Chapter 9 Introduction to the Autonomic Nervous System
| Abbreviations | |
|---|---|
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| ANS | Autonomic nervous system |
| cAMP | Cyclic adenosine monophosphate |
| CNS | Central nervous system |
| COMT | Catechol-O-methyltransferase |
| DA | Dopamine |
| DOPA | Dihydroxyphenylalanine |
| Epi | Epinephrine |
| GI | Gastrointestinal |
| MAO | Monoamine oxidase |
| NE | Norepinephrine |
| PNS | Peripheral nervous system |
| SA | Sinoatrial |
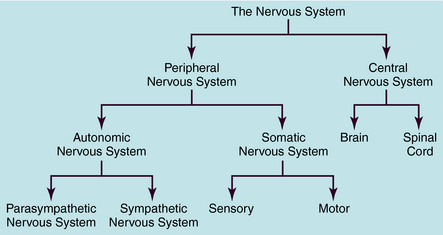
The human nervous system is the most complex of all systems in the body and is responsible for perceiving, processing, and transmitting information throughout the organism and generating responses to the information. The nervous system is divided into the peripheral nervous system (PNS) and the central nervous system (CNS) (Fig. 9-1). The PNS is subdivided into the autonomic nervous system (ANS), which controls automatic functioning, like breathing and heart rate, and the somatic nervous system, which receives sensory information and sends information to the CNS and from the CNS to skeletal muscles. The CNS is comprised of the brain and spinal cord and integrates and controls all bodily functions as well as thought processes. All these systems are interconnected and work together.
DIVISIONS OF THE AUTONOMIC NERVOUS SYSTEM
dilation of the pupil (mydriasis). In contrast, stimulation of the parasympathetic system conserves energy (“rest and digest”) and leads to responses characterized by decreased heart rate, blood pressure, and respiration; increased secretions; and constriction of the pupil (miosis).
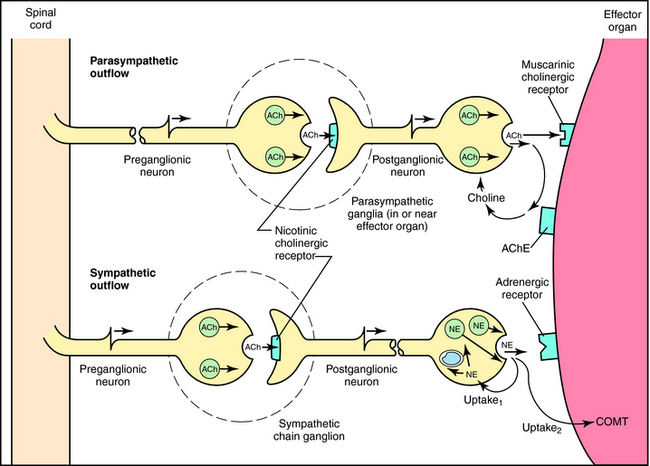
Although the parasympathetic and sympathetic systems differ both anatomically and functionally, they also share some features. The outflow of both divisions from the CNS consists of two neuron relays named after their anatomical location relative to the autonomic ganglia, or relay centers. Preganglionic neurons have their cell bodies in the spinal cord and the brainstem and their nerve terminals at autonomic ganglia, where they relay information to cell bodies of postganglionic neurons. Postganglionic neurons send their axons directly to effector organs (heart, blood vessels, visceral organs, and glands), where they relay information to these cells; these synapses are often referred to as neuroeffector junctions. Thus the preganglionic fibers of both the sympathetic and parasympathetic systems synapse with postganglionic fibers at autonomic ganglia. However, the location of the ganglia differs for the two systems, with parasympathetic ganglia located close to the organ innervated and most sympathetic ganglia located near the spinal cord. Due to the different ganglionic locations, the lengths of the preganglionic fibers relative to the postganglionic fibers also differ. Both sympathetic and parasympathetic preganglionic neurons release acetylcholine (ACh) as the neurotransmitter at ganglia. However, parasympathetic postganglionic neurons release ACh to relay their information at the neuroeffector junction, whereas most sympathetic postganglionic fibers release norepinephrine (NE, also called noradrenaline).
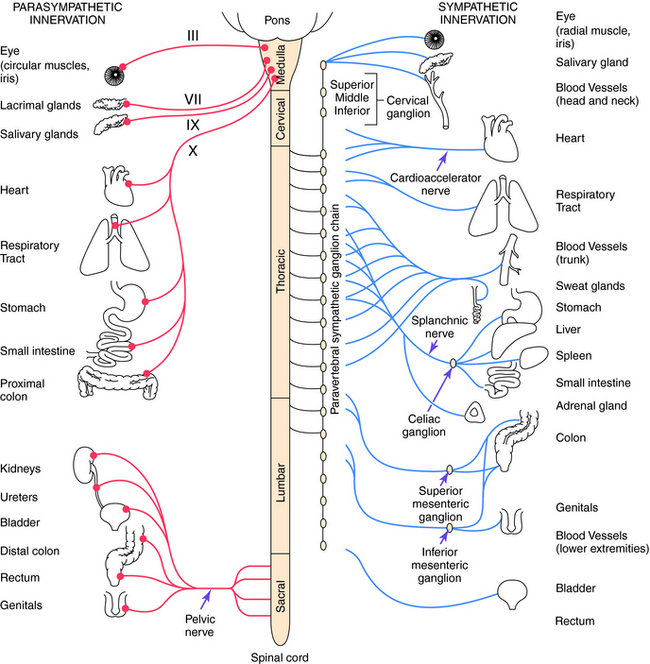
Parasympathetic and sympathetic neurons are defined anatomically, with parasympathetic neurons arising from the sacral region of the spinal cord and from the brainstem and sympathetic neurons arising from thoracic and lumbar regions of the spinal cord. They are not defined by the neurotransmitter released, and sympathetic fibers that innervate some sweat glands release ACh rather than NE. An anatomical representation of the sympathetic and parasympathetic systems is shown in Figure 9-2, and the commonalities and differences between the systems are summarized in Table 9-1.
TABLE 9–1 Comparison of the Parasympathetic and Sympathetic Divisions of the Autonomic Nervous System (ANS)

Parasympathetic Nervous System
Cell bodies giving rise to preganglionic parasympathetic nerves exit the CNS at cranial and sacral levels (see Fig. 9-2). The cranial portion of the parasympathetic outflow (cranial nerves III, VII, IX, and X) innervates structures in the head, neck, thorax, and abdomen. The sacral division of the parasympathetic nervous system forms the pelvic nerve and innervates the remainder of the GI tract and the pelvic viscera, including the bladder and reproductive organs.
Cell bodies for preganglionic sympathetic neurons originate in the intermediolateral cell column of the spinal cord at the thoracic and lumbar levels from T1 to L2 (see Fig. 9-2). Relatively short preganglionic, usually myelinated, neurons project to the sympathetic ganglia outside the spinal vertebrae. Most of these neurons synapse in the 22 segmentally arranged ganglia that form two chains located bilaterally adjacent to the spinal cord, and are often called the paravertebral chain. Postganglionic sympathetic neurons are generally unmyelinated and send long postganglionic fibers to their effector organs. Although most preganglionic sympathetic neurons synapse in the paravertebral sympathetic ganglia, several are prevertebral and lie near the bony vertebral column in the abdomen and pelvis (celiac, superior and inferior mesenteric, and aorticorenal), while a few (cervical ganglia and ganglia connected to urinary bladder and rectum) lie near the organs innervated.
Autonomic Regulation of Peripheral Organs
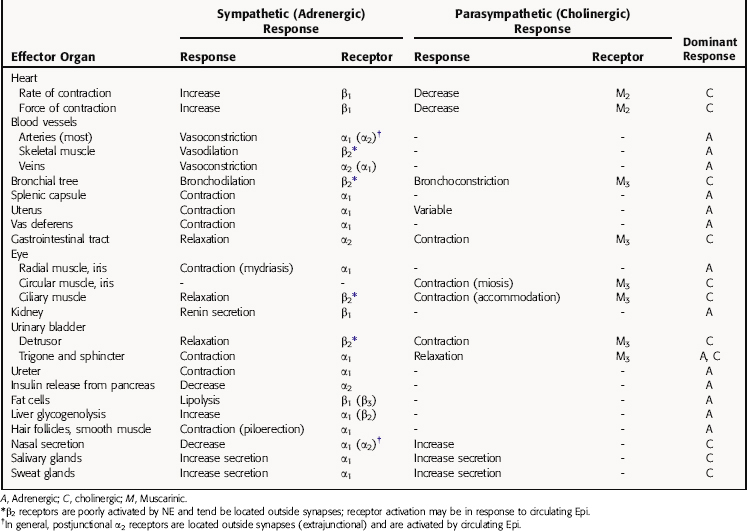
Both parasympathetic and sympathetic nerves innervate most organs of the body. Generally, these two branches of the ANS produce opposing responses in effector organs. There is generally a balance between sympathetic and parasympathetic effects on most organs, such that inhibition of one often leads to an increase in the response mediated by the other. However, there are some exceptions where the two systems cause similar responses. Some organs, such as the vasculature and spleen, receive only one type of innervation, which, in these cases, is sympathetic.
The importance of the dual innervation of most organs is evidenced by hypertension, which may involve an increase in sympathetic relative to parasympathetic control of the heart. Increased sympathetic effects can be produced by changes in neural firing rate, catecholamine concentrations at the neuroeffector junction or postjunctional receptors, or signal transduction pathways. Although there is support for each of these mechanisms, the first two are likely most important. Thus drugs that inhibit sympathetically mediated cardiovascular effects are useful for treating hypertension (see Chapters 11 and 12).
NEUROTRANSMISSION AND NEUROTRANSMITTERS IN THE AUTONOMIC NERVOUS SYSTEM
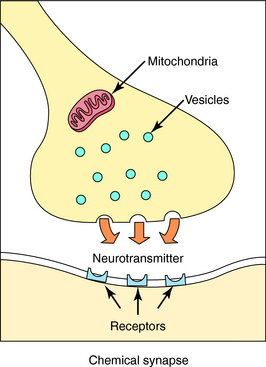
Neurotransmission is the process of effective transfer and integration of information in the nervous system. Neurotransmitters are endogenous substances released from nerve terminals, which act on receptors present on the membrane of postsynaptic cells. It is the interaction of the released neurotransmitter with the receptor that produces a functional change in the cell (Fig. 9-3).
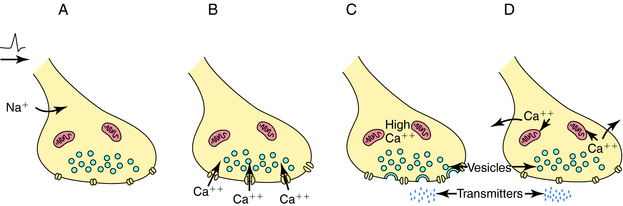
Depolarization of a presynaptic nerve terminal leads to release of a neurotransmitter into the extracellular fluid between the presynaptic and postsynaptic cells (the synaptic cleft). Calcium (Ca++) provides the essential link between depolarization and transmitter release. When a nerve terminal is depolarized, there is a large influx of Ca++ caused by opening of voltage-dependent Ca++ channels in the membrane. This influx promotes fusion of transmitter-containing synaptic vesicles with the plasma membrane resulting in exocytosis, which releases neurotransmitter into the synapse. After exocytosis, the voltage-dependent Ca++ channels inactivate rapidly, and the intracellular Ca++ concentration returns to normal by sequestration into intracellular compartments and active extrusion from the cell. The steps linking the arrival of an action potential to neurotransmitter release are summarized in Figure 9-4.
It is important to understand that voltage-dependent Ca++ channels in nerve terminals differ from those in other tissues. The Ca++ channel antagonists are an important class of drugs that block voltage-dependent Ca++ channels in cardiac and smooth muscle (see Chapter 20). However, there are distinct subtypes of these channels that can be distinguished by their electrical and pharmacological properties. The Ca++ channel antagonists block the channels most often found in cardiac and smooth muscle (L type) and have no effect on most of the voltage-dependent Ca++ channels found in nerve terminals (N type). This is fortunate, because if Ca++ channel antagonists also blocked neurotransmitter release, their toxicity would undoubtedly prevent them from being useful therapeutically.
After release, the neurotransmitter diffuses across the synaptic cleft to interact with specific receptors on the dendrites and cell body of the postganglionic neuron or on cells of the effector organ. The postsynaptic cell responds in an appropriate fashion to the received message. Thus the nerve terminal has mechanisms for storing and releasing neurotransmitters in response to depolarization, and the postsynaptic cell has receptors for detecting the presence and identity of different neurotransmitters and initiating appropriate changes in physiology or metabolism. Nerve terminals also have efficient mechanisms for the degradation and reutilization (reuptake) of neurotransmitters to ensure the rapid termination of arriving messages. These processes, neurotransmitter synthesis, storage, release, reuptake, and degradation, represent the sites of action of many drugs.
Neurotransmission at autonomic ganglia and neuroeffector junctions is illustrated in Figure 9-5. In both sympathetic and parasympathetic ganglia, preganglionic stimulation leads to the release of ACh, which activates postjunctional nicotinic acetylcholine receptors on postganglionic neurons to increase ion permeability, resulting in generation of an action potential that is propagated down the postganglionic nerve. When the action potential reaches the neuroeffector junction, either ACh or NE is released to activate muscarinic cholinergic or adrenergic receptors, respectively, on cells of the effector organ to produce an appropriate response.
Neurotransmitter Synthesis, Storage, and Inactivation
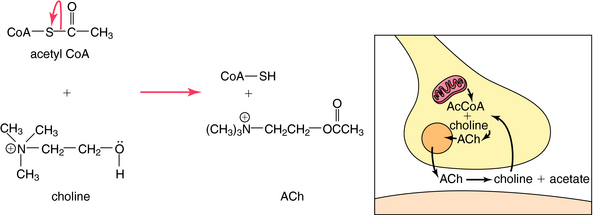
ACh is synthesized in cholinergic nerve terminals by the acetylation of choline, a process catalyzed by the enzyme choline acetyltransferase. As shown in Figure 9-6, acetyl coenzyme A provided by mitochondria serves as the acetyl donor; choline is provided by both high-affinity uptake after ACh hydrolysis and phospholipid hydrolysis within the neuron.
Norepinephrine and Epinephrine
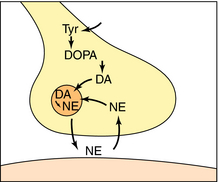
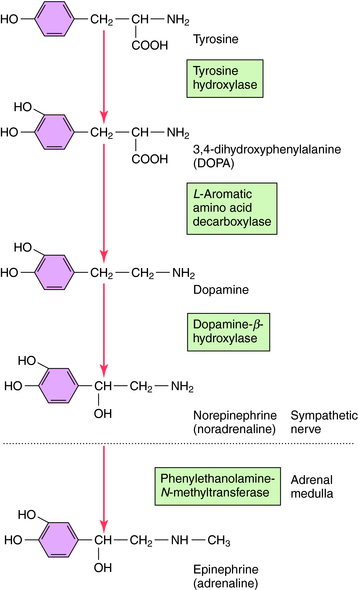
NE is synthesized in adrenergic nerve terminals by a series of enzymatic reactions beginning with the precursor tyrosine, as depicted schematically in Figure 9-7 and chemically in Figure 9-8. The first step, which takes place in the cytoplasm of postganglionic sympathetic nerve terminals, involves hydroxylation of tyrosine in the meta position by tyrosine hydroxylase to form the catechol derivative 3,4-dihydroxyphenylalanine (DOPA). Tyrosine hydroxylase is rate-limiting for the biosynthesis of all catecholamines including NE, Epi, and dopamine (DA). DOPA is subsequently decarboxylated by L-aromatic amino acid decarboxylase to form DA, which is actively taken up into storage vesicles in the sympathetic nerve terminals. Inside the vesicle, a hydroxyl group is added by dopamine-β-hydroxylase to form NE, which is retained in the vesicle associated with ATP until released by arrival of an action potential. Dopamine-β-hydroxylase is the last enzyme in the biosynthesis of NE in postganglionic sympathetic neurons, which release only NE. In the adrenal medulla and some neurons within the CNS, NE and Epi coexist. The synthesis of Epi from NE occurs by the action of phenylethanolamine-N-methyltransferase, which methylates NE. In adult humans, Epi constitutes approximately 80% of the catecholamines in the adrenal medulla, with NE making up the remainder.
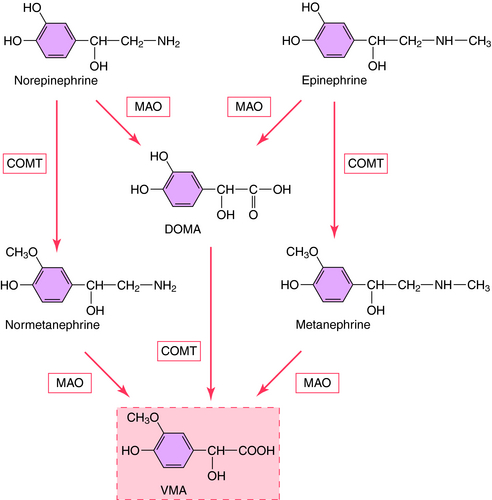
The action of NE is terminated by a combination of neuronal reuptake into the sympathetic nerve terminal by an energy-dependent pump (uptake1) and diffusion and uptake by an extraneuronal process (uptake2), as depicted in Figure 9-5. NE taken back up into sympathetic nerves may be oxidatively deaminated by the enzyme monoamine oxidase (MAO) present in mitochondria within nerve terminals, or it may be sequestered in vesicles for subsequent release. NE that diffuses to the extraneuronal uptake site may be inactivated by the enzyme catechol–O-methyltransferase (COMT). The metabolism of NE and Epi by MAO and COMT is depicted in Figure 9-9; numerous inactive metabolites are formed and excreted in blood and urine.
NEUROTRANSMITTER RECEPTORS IN THE AUTONOMIC NERVOUS SYSTEM
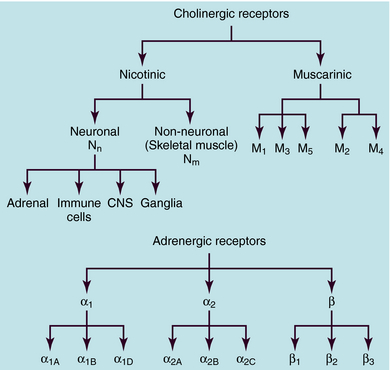
ACh and NE use different receptors to mediate their end-organ responses, and each neurotransmitter may interact with many distinct receptor subtypes (see Chapter 1). The many receptor subtypes are classified by pharmacological studies using selective agonists and antagonists and by their amino acid sequences. The genes that code for each of these receptors have now been cloned. It should be emphasized that the end-organ response is as much a function of the type of receptor present as of the neurotransmitter involved. Classification of cholinergic and adrenergic receptor subtypes is presented in Figure 9-10.
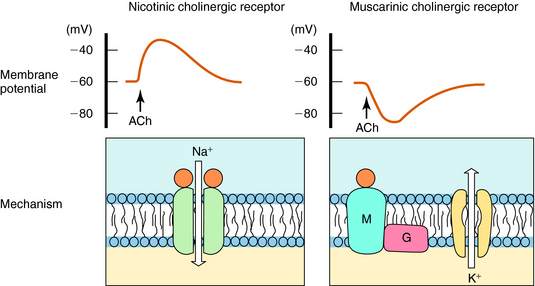
ACh produces different effects at various sites throughout the body, often resulting from differences in the cholinergic receptors involved. The actions of ACh are mimicked in certain organs, including autonomic ganglia, by the plant alkaloid nicotine, hence the name nicotinic. The response to ACh in effector organs is more closely mimicked by the plant alkaloid muscarine, leading to the name muscarinic. Thus responses to exogenous ACh or activation of the parasympathetic nervous system are described as being mediated by nicotinic or muscarinic receptors. Nicotinic receptors are ligand-gated ion channels, which, when activated by ACh, allow the influx of Na+ and Ca++. In contrast, muscarinic receptors are G-protein–coupled receptors which, when activated by ACh, activate G-proteins to induce downstream effects. These actions are illustrated schematically in Figure 9-11.
The primary actions of ACh at both parasympathetic and sympathetic ganglia are mediated by activation of ganglionic nicotinic cholinergic receptors. These receptors are similar structurally and functionally to the nicotinic receptors in the CNS and on immune cells but differ from nicotinic receptors in skeletal muscle at the neuromuscular junction. These different types of nicotinic receptors can be stimulated or blocked selectively by different agonists and antagonists. Drugs affecting ganglionic nicotinic receptors are discussed in Chapter 10, and agents that affect skeletal muscle nicotinic receptors are discussed in Chapter 12.
In general, M1 receptors are located in autonomic ganglia (where they modulate effects of nicotinic receptor activation), M2 receptors are located in the heart, and M3 receptors are located in many glands and smooth muscles. The locations of the M4 and M5 receptors are less certain, although all five muscarinic receptor subtypes are found in the CNS. Drugs affecting muscarinic receptors are discussed in Chapter 10.
Subsequent studies have shown that there are many adrenergic receptor subtypes (see Fig. 9-10). β adrenergic receptors can be subdivided into three subtypes, β1, β2, and β3, and selective agonists and antagonists have been developed with different potencies at these receptors. Stimulation of each β adrenergic receptor subtype leads to activation of Gs, an increase in cAMP, and activation of cAMP-dependent protein kinase, leading to the phosphorylation of various intracellular proteins.
Pharmacological studies further subdivided α adrenergic receptors into two major types, α1 and α2, each of which is now known to comprise three additional subtypes (see Fig. 9-10), a classification that has been confirmed by cloning and comparing genes for these proteins. Thus, there are a total of nine adrenergic receptors, subdivided into three subfamilies (α1, α2, and β), each of which contains three distinct subtypes encoded by separate genes. Amino acid sequences are very similar among the subtypes within a given subfamily.
FUNCTIONAL RESPONSES MEDIATED BY THE AUTONOMIC NERVOUS SYSTEM
The responses to parasympathetic and sympathetic stimulation that occur in many important organs of the body are presented in Table 9-2. In most instances sympathetic and parasympathetic nerves mediate physiologically opposing effects. Thus, if one system inhibits a certain function, the other enhances it. It is important to note that the responses listed represent only those mediated by stimulation of nerves in innervated tissues, and that activation of autonomic receptors located in tissues lacking nerve innervation can also lead to responses. For example, although vascular smooth muscle has no parasympathetic innervation, it expresses muscarinic cholinergic receptors, which are functional and mediate responses to exogenously administered drugs; they probably play little or no normal physiological role.
TABLE 9–2 Responses Elicited in Effector Organs by Stimulation of Sympathetic and Parasympathetic Nerves
Drugs Acting on Autonomic Nerves and Receptors
Drugs that alter the sympathetic and parasympathetic systems and autonomic ganglia are discussed in detail in Chapters 10 11. This introduction will briefly review the types of pharmacological interventions possible and provide a few examples of drugs that act by different mechanisms.
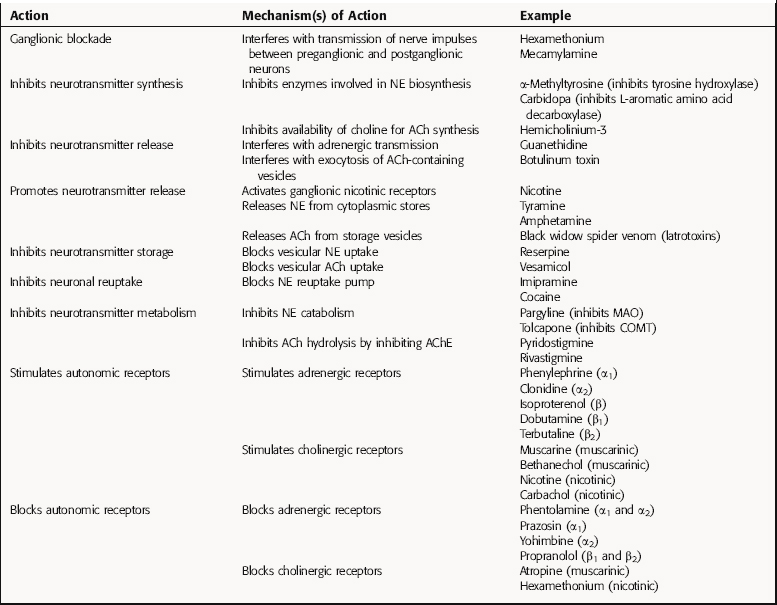
Drugs that block autonomic ganglia interfere with the transmission of nerve impulses from preganglionic nerve terminals to the cell bodies of postganglionic neurons. Because the neurotransmitter (ACh) and receptor (nicotinic) are identical in sympathetic and parasympathetic ganglia, ganglionic blockers inhibit both divisions of the ANS equally. However, the end-organ response may show a predominant adrenergic or cholinergic effect, because one system is generally dominant in a given organ (Table 9-3). Therefore interruption of ganglionic transmission selectively eliminates the dominant component, leading to a response characteristic of the less dominant component. For example, in the heart, the cholinergic system generally dominates at the level of the sinoatrial (SA) node. When a ganglionic blocker is administered, its greatest effect is on this cholinergic component, resulting in an apparent adrenergic effect (tachycardia). The classic ganglionic blockers are hexamethonium and mecamylamine (Table 9-4), which have limited clinical use because of their many actions.
TABLE 9–3 Effects of Ganglionic Blockade on Major Organ Systems
| Organ System | Predominant Tone | Effect of Ganglionic Blockade |
|---|---|---|
| Cardiovascular System | ||
| Atria; SA node | Parasympathetic | Tachycardia |
| Ventricle | Sympathetic | Reduced force of contraction |
| Blood vessels | Sympathetic | Vasodilation; decreased venous return; hypotension |
| Eye | ||
| Ciliary muscle | Parasympathetic | Focused for far vision |
| Iris | Parasympathetic | Mydriasis |
| Glands | ||
| Sweat | Sympathetic | Decreased sweat |
| Salivary | Parasympathetic | Dry mouth |
| Gastrointestinal Tract | ||
| Smooth muscle | Parasympathetic | Decreased contractions; constipation |
| Secretions | Parasympathetic | Decreased gastric and pancreatic secretions |
TABLE 9–4 Mechanisms of Action of Pharmacological Compounds Affecting the Autonomic Nervous System (ANS)
Drugs that Inhibit Neurotransmitter Synthesis
Several enzymes in the biosynthesis of NE and Epi from tyrosine may be inhibited. Tyrosine hydroxylase, the rate-limiting enzyme, is inhibited by α-methyltyrosine; l-aromatic amino acid decarboxylase is inhibited by carbidopa, which is used for the treatment of Parkinson’s disease (see Chapter 28); and α-methyldopa, which is also a substrate for the decarboxylase, converting it to α-methyl-DA and α-methyl-NE, the latter a selective α2 adrenergic receptor agonist. Fusaric acid is a selective inhibitor of dopamine-β-hydroxylase and significantly reduces NE concentrations. In the adrenal medulla, phenylethanolamine-N-methyltransferase catalyzes the formation of Epi and can be inhibited by agents such as 2,3-dichloro-α-methylbenzylamine.
Although ACh is synthesized by the acetylation of choline through the action choline acetyltransferase, no potent and specific inhibitors of this enzyme are available. However, the biosynthesis of ACh can be inhibited with hemicholinium-3, which blocks the high-affinity transport of choline that provides substrate for ACh synthesis. This results in depletion of ACh in cholinergic neurons.
Drugs that Affect the Duration of Action of Neurotransmitter
After the exocytotic release of NE from postganglionic sympathetic nerve terminals, most of the released catecholamine is actively reaccumulated in the nerve terminal by uptake1, a process that represents the primary mechanism terminating the action of NE. Agents such as imipramine and cocaine block this uptake pump, thereby increasing synaptic concentrations of NE and enhancing adrenergic neurotransmission. Enzymes involved in the metabolism of the catecholamines (see Fig. 9-9) may also be inhibited, resulting in higher concentrations of NE in peripheral tissues. MAO is inhibited by pargyline, a compound used for the treatment of major depression (see Chapter 30), and COMT is inhibited by tolcapone, an agent used for the treatment of Parkinson’s disease (see Chapter 28).
The action of ACh at synapses is terminated by its hydrolysis by the enzyme AChE. By inhibiting AChE, the hydrolysis of ACh is prevented, increasing the magnitude and duration of effects elicited by stimulation of cholinergic neurons. Numerous compounds inhibit AChE, including agents such as edrophonium and pyridostigmine, which are used for the diagnosis and treatment of myasthenia gravis (see Chapter 10), and donepezil and rivastigmine, which are used for the treatment of Alzheimer’s disease (see Chapter 28).
Drugs that Stimulate Autonomic Receptors
ACh activates all subtypes of muscarinic and nicotinic cholinergic receptors. Muscarinic receptors are stimulated selectively by the alkaloid muscarine or by synthetic agonists such as bethanechol. Nicotinic receptors are stimulated by the alkaloids nicotine and epibatidine and by the cholinomimetic carbachol (see Chapter 10).
New Horizons
With the sequencing of the human genome essentially complete, a major focus of research currently centers on defining polymorphisms in proteins involved in autonomic processes (enzymes, receptors, and transporters). Pharmacogenomic differences in both cholinergic and noradrenergic systems have been observed including both muscarinic and adrenergic receptor variants. Single nucleotide polymorphisms are associated with certain abnormal phenotypes (e.g., cardiovascular abnormalities). However, critical genetic determinants and their role in human pathophysiology and pharmacology remain to be established.
Eisenhofer G, Irwin J, Kopin IJ, et al. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331-349.
Kirstein SL, Insel PA. Autonomic nervous system pharmacogenomics: A progress report. Pharmacol Rev. 2004;56(1):31-52.
Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol. 2003;43:381-411.
Wess J. Allosteric binding sites on muscarinic acetylcholine receptors. Mol Pharmacol. 2005;68(6):1506-1509.
Zimmerman G, Soreq H. Termination and beyond: Acetylcholinesterase as a modulator of synaptic transmission. Cell Tissue Res. 2006;326(2):655-669.