CHAPTER 226 Intrathecal Baclofen Therapy for Cerebral Palsy
Baclofen, chlorophenyl, cyclohexyl γ-aminobutyric acid, is a GABAB agonist originally synthesized for evaluation as an anticonvulsant. It was found to have no significant anticonvulsant activity but did diminish spasticity and was given orally for perhaps 3 decades before it was administered intrathecally. The first intrathecal baclofen (ITB) administration in adults was reported by Penn and Kroin in 1984.1 The first intrathecal administration in a child was reported the following year, when Dralle and colleagues used it to treat a 4-year-old with hypertonia after near drowning.2 The first reported use of ITB in patients with cerebral palsy (CP) was probably in 1991 in a double-blind controlled trial in which it was shown that spasticity in the lower extremities was significantly decreased 4 hours after bolus lumbar injections and remained decreased for 8 hours afterward.3 In 1992, Müller reported the results of a European multicenter study in which continuous ITB infusion was shown to improve spasticity in approximately 20 children.4 Since then, multiple publications have confirmed the effectiveness of ITB for treating spasticity in individuals with CP, predominantly children but younger adults as well.
The half-life of an ITB bolus is 4 to 5 hours, so approximately 24 hours is required to achieve a steady-state concentration after a change in infusion dosage.5 Virtually no baclofen infused intrathecally is detectable in the systemic circulation; baclofen infusions of 77 to 400 µg/day were associated with serum concentrations that were at or below the level of quantification, 10 ng/mL.6 ITB’s site of action in treating spasticity appears to be in superficial layers of the spinal cord (Rexed layers I, II), where it in essence replaces deficient GABA that is not released by descending inhibitory axons. ITB’s site of action in treating dystonia is less clear, but it may be at a cortical level, where it inhibits the premotor and supplementary motor cortex, regions that are excessively stimulated in patients with dystonia. The rationale for thinking that the site of action is cortical is based on clinical observations: (1) in dystonia, bolus ITB doses often cause no appreciable change within 4 hours (changes that are seen when treating spasticity); (2) continuous ITB infusions often take 24 to 48 hours to decrease dystonia, long enough for baclofen to ascend and enter cerebrospinal (CSF) over the cerebral convexities; and (3) higher catheter tips, which should result in higher intracranial CSF baclofen levels, are associated with a greater reduction in dystonia scores than those seen with lower catheter placement.
Patient Selection
Candidates for ITB usually have spastic quadriparesis with mean Ashworth scores of 3 or greater in the upper and lower extremities (Table 226-1). Most have had no significant improvement with oral medications and botulinum toxin injections, but ITB is occasionally appropriate for children with severe spastic quadriparesis who have had no previous treatments because the likelihood of sufficiently improving severe spasticity with oral medications is low. Their spasticity is either impeding caregiving, causing progressive contractures, interfering with function, or causing pain. ITB is used most often in children who weigh 15 kg or more and are 4 years or older, but with the smaller pumps that are now available, there are virtually no age or size limitations. Palisano and coworkers developed the Gross Motor Function Classification System (GMFCS) to broadly categorize levels of disability of children with CP.7 Most children who are candidates for ITB are GMFCS level IV or V, occasionally level III, and rarely level II.
| SCALE | FINDING |
|---|---|
| Ashworth Scale | |
| 1 | Normal muscle tone |
| 2 | Slight increase in resistance “Catch” with movement |
| 3 | Moderately increased resistance |
| 4 | Markedly increased resistance |
| 5 | No range of motion |
| Modified Ashworth Scale | |
| 0 | Normal muscle tone |
| 1 | Slight increase in resistance “Catch” with movement |
| 1+ | Catch, plus minimal resistance through a range of motion |
| 2 | More marked increase but limb easily flexed |
| 3 | Considerable increase in tone through range of motion |
| 4 | Affected part rigid |
Screening
If a screening trial is done, however, measurement of opening pressure may be helpful because the pressure is often abnormally increased, and in such cases the risk for CSF leakage is enhanced when the baclofen pump catheter is inserted.8 Alternatively, it is reasonable to perform a quick brain magnetic resonance imaging or computed tomographic scan of the head to see whether ventriculomegaly is present; if it is, insertion of an intracranial pressure monitor for 24 hours will provide a better evaluation of intracranial pressure than a single pressure measurement via spinal tap. If pressures are abnormally high, the likelihood of a CSF leak with the baclofen catheter can be diminished either by inserting a ventriculoperitoneal shunt before pump implantation or by inserting the intrathecal catheter with open techniques (laminotomy and purse-string suture around the entry site).
If improving gait is a therapeutic goal, the effects of ITB on gait can be evaluated before pump placement with an intrathecal catheter connected to an external microinfusion pump. Bleyenheuft and coworkers evaluated gait with continuous infusions of baclofen, 45 to 150 µg/day, in nine patients whose gait was deteriorating.9 Gait improved in seven of nine as evaluated on the Observation Gait Scale, and the improvement was present after pump implantation.
Pump and Catheter Implantation
Operations to implant a baclofen pump in patients with CP are done under general anesthesia and usually last 1 to 1.5 hours.10 Programmable pumps are needed when treating patients with spasticity of cerebral origin because of the frequent need to adjust the ITB dosage. At present, the only commercially available programmable pump is the Medtronic SynchroMed pump, a battery-powered pump with a 7-year battery life. The pump is usually implanted in the abdominal wall, subfascially in most children and thin patients and on the fascia in patients with more subcutaneous fat. In patients with no abdominal site for pump implantation (e.g., because of multiple scars, ostomy sites), the infraclavicular region is an alternative site. The baclofen catheter is connected to the pump and tunneled posteriorly to the lumbar region, where it is inserted via a Tuohy needle into the thecal sac and advanced cephalad to a site that depends on the distribution of the patient’s spasticity: T10-11 for patients with spastic diplegia and C7-T4 for those with spastic quadriparesis. For patients with spine fusions or anticipated spine fusions, catheters can be tunneled obliquely cephalad and inserted into the thecal sac via a small laminectomy at C6-7 above the spine fusion, which usually extends from T1-2 to the sacrum (Fig. 226-1).
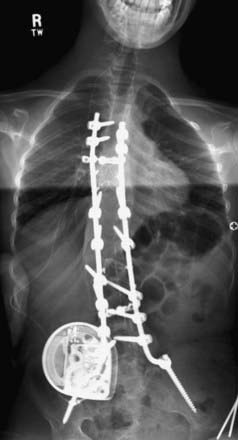
FIGURE 226-1 Baclofen pump and the catheter tunneled to enter the thecal sac above the spine fusion.
The initial reports of ITB for the treatment of spinal spasticity associated with spinal cord injury or multiple sclerosis positioned the catheter tip in the T10-12 region. However, Kroin and associates subsequently demonstrated that the concentration of radioisotope administered in the lumbar region was 4 times higher than the concentration at the craniovertebral junction.11 To increase the baclofen concentrations in the cervical region and obtain better reductions in upper extremity spasticity, catheter tips are now positioned higher when treating spastic quadriparesis. Grabb and coauthors reported that higher catheter positions were associated with a better reduction in upper extremity spasticity than was observed with lower catheters.12 Cervical catheter tip positions do not result in increased complications or adverse side effects and are associated with significantly decreased spasticity in both the upper and lower extremities.
Outcomes of Intrathecal Baclofen in Patients with Spastic Cerebral Palsy
The efficacy of ITB in reducing spasticity in children and young adults with CP has been documented in multiple publications from both single institutions and multicenter trials.13,14 Butler and Campbell reviewed the 12 publications on ITB that had been published by 2000 and concluded that spasticity is improved and function is probably improved.15 Spasticity is significantly decreased in the lower and upper extremities and remains decreased during the years of infusion, probably indefinitely; a multicenter study in 2003 reported sustained decreases in Ashworth scores in the lower and upper extremities during 6 years of follow-up.14 Although the reduction in spasticity in the upper extremities is typically less than that in the lower extremities (if the catheters tips are at the midthoracic level or lower), Motta and colleagues evaluated the effect of ITB on the upper extremities of 20 patients with quadriparetic CP and a mean age of 11.4 years with the Melbourne Assessment of Unilateral Upper Limb Function scale.16 Upper extremity spasticity was decreased, P < .05, and range of movement and target accuracy improved significantly.
ITB is associated with many other effects besides decreased spasticity. The effect of ITB on function has been evaluated with the Gross Motor Function Measure (GMFM). Krach and coworkers determined GMFM scores in 31 subjects 4 to 29 years old, most of whom had CP, before ITB and 1 year afterward and found that GMFM scores increased 3.7 to 4.1 points in all age groups; the improvements occurred mainly in the domains of transfers, dressing, and rolling.17 GMFM scores improved in patients who were at GMFCS level II (6.2) and level V (2.9). This group also questioned 100 patients and caregivers after ITB therapy for 1 year. They reported that positioning improved in 60%, transfers in 58%, dressing in 69%, sleep in 43%, and comfort in 53%; 88% indicated that they would undergo the surgery again despite the fact that 32 complications occurred in 22 subjects.
The effect of ITB on caregiving was evaluated with a caregiver questionnaire by Gooch and colleagues in 80 patients who had received ITB therapy for 1 year.18 The mean age of the patients was 11 years, and all were GMFCS level IV or V (severely motor impaired). Caregivers selected the treatment goals before therapy; the goals of decreased pain and prevention of worsening deformity were achieved in 91% of the patients, and easier caregiving was achieved in 88%.
The effect of ITB on oral motor function, communication, and nutritional status was evaluated by Bjornson and coworkers in 30 children.19 Speech improved in 10 (including 5 at GMFCS level V), worsened in 2, and was unchanged in the remainder. Use of assistive technology improved in 6, including 5 at GMFCS level V, for which communication is perhaps the most important function that can be improved. In a single case study, Leary and associates found that speech intelligibility was improved after ITB therapy as evaluated by the Assessment of Intelligibility of Dysarthric Speech Scale.20
The reduction in spasticity with ITB is associated with increased range of motion in the lower extremities and may be accompanied by improved gait. Gerstzen and colleagues graded gait on four functional levels (community, household, nonfunctional, and nonambulatory) in 24 patients with some ambulation before ITB therapy.21 The level of ambulation improved by one level in 9, worsened in 3, and was unchanged in the remainder. Hip subluxation, which is related to adductor spasticity and is common in children with CP, appears to be diminished by ITB therapy. Subluxation in 33 children who received ITB for 1 year was either improved (in 5%) or unchanged (in 91%).22
Intrathecal Baclofen for Dystonic Cerebral Palsy
Dystonic CP can be either focal, hemidystonic, or generalized; the most common form by far is generalized. ITB can be used for either generalized dystonia or hemidystonia but is rarely used for focal dystonia. ITB is probably the treatment of choice for severe, generalized secondary dystonia. If patients do not respond adequately to ITB, deep brain stimulation can be considered. The effects of ITB on dystonia can be graded with either the Barry-Albright Dystonia (BAD) scale, a validated scale that was developed to evaluate secondary dystonia, or the Burke-Fahn-Marsden (BFM) scale, a validated scale that was developed for adults with primary dystonia.23,24
Given the postulate that the site of action of ITB when treating dystonia is at the cortical level, catheter tips are often positioned higher, such as at C1-4, than when treating spasticity. Infusion at these levels is not associated with lethargy or respiratory depression. Recently, intraventricular baclofen infusion has been described to treat dystonic CP, particularly in patients with spine fusions or cervical anatomy that make intrathecal implantation difficult.25 Dystonia may respond to intraventricular baclofen at lower doses than needed with intrathecal administration, but insufficient data are available to confirm this observation.
The effects of ITB on children and young adults with severe, generalized dystonic CP were described in a large single-institution study.26 Dystonia scores on the BAD scale decreased significantly within 3 months after ITB therapy began, and the decreases continued during 36 months of follow-up. In that study, ITB infusions through catheters positioned at T6 or higher were associated with BAD scores that were significantly lower (4.9 ± 4.1) than those achieved with catheters positioned at T8 or lower (10.4 ± 7.0). Continued improvement in secondary dystonia with ITB has been observed for up to 11 years. Motta and coauthors recently reported 19 patients with dystonic CP, a mean age of 8.5 years, and GMFCS level V who were treated with ITB and evaluated with both the BAD and BFM scales.27 Dystonia scores on both scales were significantly improved at 3 and 12 months after ITB therapy, and significant improvement occurred in posture and caregiving.
Complications of Intrathecal Baclofen for Cerebral Palsy
ITB therapy is associated with frequent complications. Gooch and coworkers reported the complications that occurred in 100 children, primarily with CP, who had undergone 117 pump operations.28 Twenty-four children had 48 complications, most commonly catheter-related problems. Motta and coauthors reported that complications occurred in 31% of 200 patients treated with ITB: 11% had CSF leaks, 7% had catheter problems, 7.5% had infections, and 5.5% had more than one complication.29 Ashworth scores of 3 or greater and age younger than 10 years were associated with an increased risk for complications. Despite the frequent complications that are reported in virtually every series, approximately 90% of individuals with baclofen pumps at the end of battery life prefer to have a new pump implanted and to continue ITB therapy.30
CSF leaks occur after baclofen catheter insertion in 5% to 15% of patients with CP (most of whom are children), in contrast to the 3% leak rate reported in adults.31 The difference is probably attributable both to the smaller size, thinner tissues, and malnutrition of chronically disabled children and to the presence of higher CSF pressures associated with occult hydrocephalus. Postoperative CSF leaks can be manifested as symptoms of low pressure (postural headache and nausea), accumulation of CSF under the lumbar or abdominal incisions, or both. Mild leaks may respond to bed rest; others require injection of epidural blood at the catheter entry site (blood patches), insertion of lumbar drains, or rarely, open exploration and insertion of a purse-string suture and fibrin glue around the catheter exit site.
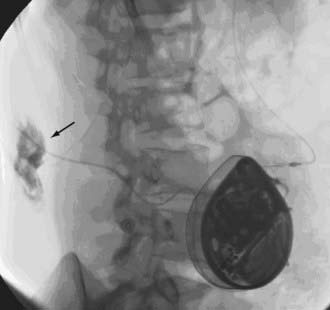
As catheter technology has improved, the frequency of catheter malfunctions has decreased from 25% to about 5% to 10%. Malfunctions include disconnection of the catheter from the pump or a leak at the site of catheter connection to the pump, development of a leak through the catheter wall, migration of the catheter out of the thecal sac, and migration of the catheter tip through the arachnoid so that it lies in—and infuses into—the subdural space. Catheter malfunctions can be evaluated by aspirating CSF through the catheter via the pump side port, by injecting contrast material through the catheter and observing its passage with fluoroscopy (Fig. 226-2), by computed tomography around the catheter tip after the instillation of contrast medium, and by injection of radioisotope into the pump to identify small leaks that may not be visible after bolus injection of contrast material.
The relationship between ITB and scoliosis was recently clarified. Although several small series reported previously that ITB caused more rapid progression of scoliosis than was observed before pump implantation, those series had no controls. Severan and associates evaluated 107 children treated with ITB for 2 or more years and found that progression of scoliosis developed or was present in 26 of them.32 These 26 patients were matched by age, gender, and GMFCS level with 26 controls who did not receive ITB therapy. Average curve progression after ITB was 16.3 degrees per year, and in the control group it was 16.1 degrees per year. Scoliosis developed in 21% of 57 patients after pump implantation during 3.6 years of follow-up and in 32% of control patients by the time of maturity.
The effect of ITB on seizures has also been clarified. Authors of small series had reported either increased seizure frequency or no change in frequency. In a substantive study, Bounaguro and colleagues evaluated seizures in 150 children undergoing ITB therapy, 40% of whom had seizures before ITB.33 After ITB therapy, seizures decreased in 13% and increased in 2% of the children who had seizures before ITB. In children who had no seizures before ITB, only 1 child had a first seizure after ITB.
Abrupt cessation of ITB infusion is followed within a few hours by symptoms of withdrawal, including generalized itching, increased spasticity, and agitation.34 Symptoms range in severity from mild to severe. Mild withdrawal may not be recognized by the patient and the interruption in therapy may go undetected for several months. Severe withdrawal is a serious condition and must be treated urgently. In its extreme form it is associated with fever (106°F), rhabdomyolysis, sometimes multisystem organ failure, and rarely death. Moderate withdrawal is treated with high doses of oral baclofen (e.g., 10 to 20 mg every 4 hours) and may be supplemented with intravenous benzodiazepines (diazepam, 2 to 5 mg every 6 hours) or cyproheptadine (6 mg every 6 hours for 24 hours).35,36 Bolus baclofen injections via lumbar puncture provide additional improvement until the malfunction can be corrected surgically. Withdrawal is most often the result of malfunction of the catheter, such as disconnection of the catheter from the pump, development of a leak in the catheter, or migration of the catheter out of the thecal sac.
Albright AL. Intraventricular baclofen infusion for dystonia. Report of two cases. J Neurosurg. 2006;105:101-104.
Albright AL, Barron WB, Fasick MP, et al. Continuous intrathecal baclofen infusion for spasticity of cerebral origin. JAMA. 1993;270:2475-2477.
Albright AL, Barry MJ, Shafron DH, et al. Intrathecal baclofen for generalized dystonia. Dev Med Child Neurol. 2001;43:652-657.
Albright AL, Cervi A, Singletary J. Intrathecal baclofen for spasticity in cerebral palsy. JAMA. 1991;265:1418-1422.
Albright AL, Ferson S, Carlos S. Occult hydrocephalus in children with cerebral palsy. Neurosurgery. 2005;56:93-96.
Albright AL, Gilmartin R, Swift D, et al. Long-term intrathecal baclofen therapy for severe spasticity of cerebral origin. J Neurosurg. 2003;98:291-295.
Albright AL, Shultz BL. Plasma baclofen levels in children receiving continuous intrathecal baclofen infusion. J Child Neurol. 1999;14:408-409.
Albright AL, Turner M, Pattissapu J. “Best practice” surgical techniques for intrathecal baclofen therapy. J Neurosurg. 2006;104:233-239.
Barry MJ, Van Swearingen JM, Albright AL. Reliability and responsiveness of the Barry-Albright Dystonia Scale. Dev Med Child Neurol. 1999;41:404-411.
Bjornson KF, McLaughlin JF, Loeser JD, et al. Oral motor, communication and nutritional status of children during intrathecal baclofen therapy: a descriptive pilot study. Arch Phys Med Rehabil. 2003;84:500-506.
Bleyenheuft C, Filipetti P, Caldas C, et al. Experience with external pump trial prior to implantation for intrathecal baclofen in ambulatory patients with spastic cerebral palsy. Neurophysiol Clin. 2007;37:23-28.
Bounaguro V, Scelsa B, Curci D, et al. Epilepsy and intrathecal baclofen therapy in children with cerebral palsy. Pediatr Neurol. 2005;33:110-113.
Burke RE, Fahn S, Marsden CD. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35:73-77.
Butler C, Campbell S. Evidence of the effects of intrathecal baclofen for spastic and dystonic cerebral palsy. Dev Med Child Neurol. 2000;42:634-645.
Coffey RJ, Cahill D, Steers W, et al. Intrathecal baclofen for intractable spasticity of spinal origin; results of a long-term multicenter study. J Neurosurg. 1993;78:226-232.
Cruikshank M, Eunson P. Intravenous diazepam infusion in the management of planned intrathecal baclofen withdrawal. Dev Med Child Neurol. 2007;49:626-628.
Dralle D, Muller H, Zierski J, et al. Intrathecal baclofen for spasticity. Lancet. 1985;2:1003.
Gerszten PC, Albright AL, Barry MJ. Effect on ambulation of continuous intrathecal baclofen infusion. Pediatr Neurosurg. 1997;27:40-44.
Gooch JL, Obertg WA, Grams B, et al. Care provider assessment of intrathecal baclofen in children. Pediatr Neurosurg. 2003;39:1-6.
Gooch JL, Overg WA, Grams B. Complications of intrathecal baclofen pumps in children. Pediatr Neurosurg. 2003;39:1-6.
Grabb PA, Guin-Renfroe S, Meythaler JM. Midthoracic catheter tip placement for intrathecal baclofen administration in children with quadriparetic spasticity. Neurosurgery. 1999;45:833-837.
Krach LE, Kriel RL, Gilmartin RC, et al. GMFM 1 year after continuous intrathecal baclofen infusion. Pediatr Rehabil. 2005;8:207-213.
Krach LE, Kriel RL, Gilmartin RC, et al. Hip status in cerebral palsy after one year of continuous intrathecal baclofen infusion. Pediatr Neurol. 2004;30:163-168.
Krach LE, Nettleton A, Klempka B. Satisfaction of individuals treated long-term with continuous infusion of intrathecal baclofen by implanted programmable pump. Pediatr Rehabil. 2006;9:210-218.
Kroin JS, Ali A, York M, et al. The distribution of medication along the spinal cord after chronic intrathecal administration. Neurosurgery. 1993;33:226-230.
Leary SM, Gilpin P, Lockley L, et al. Intrathecal baclofen therapy improves functional intelligibility of speech in cerebral palsy. Clin Rehabil. 2006;20:228-231.
Motta F, Buonaguro V, Stignani C. The use of intrathecal baclofen pump implants in children and adolescents: safety and complications in 200 consecutive cases. J Neurosurg. 2007;107:32-35.
Motta F, Stignani C, Antonello CE. Effect of intrathecal baclofen on dystonia in children with cerebral palsy and the use of functional scales. J Pediatr Orthop. 2008;28:213-217.
Motta F, Stignani C, Antonello CE. Upper limb function after intrathecal baclofen treatment in children with cerebral palsy. J Pediatr Orthop. 2008;28:91-96.
Müller H. Treatment of severe spasticity: results of a multicenter trial conducted in Germany involving the intrathecal infusion of baclofen by an implantable drug delivery system. Dev Med Child Neurol. 1992;34:739-745.
Müller H, Zierski J, Dralle D, et al. Pharmacokinetics of intrathecal baclofen. In: Müller H, Zierski J, Penn RD, editors. Local-Spinal Therapy of Spasticity. New York: Springer-Verlag; 1988:223-226.
Palisano R, Rosenbaum P, Russell D, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214-223.
Penn RD, Kroin JS. Intrathecal baclofen alleviates spinal cord spasticity. Lancet. 1984;1:1078.
Saveika JA, Shelton JE. Cyproheptadine for pediatric intrathecal baclofen withdrawal: a case report. Am J Phys Med Rehabil. 2007;86:994-997.
Severan H, Shah SA, Presedo A. The risk of progression of scoliosis in cerebral palsy patients after intrathecal baclofen. Spine. 2007;32:2348-2354.
Zuckerbraun NS, Ferson SS, Albright AL, et al. Intrathecal baclofen withdrawal: emergent recognition and management. Pediatr Emerg Care. 20, 2004. 759-564
1 Penn RD, Kroin JS. Intrathecal baclofen alleviates spinal cord spasticity. Lancet. 1984;1:1078.
2 Dralle D, Muller H, Zierski J, et al. Intrathecal baclofen for spasticity. Lancet. 1985;2:1003.
3 Albright AL, Cervi A, Singletary J. Intrathecal baclofen for spasticity in cerebral palsy. JAMA. 1991;265:1418-1422.
4 Müller H. Treatment of severe spasticity: results of a multicenter trial conducted in Germany involving the intrathecal infusion of baclofen by an implantable drug delivery system. Dev Med Child Neurol. 1992;34:739. 745
5 Müller H, Zierski J, Dralle D, et al. Pharmacokinetics of intrathecal baclofen. In: Müller H, Zierski J, Penn RD, editors. Local-Spinal Therapy of Spasticity. New York: Springer-Verlag; 1988:223-226.
6 Albright AL, Shultz BL. Plasma baclofen levels in children receiving continuous intrathecal baclofen infusion. J Child Neurol. 1999;14:408-409.
7 Palisano R, Rosenbaum P, Russell D, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214-223.
8 Albright AL, Ferson S, Carlos S. Occult hydrocephalus in children with cerebral palsy. Neurosurgery. 2005;56:93-96.
9 Bleyenheuft C, Filipetti P, Caldas C, et al. Experience with external pump trial prior to implantation for intrathecal baclofen in ambulatory patients with spastic cerebral palsy. Neurophysiol Clin. 2007;37:23-28.
10 Albright AL, Turner M, Pattissapu J. “Best practice” surgical techniques for intrathecal baclofen therapy. J Neurosurg. 2006;104:233-239.
11 Kroin JS, Ali A, York M, et al. The distribution of medication along the spinal cord after chronic intrathecal administration. Neurosurgery. 1993;33:226-230.
12 Grabb PA, Guin-Renfroe S, Meythaler JM. Midthoracic catheter tip placement for intrathecal baclofen administration in children with quadriparetic spasticity. Neurosurgery. 1999;45:833-837.
13 Albright AL, Barron WB, Fasick MP, et al. Continuous intrathecal baclofen infusion for spasticity of cerebral origin. JAMA. 1993;270:2475-2477.
14 Albright AL, Gilmartin R, Swift D, et al. Long-term intrathecal baclofen therapy for severe spasticity of cerebral origin. J Neurosurg. 2003;98:291-295.
15 Butler C, Campbell S. Evidence of the effects of intrathecal baclofen for spastic and dystonic cerebral palsy. Dev Med Child Neurol. 2000;42:634-645.
16 Motta F, Stignani C, Antonello CE. Upper limb function after intrathecal baclofen treatment in children with cerebral palsy. J Pediatr Orthop. 2008;28:91-96.
17 Krach LE, Kriel RL, Gilmartin RC, et al. GMFM 1 year after continuous intrathecal baclofen infusion. Pediatr Rehabil. 2005;8:207-213.
18 Gooch JL, Obertg WA, Grams B, et al. Care provider assessment of intrathecal baclofen in children. Pediatr Neurosurg. 2003;39:1-6.
19 Bjornson KF, McLaughlin JF, Loeser JD, et al. Oral motor, communication and nutritional status of children during intrathecal baclofen therapy: a descriptive pilot study. Arch Phys Med Rehabil. 2003;84:500-506.
20 Leary SM, Gilpin P, Lockley L, et al. Intrathecal baclofen therapy improves functional intelligibility of speech in cerebral palsy. Clin Rehabil. 2006;20:228-231.
21 Gerszten PC, Albright AL, Barry MJ. Effect on ambulation of continuous intrathecal baclofen infusion. Pediatr Neurosurg. 1997;27:40-44.
22 Krach LE, Kriel RL, Gilmartin RC, et al. Hip status in cerebral palsy after one year of continuous intrathecal baclofen infusion. Pediatr Neurol. 2004;30:163-168.
23 Barry MJ, Van Swearingen JM, Albright AL. Reliability and responsiveness of the Barry-Albright Dystonia Scale. Dev Med Child Neurol. 1999;41:404-411.
24 Burke RE, Fahn S, Marsden CD. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35:73-77.
25 Albright AL. Intraventricular baclofen infusion for dystonia. Report of two cases. J Neurosurg. 2006;105:101-104.
26 Albright AL, Barry MJ, Shafron DH, et al. Intrathecal baclofen for generalized dystonia. Dev Med Child Neurol. 2001;43:652-657.
27 Motta F, Stignani C, Antonello CE. Effect of intrathecal baclofen on dystonia in children with cerebral palsy and the use of functional scales. J Pediatr Orthop. 2008;28:213-217.
28 Gooch JL, Overg WA, Grams B. Complications of intrathecal baclofen pumps in children. Pediatr Neurosurg. 2003;39:1-6.
29 Motta F, Buonaguro V, Stignani C. The use of intrathecal baclofen pump implants in children and adolescents: safety and complications in 200 consecutive cases. J Neurosurg. 2007;107:32-35.
30 Krach LE, Nettleton A, Klempka B. Satisfaction of individuals treated long-term with continuous infusion of intrathecal baclofen by implanted programmable pump. Pediatr Rehabil. 2006;9:210-218.
31 Coffey RJ, Cahill D, Steers W, et al. Intrathecal baclofen for intractable spasticity of spinal origin; results of a long-term multicenter study. J Neurosurg. 1993;78:226-232.
32 Severan H, Shah SA, Presedo A. The risk of progression of scoliosis in cerebral palsy patients after intrathecal baclofen. Spine. 2007;32:2348-2354.
33 Bounaguro V, Scelsa B, Curci D, et al. Epilepsy and intrathecal baclofen therapy in children with cerebral palsy. Pediatr Neurol. 2005;33:110-113.
34 Zuckerbraun NS, Ferson SS, Albright AL, et al. Intrathecal baclofen withdrawal: emergent recognition and management. Pediatr Emerg Care. 20, 2004. 759-564
35 Cruikshank M, Eunson P. Intravenous diazepam infusion in the management of planned intrathecal baclofen withdrawal. Dev Med Child Neurol. 2007;49:626-628.
36 Saveika JA, Shelton JE. Cyproheptadine for pediatric intrathecal baclofen withdrawal: a case report. Am J Phys Med Rehabil. 2007;86:994-997.