Chapter 27 Introduction to the Central Nervous System
| Abbreviations | |
|---|---|
| ACh | Acetylcholine |
| BBB | Blood-brain barrier |
| CNS | Central nervous system |
| CO | Carbon monoxide |
| DA | Dopamine |
| Epi | Epinephrine |
| GABA | γ-Aminobutyric acid |
| Glu | Glutamate |
| 5-HT | Serotonin |
| l-DOPA | 3,4-dihydroxy-phenylalanine |
| NE | Norepinephrine |
| NMDA | N-methyl-D-aspartate |
| NO | Nitric oxide |
Drugs acting on the central nervous system (CNS) are among the most widely used of all drugs. Humankind has experienced the effects of mind-altering drugs throughout history, and many compounds with specific and useful effects on brain and behavior have been discovered. Drugs used for therapeutic purposes have improved the quality of life dramatically for people with diverse illnesses, whereas illicit drugs have altered the lives of many others, often in detrimental ways.
Discovery of the general anesthetics was essential for the development of surgery, and continued advances in the development of anesthetics, sedatives, narcotics, and muscle relaxants have made possible the complex microsurgical procedures in use today. Discovery of the typical antipsychotics and tricyclic antidepressants in the 1950s and the introduction of the atypical antipsychotics and new classes of antidepressants within the past 20 years have revolutionized psychiatry and enabled many individuals afflicted with these mind-paralyzing diseases to begin to lead productive lives and contribute to society. Similarly, the introduction of 3,4-dihydroxy-phenylalanine (l-DOPA) for the treatment of Parkinson’s disease in 1970 was a milestone in neurology and allowed many people who had been immobilized for years the ability to move and interact with their environment. Other advances led to the development of drugs to reduce pain or fever, relieve seizures and other movement disorders associated with neurological diseases, and alleviate the incapacitating effects associated with psychiatric illnesses, including bipolar disorder and anxiety. Major neuropsychiatric disorders and the classes of drugs available for treatment are summarized in Table 27-1.
TABLE 27–1 Major Neuropsychiatric Disorders and Classes of Drugs Used for Treatment
| Disorder or Indication | Drug Group/Class |
|---|---|
| Neurodegenerative Disorders | |
| Parkinson’s disease | Dopamine A-enhancing compounds |
| Alzheimer’s disease | Acetylcholinesterase inhibitors |
| NMDA receptor antagonists | |
| Psychiatric Disorders | |
| Psychotic disorders (schizophrenia) | Typical and atypical antipsychotics |
| Major depression | Antidepressants |
| Bipolar disorder | Mood stabilizers, anticonvulsants, atypical antipsychotics |
| Anxiety | Anxiolytics |
| Sleep disorders | Anxiolytics and sleep-promoting drugs |
| Anorexia nervosa and bulimia nervosa | Antidepressants, antipsychotics |
| Anorexia/cachexia | Corticosteroids, progestational agents |
| Obesity | Appetite suppressants, fat absorption inhibitors |
| Neurological Disorders | |
| Seizures | Anticonvulsants |
To induce CNS effects, drugs must obviously be able to reach their targets in the brain. Because the brain is protected from many harmful and foreign blood-borne substances by the blood-brain barrier (BBB), the entry of many drugs is restricted. Therefore it is important to understand the characteristics of drugs that enable them to enter the CNS. This chapter covers basic aspects of CNS function, with a focus on the cellular and molecular processes and neurotransmitters thought to underlie CNS disorders. The mechanisms through which drugs act to alleviate the symptoms of these disorders are emphasized.
NEUROTRANSMISSION IN THE CENTRAL NERVOUS SYSTEM
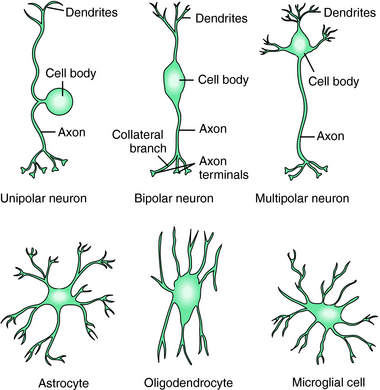
The CNS is composed of two predominant cell types, neurons and glia, each of which has many morphologically and functionally diverse subclasses. Glial cells outnumber neurons and contain many neurotransmitter receptors and transporters. For many years these cells were thought to play a supportive role, but recent studies indicate that glial cells play a key role in CNS function. There are three types of glial cells: astrocytes, oligodendrocytes, and microglia (Fig. 27-1). Astrocytes physically separate neurons and multineuronal pathways, assist in repairing nerve injury, and modulate the metabolic and ionic microenvironment. These cells express ion channels and neurotransmitter transport proteins and play an active role in modulating synapse function. Oligodendrocytes form the myelin sheath around axons and play a critical role in maintaining transmission down axons. Interestingly, polymorphisms in the genes encoding several myelin proteins have been identified in tissues from patients with both schizophrenia and bipolar disorder and may contribute to the underlying etiology of these disorders. Microglia proliferate after injury or degeneration, move to sites of injury, and transform into large macrophages (phagocytes) to remove cellular debris. These antigen-presenting cells with innate immune function also appear to play a role in endocrine development.
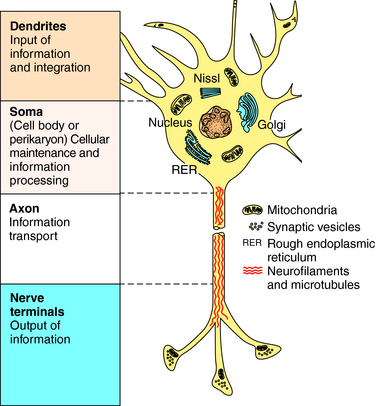
Neurons are the major cells involved in intercellular communication because of their ability to conduct impulses and transmit information. They are structurally different from other cells, with four distinct features (Fig. 27-2):
Neurons are often shaped according to their function. Unipolar or pseudounipolar neurons have a single axon, which bifurcates close to the cell body, with one end typically extending centrally and the other peripherally (see Fig. 27-1). Unipolar neurons tend to serve sensory functions. Bipolar neurons have two extensions and are associated with the retina, vestibular cochlear system, and olfactory epithelium; they are commonly interneurons. Finally, multipolar neurons have many processes but only one axon extending from the cell body. These are the most numerous neurons and include spinal motor, pyramidal, and Purkinje neurons.
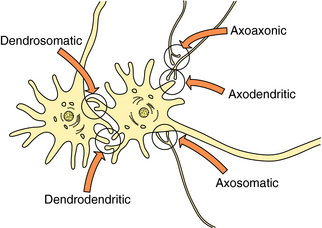
Most neurotransmission involves communication between nerve terminals and dendrites or perikarya on the postsynaptic cell, called axodendritic or axosomatic synapses, respectively. However, other areas of the neuron may also be involved in both sending and receiving information. Neurotransmitter receptors are often spread diffusely over the dendrites, perikarya, and nerve terminals but are also commonly found on glial cells, where they likely serve a functional role. In addition, transmitters can be stored in and released from dendrites. Thus transmitters released from nerve terminals may interact with receptors on other axons at axoaxonic synapses; transmitters released from dendrites can interact with receptors on either “postsynaptic” dendrites or perikarya, referred to as dendrodendritic or dendrosomatic synapses, respectively (Fig. 27-3).
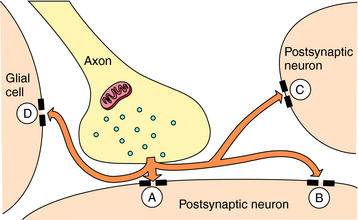
In addition, released neurotransmitters may diffuse from the synapse to act at receptors in extrasynaptic regions or on other neurons or glia distant from the site of release. This process is referred to as volume transmission (Fig. 27-4). Although the significance of volume transmission is not well understood, it may play an important role in the actions of neurotransmitters in brain regions where primary inactivation mechanisms are absent or dysfunctional.
The Life Cycle of Neurotransmitters
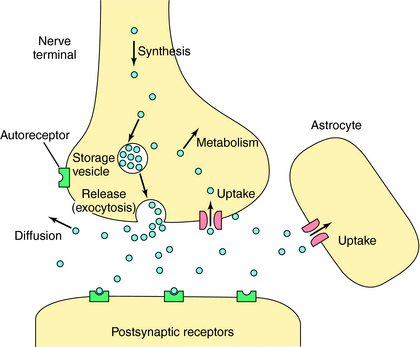
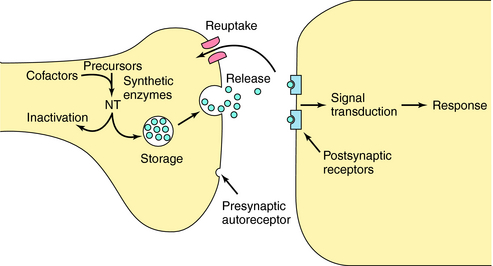
Neurotransmitters are any chemical messengers released from neurons. They represent a highly diverse group of compounds including amines, amino acids, peptides, nucleotides, gases, and growth factors (Table 27-2). Most classical neurotransmitters, first identified in peripheral neurons, play a major role in central transmission including acetylcholine (ACh), dopamine (DA), norepinephrine (NE), epinephrine (Epi), and serotonin (5-HT). Recently it has become clear that histamine is also an important neurotransmitter in the brain. The amino acid neurotransmitters include the excitatory compounds glutamate and aspartate and the inhibitory compounds GABA and glycine. All of these molecules are synthesized in nerve terminals and are generally stored in and released from small vesicles (Fig. 27-5). In addition to these small molecules, it is now clear that many peptides function as neurotransmitters. Peptide neurotransmitters are cleaved from larger precursors by proteolytic enzymes and packaged into large vesicles in neuronal perikarya. The most recent and surprising group of neurotransmitters identified are often referred to as unconventional neurotransmitters and include the gases nitric oxide (NO) and carbon monoxide (CO), along with several growth factors including brain-derived neurotrophic factor and nerve growth factor. The gaseous neurotransmitters are synthesized and released upon demand and thus are not stored in vesicles. The growth factors are stored in vesicles and released constitutively from both perikarya and dendrites.
| Category | Subcategory | Neurotransmitter |
|---|---|---|
| Primary amines | Quaternary amines | Acetylcholine |
| Catecholamines |
Because many centrally acting drugs act by altering the synthesis, storage, release, or inactivation of specific neurotransmitters, it is critical to understand these processes. For neurons to fire rapidly and repetitively, they must maintain sufficient supplies of neurotransmitter. Most neurons synthesize neurotransmitters locally in the nerve terminal (with the exception of peptides) and have complex mechanisms for regulating this process. Synthesis is usually controlled by either the amount and activity of synthetic enzymes or the availability of substrates and cofactors. For example, ACh synthesis is regulated primarily by substrate availability (see Chapters 9 and Chapters 10), whereas DA, NE, and Epi syntheses are regulated primarily by the activity of the synthetic enzyme tyrosine hydroxylase, and that of 5-HT by tryptophan hydroxylase (see Chapters 9 and Chapters 11).
The arrival of an action potential causes the nerve terminal membrane to depolarize, resulting in release of neurotransmitter into the synaptic cleft. This process is initiated by opening voltage-dependent Ca++ channels in the membrane, enabling Ca++ to enter the cell. Ca++ influx leads to a complex sequence of events resulting in translocation and fusion of vesicles with the plasma membrane, releasing their contents into the synaptic cleft by exocytosis (Fig. 27-5).
After receptor activation, neurotransmitters must be inactivated to terminate their actions and allow for further information transfer. Rapid enzymatic hydrolysis of ACh terminates its action (see Chapters 9 and Chapters 10), while the actions of biogenic amines are terminated primarily by reuptake into presynaptic terminals by specific energy-dependent transporters (see Chapters 9 and Chapters 11). The amino acid neurotransmitters are taken up primarily by astrocytes, and recent data suggest that biogenic amines may also be taken up by these cells, a process that may play an important role in some disease states and in the action of the antidepressant agents. Inside the terminal, neurotransmitters can be repackaged into vesicles and rereleased. All inactivation processes are important targets for drug action.
As discussed in Chapter 1, receptors are sensors by which cells detect incoming messages. Many different types of receptors can coexist on cells, including receptors for different transmitters and multiple subtypes for a single transmitter. The response of a particular neuron to a neurotransmitter depends as much on the type of receptors present as on the type of transmitter released. Because each transmitter can activate a receptor family composed of different receptor subtypes associated with distinct signal transduction mechanisms, a single transmitter may cause completely different effects on different cells (see Chapter 1). The function of the neuron is to integrate these multiple messages, from a single transmitter or from multiple transmitters, to control the impulse activity of its own axon.
ORGANIZATION OF THE CENTRAL NERVOUS SYSTEM
Anatomical and Functional Organization
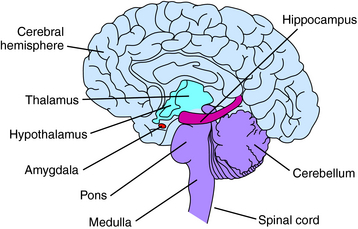
The gross anatomy of the brain includes the cerebrum or cerebral hemispheres; subcortical structures including the thalamus and hypothalamus (the diencephalon); the midbrain; and the hindbrain, composed of the pons, medulla, and cerebellum (Fig. 27-6).
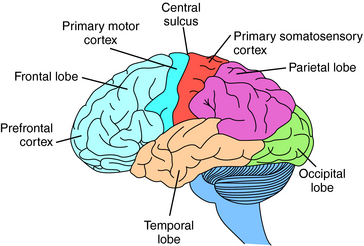
The cerebral cortex contains four regions, the frontal, parietal, occipital, and temporal lobes (Fig. 27-7). The frontal lobe extends anterior from the central sulcus and contains the motor and prefrontal cortices. It is associated with higher cognitive functions and long-term memory storage; the posterior portion is the primary motor cortex and controls fine movements. The parietal lobe, between the occipital lobe and central sulcus, is associated with sensorimotor integration and processes information from touch, muscle stretch receptors, and joint receptors. This area contains the primary somatosensory cortex. The temporal lobe is located laterally in each hemisphere and is the primary cortical target for information originating in the ears and vestibular organs; it is involved in vision and language. The occipital lobe is located in the posterior cortex and is involved in visual processing. It is the main target for axons from thalamic nuclei that receive inputs from the visual pathways and contains the primary visual cortex.
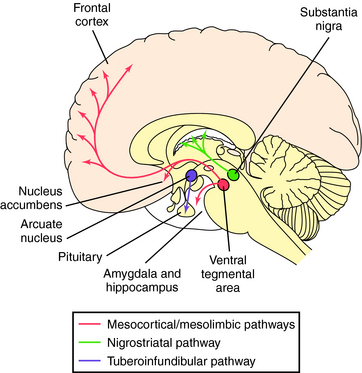
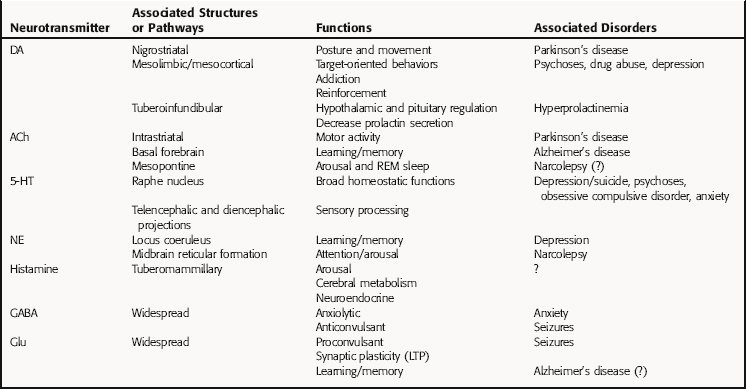
Neurons synthesizing DA have their cell bodies primarily in two brain regions, the midbrain, containing the substantia nigra and adjacent ventral tegmental area, and the hypothalamus (Fig. 27-8). Nigrostriatal DA neurons project to the striatum (caudate nucleus and putamen) and are involved in control of posture and movement; these neurons degenerate in Parkinson’s disease. The ventral tegmental neurons extend to the cortex and limbic system, referred to as the mesocortical and mesolimbic pathways, respectively, and are important for complex target-oriented behaviors, including psychotic behaviors. Those neurons projecting from the ventral tegmental area to the nucleus accumbens septi are believed to be involved in addiction. DA is also synthesized by much shorter neurons originating in the arcuate and periventricular nuclei of the hypothalamus that extend to the intermediate lobe of the pituitary and into the median eminence, known as the tuberoinfundibular pathway. These neurons regulate pituitary function and decrease prolactin secretion. Drugs used for the treatment of Parkinson’s disease (see Chapter 28) stimulate these DA systems, whereas drugs used for the treatment of psychotic disorders such as schizophrenia (see Chapter 29) block them.
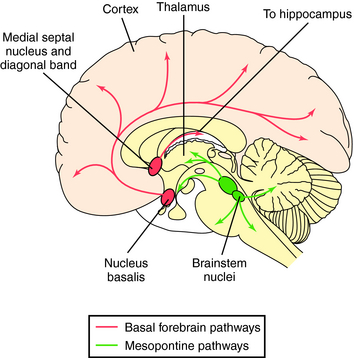
Three primary groups of cholinergic neurons are found in the brain, those originating in ventral areas of the forebrain (nucleus basalis and nuclei of the diagonal band and medial septum), the pons, and the striatum (Fig. 27-9). Neurons from the nucleus basalis project to large areas of the cerebral cortex, while septal and diagonal band neurons project largely to the hippocampus. These pathways are important in learning and memory and degenerate in Alzheimer’s disease. Thus treatment of this disorder involves the use of acetylcholinesterase inhibitors in attempts to alleviate this cholinergic deficit (see Chapter 28). Neurons originating in the pons project to the thalamus and basal forebrain and have descending pathways to the reticular formation, cerebellum, vestibular nuclei, and cranial nerve nuclei; they are involved in arousal and REM sleep. Finally, there are small cholinergic interneurons in the striatum that are inhibited by nigrostriatal DA neurons, forming the basis for the use of muscarinic receptor antagonists in treating Parkinson’s disease (see Chapter 28).
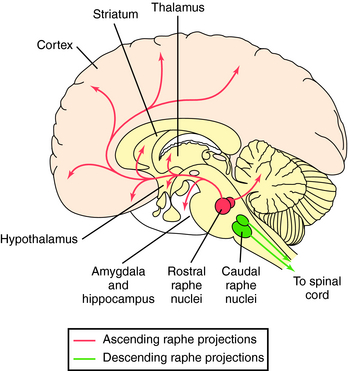
Serotonergic neurons originate primarily in the raphe nucleus and have widespread projections (Fig. 27-10). Neurons from the rostral raphe project to the limbic system, thalamus, striatum, and cerebral cortex, whereas caudal raphe neurons descend to the spinal cord. Serotonergic pathways have broad influences throughout the brain and are important for sensory processing and homeostasis. They play a role in psychotic behaviors (see Chapter 29), depression and obsessive-compulsive disorder (see Chapter 30), and eating behavior (see Chapter 33) and are major targets for drugs used to treat these diseases.
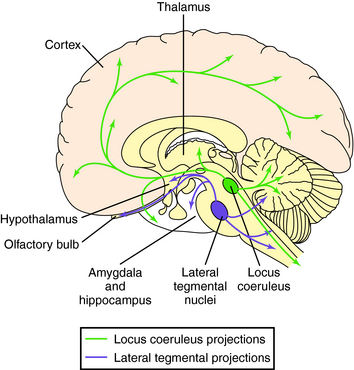
Neurons synthesizing NE have their cell bodies primarily in the locus coeruleus in the pons and project anteriorly to large areas of the cerebral cortex, thalamus, hypothalamus, and olfactory bulb (Fig. 27-11). Other noradrenergic neurons originate in the midbrain (lateral tegmental region) and have ascending pathways to the limbic system and descending projections to the cerebellum and spinal cord, with fibers passing in the ventrolateral column. Noradrenergic pathways are involved in controlling responses to external sensory and motor stimuli, arousal and attention, and learning and memory and may be important in major depression (see Chapter 30). Midbrain neurons also play important roles in control of autonomic and neuroendocrine function.
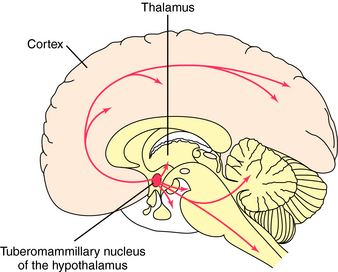
All known histaminergic neurons originate in magnocellular neurons in the posterior hypothalamus, referred to as the tuberomammillary nucleus (Fig. 27-12). These neurons form long ascending connections to many telencephalic areas, including all areas of the cerebral cortex, the limbic system, caudate putamen, nucleus accumbens septi and globus pallidus. Also, long descending neurons project to mesencephalic and brainstem structures including cranial nerve nuclei, the substantia nigra, locus coeruleus, mesopontine tegmentum, dorsal raphe, cerebellum, and spinal cord. Histaminergic neurons play a major role in arousal, in coupling neuronal activity with cerebral metabolism, and in neuroendocrine regulation.
Amino Acid Neurotransmitter Systems
Amino acid neurotransmitters are not restricted to specific pathways but are widespread throughout the brain and spinal cord. GABAergic neurons play a major inhibitory role in most brain regions and are important in anxiety and insomnia. Drugs for treating these disorders function to increase GABAergic activity (see Chapter 31). Glutamate is also widely distributed in the brain and functions opposite to GABA; that is, it is primarily excitatory. Recently, antagonists of specific glutamate N-methyl-D-aspartate (NMDA) receptors have been introduced for the treatment of Alzheimer’s disease, although the underlying rationale is somewhat unclear (see Chapter 28).
A summary of major neurotransmitter pathways and the specific brain disorders in which they play important roles is presented in Table 27-3.
DRUG ACTION IN THE CENTRAL NERVOUS SYSTEM
As mentioned, drugs acting on the brain must be able to gain access to their targets. Because of its unique importance, the brain is “protected” by a specialized system of capillary endothelial cells known as the BBB. Unlike peripheral capillaries that allow relatively free exchange of substances between cells, the BBB limits transport through both physical (tight junctions) and metabolic (enzymes) barriers (see Fig. 2-6). The primary BBB is formed by firmly connected endothelial cells with tight junctions lining cerebral capillaries. The secondary BBB surrounds the cerebral capillaries and is composed of glial cells.
Although the action of many CNS-active drugs is based on their ability to cross the BBB, it may also be advantageous for a drug to be restricted from entering the brain. For example, l-DOPA, used for the treatment of Parkinson’s disease, must enter the brain to be effective. When administered alone, only 1% to 3% of the dose reaches the brain; the rest is metabolized by plasma DOPA decarboxylase to DA, which cannot cross the BBB. Thus l-DOPA is administered in combination with carbidopa, which inhibits DOPA decarboxylase and does not itself cross the BBB, thereby increasing the amount of l-DOPA available in the circulation to enter the brain (see Chapter 28).
Many drugs interact specifically with the macromolecules involved in the synthesis, storage, release, receptor interaction, and inactivation processes associated with particular neurotransmitters. The targets for these transmitter-specific drugs are expressed only by neurons synthesizing or responding to specific neurotransmitters; consequently, these drugs have more discrete and limited actions. The targets for transmitter-specific drugs can be any of the macromolecules involved in the life cycle of specific transmitter molecules (Fig. 27-13).
It is important to remember that all drugs have multiple actions. No drug causes only a single effect, because few, if any, drugs bind to only a single target. At higher concentrations most drugs can interact with a wide variety of molecules, often resulting in cellular alterations. Some drugs have potent actions on so many different processes in the CNS that it is difficult to identify their primary targets. Although some drugs may cause their therapeutic effects by combinations of specific actions, others may exert their primary effects through their interaction with a single cellular target. The window of selectivity of any particular drug will dramatically influence its incidence of unwanted side effects.
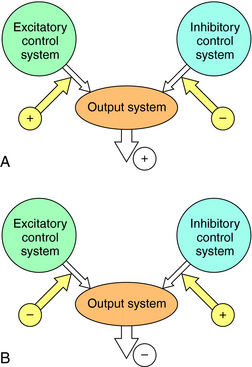
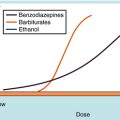
The activity of a tonically active neural network can be increased or decreased by excitatory or inhibitory control systems, respectively. This type of bidirectional regulation implies that the effect of a drug cannot be predicted solely on the basis of its effect on isolated neurons. A drug that reduces neuronal firing can activate a neural system by reducing a tonically active inhibitory input. Conversely, a drug that increases neuronal firing can inhibit a neural system by activating an inhibitory input (Fig. 27-14). Thus in some circumstances a “depressant” drug may cause excitation and a “stimulant” drug may cause sedation. A well-known example is the stimulant phase that is observed frequently after ingestion of ethanol (see Chapter 32), a general neuronal depressant. The initial stimulation is attributable to the depression of an inhibitory control system, which occurs only at low concentrations of ethanol. Higher concentrations cause a uniform depression of nerve activity. A similar “stage of excitement” can be observed during induction of general anesthesia, which is also caused by the removal of tonically active inhibitory control systems (see Chapter 35).
Adaptive mechanisms exist in all cells to control signaling. Adaptation can occur at several levels, predominantly at the receptors themselves. Two mechanisms are involved, sensitization and desensitization. As discussed in Chapter 1, sensitization is a process whereby a cell becomes more responsive to a given concentration of compound, whereas desensitization is a process whereby a cell becomes less responsive. Receptor sensitization and desensitization play a major role in the action of drugs in the CNS, in terms of both therapeutic effects and side effects induced.
Chronic activation of receptors, as occurs typically after long-term agonist administration, decreases the density of receptors in the postsynaptic cell membrane, whereas chronic decreases in synaptic activation, as a result of long-term antagonist administration, increases receptor density (see Fig. 1-16). Such changes occur slowly and are only slowly reversible, because increasing receptor density requires synthesis of new receptors, and reversing such an increase requires degrading these new receptors. Thus changing receptor density usually represents a long-term (days to weeks) adaptive response to changes in synaptic input.
Postsynaptic cells can also regulate the efficiency with which receptor activation is coupled to changes in cell physiology. These changes usually occur at the level of the coupling of a receptor to channel opening or second-messenger production and can be extremely rapid in onset. Often, a change in coupling efficiency results from increases or decreases in covalent modifications of the receptors, G proteins, channels, or enzymes responsible for signal transduction (see Chapter 1).
Adaptive responses to long-term drug administration are thought to underlie many desired therapeutic effects and unwanted side effects. For example, the therapeutic effects of antidepressants take several weeks to develop, corresponding to the time it takes for adrenergic and serotonergic receptor systems to adapt to the enhanced levels of the biogenic amines (see Chapter 30). Similarly, evidence suggests that antipsychotic-induced tardive dyskinesia may result from the up regulation of a subtype of DA receptors caused by chronic receptor antagonism (see Chapter 29).