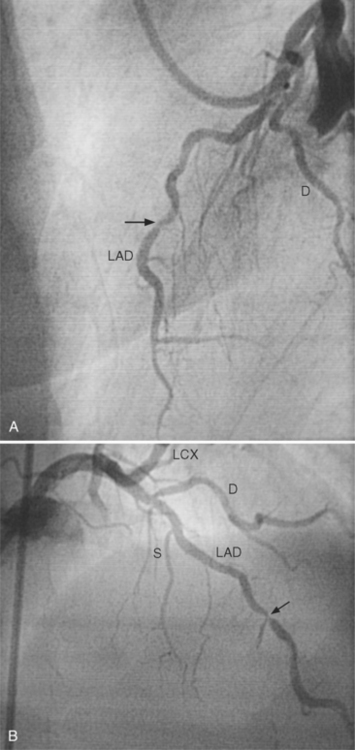
Because atherosclerotic plaques tend to be eccentric, coronary angiography must be performed in two orthogonal projections so the maximal arterial narrowing can be identified (Fig. 7-12). This system has many limitations. The normal-appearing artery may itself be diffusely diseased. A similar percentage of stenosis in a smaller distal artery is ascribed the same physiologic consequence, even though flow through a larger proximal arterial segment must be quite different. In a large artery with a greater cross-sectional area, the amount of myocardium supplied by its coronary flow is proportionate to the smaller area supplied by a distal coronary artery. A similar degree of narrowing of a small distal coronary artery produces the same profusion deficit as does the same percentage of stenosis in a large or proximal artery. The length of a coronary stenosis is important but is difficult to subjectively evaluate as to severity of a lesion reducing distal flow. Given these limitations, a 50% or greater stenosis in a patient with ischemic heart disease is defined as a significant stenosis.
Determinants of Coronary Blood Flow
Normal Flow
where Q. is flow per unit time, r is radius, P is pressure, L is length, and μ is viscosity. This equation is strictly valued for nonpulsatile, streamline flow and a uniform viscosity. With some allowance for the transfer of this mathematical principle to a biologic system, the equation helps explain some of the determinants of coronary flow. Under normal conditions, all the variables in the equation are constant except for the radius of the vessel. However, a number of factors act on the major site of vascular resistance: the precapillary arteriole.
Flow Reserve
Coronary flow reserve is the maximal flow divided by the resting flow. The “50% significant stenosis” is then a rough approximation to this physiologic model. The maximal flow is that which occurs when the coronary vascular bed has undergone maximal vasodilatation. Fig. 7-13 shows the relation between coronary blood flow and a focal stenosis in an artery at rest and after maximal vasodilatation.
Fractional Flow Reserve
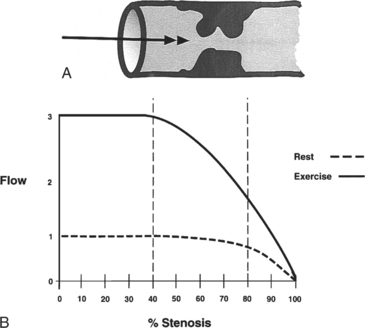
Fractional flow reserve (FFR) is an invasive index of the functional severity of a stenosis determined from coronary pressure measurement during coronary catheterization. A 0.014-in pressure wire is advanced, after calibration, into the relevant coronary segment. It is positioned distally to the stenosis. Adenosine is administered intravenously to induce maximal hyperemia. FFR is calculated as the ratio of mean hyperemic distal coronary pressure measured by the pressure wire to mean aortic pressure measured through the guiding catheter. In patients with coronary stenosis of moderate severity, FFR appears to be a useful index of the functional severity of the stenosis and the need for coronary revascularization. An FFR value of less than 0.75 demonstrates a reversible myocardial ischemia (Fig. 7-14) with a sensitivity of about 90% and a specificity of 100%.
Effects of Stenosis Length and Diameter
The length of the coronary stenosis also determines blood flow, although its effect is complex. When the diameter is constricted by less than 50%, stenoses of up to 15 mm in length have little effect on flow during reactive hyperemia. As the diameter of a stenosis is increased to 70%, a stenosis 10 mm long results in a reduction in distal flow. Long stenoses in the presence of borderline reduction in diameter (40% to 70%) may greatly alter coronary hemodynamics during stress. Although Poiseuille’s equation suggests that the length of a stenosis would decrease flow linearly, this cannot be precisely observed because there are neural and biochemical factors and collateral flow that also regulate blood flow. Moreover, the distal coronary vascular bed may vasodilate in a nonuniform manner from endocardium to epicardium. Because a proximal stenosis reduces coronary pressure, the endocardium becomes anoxic before the epicardium does, and there is increased lactate production and a fall in highenergy phosphate mediators. The end result is that vascular resistance distal to a stenosis in an ischemic myocardium varies in a complex way.
Angiographic Appearance
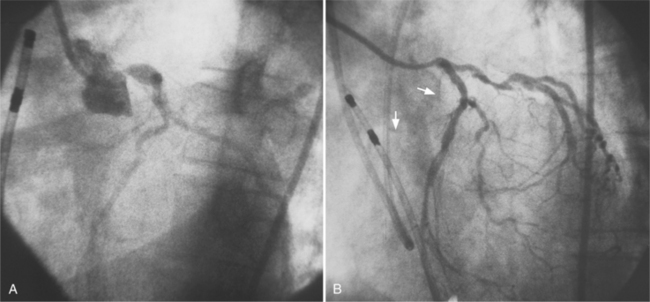
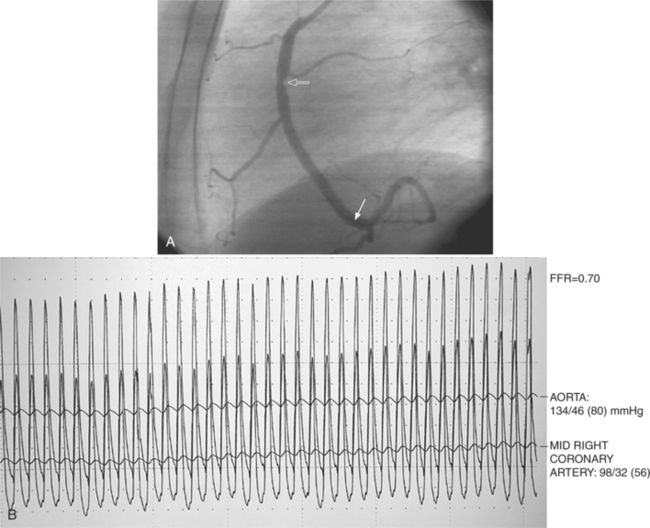
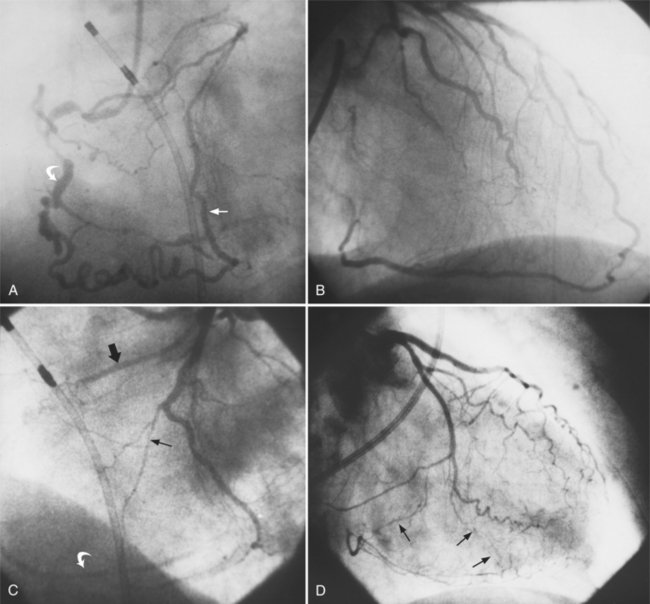
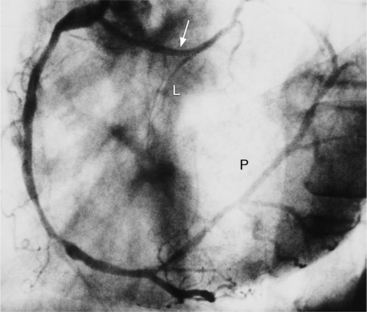
The angiographic appearance of an epicardial collateral is usually a serpentine, corkscrew artery that goes from the end of an adjacent artery to the end of the occluded artery. Collaterals through the interventricular septum appear differently: They are straight and connect the septal branches from the anterior descending to the posterior descending artery (Fig. 7-15).
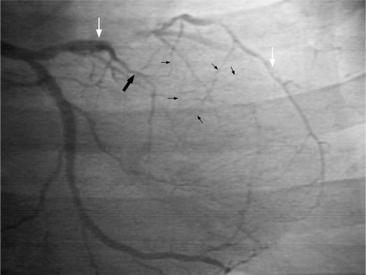
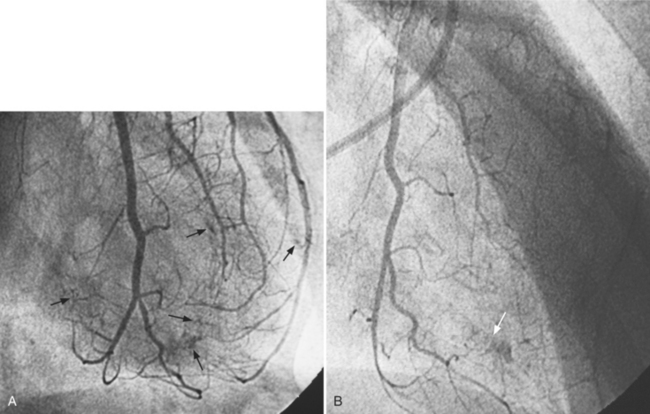
There are many pathways in the heart for collateral vessels. Intracoronary collaterals bridge an occluded artery from its proximal to its distal end (Fig. 7-16). This kind may be difficult to distinguish from a recanalized thrombus. Intercoronary collaterals develop between the terminal branches of different arteries (Fig. 7-17). Frequent collateral pathways are from the conus artery of the right coronary artery to the left anterior descending artery (Vieussens’ ring). Kugel’s artery is a collateral that connects the sinoatrial nodal artery to the atrioventricular nodal artery through the interatrial septum. Because the sinoatrial nodal artery can come from either the right coronary or left circumflex artery, and the atrioventricular nodal artery can originate from either a right or left dominant posterior left ventricular artery, there are four pathways that collaterals can take through the atria that can be called Kugel’s artery (Fig. 7-18).
Coronary Artery Spasm
Dynamic forms of coronary obstruction have been recognized for centuries as causing angina and myocardial infarction. In his original description of angina pectoris Heberden wrote in 1768 that chest pain occasionally occurs without exertion. In 1910, Osler speculated that spasm of a coronary artery could cause anginal pain. In 1959, Prinzmetal described an unusual syndrome of cardiac pain that occurs almost exclusively at rest, is not precipitated by exertion, and is associated with electrocardiographic ST-segment elevations. Myocardial perfusion scanning and coronary arteriography have shown that spasm may reduce or stop segmental myocardial blood flow.
Angiographic Appearance
Coronary spasm is one of a number of causes of myocardial infarction with angiographically normal coronary arteries. The angiographic definition of spasm is a focal stenosis that changes during sequential arterial injections (Fig. 7-19). Occasionally an entire artery will have severe vasoconstriction, but spasm typically is a focal, changeable stenosis. Coronary artery spasm in Prinzmetal variant angina has been seen in the left main, left anterior descending, and circumflex arteries, but spasm in the right coronary artery is more frequent.
Catheter-Induced versus Prinzmetal Syndrome
The distinction between catheter-induced spasm and that in Prinzmetal syndrome is difficult, but one caused by a catheter occurs proximally, adjacent to the catheter tip (Fig. 7-20). Catheter-induced spasm is usually seen at the origin of the right coronary artery as a short area with smooth fusiform narrowing. Focal spasm frequently occurs at the site of an atherosclerotic plaque, which may make it difficult to distinguish from fixed disease. Spasm may be slight or it may completely occlude the artery. Because the coronary endothelium has potent factors that can cause spasm and thrombus after intimal injury, spasm is frequently seen after percutaneous coronary angioplasty. In this situation, the artery has a sawtooth appearance, which is reversed with nitroglycerin.
Coronary Aneurysms
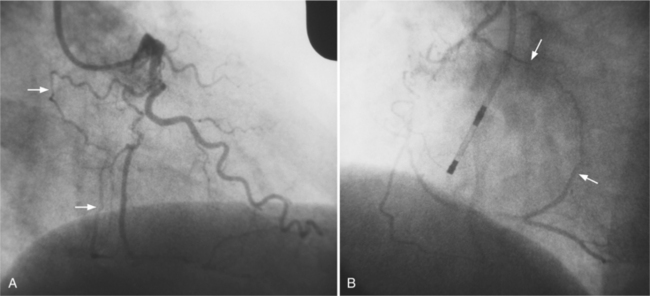
Coronary artery aneurysms are occasionally discovered during angiographic evaluation of ischemic heart disease and are usually a variant of coronary atherosclerosis (Fig. 7-21). This lesion will frequently be found in the same artery with stenoses and occlusions. Atherosclerosis can produce diffuse ectasia from degeneration of the media and associated elastic elements (Fig. 7-22). To distinguish an ectatic artery or poststenotic dilatation from an aneurysm, a useful definition of coronary aneurysm is a dilatation of 50% greater than the preceding normal artery. Most atherosclerotic coronary aneurysms have an adjacent severe stenosis. The saccular aneurysm may overlap and hide the stenosis during angiography so that at least one angiographic projection should profile the neck of the aneurysm.
Causes
Kawasaki disease is a childhood acute multisystem vasculitis that includes cervical lymphadenopathy, fever, and a rash in the mouth and on the hands and feet. Also called mucocutaneous lymph node syndrome, Kawasaki disease involves a myocarditis and pericarditis that may cause congestive heart failure. Aneurysms of the coronary arteries are frequent (Fig. 7-23) and may cause coronary thrombosis, myocardial infarction, and sudden death. About half of the children with coronary aneurysms have angiographically normal-appearing arteries 2 years later. Some aneurysms may be quite large, and rupture is a fatal complication. See Table 7-1 for prognosis of aneurysms in Kawasaki disease.
| Size | Prognosis |
|---|---|
| <4 mm | Regress to normal size |
| 4-8 mm | Tend to become smaller |
| >8 mm | Progress to obstruction or stenosis |
Myocardial Bridging
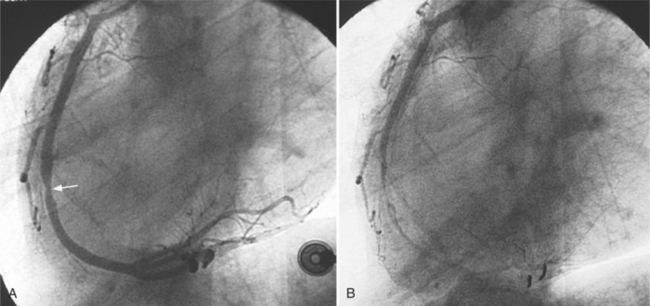
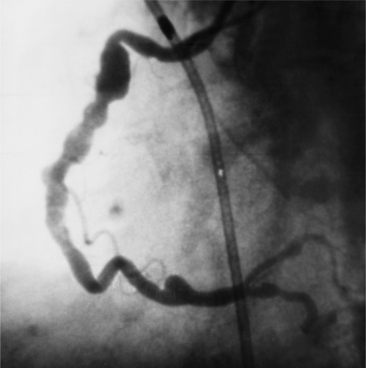
An intramyocardial bridge is a coronary artery that dips into the myocardium and is completely surrounded by cardiac muscle (Fig. 7-24). This segment of the coronary artery narrows dynamically during systole and returns to its baseline size during diastole. Bridges are seen in about 0.5% to 7.5% of angiographic studies, usually in the left anterior descending artery, although diagonal and marginal arteries may be affected. The bridges may be as deep as 10 mm and usually are 10 to 30 mm long. The bridge segment rarely has atherosclerosis, but plaques are seen both proximately and distally. Because narrowing takes place during systole and most coronary flow occurs in diastole, mural coronary arteries were initially considered a minor anomaly. However, chest pain and myocardial infarction may occur when the bridge narrows more than 75% in systole. Because the bridge is usually eccentric, views in at least two orthogonal projections should be obtained.
Coronary Dissection
The angiographic appearance of dissection is a linear or spiral lucency within the arterial lumen, which represents the intimal flap (Fig. 7-25). The dissection may occlude the distal artery or, as in the aorta, have a false channel with stasis of the contrast medium. Spontaneous dissection occurs in the coronary arteries as it does in most medium-sized arteries (Fig. 7-26). It is seen in pregnancy, Marfan syndrome, and chest trauma. Coronary dissection may occur secondary to an aortic dissection with a retrograde tear. The angiographic catheter may lift a plaque and initiate the downstream tear. After transluminal angioplasty, a short dissection cleft is frequently seen but rarely extends beyond the length of the balloon.
CONGENITAL ANOMALIES OF THE CORONARY ARTERIES
The morphologic classification is used in this discussion.
Anomalies of Origin
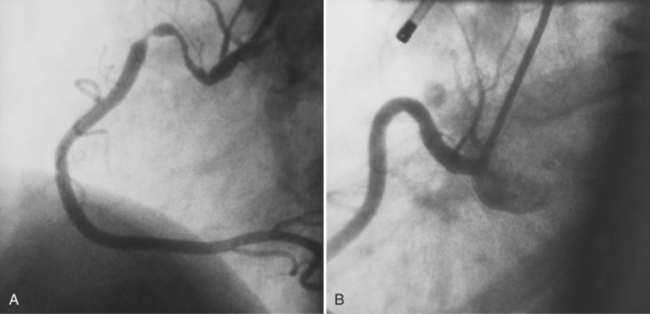
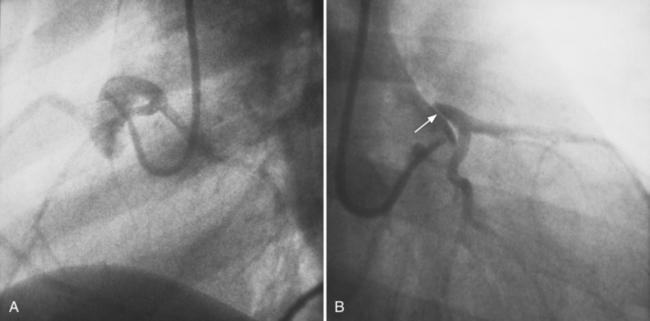
Although the usual origin of the left and right coronary arteries is the left and right sinuses of Valsalva, respectively, ectopic coronary arteries can arise above the sinuses in the ascending aorta, within the posterior (noncoronary) sinus, low within the sinus adjacent to the leaflet, within the commissure, or in a subvalvular location. The conus artery frequently originates as a separate ostium in the right sinus of Valsalva. There may be no left main artery if the left anterior descending and circumflex arteries arise separately from the left sinus of Valsalva. A common anomaly is the left circumflex artery arising from the right sinus of Valsalva and passing behind the aorta into the left atrioventricular groove (Fig. 7-27). When the left coronary artery originates from the right sinus of Valsalva, it may:
Similarly, when the right coronary artery originates from the left sinus of Valsalva by passing between the aorta and the right ventricular infundibulum, it forms an acute angle at its ostium and may be compressed by the two great vessels. If the anomalous right coronary, originating from the left sinus of Valsalva, courses anteriorly to the aorta, it should not produce any symptom, unless it is diseased (Fig. 7-29). Rarely a left coronary artery arises from the right sinus of Valsalva, assumes an intramyocardial course by traversing through the crista supraventricularis, then continues as the left anterior descending and circumflex arteries in their usual locations.
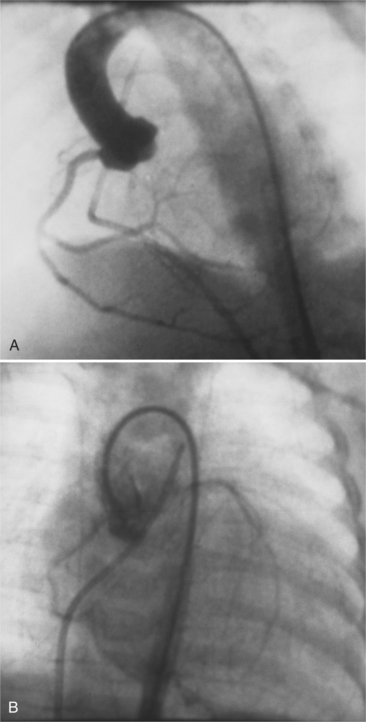
The most common of these is the left coronary origin from the pulmonary artery. This entity, the Bland-White-Garland syndrome, is seen in infants who have a myocardial infarction in the first few months of life. Coronary arteriography shows an empty left sinus of Valsalva. The right coronary artery supplies collaterals to the left coronary artery, which fills in a retrograde direction to opacify the pulmonary arteries (Fig. 7-30). The infantile left ventricle may have a segmental wall motion abnormality like that seen in coronary disease in the adult. Left ventricular aneurysms and mitral regurgitation can produce congestive heart failure. Left ventricular aneurysms may completely regress after corrective coronary surgery.
Anomalies of Origin and Course
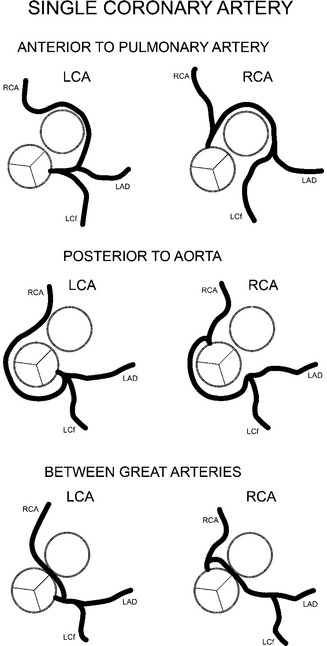
Major coronary anomalies of location are those that go behind the aorta, between the aorta and the pulmonary artery, or anterior to the right ventricular infundibulum (Fig. 7-31). The anomaly that can cause angina and sudden death is the aberrant coronary artery that goes between the aorta and the pulmonary artery. These two great arteries expand during systole and may compress the aberrant coronary artery and constrict its flow. This type of anomaly usually has a stenosis at its origin because of an acute angle with the sinus of Valsalva. A prime example of this group is the isolated single coronary artery, which can originate from either the left or right sinus of Valsalva (Fig. 7-32).
The most common anomaly is origin of the left circumflex artery from the right coronary artery with a course that goes behind the aorta before supplying the usual circumflex territory. You can usually see this retroaortic course on a right anterior oblique left ventriculogram, and it will alert you to the anomaly. Other common variations include the left anterior descending artery coming from the conus artery and the posterior descending artery continuing around the apex as a long left anterior descending artery (Fig. 7-33).
Anomalies of Termination
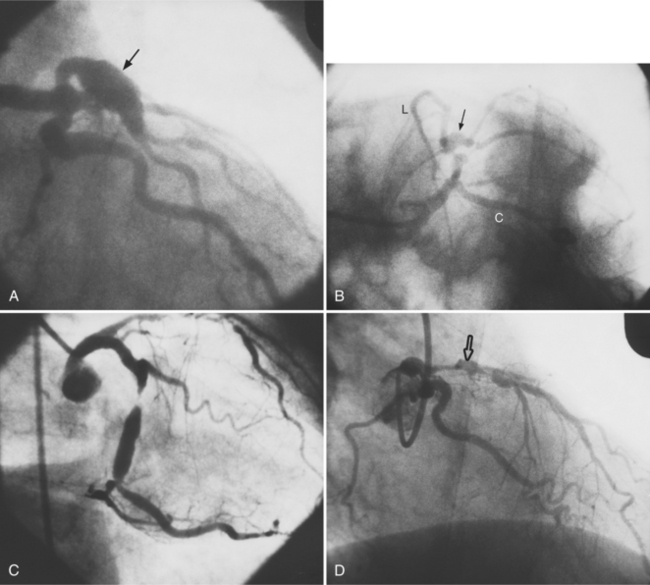
Coronary artery fistula is a rare congenital anomaly but is significant in the differential diagnosis of a continuous murmur. A coronary fistula may occur to any adjacent vascular bed. Fistulas may connect to all four cardiac chambers, the coronary sinus, the right or left superior vena cava, the pulmonary artery, and the systemic arteries (Fig. 7-34). In aortic atresia and pulmonary atresia, there are frequently large coronary artery sinuses and fistulas to the ventricles.
Complications
These fistulas are best studied with selective coronary arteriography. Congenital coronary arteriovenous fistulas have a large tortuous artery and vein down to the point of termination (Fig. 7-35). In contrast, acquired fistulas from either trauma or endocarditis have a normal or slightly dilated artery proximal to the fistula. The arterial bed distal to the fistula may be hypoperfused (a vascular steal), and if long-standing, may have collaterals supplying this lesion. Acute traumatic fistulas initially have normal-sized arteries and veins, but if they are uncorrected both these vessels will dilate. If a fistula opens into the right heart, a left-to-right shunt is present.
CORONARY BYPASS GRAFTING
Internal Mammary Artery Grafts
The most common procedure is to attach the left internal mammary artery to the proximal left anterior descending artery (Fig. 7-36). Either the right or left internal mammary artery may be used for grafting, but usually not both together, to prevent sternal osteonecrosis. Occasionally these arteries are sequentially grafted to the left anterior descending and to the diagonal artery. The patency rate of the internal mammary grafts is higher than that of saphenous vein grafts, even though the arterial graft has a slower flow than the venous graft. Unlike the vein graft, the internal mammary arteries do not form intimal hyperplasia, which occurs in all vein grafts during the first year after surgery.
Saphenous Vein Grafts
Saphenous vein grafts have a proximal anastomosis to the ascending aorta several centimeters above the aortic valve. Vein grafts may be constructed so that one or more side-to-side anastomoses are connected with diagonal and marginal arteries that lie in proximity. A side limb can be placed to a saphenous vein graft so that several coronary arteries at a distance from one another may be supplied by a single proximal graft (Fig. 7-37). This type of graft resembles an inverted Y.
Complications
Complications in venous grafts are usually at the proximal aortic anastomosis or the distal coronary anastomosis. Graft complications in coronary surgery are the same as complications to grafts elsewhere. Occlusion and focal or diffuse stenosis are common (Fig. 7-38), whereas pseudoaneurysm at either anastomosis is unusual. Over time, saphenous grafts develop a diffuse stenosis from intimal hyperplasia (Fig. 7-39). Degenerated venous grafts can look like severe atherosclerosis with ragged ulcerated plaques. Unlike coronary artery stenoses, which can be graded by a percentage reduction in diameter, this method is not accurate when applied to a stenosis in a vein graft because vein grafts are larger than the supplied coronary artery. In a graft four times the size of the native artery a stenosis must have an 80% diameter before it reduces distal flow, but in a graft of the same size as the coronary artery, distal flow would be reduced by about 50% at rest. Because of these difficulties, a thallium exercise test is required to assess the severity of a graft stenosis.
Imaging Techniques
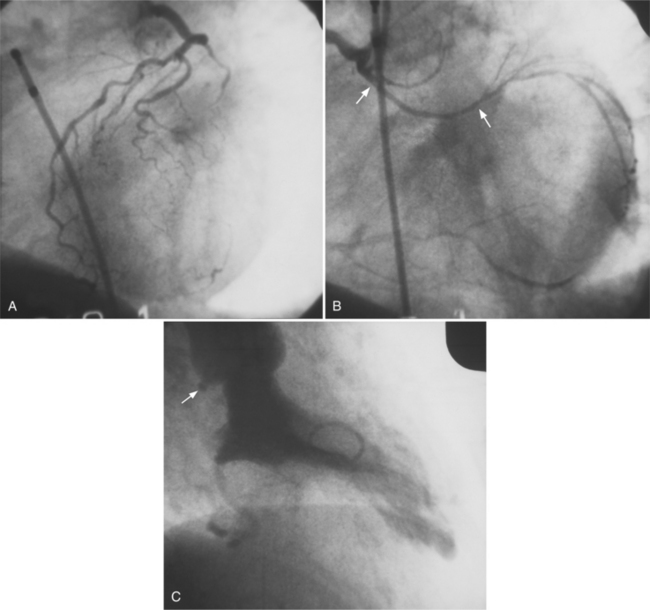
Bypass grafts can be visualized by angiography, multidetector computed tomography (MDCT), and magnetic resonance imaging (MRI). Angiography of bypass grafts is performed by selective injection in the internal mammary artery or in the aortic anastomosis of the vein graft. The flow into the native coronary artery from a graft should occur in both antegrade and retrograde directions (Fig. 7-40). If there is no flow into the proximal coronary artery from the graft, that artery is usually occluded. Injection into a coronary artery that has a distal graft may show an apparent occlusion in the “watershed” region between the proximal coronary artery and graft insertion. When the coronary artery is stenotic and not occluded before the graft, the graft will fill in a retrograde direction. These complex flow patterns in the graft and the adjacent coronary arteries depend on how much pressure is applied by the angiographer when injecting the graft or artery, and the hemodynamic variables in the arterial system. Collaterals to a grafted artery are not seen when the graft is functioning. Collateral vessels that were present before surgery, unless quite large, no longer are visualized, presumably because the collaterals have involuted or because the graft reduces the pressure gradient between the ends of the collateral channels, thereby slowing flow to the collateral.
LEFT VENTRICULAR ABNORMALITIES IN CORONARY DISEASE
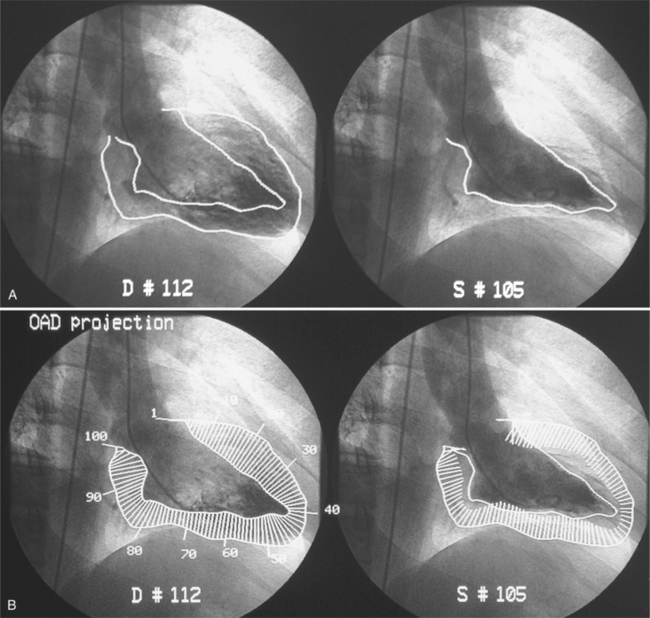
The evaluation of segmental and global left ventricular function is a standard part of the imaging evaluation for ischemic heart disease. Ventricular wall motion can be analyzed on tomographic methods such as echocardiography or cine magnetic resonance (Fig. 7-41). Because the angiographic left ventriculogram is a projectional and not a tomographic image, only those wall segments that are tangential to the x-ray beam can be evaluated (Fig. 7-42). Biplanar, rather than single-plane, left ventriculograms generally give a more accurate evaluation of segmental wall motion and a better estimate of the ejection fraction.
Ventriculogram Analysis
The ventriculogram is a classic example of “information overload.” It images many structures that are usually moving in different directions at different velocities. Even among experienced observers, one person’s dyskinetic wall motion may be another’s normal motion. Therefore, it is useful to develop a schema for interpreting the ventriculogram by isolating the components and separately judging their shape and motion. The basis for analyzing the left ventriculogram follows.
Projection
A few principles of geometry and perception are important to consider, particularly when comparing one examination with another. First, even though most laboratories have a standard examination (30 degrees right anterior oblique projection and 70 degrees left anterior oblique projection), the same projection of a wall segment is not always the same part of the heart. The actual wall seen is a function of the rotation of the heart in the thorax. For example, the “anterolateral wall” in a patient with right ventricular enlargement that rotates the heart posteriorly may be the anterior segment of the left ventricle. A heart that lies horizontally in the thorax may not project its wall segments exactly as does a heart with a more vertical orientation.
SEGMENTAL WALL MOTION ABNORMALITIES WITH AND WITHOUT CORONARY DISEASE
Left Ventricular Thrombus
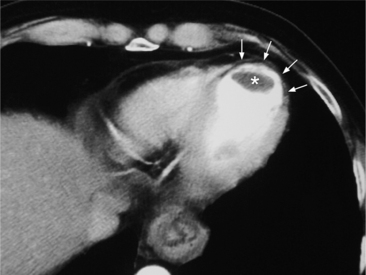
Approximately half of all patients with postinfarction ventricular aneurysm have left ventricular thrombi that are seen at surgery or on necropsy study; however, the angiographic detection of these thrombi is only moderately sensitive and specific. Simpson and colleagues found that roughly 74% of mural thrombi found at surgery are not detected by angiography and in 10% of patients diagnosed as having thrombi no thrombi are present at operation. Even during echocardiography of the left ventricle, it can be difficult to diagnose mural thrombi. Gadolinium magnetic resonance angiography or ECG-gated multirow detector CT can precisely characterize or delineate the thrombus (Fig. 7-43). Furthermore, echocardiography and noninvasive imaging techniques do not have the risk of dislodging mural thrombi when compared to angiography.
Calcifications
Left ventricular thrombi may be calcified, particularly when there is calcification of adjacent structures, such as an aneurysm or infarcted papillary muscle. Filling defects that are typical of thrombus may have several appearances (Fig. 7-44). A polypoid shape usually arises in an akinetic apex. This angiographic configuration is a harbinger of systemic embolization. A truncated apex with an adjacent shaggy wall may also indicate thrombus formation. Because the wall of an aneurysm is thin, the presence of a normal or thick wall to the aneurysm indicates a thrombus within the aneurysm.
Cardiac Rupture
Although rupture of the heart may be catastrophic, certain types of rupture may be contained so that the patient can have diagnostic imaging and undergo surgical repair (Box 7-3). Cardiac rupture usually occurs after a myocardial infarct; trauma is less common. Free rupture into the pericardium typically is fatal. Rupture through the interventricular septum, of a papillary muscle, or development of a false aneurysm is a serious complication but may have a longer time course to allow treatment.
Left Ventricular True and False Aneurysm
Definition and Pathologic Correlation
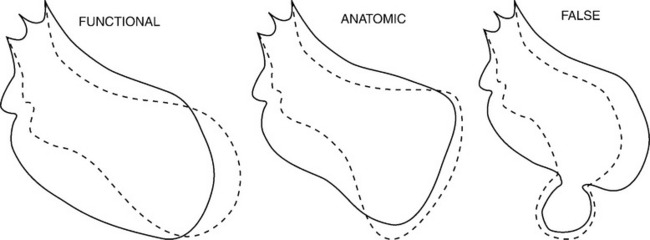
A left ventricular aneurysm (Box 7-4) is a segment of a left ventricular wall that protrudes from the expected diastolic outline of the ventricular chamber. The wall motion may be either akinetic or dyskinetic. The wall of the aneurysm is generally smooth without the usual trabecular pattern. This definition of a left ventricular aneurysm overlaps with several features of reversible ischemia. During an acute myocardial infarction, the wall segment involved has a dyskinetic motion, which may return to normal if a transmural infarction does not occur.
When the wall thins, a large myocardial scar may develop the appearance of an aneurysm. On imaging studies, a large scar or a small aneurysm may have the same appearance. A functional aneurysm has a normal diastolic contour but a dyskinetic systolic bulge in the region of a large acontractile segment and may contain either reversible ischemic myocardium or scar. In contrast, an anatomic aneurysm has an abnormal protrusion during both systole and diastole because the wall is entirely a scar that has stretched (Fig. 7-45).
Imaging Signs of Aneurysm
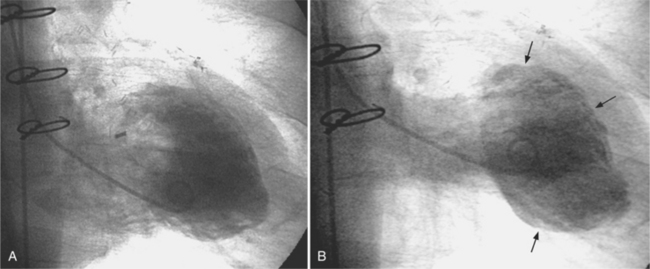
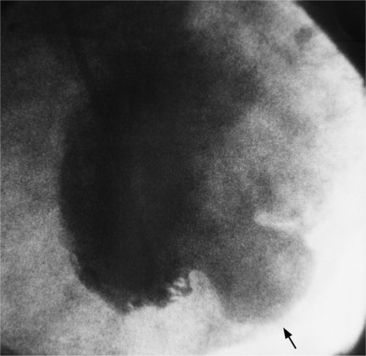
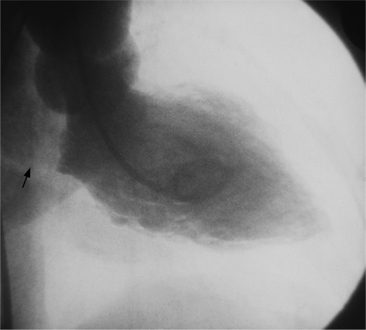
Anatomic aneurysms that have been present for more than several months may have a thin rim of calcium outlining their extent. This type of aneurysm may be either akinetic or dyskinetic. The wall of a true aneurysm is quite thin, reflecting the fact that the scar extends from the endocardium through to the pericardium (Fig. 7-46). The aneurysmal segment has a large neck, comparable in size to its internal circumference. Within the aneurysm, the wall is usually smooth unless a thrombus is present. The boundaries of the aneurysm are usually discrete and easily discernible.
The chest film is not a sensitive test to screen for left ventricular aneurysm. Only one third of angiographically diagnosed aneurysms show contour abnormalities on the chest film. Part of the explanation for this discrepancy is that the septum is not visible on the chest film and small aneurysms at the apex are obscured by the fat pad. The left ventricle is usually dilated and the remaining wall is hyperkinetic to maintain cardiac output. The uninvolved ventricular wall is frequently hypertrophied, which probably reflects the fact that about half of patients with aneurysms also have systemic hypertension. Most true aneurysms are in the anterolateral and apical walls (Fig. 7-47), although the inferior and posterolateral walls occasionally have isolated aneurysms. Echocardiography depicts left ventricular aneurysm quite well and should be the first modality of choice.
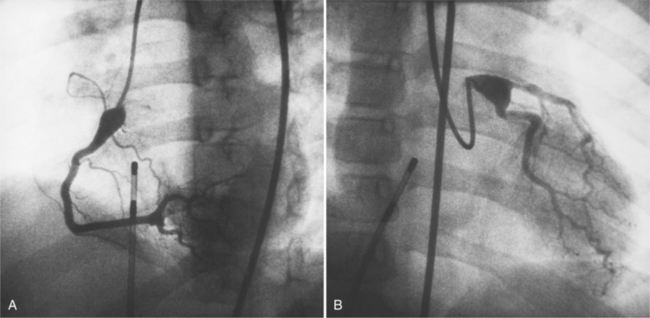
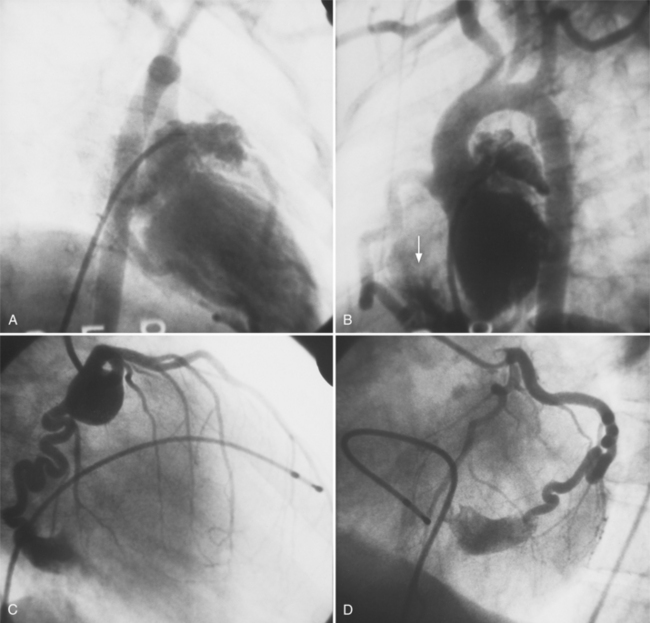
False Aneurysm
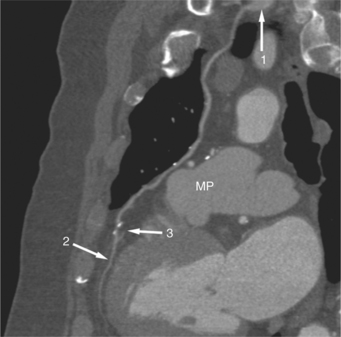
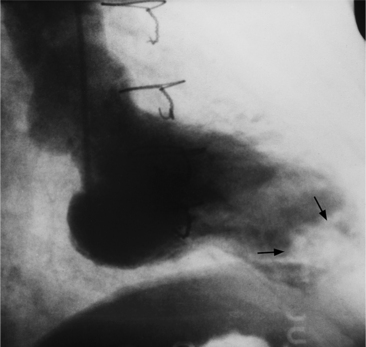
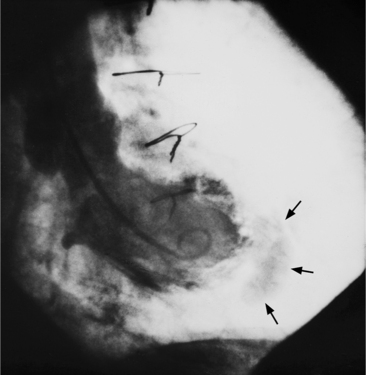
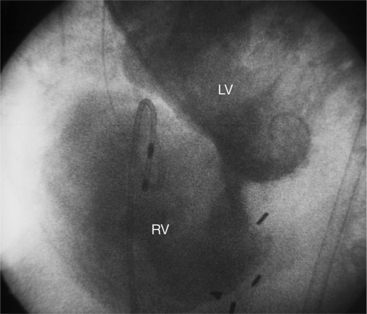
When the pericardium rather than myocardium composes the wall of the aneurysm, it is a false aneurysm or pseudoaneurysm. The usual cause is a rupture of the left ventricle into the pericardial space after myocardial infarction. Because of adhesions from previous pericarditis, the pericardium attaches locally to the epicardium. This opportunely prevents the ventricular blood from extending into the remaining pericardial space and causing tamponade. Causes that are less common but have a nearly identical appearance include abscess from bacterial endocarditis, surgical ventriculotomy (Fig. 7-48), and penetrating trauma. In contrast to true aneurysms, most false aneurysms resulting from myocardial infarct are on the posterolateral and diaphragmatic sides of the left ventricle (Fig. 7-49).
The left ventriculogram demonstrates a discrete saccular aneurysm whose neck is considerably smaller than the internal circumference of the saccule. Contrast material may not flow into the false aneurysm until late in systole, and it may oscillate into and out of the neck of the aneurysm without exiting from the left ventricle. Because of the narrow communication with the left ventricular chamber, the opacification of the false aneurysm may persist for many seconds after the injection has ended (Fig. 7-50). Frequently the false aneurysm is bigger than the left ventricle, and in fact, may enlarge over serial examinations. This has prognostic significance in that false aneurysms have a high propensity to rupture. Rupture of a true aneurysm is rare, occurring in less than 4% of several necropsy series. In contrast, postmortem series indicate that approximately 45% of false aneurysms rupture. Echocardiography is also the first modality of choice when a false aneurysm is suspected.
RUPTURE OF THE INTERVENTRICULAR SEPTUM
Clinical Presentation
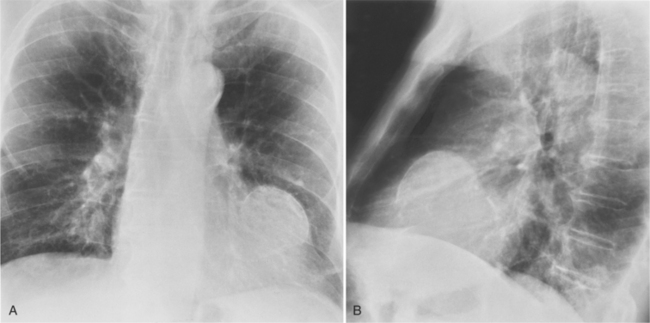
Common abnormalities in chest radiographs of patients with a ruptured septum are signs of interstitial or alveolar pulmonary edema and left ventricular enlargement (Table 7-2). Enlargement of other chambers is inconstant. Almost half will show pleural effusions, enlargement of the pulmonary arteries, and signs of pulmonary venous hypertension. Occasionally, an unusual configuration of the heart will suggest the presence of an aneurysm in addition to the septal rupture (Fig. 7-51).
TABLE 7-2 Chest radiographic abnormalities in rupture of the interventricular septum
| Abnormality | Percentage |
|---|---|
| PULMONARY | |
| Normal | 12 |
| Pulmonary venous hypertension | 9 |
| Interstitial edema | 42 |
| Alveolar edema | 37 |
| PLEURAL EFFUSIONS | |
| None | 37 |
| Right side | 3 |
| Left side | 30 |
| Both sides | 30 |
| Pulmonary artery enlargement | 39 |
| CARDIAC ENLARGEMENT | |
| Left ventricle | 82 |
| Left atrium | 7 |
| Right ventricle | 3 |
| Right atrium | 12 |
Modified with permission from Miller SW, Dinsmore RE, Greene RE, et al.: Coronary, ventricular, and pulmonary abnormalities associated with rupture of the interventricular septum complicating myocardial infarction, Am J Roentgenol 131:571, 1978.
Left Ventricular Signs
Doppler echocardiography is a good noninvasive technique to demonstrate ventricular septal rupture. During left ventriculography, almost all patients have at least one segment with akinesis or dyskinesis. The posterolateral wall is hyperkinetic in some patients, suggesting that the exaggerated motion is partly compensating for the other segments of the ventricle. The septum is always akinetic or dyskinetic.
In the right anterior oblique view, you will see opacification of the pulmonary artery during left ventriculography. This view superimposes the two ventricles. The most inferior wall visible is the diaphragmatic wall of the right ventricle. If the left ventricular diaphragmatic wall is akinetic, the adjacent right ventricular wall behaves similarly. In the left anterior oblique projection, contrast material passes through the rupture to opacify the right ventricle (Fig. 7-52). The margins of the rupture may be difficult to identify, but they always are in a region of severe segmental wall motion abnormality. Occasionally, the right ventricular anterior wall will also be akinetic and represent an extension of the diaphragmatic infarct.
Left ventricular aneurysm, mitral regurgitation, or both are found in roughly three quarters of patients with septal rupture. These exaggerate extensive wall motion abnormalities. The aneurysm may be in the region of the septal rupture or in a separate portion of the ventricle. The mitral regurgitation is the result of a variety of factors, including left ventricular dilatation and papillary muscle dysfunction. Both these lesions contribute to poor left ventricular performance and make catheterization difficult. However, their recognition is essential for proper surgical management.
MITRAL REGURGITATION RESULTING FROM CORONARY ARTERY DISEASE
Pathologic Causes
The spectrum of papillary muscle disorders includes ischemic processes involving both the papillary muscle and its adjacent left ventricular free wall. For example, hypokinesis, akinesis, or aneurysm may produce any degree of mitral regurgitation (Fig. 7-53).
ACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines). J Am Coll Cardiol. 2001;37:2215-2238.
AHA Statistical Update. Heart disease and stroke statistics—2008. Circulation. 2008;117:e25-e147.
Ambrose JA, Winters SL, Arora RR, et al. Angiographic evolution of coronary artery morphology in unstable angina. J Am Coll Cardiol. 1987;7:472-478.
Ambrose JA. Plaque disruption and the acute coronary syndromes of unstable angina and myocardial infarction: if the substrate is similar, why is the clinical presentation different? J Am Coll Cardiol. 1992;19:1753-1758.
Attili AK, Cascade PN. CT and MRI of coronary artery disease: evidence-based review. Am J Roentgenol. 2007;187:S483-S499.
Bogaty P, Brecker SJ, White SE, et al. Compa rison of coronary angiographic findings in acute and chronic first presentation of ischemic heart disease. Circulation. 1993;87:1938-1947.
Bolli R. Myocardial “stunning” in man. Circulation. 1992;87:1771-1791.
Brown BG, Zhao ZQ, Sacco DE, et al. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993;87:1781-1791.
Cabins HS, Roberts WC. Left ventricular aneurysm, intra-aneurysmal thrombus and systemic embolus in coronary heart disease. Chest. 1980;77:587-590.
Cabins HS, Roberts WC. True left ventricular aneurysm and healed myocardial infarction. Clinical and necropsy observations including quantification of degrees of coronary arterial narrowing. Am J Cardiol. 1980;47:754-773.
Datta J, White CS, Gilkeson RC, et al. Anomalous coronary arteries in adults: depiction at multidetector row CT angiography. Radiology. 2005;235:812-818.
Davies MJ. A macro and micro view of coronary vascular insult in ischemic heart disease. Circulation. 1990;82(Suppl. 2):II-38-II-47.
Davies SW, Marchant B, Lyons JP, et al. Coronary lesion morphology in acute myocardial infarction: demonstration of early remodeling after streptokinase treatment. J Am Coll Cardiol. 1990;17:1079-1087.
Dodd JD, Ferencik M, Liberthson RR, et al. Congenital anomalies of coronary artery origin in adults: 64-MDCT appearance. Am J Roentgenol. 2007;188:W138-W146.
Faxon DP, Ryan TJ, Davis KB, et al. Prognostic significance of angiographically documented left ventricular aneurysm from the coronary artery surgery study (CASS). Am J Cardiol. 1982;50:157-174.
Feldman RL, Nichols WW, Pepine CJ, et al. Hemodynamic significance of the length of a coronary arterial narrowing. Am J Cardiol. 1978;41:871-875.
Fellows KE, Freed MD, Keane JF, et al. Results of routine preoperative coronary angiography in tetralogy of Fallot. Circulation. 1975;51:571-577.
Fuster V. Assessing and modifying the vulnerable atherosclerotic plaque. Boston: Blackwell Publishing, 2002.
Fuster VF, Badimon L, Badimon JJ, et al. The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med. 1992;327:242-250. 310–318
Gould KL, Kirkeeide RL, Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol. 1990;15:459-474.
Greenberg MA, Fish BG, Spindola-Franco H. Congenital anomalies of the coronary arteries: classification and significance. Radiol Clin North Am. 1989;27:1127-1147.
Harding MB, Leither ME, Mark DB, et al. Ergonovine maleate testing during cardiac catheterization: a 10 year perspective in 3,447 patients without significant coronary artery disease or Prinzmetal’s variant angina. J Am Coll Cardiol. 1992;20:107-111.
Higgins CB, Lipton MJ. Radiography of acute myocardial infarction. Radiol Clin North Am. 1980;18:359-378.
Hauser M. Congenital anomalies of the coronary arteries. Heart. 2005;91:1240-1245.
Irvin RG. The angiographic prevalence of myocardial bridging in man. Chest. 1982;81:198-202.
Jacoby DS, Mohler ERIII, Rader DJ. Noninvasive atherosclerotic imaging for predicting cardiovascular events and assessing therapeutic interventions. Curr Atheroscler Rep. 2004;7:20-27.
Kahn K, Fisher MR. MRI of cardiac pseudoaneurysm and other complications of myocardial infarction. Magn Reson Imaging. 1991;9:159-174.
Kaski JC, Rosano GM C, Collins P, et al. Cardiac syndrome X. Clinical characteristics and left ventricular function. J Am Coll Cardiol. 1995;25:807-814.
Kereiakes DJ, Topol EJ, George BS, et al. Myocardial infarction with minimal coronary atherosclerosis in the era of thrombolytic reperfusion. J Am Coll Cardiol. 1991;17:304-312.
Kim SY, Seo JB, Do K, et al. Coronary artery anomalies: classification and ECG-gated multidetector row CT findings with angiographic correlation. Radiographics. 2007;27:317-333.
Kimbiris JB, Iskandrian AS, Segal BL, et al. Anomalous aortic origin of coronary arteries. Circulation. 1978;58:707-715.
Kloner RA, Przyklenk K, Patel B. Altered myocardial states: the stunned and hibernating myocardium. Am J Med. 1987;80(Suppl. 1A):14.
Konen E, Merchant N, Gutierrez C, et al. True versus false left ventricular aneurysm: differentiation with MR imaging-initial experience. Radiology. 2005;237:75-76.
Lee J, Choe YH, Kim HJ, et al. Magnetic resonance imaging demonstration of anomalous origin of the right coronary artery from the left coronary sinus associated with acute myocardial infarction. J Comput Assist Tomogr. 2003;27:289-291.
Leschka S, Koepfli P, Husmann L, et al. Myocardial bridging: depiction rate and morphology at CT coronary angiography-comparison with conventional coronary angiography. Radiology. 2008;247:754-772.
Levin DC, Gardiner GAJr. Complex and simple coronary artery stenoses: a new way to interpret coronary angiograms based on morphologic features of lesions. Radiology. 1987;174:775-780.
Liberthson RR, Dinsmore RE, Bharatic S, et al. Aberrant coronary artery origin from the aorta. Diagnosis and clinical significance. Circulation. 1974;50:774-779.
Liberthson RR, Sagar K, Berkoben JP, et al. Congenital coronary arteriovenous fistula. Report of 13 patients, review of the literature and delineation of management. Circulation. 1979;59:849-854.
Manghat NE, Morgan-Hughes GJ, Marshall AJ, et al. Multidetector row computed tomography: imaging congenital coronary anomalies in adults. Heart. 2005;91:1515-1522.
Marcus ML, Schelbert HR, Skorton DJ, et al. Cardiac imaging: a companion to Braunwald’s heart disease. Philadelphia: WB Saunders, 1997.
Massie BM, Botvinick EH, Brundage BH, et al. Relationship of regional myocardial perfusion to segmental wall motion. A physiologic basis for understanding the presence and reversibility of asynergy. Circulation. 1978;58:1154-1173.
Megnien JK L, Sene V, Jeannin S, et al. Coronary calcification and its relation to extracoronary atherosclerosis in asymptomatic hypercholesterolemic men. Circulation. 1992;85:1799-1807.
Meizlish JL, Berger HJ, Plankey M, et al. Functional left ventricular aneurysm formation after acute anterior transmural myocardial infarction. N Engl J Med. 1984;311:1001-1007.
Miller SW, Boucher CA. Assessing the adequacy of myocardial perfusion in man: anatomic and functional techniques. Radiol Clin North Am. 1985;23:589-597.
Miller SW, Dinsmore RE, Greene RE, et al. Coronary, ventricular, and pulmonary abnormalities associated with rupture of the interventricular septum complicating myocardial infarction. Am J Roentgenol. 1978;131:571-577.
Mizuno K, Horiuchi K, Matui H, et al. Role of coronary collateral vessels during transient coronary occlusion during angioplasty assessed by hemodynamic, electrocardiographic and metabolic changes. J Am Coll Cardiol. 1988;12:724-728.
Motoyasu M, Sakuma H, Ichikawa Y, et al. Prediction of regional functional recovery after acute myocardial infarction with low dose dobutamine stress cine MR imaging and contrast enhanced MR imaging. J Cardiovasc Magn Reson. 2003;5:573-574.
Mohlenkamp S, Hort W, Ge J, et al. Update of myocardial bridging. Circulation. 2002;107:2717-2731.
Nassir K, Budoff MJ, Post WS, et al. Electron beam CT versus helical CT scans for assessing coronary calcification: current utility and future directions. Am Heart J. 2003;147:977-979.
Petersen SE, Voigtlander T, Kreitner KF, et al. Late improvement of regional wall motion after the subacute phase of myocardial infarction treated by acute PTCA in a 7-month follow-up. J Cardiovasc Magn Reson. 2003;5:487-495.
Pierard LA, Albert A, Henrard L, et al. Incidence and significance of pericardial effusion in acute myocardial infarction as determined by two-dimensional echocardiography. J Am Coll Cardiol. 1987;8:517-520.
Pijls NH J, de Bruyne B, Bech JW, et al. Coronary pressure measurement to assess the hemodynamic significance of serial stenoses within one coronary artery. Circulation. 2000;102:2371-2377.
Pijls NH J, de Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary artery stenoses. N Engl J Med. 1997;334:1703-1708.
Reddy K, Gupta M, Hamby RI. Multiple coronary arteriosystemic fistulas. Am J Cardiol. 1974;33:304-307.
Rubins HB, Nelson DB, Noorbaloochi S, et al. Effectiveness of lipid-lowering medications in outpatients with coronary heart disease in the Department of Veterans Affairs System. Am J Cardiol. 2003;92:1177-1182.
Sandstede JJ. Assessment of myocardial viability by MR imaging. Eur Radiol. 2003;13:52-71.
Schwartz H, Leiboff RH, Bren GB, et al. Temporal evolution of the human coronary collateral circulation after myocardial infarction. J Am Coll Cardiol. 1984;4:1088-1093.
Sharifi M, Frohlich TG, Silverman IM. Myocardial infarction with angiographically normal coronary arteries. Chest. 1995;107:37-40.
Simpson MT, Oberman A, Kouchoukos NT, et al. Prevalence of mural thrombi and systemic embolization with left ventricular aneurysm. Effect of anticoagulation therapy. Chest. 1980;77:473-479.
Sleight P. Current options in the management of coronary artery disease. Am J Cardiol. 2003;92:4N-8N.
Stanford W, Thompson BH, Weiss RM. Coronary artery calcification: clinical significance and current methods of detection. Am J Roentgenol. 1993;171:1139-1147.
Syed M, Lesch M. Coronary artery aneurysm. A review. Prog Cardiovasc Dis. 1997;40:77-84.
Tunick PA, Slater J, Kronzon I, et al. Discrete atherosclerotic coronary artery aneurysms: a study of 20 patients. J Am Coll Cardiol. 1990;15:279-282.
Virmani R, Forman MB, editors. Nonatherosclerotic ischemic heart disease. New York: Raven Press, 1989.
Wicky S, Lyon X, Kappenberger L. Silent single coronary artery anomaly depicted by magnetic resonance angiography. J Invasive Cardiol. 2002;14:328-330.
Wijns W, Vatner SF, Camici PG. Hibernating myocardium. New Engl J Med. 1998;339:173-181.
Yacoe ME, Dake MD. Development and resolution of systemic and coronary artery aneurysms in Kawasaki disease. Am J Roentgenol. 1992;159:708-710.